Abstract
Individuals with chronic HCV infection have impaired response to vaccine, though the etiology remains to be elucidated. Dendritic cells (DC) and monocytes (MN) provide antigen uptake, processing, presentation, and costimulatory functions necessary to achieve optimal immune responses. The integrity of antigen processing and presentation function within these antigen presenting cells (APC) in the setting of HCV infection has been unclear. We used a novel T cell hybridoma system that specifically measures MHC-II antigen processing and presentation function of human APC. Results demonstrate MHC-II antigen processing and presentation function is preserved in both myeloid DC (mDC) and MN in the peripheral blood of chronically HCV-infected individuals, and indicates that an alteration in this function does not likely underlie the defective HCV-infected host response to vaccination.
Keywords: hepatitis C, immunology, dendritic cells, monocytes, antigen presentation, MHC-II
1. Introduction
Hepatitis C virus (HCV) is a leading cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma worldwide [1; 2]. It is estimated that over 120 million people are HCV-infected, with particularly higher rates in Asia where hepatitis A (HAV) and B (HBV) co-infection is common [3]. It is currently recommended that individuals with chronic HCV infection receive vaccination against HAV and HBV. The rationale for these vaccines derives from an increase in mortality with acute HAV co-infection, and accelerated progression to cirrhosis in HBV co-infection [4; 5]. However, impaired antibody titers to HAV [6; 7] and antibody titers and seroconversion to HBV vaccination [8; 9] has been observed in the setting of chronic HCV infection. One possible explanation is a defect in APC function. DC are the most potent APC to elicit naive T-cell activation. In peripheral blood, two major DC populations can be identified: CDllc+ mDC and CD123+ plasmacytoid DC. mDC are felt to be the primary DC subpopulation that provides both antigen presentation and costimulation necessary for naïve T cell activation. Our group and others have observed intact ability of mDC from HCV-infected subjects to activate allogeneic T cells [10; 11; 12]. Other groups however have found mDC from chronic HCV-infected subjects to be defective in activating T cells [13; 14]. Notably, all of these studies used assays of allogeneic T cell activation as a measure of DC function. In particular, such assays assess the ability of DC that are presenting endogenous antigen already present on the APC surface to costimulate T cells, and do not evaluate the ability of APC to process and present exogenous antigens [15; 16].
In this study we have focused specifically on the antigen processing and presentation ability of mDC and MN from HCV-infected individuals. We use a novel HLA-DR matched T cell hybridoma system that is costimulation independent to specifically ask if mDC and MN from HCV-infected individuals have a defect in MHC-II antigen processing and presentation. Results indicate that although there is heterogeneity in responses elicited by APC from both HCV-infected subjects and healthy controls, both groups have similar overall antigen processing and presentation capability.
2. Materials and Methods
2.1. Study subjects
Recruitment and participation of healthy and HCV-infected subjects was approved by University Hospitals Case Medical Center and Cleveland VA Medical Center Institutional Review Boards, and written informed consent was obtained from all subjects for peripheral blood sampling. Subjects were screened for HLA-DR15.01 expression initially by analysis of whole blood for HLA-DR15 expression by flow cytometry (biotin anti-HLA-DR15 antibody (One Lambda), followed by streptavidin-APC (InVitrogen)). Subject cells expressing HLA-DR15 using this low resolution assay underwent high-resolution analysis by PCR (Biosynthesis) to identify individuals that were HLA-DR15.01 subtype positive as previously described [17]. 14 HCV infected individuals and 13 healthy controls that all screened positive for HLA-DR15.01 were enrolled in the study. Clinical chemistry values, platelet counts, HCV genotypes, and HCV viral loads were obtained from the Cleveland VA clinical laboratory.
2.2. Cell purification
All blood was collected in heparin containing green top tubes and rocked overnight. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll Paque Plus (Amersham Biosciences) density gradient centrifugation. MDC were isolated from PBMC using the BDCA-1 kit (Miltenyi Biotec) according to the manufacture's instructions. MN were isolated using the Monocyte Isolation Kit II kit (Miltenyi Biotec) and PBMC were additionally incubated with anti-CDlc antibody (Miltenyi) and anti-CD2 beads (Miltenyi) to minimize contamination of MN by mDC and T cells respectively. HLA-DR expression was determined using anti-CDllc-APC (Biolegend) and anti-HLA-DR-PECY5 (BDBiosciences) for mDC and anti-CD14-PE (BDBiosciences) and anti-HLA-DR-APC (BDBiosciences) for MN.
2.3. Antigen processing and presentation assays
Antigen processing and presentation assays were performed as previously described [18; 19]. Briefly, freshly prepared MN (5×104) or mDC (5×103)/well were cultured in 96 well plates with the HLA-DR15.01- restricted HEL-specific T cell hybridoma clone (15HEL, 1×105/well) and incubated with titrated concentrations of hen egg lysozyme (HEL, Roche) or HEL peptide (aa 14–37, Genemed Synthesis) in DMEM based medium with 10% FCS. After 22 hrs, supernatants were harvested and IL-2 levels measured by ELISA (eBioscience). Aliquots of a single batch of 15HEL T cell hybridoma cells were frozen so that freshly prepared mDC and MN could be tested with an identical T cell cell line readout.
2.4. Statistical Analysis
As the data were not normally distributed and the sample size was small nonparametric procedures were used. For continuous outcomes, comparisons between the groups were made with Mann-Whitney U test. Spearman rank correlations were used to assess the relationships between continuous variables. SPSS 17 and GPower (3.1.0)were used to perform the statistical and power calculations respectively.
3. Results
3.1. Subjects
We obtained samples from 14 individuals with chronic-HCV infection without prior HCV treatment that were HLA-DR15.01 positive. Table 1 summarizes clinical information. None of the subjects had overt signs or symptoms of hepatic decompensation and only one had a low serum albumin (2.9 g/dL). Aspartate aminotransferase/platelet ratio index (APRI, calculated as aspartate aminotransferase (AST) (U/L)/upper normal × 100/platelet count (109/L)]) is associated with liver fibrosis in HCV infected individuals [Shaheen, 2007 #2926] and has been shown to correlate with progression of disease and mortality [Sinn, 2008 #2950][Ngo, 2006 #2948][Nunes, 2010 #2949]. Eight of 14 subjects had an APRI<0.5 (associated with lower likelihood of advanced stage liver disease), 6 of 14 subjects had an APRI > 0.5, and 4 of 14 subjects had an APRI >1 (associated with a greater likelihood of advanced stage liver disease) [20].
Table 1.
Clinical Characteristics of HCV-infected subjects
| HCV viral load | ||||||||
|---|---|---|---|---|---|---|---|---|
| subject | age | genotype | ALT | AST | PLT | ALB | APRI | |
| 1 | 53 | 1,782,120 | 3a | 29 | 22 | 278 | 3.8 | 0.18 |
| 2 | 49 | 125,419 | 1a | 21 | 19 | 215 | 3.7 | 0.20 |
| 3 | 46 | 4,608,270 | 1a | 73 | 136 | 243 | 4 | 1.24 |
| 4 | 65 | 632,416 | 2a | 56 | 54 | 181 | 4.1 | 0.66 |
| 5 | 58 | 783 | NA | 36 | 28 | 332 | 4.2 | 0.19 |
| 6 | 57 | 464,082 | 1a | 95 | 115 | 135 | 2.9 | 1.89 |
| 7 | 63 | 1,924,230 | 1a | 54 | 39 | 197 | 4 | 0.44 |
| 8 | 60 | 185,662 | 1a | 45 | 30 | 262 | 4.1 | 0.25 |
| 9 | 53 | 110,741 | 1b | 58 | 30 | 209 | 4.1 | 0.32 |
| 10 | 49 | 1,168,790 | 3 | 272 | 207 | 248 | 3.8 | 1.85 |
| 11 | 62 | 332,882 | 1a | 94 | 111 | 134 | 3.6 | 1.84 |
| 12 | 52 | 2,003,560 | 1b | 95 | 75 | 132 | 4 | 1.26 |
| 13 | 62 | 132,541 | 1 | 50 | 49 | 229 | 4.3 | 0.48 |
| 14 | 60 | 2,070,000 | 1a | 30 | 33 | 322 | 4.1 | 0.23 |
HCV viral load, ALT U/L, AST U/L, PLT in thousands, ALB g/dL, APRI (aspartate aminotransferase/platelet ratio index)
Control subjects were healthy individuals from the university and hospital employee community. Mean age of controls was 47, and this is statistically lower than the mean age of 56 for HCV infected subjects (p<0.003). Four of 13 controls were female, while 1 of 14 HCV infected individuals were female. This reflects the general pool of healthy control donors that are a somewhat younger population and the HCV-infected subjects in the VA system that are overwhelmingly males from the Vietnam era of military service. Only one subject was African American as the HLA-DR15.01 subtype is uncommon in persons of African descent.
3.2. Antigen processing and presentation assays
The goal of these studies was to specifically determine if there is an MHC-II antigen processing and presentation defect in APC from HCV-infected individuals. HLA-DR-15.01 restricted CD4+ T cell hybridomas were used to study MHC-II antigen processing and presentation. Hybridomas are stable immortal cell lines that provide quantitative and reproducible responses well suited for comparative studies [18; 19; 21]. They were generated in HLA-DR transgenic mice, but respond to human APC as previously described [18]. The specific T cell hybridoma clone was selected as it secretes IL-2 in proportion to amount of specific peptide-MHC on the APC surface. This DR15-restricted T cell hybridoma clone was previously shown to be CD80 and CD86 costimulation independent and therefore provides a readout that is dependent on levels of expression of specific peptide:MHC-II complexes [17]. T cell hybridomas respond immediately after thawing from cryopreservation, permitting utilization of a single stock of T cells for analysis of APC isolated freshly at different time points. The APC therefore becomes the main variable in the experimental system. We have used this HLA-DR-restricted T cell hybridoma system to identify a defect in antigen presentation by cord blood MN [19] and preserved antigen presentation function in MN from HIV-infected individuals [17].
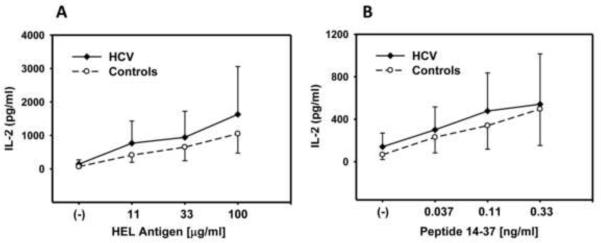
mDC purified from PBMC by positive selection using immuno-magnetic beads from 9 HCV-infected individuals and 10 healthy controls were studied using this experimental system. Fig. 1A and 1B demonstrates the amount antigen presentation at varying concentrations of soluble HEL or peptide for the entire group of HCV infected individuals. Soluble HEL must be taken up, processed, and presented, while peptide can bind HLA-DR on the mDC surface and does not require uptake and intracellular processing to be presented. There is heterogeneity in the ability to present both soluble and peptide antigen, but there is no significant difference in presentation function at any concentration of HEL or peptide comparing mDC from controls vs. the total group of HCV-infected subjects (p>0.35).
Fig. 1. mDC antigen presentation.
Purified mDC (5×103/well) from healthy controls and HCV-infected individuals were incubated with soluble HEL (A) or peptide (B) and T cell hybridoma (1×105/well) for 22 hrs. IL-2 levels were determined by ELISA.
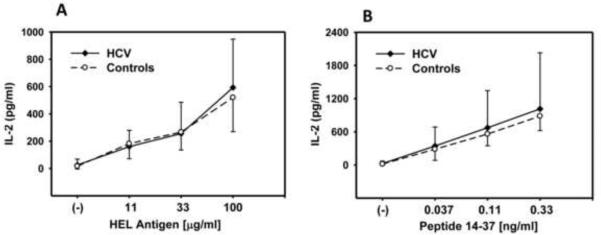
MN were purified from PBMC of 14 HCV-infected individuals and 13 healthy control by negative selection using immuno-magnetic beads s. Fig. 2 demonstrates no significant difference in presentation at any concentration of soluble HEL or specific peptide antigen comparing MN from the total group of HCV-infected individuals (Fig 2A and B, p>0.4) vs. healthy controls.
Fig. 2. MN antigen presentation.
Purified MN (5×104/well) from healthy controls and HCV-infected individuals were incubated with soluble HEL (A) or peptide (B) and T cell hybridoma (1×105/well) for 22 hrs. IL-2 levels were determined by ELISA.
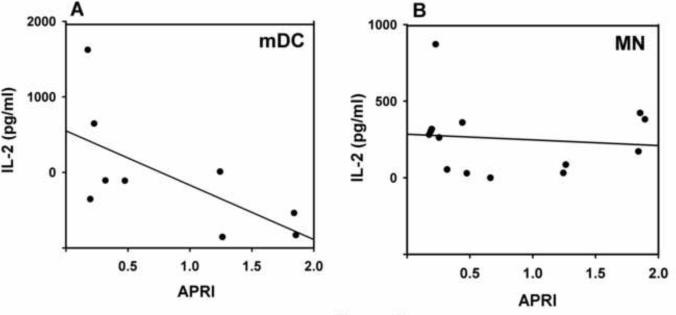
While antigen processing and presentation is preserved in HCV-infected individuals, we sought to determine if there were relationships between the clinical course of the HCV infection and APC antigen presentation that may explain the observed heterogeneity in activity in the HCV infected subjects. We evaluated APRI as a continuous variable, serum HCV level, and albumin. mDC antigen presentation negatively correlated with APRI (Fig. 3A, r=−0.71, p<=0.03) while there was no relationship for MN (Fig. 3B, r=−0.09, p=0.76). With an effect size (0.71) this large, the sample size of 9 should be sufficient for a correlation with a one-tail test, an alpha of 0.05 and a power of 0.86. HCV levels and albumin did not correlate with antigen presentation by mDC or MN (data not shown).
Fig. 3. Relationship between antigen presentation and APRI score.
The relationship between IL-2 levels at the 33 μg/ml concentration of HEL and the APRI score are presented for mDC (A) and MN (B).
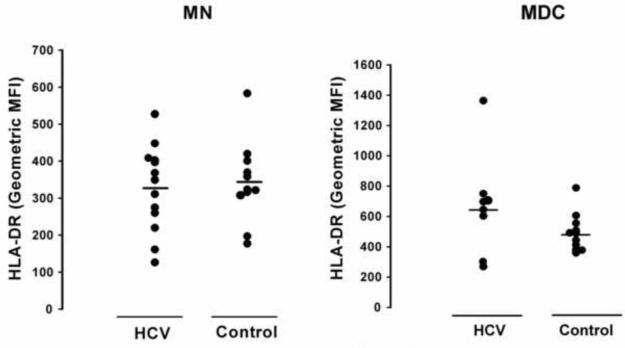
During the latter stages of antigen processing the HLA-DR molecule is loaded with processed HEL peptide to be presented. Therefore the HLA-DR expression levels could directly affect antigen presentation. In the past we have found only a weak correlation between HLA-DR expression and antigen presentation in one study, and none in another [17; 19]. We have taken this to suggest that within the physiologic levels of HLA-DR normally present, that the intracellular events of processing and peptide loading are more limiting than surface HLA-DR expression in determining presentation and T cell activation. Here we found no statistical difference in the HLA-DR levels in mDC or MN comparing HCV-infected and healthy control groups (Fig. 4). Also, we did not find a correlation between HEL or peptide presentation and HLA-DR levels in the HCV-infected or control subjects.
Fig. 4. HLA-DR levels on mDC and MN.
Purified mDC and MN were stained with anti-CD14, CD11c, and HLA-DR. HLA-DR MFI of the purified CD14+ MN and CD11c+ mDC and are shown.
4. Discussion
In the current study we observed intact MHC-II antigen processing and presentation function in ex vivo blood mDC and MN from individuals with untreated chronic HCV infection. To our knowledge this is the first report specifically analyzing levels of peptide-MHC complexes on APC of untreated chronic HCV individuals. We have shown previously that our system using T cell hybridomas is not costimulation (CD80 or CD86) dependent [17; 19]. This MHC class II antigen processing and presentation assay encompasses a number of steps that lead up to presentation of the specific peptide on HLA-DR of an APC. These include uptake of HEL antigen via receptor-mediated endocytosis or macropinocytosis, trafficking to an acidified compartment for degradation, loading on intracellular HLA-DR, and trafficking to the cell surface. Adhesion molecule interactions are also necessary for a successful T cell response. It is of course possible that one part of this process could be partially defective while another part of this process compensates for the defect, resulting in overall intact function.
We have previously observed mDC to be in an increased state of activation, as reflected by increased CD83 and CD86 expression, in the setting of HCV infection [12]. Additionally, expression of these markers tended to correlate with serum AST and ALT, indicating a relation between degree of liver inflammation and circulating mDC activation/maturation state. Here we observed APRI level (a function of AST and platelet count) negatively correlated with mDC antigen processing and presentation function within the HCV infected group. Interestingly there is a trend toward better mDC antigen presentation in the HCV infected subjects with lower APRI. It is therefore possible that in earlier stage liver disease there is in fact enhanced antigen processing and presentation function.
Reduced response to HAV and HBV vaccination in the setting of chronic HCV infection may be due to a number of factors. Elements necessary for a successful antibody mediated T dependent vaccine response include B cells, helper CD4+ T cells, and primarily DC as APC. There are multiple functions of APC necessary to shape the helper CD4+ T cell and optimal antibody producing B cell responses, including APC maturation and migration, production of specific chemokines and cytokines, and contact dependent costimulation. Alterations in some of these mDC functions, including maturation state, chemokine and cytokine release have been observed [22; 23; 24; 25; 26]. Previous analyses of mDC ability to activate T cells have selectively utilized assays of allogenic T cell activation, and there has been some controversy regarding results, with one study observing a defect in mDC [14] and others observing no defect [10; 11; 12]. As a result, it has been unclear whether on a per cell basis mDC from HCV infected individuals are able to prime naïve T cells. Reduced numbers of peripheral blood mDC have been observed in HCV-infected individuals as well [11; 14; 27; 28] and may play a role in the defective response to vaccine. Here we specifically focused on analysis of peptide-MHC complex formation and presentation, the culmination of uptake, degradation and trafficking of antigen. We found that this specific APC function is preserved in the setting of chronic HCV infection.
It is quite possible that the mechanisms for the defects in HAV and HBV vaccination are multi-factorial. There have been a paucity of data concerning global T or B cell defects in HCV-infected individuals. Defects in either or both of these compartments could certainly hamper optimal vaccine induced antibody responses. Dolganiuc et al found that mDC induced more Tregs in HCV infected individuals, which could hamper T helper responses [29]. Nisii et al found an accumulation of dysfunctional CD8+ T cells in livers of subjects with chronic HCV suggesting global T cell defects may be present [30]. Defects in CD4+ helper T cells have not been reported. A better understanding of the nature and scope of immune defects in HCV-infected individuals is warranted as the morbidity of HAV and HBV infection in individuals with chronic HCV is significant. Such an understanding should guide development of strategies to obtain optimal vaccine-induced protection against hepatitis viruses.
5. Conclusions
Overall there is no antigen presentation defect in mDC and MN from chronically HCV infected individuals at the stage of disease prior to significant hepatic decompensation. The data does, however, suggest a relationship between milder stage HCV disease and better antigen processing function. The implications of these findings are that in chronically HCV infected individuals prior to hepatic decompensation that a defect in antigen presentation is not the likely etiology of the reduced vaccine responses that others have observed in this group.
Acknowledgements
This work was supported by AI-73217, AI-70022, Cleveland VA GRECC (Canaday), DK068361 (Anthony), and CWRU/UHC Center for AIDS Research, AI-36219. Dr. Woc-Colburn was supported by NIH Pulmonary training grant HL-07889.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, Marinos G, Kaldor JM. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–16. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- [2].Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- [3].Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- [4].Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–90. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- [5].Keeffe EB. Acute hepatitis A and B in patients with chronic liver disease: prevention through vaccination. Am J Med. 2005;118(Suppl 10A):21S–27S. doi: 10.1016/j.amjmed.2005.07.013. [DOI] [PubMed] [Google Scholar]
- [6].Keeffe EB, Iwarson S, McMahon BJ, Lindsay KL, Koff RS, Manns M, Baumgarten R, Wiese M, Fourneau M, Safary A, Clemens R, Krause DS. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology. 1998;27:881–6. doi: 10.1002/hep.510270336. [DOI] [PubMed] [Google Scholar]
- [7].Lee SD, Chan CY, Yu MI, Wang YJ, Chang FY, Lo KJ, Safary A. Safety and immunogenicity of inactivated hepatitis A vaccine in patients with chronic liver disease. J Med Virol. 1997;52:215–8. doi: 10.1002/(sici)1096-9071(199706)52:2<215::aid-jmv16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- [8].Wiedmann M, Liebert UG, Oesen U, Porst H, Wiese M, Schroeder S, Halm U, Mossner J, Berr F. Decreased immunogenicity of recombinant hepatitis B vaccine in chronic hepatitis C. Hepatology. 2000;31:230–4. doi: 10.1002/hep.510310134. [DOI] [PubMed] [Google Scholar]
- [9].Buxton JA, Yu A, Kim PH, Spinelli JJ, Kuo M, Alvarez M, Gilbert M, Krajden M. HCV co-infection in HIV positive population in British Columbia, Canada. BMC Public Health. 2010;10:225. doi: 10.1186/1471-2458-10-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Piccioli D, Tavarini S, Nuti S, Colombatto P, Brunetto M, Bonino F, Ciccorossi P, Zorat F, Pozzato G, Comar C, Abrignani S, Wack A. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42:61–7. doi: 10.1016/j.jhep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- [11].Longman RS, Talal AH, Jacobson IM, Rice CM, Albert ML. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- [12].Yonkers NL, Rodriguez B, Milkovich KA, Asaad R, Lederman MM, Heeger PS, Anthony DD. TLR ligand-dependent activation of naive CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection. J Immunol. 2007;178:4436–44. doi: 10.4049/jimmunol.178.7.4436. [DOI] [PubMed] [Google Scholar]
- [13].Tsubouchi E, Akbar SM, Horiike N, Onji M. Infection and dysfunction of circulating blood dendritic cells and their subsets in chronic hepatitis C virus infection. J Gastroenterol. 2004;39:754–62. doi: 10.1007/s00535-003-1385-3. [DOI] [PubMed] [Google Scholar]
- [14].Kanto T, Inoue M, Miyatake H, Sato A, Sakakibara M, Yakushijin T, Oki C, Itose I, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–26. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- [15].Tzachanis D, Berezovskaya A, Nadler LM, Boussiotis VA. Blockade of B7/CD28 in mixed lymphocyte reaction cultures results in the generation of alternatively activated macrophages, which suppress T-cell responses. Blood. 2002;99:1465–73. doi: 10.1182/blood.v99.4.1465. [DOI] [PubMed] [Google Scholar]
- [16].Tan P, Anasetti C, Hansen JA, Melrose J, Brunvand M, Bradshaw J, Ledbetter JA, Linsley PS. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993;177:165–73. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Woc-Colburn L, Smultea L, Ramachandra L, Canaday DH. Preserved MHC Class II Antigen Processing in Monocytes from HIV-Infected Individuals. PLoS One. 2010;5:e9491. doi: 10.1371/journal.pone.0009491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Canaday DH, Gehring A, Leonard EG, Eilertson B, Schreiber JR, Harding CV, Boom WH. T-cell hybridomas from HLA-transgenic mice as tools for analysis of human antigen processing. J Immunol Methods. 2003;281:29–42. doi: 10.1016/j.jim.2003.07.004. [DOI] [PubMed] [Google Scholar]
- [19].Canaday DH, Chakravarti S, Srivastava T, Tisch DJ, Cheruvu VK, Smialek J, Harding CV, Ramachandra L. Class II MHC antigen presentation defect in neonatal monocytes is not correlated with decreased MHC-II expression. Cell Immunol. 2006;243:96–106. doi: 10.1016/j.cellimm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912–21. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- [21].Jones L, McDonald D, Canaday DH. Rapid MHC-II antigen presentation of HIV type 1 by human dendritic cells. AIDS Res Hum Retroviruses. 2007;23:812–6. doi: 10.1089/aid.2006.0280. [DOI] [PubMed] [Google Scholar]
- [22].Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–6. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- [23].Anthony DD, Yonkers NL, Post AB, Asaad R, Heinzel FP, Lederman MM, Lehmann PV, Valdez H. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–16. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- [24].Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–91. [PubMed] [Google Scholar]
- [25].Rodrigue-Gervais IG, Jouan L, Beaule G, Sauve D, Bruneau J, Willems B, Sekaly RP, Lamarre D. Poly(I:C) and lipopolysaccharide innate sensing functions of circulating human myeloid dendritic cells are affected in vivo in hepatitis C virus-infected patients. J Virol. 2007;81:5537–46. doi: 10.1128/JVI.01741-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rodrigue-Gervais IG, Rigsby H, Jouan L, Sauve D, Sekaly RP, Willems B, Lamarre D. Dendritic cell inhibition is connected to exhaustion of CD8+ T cell polyfunctionality during chronic hepatitis C virus infection. J Immunol. 2010;184:3134–44. doi: 10.4049/jimmunol.0902522. [DOI] [PubMed] [Google Scholar]
- [27].Kanto T, Inoue M, Miyazaki M, Itose I, Miyatake H, Sakakibara M, Yakushijin T, Kaimori A, Oki C, Hiramatsu N, Kasahara A, Hayashi N. Impaired function of dendritic cells circulating in patients infected with hepatitis C virus who have persistently normal alanine aminotransferase levels. Intervirology. 2006;49:58–63. doi: 10.1159/000087264. [DOI] [PubMed] [Google Scholar]
- [28].Wertheimer AM, Bakke A, Rosen HR. Direct enumeration and functional assessment of circulating dendritic cells in patients with liver disease. Hepatology. 2004;40:335–45. doi: 10.1002/hep.20306. [DOI] [PubMed] [Google Scholar]
- [29].Dolganiuc A, Paek E, Kodys K, Thomas J, Szabo G. Myeloid dendritic cells of patients with chronic HCV infection induce proliferation of regulatory T lymphocytes. Gastroenterology. 2008;135:2119–27. doi: 10.1053/j.gastro.2008.07.082. [DOI] [PubMed] [Google Scholar]
- [30].Nisii C, Tempestilli M, Agrati C, Poccia F, Tocci G, Longo MA, D'Offizi G, Tersigni R, Lo Iacono O, Antonucci G, Oliva A. Accumulation of dysfunctional effector CD8+ T cells in the liver of patients with chronic HCV infection. J Hepatol. 2006;44:475–83. doi: 10.1016/j.jhep.2005.10.023. [DOI] [PubMed] [Google Scholar]