Abstract
Background
Although 900,000 HIV-infected South Africans receive antiretroviral therapy (ART), the majority of South Africans with HIV remain undiagnosed.
Methods
We use a published simulation model of HIV case detection and treatment to examine three HIV screening scenarios, in addition to current practice: 1) one-time; 2) every five years; and 3) annually. South African model input data include: 16.9% HIV prevalence, 1.3% annual incidence, 49% test acceptance rate, HIV testing costs of $6.49/patient, and a 47% linkage-to-care rate (including two sequential ART regimens) for identified cases. Outcomes include life expectancy, direct medical costs, and incremental cost-effectiveness.
Results
HIV screening one-time, every five years, and annually increase HIV-infected quality-adjusted life expectancy (mean age 33 years) from 180.6 months (current practice) to 184.9, 187.6 and 197.2 months. The incremental cost-effectiveness of one-time screening is dominated by screening every five years. Screening every five years and annually each have incremental cost-effectiveness ratios of $1,570/quality-adjusted life year (QALY) and $1,720/QALY. Screening annually is very cost-effective even in settings with the lowest incidence/prevalence, with test acceptance and linkage rates both as low as 20%, or when accounting for a stigma impact at least four-fold that of the base case.
Conclusions
In South Africa, annual voluntary HIV screening offers substantial clinical benefit and is very cost-effective, even with highly constrained access to care and treatment.
Keywords: HIV, screening, cost-effectiveness, South Africa
INTRODUCTION
In 2007, the World Health Organization (WHO) recommended routine HIV testing of all persons in countries, like South Africa, with generalized epidemics -- a recommendation still far from implementation.1 Of the five million South Africans currently living with HIV, over three million remain unaware of their infection and unable to access lifesaving care and counseling.2, 3 Although there are over 4,000 HIV voluntary, counseling and testing (VCT) centers throughout South Africa, only 47% of South Africans report ever having been tested for HIV infection and only an estimated 34% of all HIV-infected individuals (1.9 million patients) are receiving HIV care.4, 5
Obstacles to implementing the WHO recommendations for testing include cost, limited capacity for HIV care, and stigma.4, 6–9 Soon after the release of the WHO recommendations, the South African province of Free State suspended antiretroviral treatment admissions due to financial constraints.6 Such action implies an inability to pay for the care of persons with HIV already detected and in need of treatment, let alone to cover the costs of expanded testing and care of newly detected cases. Further, despite the tremendous progress that has been made in shifting cultural norms, both HIV testing and living with known HIV infection remain highly stigmatized.4, 7–9
Implementation of the WHO HIV testing recommendations in South Africa will inevitably be hampered by insufficient treatment resources, inadequate linkage to care and persistent stigma. Our objective was to take these challenges into explicit consideration – adopting a deliberately conservative approach with regard to the availability of funds, rates of program uptake, access to care, and not incorporating the benefits of preventing HIV transmission – to provide decision makers with a realistic assessment of the clinical impact and cost-effectiveness of HIV screening in South Africa.
METHODS
Analytic Overview
We use the Cost-Effectiveness of Preventing AIDS Complications (CEPAC)-International model to examine three HIV screening strategies (outside the context of pre-natal HIV screening), in addition to current practice: one-time screening at age 33 years, screening every five years, and annual screening (see Technical Appendix).10–13 We choose the every five years screening strategy as an intermediately aggressive and more immediately achievable approach compared to annual testing. For each strategy, we project mean quality-adjusted life expectancy, and mean per person lifetime costs (in 2006 US$) for both HIV-infected individuals and the general population. Outcome measures are assessed from a societal perspective (excluding patient travel time and lost wages), and quality-adjusted life expectancy and costs are discounted at 3% annually.14 Cost-effectiveness ratios, calculated by comparing each strategy to the next least costly, non-dominated strategy, are expressed in incremental cost per quality-adjusted life-year (QALY). Guided by recommendations from the WHO, we consider strategies with cost-effectiveness ratios less than the Gross Domestic Product (GDP) per capita – one measure of a nation’s ability to pay – in South Africa ($5,400) to be “very cost-effective.”15, 16 Sensitivity analyses examine uncertainties in model parameters, as well as criticisms of expanded routine testing, notably, limited test acceptance, incomplete ART availability, stigma, and cost.
Disease Model
The CEPAC-International model is a state-transition model of HIV disease and treatment in resource-limited settings (see Technical Appendix).10–13 In the model, a hypothetical patient’s health state (defined by CD4 count and HIV RNA) in any given monthly cycle predicts progression of HIV disease; in the absence of effective ART, HIV RNA level determines the monthly decline in CD4 cell count, resulting in increased risks of opportunistic disease and chronic HIV-related mortality.17, 18 Mortality risks accrue from opportunistic diseases, chronic-HIV-related causes, and non-HIV-related causes.18, 19
In accordance with South African treatment standards, patients with diagnosed HIV infection who are successfully linked to care attend quarterly clinic visits and receive bi-annual CD4 counts.20 When treatment criteria are met (either CD4 <350/µl or WHO Stage 4 disease), ART is initiated.20, 21 According to South African treatment guidelines, we assume all patients receive the same first-line (tenofovir/lamivudine/efavirenz) and second-line (zidovudine/lamivudine/lopinavir/ritonovir) regimens.20 Successful HIV RNA suppression leads to increased CD4 count and a concomitant reduction in the risk of opportunistic disease and death.18, 22, 23 Patients experiencing virologic suppression on these regimens have an ongoing risk of treatment failure, resulting in virologic rebound and CD4 count decline. In the absence of HIV RNA monitoring, detection of treatment failure, based on observation of a 30% CD4 decline from peak or a severe opportunistic disease while receiving ART, triggers a switch to the second (and final) available ART regimen, on which the patient remains until death.24
Screening Model
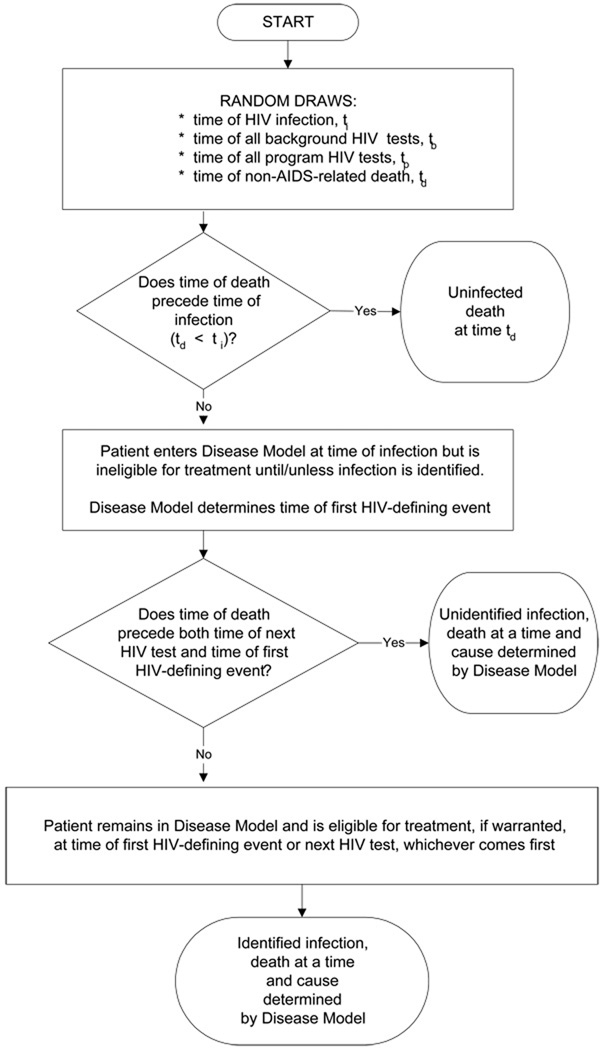
Entry into the disease model is regulated by a population-level model of HIV detection that captures HIV prevalence and incidence (see Technical Appendix).25, 26 Briefly, this model allows users to define characteristics (age, sex, CD4 count, HIV RNA level, history of opportunistic disease) of patients who are already HIV-infected at the simulation outset. Given the demographic characteristics of the HIV-negative subpopulation and the user-defined incidence of HIV infection, the model determines if and when a new HIV infection occurs.
Undiagnosed cases of HIV infection may be detected by one of three mechanisms: 1) “background testing,” as occurs currently in VCT sites, tuberculosis clinics, sexually transmitted infection centers, or antenatal clinics; 2) presentation with an AIDS-defining opportunistic disease; or 3) an expanded routine HIV screening program. In this study, “current practice” is defined as detection via mechanisms 1 and 2, but not 3. To be conservative with respect to the value of expanded screening, the model assumes that detection via either background testing or opportunistic disease development is completely accurate and always results in successful linkage to care. Expanded screening programs, in contrast, have “leakage” based on user-specified test acceptance and linkage-to-care rates. While all HIV-infected individuals enter the disease model, only those who are HIV-tested, diagnosed, linked to care, and meet eligibility criteria receive ART and opportunistic disease prophylaxis. Likewise, only patients with an HIV diagnosis who link to care accrue HIV-related costs. This is because costs captured in the model are intended to represent services that would only incur with an HIV diagnosis.
In an attempt to quantify the impact of stigma on psychosocial health, we first assign quality of life decrements to HIV testing itself (“test-associated stigma”), regardless of the HIV serostatus of the individual tested. In the base case, we assign a high degree of test-associated stigma by basing the decrement on quality of life values associated with the anxiety of waiting for a confirmatory test result following a positive screen (Table 1).27 The reported decrement in quality of life is 0.32, measured over the course of a single week. To spread this value evenly over the one-month cycle time used in the model, we used a monthly decrement of 0.32/4 = 0.08. The quality of life decrement associated with the HIV test applies only to those who are tested and only in the month of testing. Recognizing the scarcity of data on “test-associated stigma” either in the US or in South Africa, we consider in sensitivity analyses quality of life decrement values ranging from 0.0 to 1.0 in the month of testing.
Table 1.
Model input parameters.
| Variable | Base Case Value (SD) |
Range Examined | Reference |
|---|---|---|---|
| Baseline cohort characteristics | |||
| HIV prevalence (%) | |||
| Asymptomatic, chronic HIV+ | 11.4 | 2.6–17.6% | 39 |
| Symptomatic, chronic HIV+ | 5.2 | 1.2–8.0% | 39 |
| Acute, primary HIV infection | 0.3 | 0.05–0.4% | Assumption |
| Total | 16.9 | 3.9–26.0% | 5, 29 |
| Annual HIV incidence (%) | 1.3 | 0.4–1.6% | 5, 29 |
| Age, mean years ± SD | 32.8 ± 9.2 | 18 | |
| Male subjects (%) | 54.6 | 18 | |
| HIV testing protocols | |||
| Average background HIV test frequency | Every 10 years | 0–10 years, detection by OD | 39 |
| Sensitivity* (%) | 99.6 | 37 | |
| Specificity* (%) | 98.0 | 37 | |
| Test acceptance rate (%) | 48.6 | 20–100 | 32 |
| Rate of HIV-infected return for test results and linkage to care (%) | 46.8 | 20–100 | 39 |
| Distribution of initial CD4, mean cells/µl (SD) | |||
| Acute, primary HIV infection† | 534 (164) | 10 | |
| Chronic HIV infection‡ | 288 (331) | 288–320 | 39 |
| HIV RNA distribution (%) | 35 | ||
| >100,000 copies/ml | 42.5 | 0–100 | |
| 30,001 – 100,000 copies/ml | 28.3 | 0–100 | |
| 10,001 – 30,000 copies/ml | 17.9 | 0–100 | |
| 3,001 – 10,000 copies/ml | 7.8 | 0–100 | |
| 501 – 3,000 copies/ml | 2.3 | 0–100 | |
| <500 copies/ml | 1.2 | 0–100 | |
| Natural history of disease | |||
| Mean monthly CD4 cell decline by HIV RNA level (cells/µl) | 17 | ||
| >30,001 – 100,000 copies/ml | 6.4 | ||
| 10,001 – 30,000 copies/ml | 5.4 | ||
| 3,001 – 10,000 copies/ml | 4.6 | ||
| 501 – 3,000 copies/ml | 3.7 | ||
| <500 copies/ml | 3.0 | ||
| Percent monthly risk of severe opportunistic diseases§ (%) | 18 | ||
| Bacterial | 0.08–0.71 | ||
| Fungal | 0.02–2.22 | ||
| Tuberculosis | 0.21–1.96 | ||
| Toxoplasmosis | 0.00–0.06 | ||
| Non-tuberculous mycobacteriosis | 0.00–0.30 | ||
| Pneumocystis jiroveci pneumonia | 0.00–0.12 | ||
| Other WHO stage 4-defining diseases | 0.25–2.57 | ||
| Percent monthly risk of mild opportunistic diseases (%) | 18 | ||
| Fungal | 0.59–3.51 | ||
| Other | 2.51–3.10 | ||
| Efficacy of co-trimoxazole (% reduction in probability of infection) | |||
| Severe bacterial | 49.8 | 30, 38 | |
| Mild fungal infections‖ | −46.4 | 30, 38 | |
| Toxoplasmosis | 83.3 | 30, 38 | |
| Pneumocystis jiroveci pneumonia | 97.3 | 42 | |
| Other WHO stage 4-defining diseases | 17.9 | 30 | |
| Efficacy of ART (% patients with HIV RNA suppression, CD4 increase at 48 weeks) | |||
| First line (NNRTI + 2 NRTIs) | 75%, 168 cells/µl | 42%–84% | 22, 23 |
| Second line (PI + 2 recycled NRTIs) | 75%, 168 cells/µl | 35%–71% | 22, 23 |
| Stigma (quality of life decrement)¶ | |||
| Test-associated stigma | 0.08 | 0.00–1.00 | 27 |
| Diagnosis-associated stigma | 0.07 | 0.00–1.00 | 28 |
| Discount Rate | 3% | 0%–3% | 14 |
| Costs (2006 US$) | |||
| HIV testing | |||
| Rapid HIV test | 1.20 | 1–10 times base case | 32 |
| Confirmatory rapid test | 1.78 | 1–10 times base case | 32 |
| Pre-test counseling | 3.51 | 1–10 times base case | 32 |
| HIV care | |||
| Co-trimoxazole prophylaxis (monthly) | 1.02 | 34 | |
| First-line ART (monthly) | 18 | 36 | |
| Second-line ART (monthly) | 49 | 36 | |
| Minor drug toxicity | 11 | 18, 33, 34 | |
| Major drug toxicity | 1,548 | 18, 33, 34 | |
| Routine care (range by CD4, monthly) | 9.85–129.41 | 1–10 times base case | 18, 31, 33 |
| Inpatient hospital care, per day | 221.14 | 1–10 times base case | 33 |
| Outpatient hospital care, per visit | 11 | 1–10 times base case | 33 |
| CD4 count test | 9 | 1–10 times base case | 34 |
| HIV RNA test | 47 | 1–10 times base case | 34 |
SD: Standard deviation; OD: Opportunistic disease; WHO: World Health Organization;
ART: Antiretroviral therapy; NNRTI: non-nucleoside reverse transcriptase inhibitor;
NRTI: nucleoside reverse transcriptase inhibitor; PI: protease inhibitor
Sensitivity and specificity refer to the characteristics of a single rapid test, not the confirmatory process; test specificity is assumed to be zero during the acute infection window period (approximately 2 months)
Starting CD4 cell count for incident cases
Starting CD4 cell count, on average, for prevalent cases
Range indicated by CD4 count; details on risk by CD4 stratum are provided in the technical appendix.
The percent monthly risk of mild fungal infections is increased by 46.4% in the presence of co-trimoxazole.30
Quality of life decrement reduces quality of life on a 0.00–1.00 scale where 1.00 represents perfect health and 0.00 represents death. By intentionally limiting quality of life decrements to these two forms of stigma (and assuming all other health states are “perfect” = 1.00), we bias results against HIV screening.
We also consider the stigma associated with a known diagnosis of HIV infection (“diagnosis-associated stigma”). To account for potentially diminished psychosocial health, we apply a quality of life penalty -- derived from the decrement associated with a new diagnosis of asymptomatic HIV infection. The quality of life decrement associated with diagnosis-associated stigma is applied starting in the month when a patient receives an HIV diagnosis (Table 1); this monthly decrement is maintained until death.28 This penalty is imposed regardless of whether the patient links to care or is initiated on antiretroviral therapy. The impact of diagnosis-associated stigma is also examined in sensitivity analyses.
We recognize that stigma is one of many factors that may diminish quality of life in HIV disease, including opportunistic infections and treatment-related toxicities. We sought to understand the magnitude of the potential stigma-related harms due to HIV testing. Accordingly, we portrayed both the test- and disease-associated stigma effects in an isolated and deliberately unfavorable light. We therefore considered the hypothetical case where all patients who have not been tested for HIV enjoy a quality of life equivalent to perfect health (i.e., the quality adjustment value assigned to all untested health states is 1.0). This is not to deny the reality that quality of life in HIV is influenced by a host of additional considerations, including opportunistic infections and treatment-related toxicities. Rather, it is to examine just how great an impact stigma alone might reasonably exert.
Input Parameters (Table 1)
Data on the population and clinical characteristics of the simulated cohort are derived from several South African studies, including the Cape Town AIDS Cohort (CTAC).18 We first perform the simulation with 16.9% HIV prevalence and 1.3% annual incidence (adults aged 15–49 years) based on national estimates from the South Africa Human Sciences Research Council (base case).5, 29 We then repeat the analyses for province-specific prevalence and incidence estimates.5
Test acceptance (49%) and linkage-to-care rates (47%) for those identified are derived from ongoing South Africa testing programs and are, by design, lower than the 64–69% test acceptance rate found in a 2008 South African survey where voluntary HIV screening was routinely offered.5, 32, 39 The potential for accepting the offer of any given test is likely correlated with prior test acceptance behavior. Lacking any data on such conditional behavior, we assume average participation (49%) each time a test is offered and consider alternative values ranging from 0–100% in sensitivity analysis. We assume a point-of-care rapid HIV test, with 99.6% sensitivity and 98.0% specificity.37 Reactive results are confirmed via a second rapid test.32
Individuals enter the simulation with a mean age of 32.8 (± 9.2) years.18 Patients with prevalent HIV infection have CD4 count and HIV RNA distribution parameters defined by South African cohorts (Table 1).18, 35 We estimate the proportion of HIV-infected individuals in the acute and chronic stages of disease based on published reports on the natural history of HIV-infected patients in South Africa.40, 41
The median CD4 cell count of chronically HIV-infected persons in the cohort is 288/µl (SD 331/µl). Data on natural history and on cotrimoxazole prophylaxis efficacy have been previously described.17, 18, 38, 42 Mean monthly CD4 cell count decline, determined by HIV RNA level, ranges from 3.0–6.4 cells/µl.17 Persons initiate ART with a CD4 count <350/µl or if they present with an AIDS-defining opportunistic disease.20, 21 ART-eligible patients receive a first-line non-nucleoside reverse transcriptase inhibitor-based regimen followed by a second-line regimen using a boosted protease inhibitor. In the absence of clinical trial or large cohort data reporting the efficacy of 2nd-line ART in South Africa, we assumed the same efficacy for 1st and 2nd-line ART (48-week suppression of 75%).22 CD4 cell counts, among those with successful virologic suppression, increase by 168 cells/µl in the first 48 weeks, followed by an average increase of 3 cells/µl/month until virologic failure.23
We derive the direct medical costs of HIV care (clinic visits, inpatient days, and monitoring tests) using healthcare utilization and unit costs in the CTAC cohort.31, 33 ART costs are from the Clinton Foundation HIV/AIDS Initiative (CHAI) 2009 list of negotiated prices for generic drugs in resource-limited settings (Table 1).36 The costs of HIV test kits and post-test counseling and referral are applied for patients who receive testing through a routine screening program; these tests costs are not incurred for patients diagnosed with HIV via background testing or development of an opportunistic disease.32
RESULTS
Base Case
Clinical and Economic Impact of Screening
In the base case, the discounted quality-adjusted life expectancy of HIV-infected individuals is 180.6 months (15.1 years, undiscounted 285.6 months, or 23.8 years) and the average discounted life expectancy in the overall population is 213.7 months (17.8 years, undiscounted 354.3 months, or 29.5 years) (Table 2). This population life expectancy is consistent with that reported in South Africa.19 The addition of a single, one-time HIV screen increases quality-adjusted life expectancy to 184.9 months (undiscounted 291.9 months) for HIV-infected individuals and 215.7 months (undiscounted 357.6 months) in the overall population. Screening every five years or annually increases the discounted quality-adjusted life expectancy of HIV-infected persons to 187.6 and 197.2 months (undiscounted 298.3 and 317.2 months). Compared to current practice, all screening strategies increase mean CD4 count at the time of HIV detection.
Table 2.
Base case results and selected sensitivity analyses of an HIV screening analysis in South Africa.
| HIV Screening Frequency | ||||
|---|---|---|---|---|
| Current Practice* |
One-time | Every 5 years |
Annually | |
| Base Case, South Africa | ||||
| Prevalence 16.9%, Annual Incidence 1.3% | ||||
| HIV+ Population | ||||
| Undiscounted per person quality-adjusted life expectancy (months) | 285.6 | 291.9 | 298.3 | 317.2 |
| Discounted per person quality-adjust life expectancy (months) | 180.6 | 184.9 | 187.6 | 197.2 |
| Mean CD4 at detection (/µl) | ||||
| Prevalent cases | 195 | 233 | 235 | 259 |
| Incident cases | 337 | 337 | 357 | 410 |
| General Population | ||||
| Undiscounted per person quality-adjusted life expectancy (months) | 354.3 | 357.6 | 361.0 | 371.2 |
| Discounted per person quality-adjusted life expectancy (months) | 213.7 | 215.7 | 216.8 | 221.0 |
| Per person costs ($) | 2,330 | 2,570 | 2,740 | 3,330 |
| Cost-effectiveness ratio ($/QALY)§‖ | ----- | dominated† | 1,570 | 1,720 |
| Sensitivity Analysis, Western Cape | ||||
| Prevalence 5.3%, Annual Incidence 0.4% | ||||
| HIV+ Population | ||||
| Undiscounted per person quality-adjusted life expectancy (months) | 314.9 | 320.2 | 326.3 | 344.6 |
| Discounted per person life expectancy (months) | 194.7 | 198.0 | 199.7 | 209.6 |
| General Population | ||||
| Undiscounted per person life expectancy (months) | 420.3 | 421.2 | 422.2 | 424.7 |
| Discounted per person life expectancy (months) | 243.4 | 243.9 | 244.1 | 245.1 |
| Per person costs ($) | 830 | 910 | 980 | 1,220 |
| Cost-effectiveness ratio ($/QALY)§‖ | ----- | 1,650 | dominated† | 3,090 |
| Sensitivity Analysis, KwaZulu Natal | ||||
| Prevalence 25.8%, Annual Incidence 2.0% | ||||
| HIV+ Population | ||||
| Undiscounted per person life expectancy (months) | 264.6 | 271.9 | 277.9 | 297.2 |
| Discounted per person life expectancy (months) | 170.1 | 175.0 | 176.6 | 187.9 |
| General Population | ||||
| Undiscounted per person life expectancy (months) | 313.5 | 318.1 | 321.7 | 334.2 |
| Discounted per person life expectancy (months) | 194.4 | 197.5 | 198.3 | 204.8 |
| Per person costs ($) | 3,240 | 3,610 | 3,830 | 4,630 |
| Cost-effectiveness ratio ($/QALY)§‖ | ----- | dominated† | dominated† | 1,590 |
QALY: Quality-adjusted life-year
In the absence of routine screening, HIV infection is detected via background screening (on average, every ten years) or with the development of one of the following severe opportunistic infections: Isosporiasis, Mycobacterium avium complex, Toxoplasmosis, Pneumocystis jirovecii pneumonia (PCP), and other WHO stage 4-defining non-bacterial diseases.
“dominated” strategies are eliminated because they cost more and deliver fewer years of life saved than the comparative combination of strategies.14
In order to accentuate the effects of HIV-related stigma, it is assumed that all health states prior to HIV testing have quality of life equivalent to perfect health. Quality of life decrements are then applied only to states involving either an HIV test or time spent living with an HIV diagnosis.
Cost-effectiveness ratios were calculated prior to rounding quality-adjusted life expectancy and costs.
Expanded screening (one-time, every five years, and annually) increases average per person lifetime costs from $2,330 for current practice to $2,570, $2,740 and $3,330. The fraction of these total per person costs that are directly related to screening range from 0.1% (one-time screen) to 0.3% (screening every five years) to 1.0% (annual screening). The incremental cost-effectiveness of every five year screening weakly dominates one-time screening, with a ratio of $1,570/quality-adjusted life year (QALY), compared to current practice. Compared to screening every five years, annual screening has an incremental cost-effectiveness ratio of $1,720/QALY. Both of these strategies are economically attractive when compared to the WHO’s benchmark of South Africa’s per capita GDP.15
Mechanisms of Detection
Under current practice, 25% of infected persons are detected via development of a severe opportunistic disease (Figure 1). Screening once, every five years, or annually decreases the proportion of HIV-infected individuals detected via severe opportunistic disease to 22%, 20%, or 13%. The percent detected via background or expanded screening increases from 31% with current practice to 66% with annual screening.
FIGURE 1.
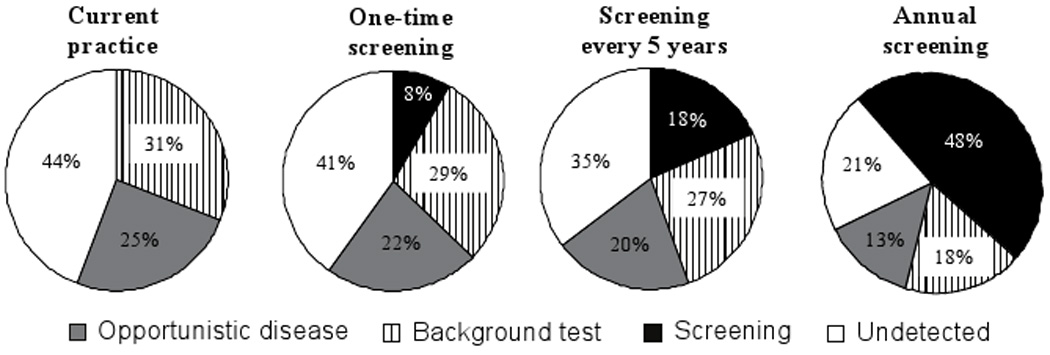
Mechanisms of HIV detection in South Africa.
Other HIV Prevalence and Incidence Populations
For any given screening strategy, the potential gains in life expectancy are lowest in areas with low baseline HIV prevalence and incidence (Table 2, bottom).5, 29 Yet even in the Western Cape, the province with the lowest rates of HIV infection, screening annually maintains a favorable cost-effectiveness ratio ($3,090/QALY).
Sensitivity Analyses
ART Initiation Thresholds as a Surrogate for Limited ART Access
For HIV-infected individuals, the discounted quality-adjusted life expectancy benefit associated with annual HIV screening is 16.6 months compared to current practice when there is adequate ART capacity to treat all those with CD4 <350/µl, but decreases to 4.1 months when there is only sufficient ART capacity to treat those with CD4 <50/µl (Figure 2, sum of black, white, and hatched bars). The synergy between effective screening, linkage-to-care and timely ART initiation is illustrated by the greater life expectancies that can be achieved via current screening practices and ART initiation at <200/µl than by annual screening with ART initiation at <50/µl as noted by the dashed, horizontal line in the figure. In a threshold analysis, annual screening compared to screening every five years remains cost-effective ($3,750/QALY) even if expanded screening leads to restricted ART availability for only those with CD4 <100/µl (data not shown).
FIGURE 2.
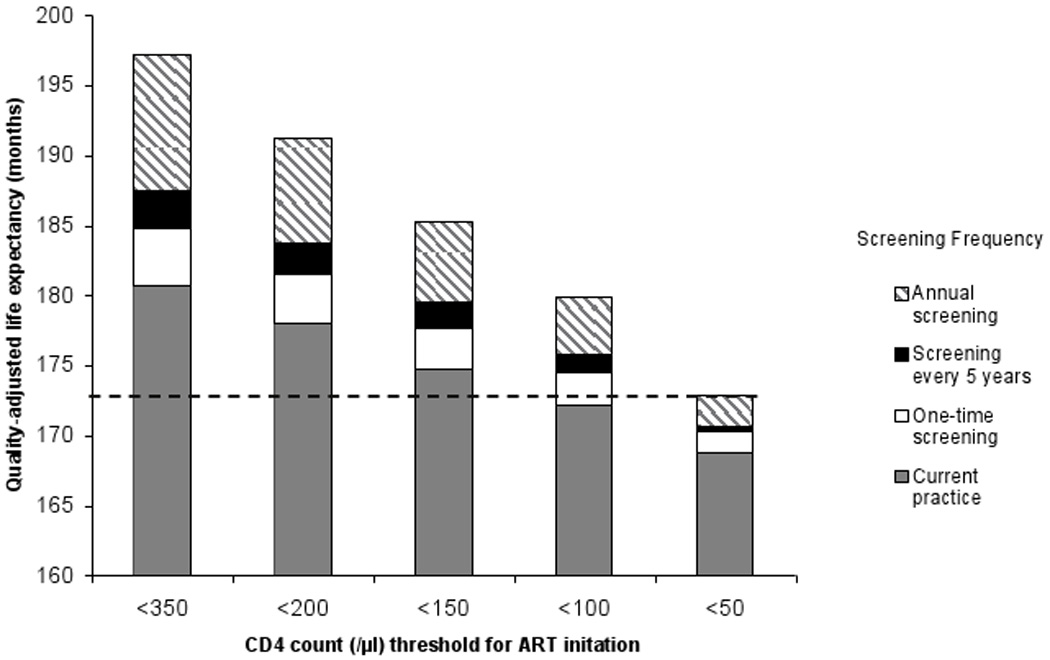
Quality-adjusted life expectancy for HIV-infected individuals under varying thresholds for ART initiation and HIV screening frequencies.
Limited Test Acceptability and Incomplete Linkage to Care
In a two-way sensitivity analysis, we vary both the test acceptance and linkage-to-care rates from 20% to 100% in 20% increments (25 unique combinations). At the combination with the poorest screening program performance (20% acceptance/20% linkage-to-care), annual HIV screening provides a discounted per person quality-adjusted life expectancy for HIV-infected individuals of 184.7 months (4.1 months more than current practice) and maintains a favorable cost-effectiveness ratio of $2,110/QALY compared to screening every five years (Technical Appendix, Table A2b).
Test-associated Stigma and Diagnosis-associated Stigma
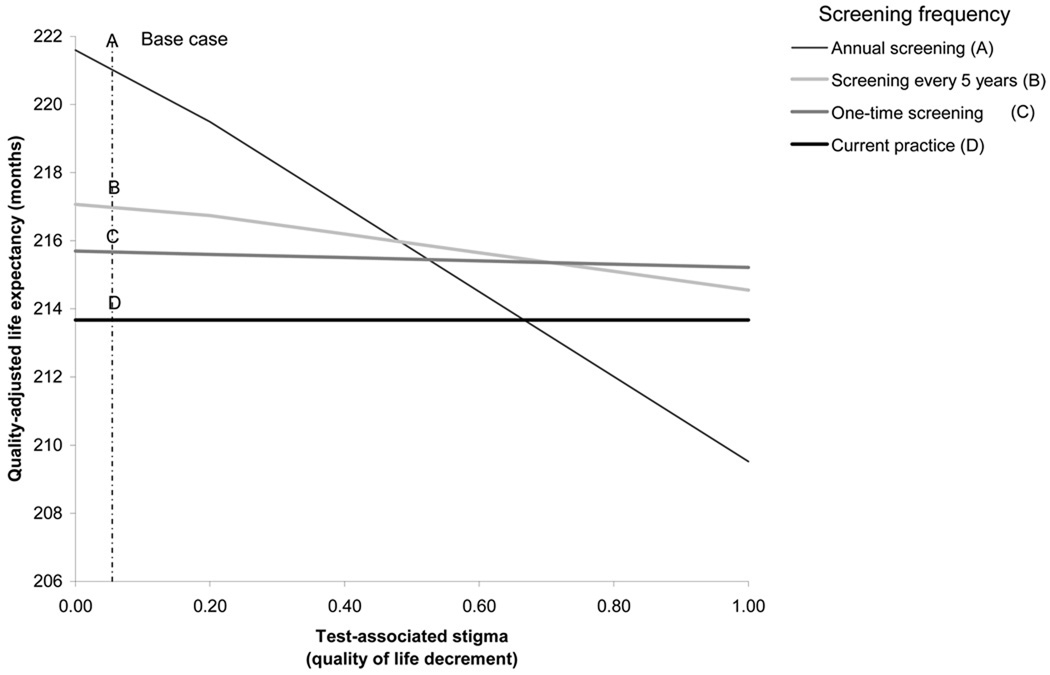
In Figure 3, we enumerate all possible combinations of test-associated and diagnosis-associated stigma that would yield the same clinical outcomes as those obtained under current practice. For example, when test-associated stigma leads to a 0.33 quality of life decrement for one month, the diagnosis-associated stigma decrement could also be as high as 0.34 before losing the clinical benefit of an annual screening program (Arrow). These quality of life decrements are approximately four- to five-fold higher than those used in the base case analysis. One-way sensitivity analysis on the impact of test-associated and diagnosis-associated stigma on quality-adjusted life expectancy are provided in Technical Appendix, Figure A2. When each stigma parameter is varied individually, screening annually remains very cost-effective provided that the test-associated stigma decrement is less than 0.35 in the month of the test ($5,390/QALY) and the monthly diagnosis-associated stigma decrement is less than 0.41, applied for the duration of the lifetime following an HIV diagnosis ($5,360/QALY).
FIGURE 3.
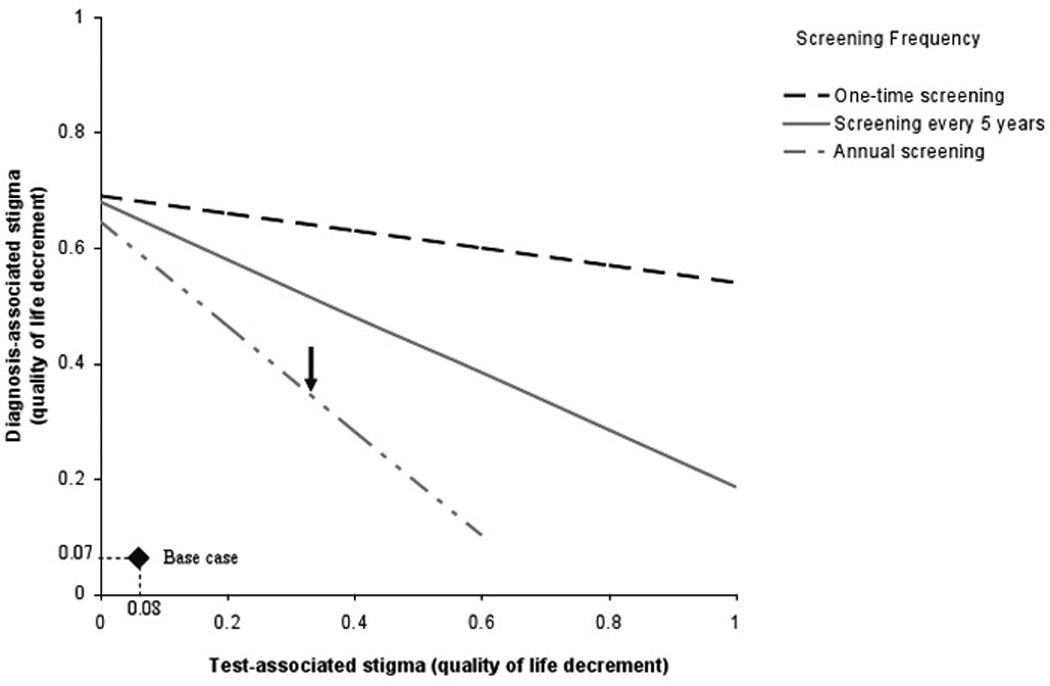
Two-way sensitivity analysis on test-associated and diagnosis-associated stigma.
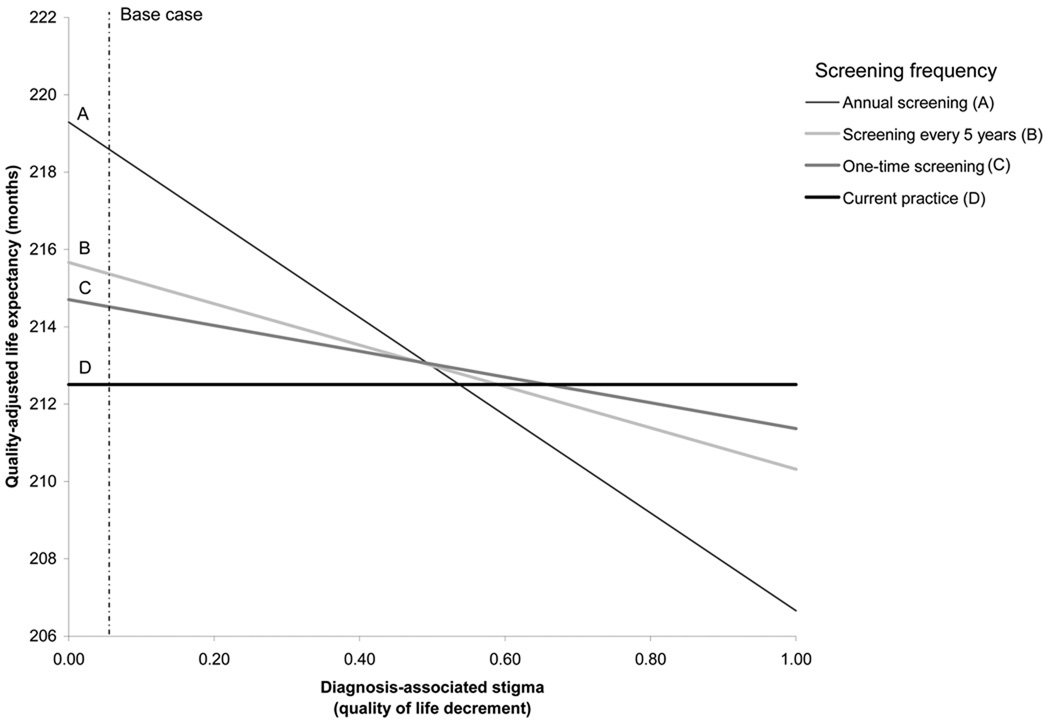
FIGURE A2.
a: One-way sensitivity analysis on the impact of test-associated stigma on quality-adjusted life expectancy.
b: One-way sensitivity analysis on the impact of diagnosis-related stigma on quality-adjusted life expectancy.
Other Sensitivity Analyses
Results remain robust in sensitivity analyses of other parameters, including increasing test costs (up to ten-fold higher than the base case) and treatment costs (up to two-fold higher than the base case); incorporating additional clinical triggers leading to HIV diagnosis in the absence of screening (including tuberculosis); increasing background testing rates (up to once every five years); changing the mean age at cohort initiation (23 years to 43 years); increasing ART efficacy; and including a third-line regimen. Please see Technical Appendix for details.
DISCUSSION
Annual routine HIV screening in South Africa increases the per person quality-adjusted life expectancy of an HIV-infected individual by 16.6 months, even when assuming highly constrained rates of acceptance and linkage-to-care. Annual screening is also very cost-effective ($1,720/QALY),15 a result that remains stable (i.e., below the “very cost-effective” South African GDP threshold of $5,400/QALY) at the lowest reported provincial rates of HIV prevalence/incidence, or when we assume rates as low as 20% for both test acceptance and linkage-to-care and with testing costs greater than ten-fold higher than in the base case.5, 29 The stability of these cost-effectiveness findings reflects the fact that it is effective antiretroviral therapy, and not screening per se, that is the major determinant of both the clinical and economic impact of screening. Thus, the cost-effectiveness ratio of annual HIV screening is only slightly higher than the cost-effectiveness ratio for routine HIV care in South Africa (~$1,200/QALY).43 While expanded screening at any level would improve upon the current status quo, for clinical outcomes, implementation of an intensive annual routine screening program is also very cost-effective, and will provide nearly as good value for money as screening once or every five years.
This analysis underscores the WHO’s recommendations that HIV screening should be associated with “assurances of linkage between the site where the test is being conducted and relevant treatment, care and other services,”44 emphasizing that case detection alone is insufficient to yield improvement in clinical outcomes. We therefore critically examine the impact of a screening program in the setting of both incomplete ART access (Figure 2) and poor rates of linkage to care. We find that annual HIV screening leading to earlier case detection – even in the setting of insufficient resources for guideline-concordant ART – still improves clinical outcomes and is cost-effective as long as ART is initiated at a CD4 count <100/µl or higher. Moreover, because the clinical benefits to those linked to care are so dramatic, linkage rates need only be 20% to continue to demonstrate cost-effectiveness.
The results are also robust to test costs up to ten-fold higher than those used in the base case. Thus, variations in screening programs by site, by testing algorithm, or by operational differences in counseling and referral are unlikely to have an impact on the general results. The stability of the results under these conditions, combined with sensitivity analyses using data from three different South African regions, with varying prevalence/incidence rates, suggests the likely generalizability of our results even outside the South African setting in countries with generalized HIV epidemics.
A persistent challenge to HIV screening is the impact of stigma on health-related quality of life, both as it pertains to the act of obtaining an HIV test and to living with known HIV infection. The very favorable cost-effectiveness findings remain unchanged, even when we assume that the psychosocial impact of testing- and HIV-related stigma on quality of life are four-fold more detrimental than those reported in the literature. Furthermore, South African data collected in the context of increasing ART availability report decreases in stigma over time; <1% of survey respondents in 2005 report fear of non-confidentiality, stigma or job loss as their reason for not being tested.45
Despite concerns regarding stigma and the ethics of routine HIV screening, when concerted efforts and resources are dedicated to HIV case identification, the results are clear. Routine HIV screening in one South African urgent care center resulted in a case identification rate of 39 cases per week (newly diagnosed prevalence of 33%).32 Other approaches to routine screening have also proven feasible in other resource-limited settings, including: large public health campaigns (Botswana);46 testing of male partners in the antenatal period (Uganda);47 home-based testing (Uganda, Zambia);48, 49 and testing in combination with other health care services (Haiti).50
There are several limitations to this analysis. First, we have not included any transmission benefits associated with HIV screening. A recent meta-analysis found that HIV VCT clients in resource limited settings were 70% less likely to engage in unprotected sex compared to those without access to VCT.51 Results of this analysis would be even more favorable if additional benefits in risk reduction and transmission were achieved among both HIV-infected and uninfected individuals. Second, cost-effectiveness analysis is an inappropriate tool with which to evaluate the ethical considerations associated with resource allocation policies for HIV prevention and treatment.52 In a resource-limited setting, affordability is as important as cost-effectiveness. While budget impact is beyond the scope of the present paper, it merits more attention than it has received to date in the HIV-related literature.53 Although many univariate and multi-way assessments were conducted, a more complete sensitivity analysis might examine the question of cost-effectiveness over a range of willingness-to-pay thresholds and examine the simultaneous interaction of all input parameters. Recognizing the difficulties of conducting this type of analysis alongside first-order, Monte Carlo microsimulation, we have adhered to the guidance of both the US Panel on Cost-effectiveness in Health and Medicine and the ISPOR Task Force on Good Modeling Practice with regard to the appropriate use of deterministic methods in sensitivity analysis.14, 54, 55
While we use lifetime projections to forecast the optimal HIV screening policy recommendations today, secular changes in South Africa will require a reevaluation of these results over a five- to ten-year time horizon. Finally, routine screening will require additional health care infrastructure. This analysis, by convention, excluded fixed costs associated with scaling up a routine screening program, but sensitivity analyses examined a possible surrogate for their inclusion -- a two-fold increase in total HIV care costs -- and still found annual testing to be very cost-effective.14
As researchers worldwide begin to address the potential of “HIV treatment as prevention” strategies, an initial critical question is whether frequent HIV screening is clinically beneficial and economically viable.56, 57 Annual screening in South Africa is likely not immediately achievable; it will require dedicated political will and financial investment. Although cost-effectiveness is just one of many criteria for policy decision-making, when used to understand the impact and value of routine HIV screening in South Africa, the conclusions are clear. Case identification is required to access life-saving antiretroviral therapy; HIV screening in South Africa would provide more clinical benefit than virtually any other screening program for any disease that has been reported.58 The unchecked HIV epidemic in South Africa warrants a major and comprehensive response. That response should include frequent routine, voluntary HIV screening, ensuring that those identified as HIV-infected receive the highly effective antiretroviral therapy that is currently the standard of HIV care in South Africa.
FIGURE A1.
Conceptual framework for the Screening Model
ACKNOWLEDGEMENTS
All listed co-authors meet criteria for authorship. Specifically, the following contributions were made by the authors listed parenthetically: Conception and design (RPW, KAF, ADP); acquisition of data (RPW, RW, NM, MDA, IVB); analysis and interpretation of data (RPW, RW, MOF, NM, EL, MDA, BLM, KAF, ADP); drafting of the manuscript (RPW, ADP); critical revision of manuscript (all); statistical analysis (RW, NM, EL); obtaining funding (RPW, KAF, ADP); administrative, technical, and material support (MOF, BLM); and supervision (RPW, KAF, ADP). No authors have financial conflicts to disclose.
We are indebted to the entire CEPAC-International team and investigators for their contributions, including Xavier Anglaret (Lead Co-Investigator: Programme PACCI, Abidjan, Côte d’Ivoire); Yazdan Yazdanpanah (Lead Co-Investigator: Service Universitaire des Maladies Infectieuses et du Voyageur, Centre Hospitalier de Tourcoing, EA 2694, Faculté de Médecine de Lille, and Laboratoire de Recherches Économiques et Sociales, Centre National de la Recherche Scientifique Unité de Recherche Associée 362, Lille, France); Nagalingeswaran Kumarasamy and Kenneth Mayer (Lead Co-Investigators: Y.R. Gaitonde Centre for AIDS Research & Education, Chennai, India); C. Robert Horsburgh (Boston University School of Public Health, Boston, MA, USA), Sue Goldie, George R. Seage III, Milton C. Weinstein (Harvard School of Public Health, Boston, MA, USA); and Caroline Sloan, Lauren Uhler (Massachusetts General Hospital, Boston, MA, USA).
This research was funded by the National Institute of Allergy and Infectious Diseases (R01 AI058736, K24 AI062476, K23 AI068458, P30 AI060354), the National Institute of Mental Health (R01 MH065869) and the Doris Duke Charitable Foundation (Clinical Scientist Development and ORACTA Awards). The funding sources had no role in the design, analysis, or interpretation of the study or in the decision to submit the manuscript for publication. Dr. Walensky had access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These data have previously been presented at the 16th Conference on Retroviruses and Opportunistic Infections (CROI), February 8–11, 2009 in Montreal, Quebec, Canada.
LITERATURE CITED
- 1.UNAIDS/WHO. Guidance on provider-initiated HIV testing and counselling in health facilities. [Accessed July 29, 2010];Geneva: World Health Organization; 2007 Available at: http://whqlibdoc.who.int/publications/2007/9789241595568_eng.pdf.
- 2.President's Emergency Plan for AIDS Relief (PEPfAR) FY 2008 country profile: South Africa. [Accessed July 30, 2010]; Available at: http://www.pepfar.gov/documents/organization/116231.pdf.
- 3.World Health Organization. Summary country profile for HIV/AIDS treatment scale-up: South Africa. [Accessed July 31, 2010];2005 Available at: http://www.who.int/hiv/HIVCP_ZAF.pdf.
- 4.AVERT. HIV and AIDS in South Africa. [Accessed March 5, 2010]; Available at: http://www.avert.org/aidssouthafrica.htm.
- 5.Shisana O, Rehle T, Simbayi LC, Parker W, Zuma K, Bhana A, Connolly C, Jooste S, Pillay-van-Wyk V, Mbelle N, Van Zyl J, Parker W, Zungu NP, Pezi S the SABSSM III Implementation Team. South African national HIV prevalence, incidence, behaviour and communication survey 2008: A turning tide among teenagers? Cape Town: HSRC Press; 2009. [Google Scholar]
- 6.News24. Still no money for ARV patients. [Accessed July 30, 2010];News24 Special Report: AIDS Focus South Africa. 2009 Available at: http://www.news24.com/News24/South_Africa/Aids_Focus/0,,2-7-659_2463652,00.html.
- 7.Campbell C, Foulis CA, Maimane S, Sibiya Z. "I have an evil child at my house": stigma and HIV/AIDS management in a South African community. Am J Public Health. 2005;95(5):808–815. doi: 10.2105/AJPH.2003.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahajan AP, Sayles JN, Patel VA, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS. 2008;22 Suppl 2:S67–S79. doi: 10.1097/01.aids.0000327438.13291.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rankin WW, Brennan S, Schell E, Laviwa J, Rankin SH. The stigma of being HIV-positive in Africa. PLoS Med. 2005;2(8):e247. doi: 10.1371/journal.pmed.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walensky RP, Wood R, Weinstein MC, et al. Scaling up antiretroviral therapy in South Africa: the impact of speed on survival. J Infect Dis. 2008;197(9):1324–1332. doi: 10.1086/587184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedberg KA, Kumarasamy N, Losina E, et al. Clinical impact and cost-effectiveness of antiretroviral therapy in India: starting criteria and second-line therapy. AIDS. 2007;21 Suppl 4:S117–S128. doi: 10.1097/01.aids.0000279714.60935.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings--the case of Côte d'Ivoire. N Engl J Med. 2006;355(11):1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 13.Walensky RP, Weinstein MC, Yazdanpanah Y, et al. HIV drug resistance surveillance for prioritizing treatment in resource-limited settings. AIDS. 2007;21(8):973–982. doi: 10.1097/QAD.0b013e328011ec53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 15.WHO. CHOosing interventions that are cost-effective (WHO-CHOICE): Cost-effectiveness thresholds. [Accessed July 31, 2010];Geneva: World Health Organization; 2009 Available at: http://www.who.int/choice/costs/CER_thresholds/en/index.html.
- 16.World development indicators online. [Accessed July 28, 2010];Washington, DC: World Bank Group; Available at: http://ddpext.worldbank.org/ext/DDPQQ/member.do?method=getMembers&userid=1&queryId=135.
- 17.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126(12):946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42(4):464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Life Tables for WHO Member States: South Africa. [Accessed March 12, 2010];2006 Available at: http://apps.who.int/whosis/database/life_tables/life_tables.cfm.
- 20.South Africa National Department of Health. The South African Antiretroviral Treatment Guidelines. [Accessed July 19, 2010];2010 http://www.doh.gov.za/docs/factsheets/guidelines/art.pdf.
- 21.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a public health approach. [Accessed July 19, 2010];2010 http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [PubMed]
- 22.Hammond R, Harry TC. Efficacy of antiretroviral therapy in Africa: effect on immunological and virological outcome measures -- a meta-analysis. Int J STD AIDS. 2008;19(5):291–296. doi: 10.1258/ijsa.2007.007248. [DOI] [PubMed] [Google Scholar]
- 23.Tuboi SH, Brinkhof MW, Egger M, et al. Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resource-constrained countries: the antiretroviral therapy in low-income countries (ART-LINC) collaboration. J Acquir Immune Defic Syndr. 2007;45(1):52–59. doi: 10.1097/QAI.0b013e318042e1c3. [DOI] [PubMed] [Google Scholar]
- 24.South Africa National Department of Health. National antiretroviral treatment guidelines. [Accessed July 30, 2010];2004 Available at: http://hivinsite.ucsf.edu/doc/cr09-sf-01.doc.
- 25.Paltiel AD, Walensky RP, Schackman BR, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145(11):797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 26.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005;352(6):586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 27.Coco A. The cost-effectiveness of expanded testing for primary HIV infection. Ann Fam Med. 2005;3(5):391–399. doi: 10.1370/afm.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352(6):570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 29.Rehle TM, Hallett TB, Shisana O, et al. A decline in new HIV infections in south africa: estimating HIV incidence from three national HIV surveys in 2002, 2005 and 2008. PLoS One. 5(6):e11094. doi: 10.1371/journal.pone.0011094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anglaret X, Chêne G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353(9163):1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 31.Badri M, Cleary S, Maartens G, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther. 2006;11(1):63–72. [PubMed] [Google Scholar]
- 32.Bassett IV, Giddy J, Nkera J, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. J Acquir Immune Defic Syndr. 2007;46(2):181–186. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleary S, Boulle A, McIntyre D, Coetzee D, Cleary S, Boulle A, McIntyre D, Coetzee D. Cost-effectiveness of antiretroviral treatment for HIV-positive adults in a South African township. [Accessed July 31, 2010];Médecins Sans Frontières and the Health Systems Trust. 2004 Available at: http://www.hst.org.za/uploads/files/arv_cost.pdf.
- 34.Gauteng Department of Health. South Africa: Gauteng Hospitals Numeric, Gauteng Province; 2004
- 35.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21(3):335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 36.The Clinton Foundation HIV/AIDS Initiative. Antiretroviral (ARV) Price List. [Accessed July 17, 2010];2009 http://www.clintonfoundation.org/files/chaiarvpricelistaugust2009english.pdf.
- 37.Sauer G, Brand T, Bester R, Beggs M, Arai HX, Janse van Rensburg E. Evaluation of the Abbott Determine HIV-1/2 rapid assay using samples from the Western Cape region, South Africa. Paper presented at: 13th International AIDS Conference; July 9–14, 2000; Durban. [abstract MoPeA2091]. [Google Scholar]
- 38.Yazdanpanah Y, Losina E, Anglaret X, et al. Clinical impact and cost-effectiveness of co-trimoxazole prophylaxis in patients with HIV/AIDS in Côte d'Ivoire: a trial-based analysis. AIDS. 2005;19(12):1299–1308. doi: 10.1097/01.aids.0000180101.80888.c6. [DOI] [PubMed] [Google Scholar]
- 39.April MD, Walensky RP, Chang Y, et al. HIV testing rates and outcomes in a South African community, 2001–2006: implications for expanded screening policies. J Acquir Immune Defic Syndr. 2009;51(3):310–316. doi: 10.1097/qai.0b013e3181a248e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glynn JR, Sonnenberg P, Nelson G, Bester A, Shearer S, Murray J. Survival from HIV-1 seroconversion in Southern Africa: a retrospective cohort study in nearly 2000 gold-miners over 10 years of follow-up. AIDS. 2007;21(5):625–632. doi: 10.1097/QAD.0b013e328017f857. [DOI] [PubMed] [Google Scholar]
- 41.Vanhems P, Hirschel B, Phillips AN, et al. Incubation time of acute human immunodeficiency virus (HIV) infection and duration of acute HIV infection are independent prognostic factors of progression to AIDS. J Infect Dis. 2000;182(1):334–337. doi: 10.1086/315687. [DOI] [PubMed] [Google Scholar]
- 42.Goldie SJ, Kaplan JE, Losina E, et al. Prophylaxis for human immunodeficiency virus-related Pneumocystis carinii pneumonia: using simulation modeling to inform clinical guidelines. Arch Intern Med. 2002;162(8):921–928. doi: 10.1001/archinte.162.8.921. [DOI] [PubMed] [Google Scholar]
- 43.Walensky RP, Wolf LL, Wood R, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151(3):157–166. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.UNAIDS/WHO. WHO policy statement on HIV testing. [Accessed July 29, 2010];Geneva: World Health Organization; 2004 Available at: http://www.who.int/ethics/topics/en/hivtestingpolicy_who_unaids_en_2004.pdf.
- 45.Shisana O, Rehle T, Simbayi LC, Parker W, Zuma K, Bhana A, Connolly C, Jooste S, Pillay V, et al. South African National HIV prevalence, HIV incidence, behaviour and communication survey, 2005. Cape Town: HSRC Press; 2005. [Google Scholar]
- 46.Steen TW, Seipone K, Gomez Fde L, et al. Two and a half years of routine HIV testing in Botswana. J Acquir Immune Defic Syndr. 2007;44(4):484–488. doi: 10.1097/QAI.0b013e318030ffa9. [DOI] [PubMed] [Google Scholar]
- 47.Homsy J, Kalamya JN, Obonyo J, et al. Routine intrapartum HIV counseling and testing for prevention of mother-to-child transmission of HIV in a rural Ugandan hospital. J Acquir Immune Defic Syndr. 2006;42(2):149–154. doi: 10.1097/01.qai.0000225032.52766.c2. [DOI] [PubMed] [Google Scholar]
- 48.Fylkesnes K, Siziya S. A randomized trial on acceptability of voluntary HIV counselling and testing. Trop Med Int Health. 2004;9(5):566–572. doi: 10.1111/j.1365-3156.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- 49.Menzies N, Abang B, Wanyenze R, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23(3):395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 50.Peck R, Fitzgerald DW, Liautaud B, et al. The feasibility, demand, and effect of integrating primary care services with HIV voluntary counseling and testing: evaluation of a 15-year experience in Haiti, 1985–2000. J Acquir Immune Defic Syndr. 2003;33(4):470–475. doi: 10.1097/00126334-200308010-00007. [DOI] [PubMed] [Google Scholar]
- 51.Denison JA, O'Reilly KR, Schmid GP, Kennedy CE, Sweat MD. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990--2005. AIDS and behavior. 2008;12(3):363–373. doi: 10.1007/s10461-007-9349-x. [DOI] [PubMed] [Google Scholar]
- 52.April M. Rethinking HIV exceptionalism: the ethics of opt-out HIV testing sub-Saharan Africa. [Accessed July 29, 2010];Bull WHO. 2010 doi: 10.2471/BLT.09.073049. http://www.who.int/bulletin/09-073049.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin EGPA, Walensky RP, Schackman BR. Expanded HIV screening in the US: What will it cost and who will pay? A budget impact analysis. Value Health. doi: 10.1111/j.1524-4733.2010.00763.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drummond M, Brown R, Fendrick AM, et al. Use of pharmacoeconomics information--report of the ISPOR Task Force on use of pharmacoeconomic/health economic information in health-care decision making. Value Health. 2003;6(4):407–416. doi: 10.1046/j.1524-4733.2003.64245.x. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein MC, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices--Modeling Studies. Value Health. 2003;6(1):9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 56.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 57.Walensky RP, Paltiel AD, Losina E, et al. Test and treat DC: forecasting the impact of a comprehensive HIV strategy in Washington DC. Clin Infect Dis. 2010;51(4):392–400. doi: 10.1086/655130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright JC, Weinstein MC. Gains in life expectancy from medical interventions--standardizing data on outcomes. N Engl J Med. 1998;339(6):380–386. doi: 10.1056/NEJM199808063390606. [DOI] [PubMed] [Google Scholar]