Abstract
Melatonin is a potent scavenger of reactive oxygen (ROS) and reactive nitrogen species (RNS). At pharmacological concentrations, however, melatonin is documented to cause ROS/RNS production, especially in cultured cancerous cells. Currently, the mechanism responsible for melatonin-induced ROS generation remains elusive. In this study, we provide evidence that melatonin, at micromolar concentrations induced rapid ROS generation by a mitochondrial dependent mechanism in primary human mesangial (HM) cells. The melatonin-induced ROS production occurred independent of changes in Ca2+ concentrations in the cytosol and/or in mitochondria. In mitochondria isolated from HM cells and mice kidney tissues, melatonin caused ROS production; this melatonin response was completely blocked by the complex III inhibitor antimycin A. In contrast, both the mitochondrial complex I inhibitor, rotenone, and another complex III inhibitor, myxothiazol, which interacts with complex III at a distinct site, had no significant inhibitory effect on melatonin-induced ROS generation. These results demonstrate that melatonin-induced rapid ROS generation via the antimycin A sensitive site of mitochondrial complex III.
Keywords: melatonin, mitochondria, mesangial cells, reactive oxygen species
Introduction
The role of melatonin as an endogenous scavenger of reactive oxygen/nitrogen species (ROS/RNS) was discovered in 1993 [1] and subsequent studies from different laboratories have clearly established that melatonin and its oxidative metabolites form a cascade of antioxidant reactions [2]. The strong antioxidant properties has made melatonin a promising candidate for treating and preventing a variety of diseases including cancer [3], neurodegenerative diseases [4], metabolic syndrome and diabetes [5] since oxidative stress is a major factor involved in the pathogenesis of these disorders. In spite of its clear antioxidant function, several recent works reported that melatonin per se can act as a pro-oxidant to induce ROS generation in several cell lines [6–11]. This is particularly obvious in cultured cancer cells. The mechanism underlining this melatonin-induced ROS production remains unclear. To date, several characteristics of this pro-oxidant action of melatonin have been identified: a) high micromolar doses of melatonin are needed; b) the ROS production is transient and calmodulin is involved; c) lipoxygenase rather than the mitochondrial respiratory chain contribute to the ROS production.
Overproduction of ROS has been hypothesized as a unifying factor responsible for diabetic complications including diabetic nephropathy [12]. Hyperglycemia-induced mitochondrial superoxide overproduction may contribute to diabetic nephropathy inhibits glyceraldehyde-3-phosphate dehydrogenase, activates protein kinase C, increases hexosamine pathway flux and advanced glycation end products [13]. Additionally, overproduction of superoxide can directly impact the kidney function and structure by eliminating nitric oxide bioavailability and overproducing peroxynitrite in type 2 diabetes. Peroxynitrite is an extremely strong oxidant that attacks all biomolecules including DNA, RNA, proteins, and lipids. Peroxynitrite targets and converts tyrosine residues into nitrotyrosine in proteins, which causes nitrosative injury to kidney tissues and impairs renal functions. Indeed increases of 3-nitrotyrosines in the plasma and kidney of diabetic patients have been observed [14–16].
Elevated non-esterified fatty acids (NEFA) due to insulin resistance or increased ingestion in type 2 diabetes may also contribute to oxidative stress. Mitochondria utilize NEFA as fuel for ATP production and for ROS generation. Through β-oxidation and sequential oxidation of NEFA in the tricarboxylic acid cycle, the electron donors (NADH and FADH2) are generated for oxidative phosphorylation and for overproduction of ROS. Therefore, the excessive NEFA in combination with hyperglycemia in diabetes exacerbates the defective mitochondria and result in more severe oxidative stress [17]. Excessive NEFA may also influence the mitochondrial ROS production by other mechanisms. For example, excessive NEFA, particularly n-6 polyunsaturated fatty acids, activate mitochondrial Ca2+ ([Ca2+]m) efflux, which may deplete [Ca2+]m, overproduce superoxide and peroxynitrite, and cause nitrosative injury [18–20]. Treatment with melatonin improves kidney functional renal indices and reduces oxidative stress in experimental diabetic models [21]. However, the transient ROS production in response to melatonin may counteract any beneficial effect against oxidative stress in vivo. A clear elucidation of the mechanism of melatonin-induced ROS generation may help to uncover a means to eliminate the prooxidant effect of melatonin.
In the current study, we investigated the mechanism underlying melatonin-induced ROS production in primary human mesangial (HM) cells. Consistent with previous findings, our results demonstrate that melatonin, at micromolar concentrations, triggered acute ROS production in HM cells. The mitochondrial electron transport complex III, specifically the antimycin A-sensitive site, mediated the ROS production in responding to melatonin.
Materials and Methods
Materials
Melatonin was a gift of Drs. Tan and Reiter (Department of Cellular and Structural Biology, University of Texas Health Science Center at San Antonio) and originally purchased from Sigma Chemical Co. (St. Louis, MO). Fura-2 AM, X-rhod-1 and 2',7'-dichlorodihydrofluorescein (DCF) diacetate were purchased from Molecular Probes (Eugene, OR, USA) and Sigma, respectively. Antimycin A, myxothiazol, and other chemicals were from Sigma.
Cell culture
Primary human kidney mesangial (HM) cells were prepared as previously described [22] and is a gift from Dr. Abboud, Department of Medicine, University of Texas Health Science Center San Antonio. The cells were cultured in 100 mm dishes with RPMI 1640 medium, supplemented with 14% fetal bovine serum (Life Technologies, Carlsbad, CA, USA), 1% penicillin and streptomycin (Gibco-Invitrogen, Grand Island, NY, USA). Cells were grown in an incubator at 37°C with humidified 5% CO2.
Measurement of intracellular Ca2+ and ROS in HM cells
HM cells grown in dishes were labeled with fura-2 AM (1 µM) and DCF (1 µM) at 37°C for 30 min in RPMI 1640 medium for measuring intracellular Ca2+ ([Ca2+]i) and ROS simultaneously. Then cells were harvested and resuspended in PBS1Ca buffer (containing 1 mM KH2PO4, 3 mM Na2HPO4, 154 mM NaCl, 1 mM CaCl2, 1mM MgSO4, pH 7.2). [Ca2+]i and ROS were measured in a fluorometer (QM-6, Photon Technology International, NJ) with a cuvette at room temperature. Using the multidye mode of the QM-6 fluorometer, the ratio of fluorescence excited at 340 and 380 nm with emission of 510 nm was recorded to index the change of intracellular Ca2+ as previously reported [18]. Fluorescence excited at 506 nm and emitted at 529 nm was measured to index the generation of ROS in HM cells. The cell density was 1×106 cells/ml in these experiments.
Measurement of mitochondrial Ca2+ and ROS in isolated mitochondria
Mitochondria were isolated from HM cells or kidney tissues of 10-month db/m mice (BKS.Cg-m +/− lepr<db>/J, Jackson Laboratories) as previously described [18] and were suspended in MB1 buffer (containing 250 mM mannitol, 75 mM succinic acid, 0.1 mM EDTA, 0.5 mM EGTA, 10 mM HEPES, pH 7.4). The samples were diluted with 10 volumes of HKG buffer (20 mM NaCl, 100 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM pyruvate, 20 mM HEPES, pH 7.4) and labeled with X-rhod-1 (2 µM) and DCF (1 µM) at 37°C for 60 min. The loaded mitochondria were resuspended in HNG buffer and the measurement was performed by diluted the mitochondrial sample to PBS0Ca buffer (containing 1 mM KH2PO4, 3 mM Na2HPO4, 154 mM NaCl, 200 µM EGTA, 1mM MgSO4, pH 7.2). Alteration of [Ca2+]m and ROS were measured with fluorescence excited at 506 or 578 nm and emitted at 529 or 602 nm in PTI QM-6 fluorometer, respectively. In these experiments, 40 µg/ml mitochondrial proteins were used.
Statistic analysis
Results are presented as mean ± S.E. Student's t-test was used to analyze the difference between two groups. Differences were considered statistically significant at P < 0.05.
Results
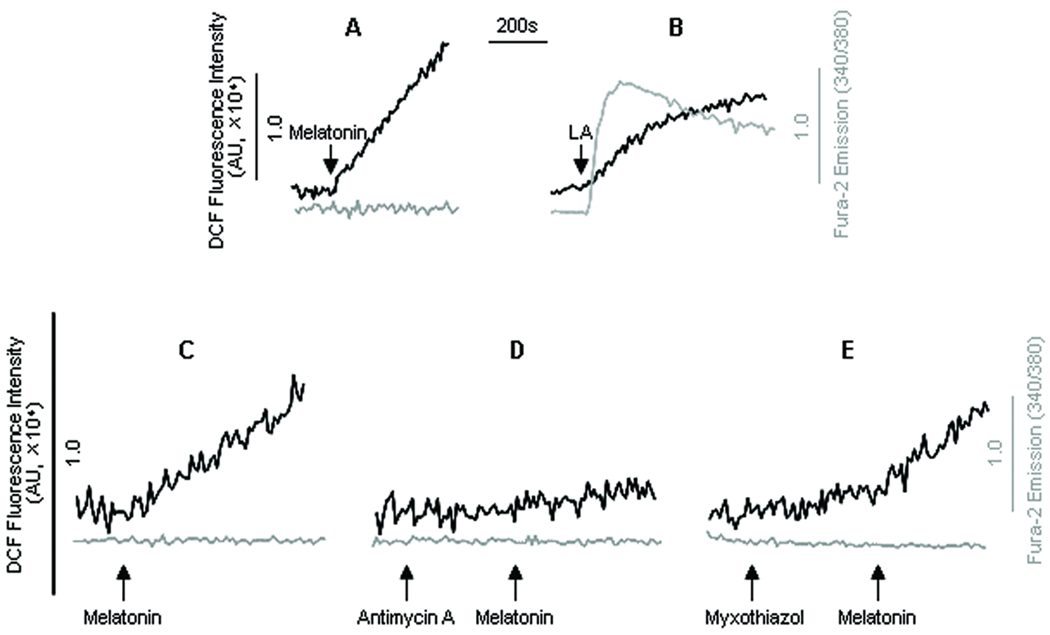
Oxidative and/or nitrosative injury to renal tissues contribute markedly to functional deficiencies of the kidney in diabetes and other prooxidant conditions. Although melatonin has been widely used as a ROS scavenger and protects the kidney from ischemia-reperfusion injuries [23], the recently documented ROS generation by melatonin at pharmacological concentrations [9,10] indicates that excessive melatonin causes ROS generation in certain in vitro conditions. As shown in figure 1A, melatonin (50 µM) caused rapid ROS generations (darker trace) in HM cells without affecting intracellular Ca2+ ([Ca2+]i, light trace). The rate of ROS generation by melatonin remained linear without measurable decay during the entire measurement time (20 min). In contrast, peroxynitrite production by linoleic acid rapidly reached a plateau (Fig. 1B, dark trace) and was accompanied by a rise in [Ca2+]i mobilization (involving contribution from [Ca2+]m efflux as previously shown [18]), consistent with our previous observations [18].
Fig. 1.
Melatonin-induced [Ca2+]i signaling and ROS generation. Human mesangial cells (HM) were labeled with fura-2 (1 µM) and DCF (1 µM) (37°C, 30 min) and [Ca2+]i signaling and ROS generation were measured simultaneously. Traces in (A) and (B) represented typical responses of [Ca2+]i (light trace) and ROS generation (dark trace) induced by melatonin (50 µM); and linoleic acid (LA, 30 µM). The effect of the mitochondrial complex III blockers were shown in traces C–E. The [Ca2+]i (light trace) and ROS generation (dark trace) induced by melatonin (50 µM) were measured in control (C) and antimycin A (D) or myxothiazol (E) pretreated HM cells. The arrows in the figure indicated the addition of melatonin, LA, antimycin A (5 µM) or myxothiazol (5 µM).
Mitochondria are the major subcellular organelle responsible for ROS production; herein, we tested whether melatonin-induced ROS generation involved mitochondria by using specific inhibitors of electron transport complexes I and III. Consistent with previous report in other cell types [8], pretreatment of HM cells with rotenone (5 µM) did not inhibit melatonin-induced ROS production (data not shown). Interestingly, prior incubation of HM cells with antimycin A (5 µM) markedly inhibited melatonin-induced ROS generation (Fig. 1 C and D). In contrast, treatment of the cells with myxothiazol (5 µM), which targets complex III at a distinct site other than that of antimycin A, did not block the ROS production in response to melatonin (Fig. 1 C and E). Antimycin A or myxothiazol per se affected neither [Ca2+]i nor ROS in HM cells.
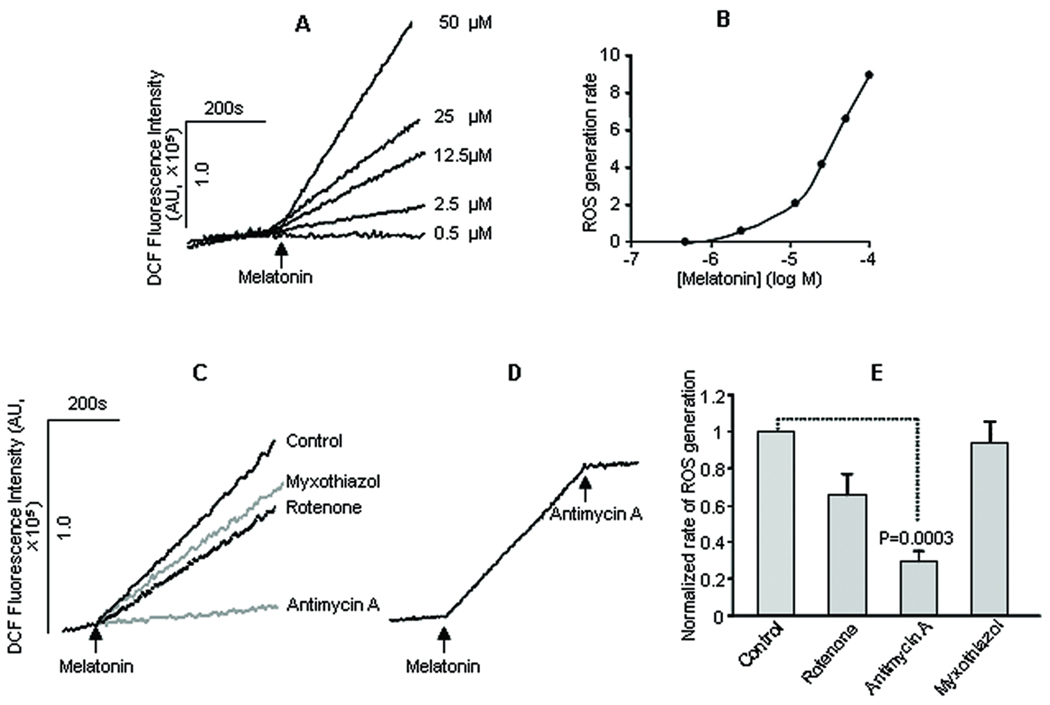
Based on the results summarized in figure 1, we next examined the role of the antimycin A-sensitive site of mitochondrial complex III in melatonin-induced ROS production in mitochondria prepared from mouse kidney tissues. As shown in figure 2 (A and B), melatonin induced ROS generation in a concentration-dependent manner in isolated kidney mitochondria. The rate of ROS production increased with increasing concentrations of melatonin and a minimal concentration of 2.5 µM was required for detectable ROS production in kidney mitochondria (Fig. 2 A and B). The ROS production rate was continuously enhanced within the concentration ranges of melatonin tested (0.5–100 µM, Fig. 2 B).
Fig. 2.
Melatonin-induced ROS generation in kidney mitochondria and the effects of mitochondrial blockers. Mitochondria were prepared from mouse kidney and labeled with DCF (1 µM) and ROS generation was indexed by the changes in DCF fluorescence. The traces in (A) represented the ROS generation in response to different concentrations of melatonin; the rate of melatonin-induced ROS formation increased with increasing melatonin concentration (B). Traces in (C) showed the effect of pretreatment of isolated kidney mitochondria with vehicle (control), the mitochondrial complex I inhibitor rotenone (5 µM), the mitochondrial complex III blockers myxothiazol (5 µM) or antimycin A (5 µM) on melatonin-induced ROS generation. Traces in (D) showed that addition of antimycin A (5 µM) after the start of melatonin-induced ROS production halted completely further ROS generations. The values in (E) were mean±SE rate of melatonin-induced ROS generation in mitochondria treated with vehicle (control), rotenone, antimycin A and myxothiazol from 3–5 experiments.
Pretreatment of the isolated mitochondria with antimycin A (5 µM) significantly inhibited the melatonin-induced ROS production (Fig. 2 C and E), confirmed earlier observation in HM cells (Fig. 1). Furthermore, antimycin A (5 µM) added after the start of melatonin-induced ROS generation completely halted ROS production (Fig. 2D). In contrast, pretreatment of the kidney mitochondria with myxothiazol (5 µM), another complex III inhibitor that binds to a distinct site other than the antimycin A-sensitive site, had no effect (Fig. 2 C and E). Furthermore, incubation of the kidney mitochondria with the complex I inhibitor rotenone (5 µM) also did not significantly affect melatonin-induced ROS production (Figure 2 C and E). These results demonstrate that the antimycin A sensitive site of mitochondrial respiratory complex III is responsible for melatonin-induced ROS generation in the kidney tissues.
Discussion
The physiological level of endogenous melatonin in plasma is usually in the picomolar although the cytosolic concentrations may reach nano to low micromolar levels in certain cells [2]. Therefore the biological significance of ROS induction by melatonin is unclear. However, increased intake from foods or food supplements rich in melatonin may dramatically increase the concentrations of melatonin in vivo. Our current in vitro findings indicate that excessive concentrations of melatonin (at micromolar levels) may actually cause ROS generation in the mitochondria and, therefore, could be harmful to the kidney and other tissues despite the well established antioxidant action of melatonin [24]; this, however, has never been documented in normal cells in vivo despite the fact that massive amounts of melatonin have often been administered. Previously, it has been demonstrated that melatonin-induced ROS generation was not associated with elevated oxidative stress [9]. The lack of apparent oxidative stress following melatonin-induced ROS generation reflects that the endogenous antioxidant mechanisms could completely clear the ROS generated by melatonin under normal conditions. However, the co-presence of other pathogenesis factors such as diabetes in the kidney, may disrupt the balance between pro- and anti-oxidant reactions and the damage to mesangial and other cells from ROS induced by high concentrations of melatonin may be exacerbated.
Our results provided evidence that the ROS production induced by micromolar melatonin in HM cells and kidney mitochondria was mainly mediated by the mitochondrial electron transport complex III (Fig. 1 and 2). The mitochondrial complex I inhibitor, rotenone, did not influence melatonin-induced ROS production, consistent with previous observations in other cell types [9]. It had been shown that in human leukocytes exposed to micromolar melatonin, ROS production was induced through a receptor-independent mechanism that involved calmodulin as a key component [6–11]. Calmodulin may bind and interact with mitochondria in a Ca2+-dependent manner [25]. The ubiquinol cytochrome C reductase core protein 2 of mitochondrial complex III may be regulated by Ca2+-calmodulin. However, in our study melatonin caused ROS production in HM cells without affecting [Ca2+]i or [Ca2+]m (Fig. 1). Therefore, the role of calmodulin in melatonin-induced ROS production in HM cells is not explored in our study.
Both the Qo (sensitive to myxothiazol) and Qi (sensitive to antimycin A) sites of mitochondrial complex III are capable of directly transferring electron to O2 to form superoxide in the mitochondrial membrane space and matrix, respectively [26]. Melatonin-induced ROS formation was significantly inhibited by antimycin A but not myxothiazol, indicating that the Qi site played a predominant role in the process. There two potential paths by which melatonin may cause ROS generation in a complex III-dependent manner: melatonin may function as an exogenous electron donor and the Qi site of complex III facilitates the electron transfer to O2 for ROS generation. If this is true, we would expect a rapid depletion of melatonin, particularly at low concentrations, together with decay in the rate of ROS generation. Our results, shown in figure 2, seemed to argue against this scenario. Alternatively, melatonin may specifically target and interact with the Qi site of complex III and divert the electrons from reducing cytochrome C to O2 for ROS generation. In this case, melatonin may not be consumed during ROS formation and the rate of ROS generation will depend on melatonin concentrations and remain constant. The persistent linearity of melatonin-induced ROS generation and the dependence of the rate of ROS formation on melatonin concentration observed (Fig. 2) provides support for this scenario.
In summary, our current work demonstrated that melatonin caused ROS formation in kidney cells and mitochondria. The myxothiazol-sensitive site of mitochondrial complex III is responsible for this ROS formation.
Acknowledgment
The work was partially supported by grants from the American Heart Asscoaition (0235065N), the NIH (HL075011) and the Department of Veterans Affairs (BXZ and CKY). The authors are grateful to Drs Tan and Reiter for their support of the work. Permanent address for Dr. Hong-Mei Zhang: Department of Oncology, Xijing Hospital, the Fourth Military Medical University, Xi’an, China.
References
- 1.Tan DX, Chen LD, Poeggeler B, et al. Melatonin: a potent endogenous hydroxyl radical scavenger. Endocrine J. 1993;1:57–60. [Google Scholar]
- 2.Tan DX, Manchester LC, Terron MP, et al. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 3.Jung-Hyues B, Reiter RJ, Ahmad N. Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J Pineal Res. 2010;48:9–19. doi: 10.1111/j.1600-079X.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olcese JM, Cao C, Mori T, et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res. 2009;47:82–94. doi: 10.1111/j.1600-079X.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 5.Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44:26–40. doi: 10.1111/j.1600-079X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 6.Albertini MC, Radogna F, Accorsi A, et al. Intracellular pro-oxidant activity of melatonin deprives U937 cells of reduced glutathione without affecting glutathione peroxidase activity. Ann N Y Acad Sci. 2006;1091:10–16. doi: 10.1196/annals.1378.050. [DOI] [PubMed] [Google Scholar]
- 7.Büyükavci M, Ozdemir O, Buck S, et al. Melatonin cytotoxicity in human leukemia cells: relation with its pro-oxidant effect. Fundam Clin Pharmacol. 2006;20:73–79. doi: 10.1111/j.1472-8206.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 8.Osseni RA, Rat P, Bogdan A, et al. Evidence of prooxidant and antioxidant action of melatonin on human liver cell line HepG2. Life Sci. 2000 Dec 15;68:387–399. doi: 10.1016/s0024-3205(00)00955-3. [DOI] [PubMed] [Google Scholar]
- 9.Radogna F, PaternosteR L, De Nicola M, et al. Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol Appl Pharmacol. 2009;239:37–45. doi: 10.1016/j.taap.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Radogna F, Sestili P, Martinelli C, et al. Lipoxygenase-mediated pro-radical effect of melatonin via stimulation of arachidonic acid metabolism. Toxicol Appl Pharmacol. 2009;238:170–177. doi: 10.1016/j.taap.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Wölfler A, Caluba HC, Abuja PM, et al. Prooxidant activity of melatonin promotes fas-induced cell death in human leukemic Jurkat cells. FEBS Lett. 2001;502:127–131. doi: 10.1016/s0014-5793(01)02680-1. [DOI] [PubMed] [Google Scholar]
- 12.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 13.Du X, Matsumura T, Edelstein D, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceriello A, Mercuri F, Quagliaro L, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia. 2001;44:834–838. doi: 10.1007/s001250100529. [DOI] [PubMed] [Google Scholar]
- 15.Shishehbor MH, Aviles RJ, Brennan ML, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 16.Thuraisingham RC, Nott CA, Dodd SM, et al. Increased nitrotyrosine staining in kidneys from patients with diabetic nephropathy. Kidney Int. 2000;57:1968–1972. doi: 10.1046/j.1523-1755.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 17.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 18.Zhang HM, Dang H, Yeh CK, et al. Linoleic Acid-Induced Mitochondrial Ca2+ Efflux Causes Peroxynitrite Generation and Protein Nitrotyrosylation. PLoS One. 2009;4:e6048. doi: 10.1371/journal.pone.0006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang BX, Ma X, Zhang W, et al. Polyunsaturated fatty acids mobilize intracellular Ca2+ in NT2 human teratocarcinoma cells by causing release of Ca2+ from mitochondria. Am J Physiol Cell Physiol. 2006;290:C1321–C1333. doi: 10.1152/ajpcell.00335.2005. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Li ZH, Zhang MQ, et al. Heat shock protein 90beta 1 is essential for polyunsaturated fatty acid-induced mitochondrial Ca2+ efflux. J Biol Chem. 2008;283:7580–7589. doi: 10.1074/jbc.M707192200. [DOI] [PubMed] [Google Scholar]
- 21.Ha H, Yu MR, Kim KH. Melatonin and taurine reduce early glomerulopathy in diabetic rats. Free Radic Biol Med. 1999;26:944–950. doi: 10.1016/s0891-5849(98)00276-7. [DOI] [PubMed] [Google Scholar]
- 22.Silver BJ, Jaffer FE, Abboud HE. Platelet-derived growth factor synthesis in mesangial cells: Induction by multiple peptide mitogens. Proc Natl Acad Sci USA. 1989;86:1056–1060. doi: 10.1073/pnas.86.3.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Nickkholgh A, Yi X, et al. Melatonin protects kidney grafts from ischemia/reperfusion injury through inhibition of NF-kB and apoptosis after experimental kidney transplantation. J Pineal Res. 2009;46(4):365–372. doi: 10.1111/j.1600-079X.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 24.Luchetti F, Canonico B, Betti M, et al. Melatonin signaling and cell protection function. FASEB J. 2010 doi: 10.1096/fj.10-154450. Published online June 16, fj.10-154450. [DOI] [PubMed] [Google Scholar]
- 25.Pardue RL, Kaetzel MA, Hahn SH, et al. The identification of calmodulin-binding sites on mitochondria in cultured 3T3 cells. Cell. 1981;23(2):533–542. doi: 10.1016/0092-8674(81)90149-5. [DOI] [PubMed] [Google Scholar]
- 26.Starkov AA, Fiskum G. Myxothiazol induces H(2)O(2) production from mitochondrial respiratory chain. Biochem Biophys Res Commun. 2001 Mar 2;281(3):645–650. doi: 10.1006/bbrc.2001.4409. [DOI] [PubMed] [Google Scholar]