Abstract
Objective
The present study was aimed at developing a new cell-permeant peptide inhibitor (MK2i) of the kinase that phosphorylates and activates HSP27 (MAPKAP kinase II), and evaluating the ability of this peptide to inhibit HSP27 phosphorylation and intimal thickening.
Design of study
The ability of MK2i to reduce HSP27 phosphorylation and cell migration was evaluated in A7R5 cells stimulated with arsenite or lysophosphatidic acid. Stable isotopic labeling using amino acids in cell culture (SILAC), in combination with liquid chromatography mass spectrometry was used to characterize the effect of MK2i on global protein expression in fibroblasts. The effect of MK2i on intimal thickening and CTGF expression was evaluated in human saphenous vein (HSV) rings maintained with 30% FBS for 14 days by light microscopy and immunoblotting.
Results
Pre-treatment of cells with MK2i (10 μM) prior to arsenite or lysophosphatidic acid stimulation decreased phosphorylation of HSP27 (36±9% and 33±10% respectively) compared to control (not pre-treated) cells. MK2i also inhibited A7R5 migration, and downregulated the TGF-induced expression of collagen and fibronectin in keloid cells, two major matrix proteins involved in the development of intimal hyperplasia. Treatment of HSV segments with MK2i enhanced relaxation, reduced HSP27 phosphorylation (40±17%), CTGF expression (17±5%) and intimal thickness (48.2±10.5%) compared to untreated segments. On the other hand, treatment with a recombinant fusion protein containing a cell permeant peptide attached to the HSP27 sequence increased intimal thickness of HSV segments by 48±14%.
Conclusion
Our results suggest that HSP27 may play a role in the development of processes leading to intimal hyperplasia in HSV, and reduction of HSP27 phosphorylation by MK2i may be a potential strategy to inhibit the development of intimal hyperplasia in HSV to prevent the autologous vascular graft failure.
Keywords: intimal hyperplasia, HSP27, intimal thickness, CTGF
INTRODUCTION
Intimal hyperplasia represents the leading cause of prosthetic and autologous vascular graft failure 1. Despite the many recent technological advances in vascular interventions, intimal hyperplasia remains an expensive, morbid, and unsolved problem 2. While incompletely understood, intimal hyperplasia involves a response to injury, and is mediated by a sequence of events that include smooth muscle cell proliferation, migration, phenotypic modulation, and extracellular matrix deposition 3.
Heat-shock protein (HSP) 27 (also termed HSPB1, HSP25) is a member of the family of small heat-shock proteins. The expression and phosphorylation of HSP27 increase with stress. Increases in the phosphorylation of HSP27 are associated with enhanced cell migration 4,5 and with abundant stress fiber formation 6,7, effects attributed to HSP27 stabilization of the actin cytoskeleton. In addition to cell migration, stabilization of the cytoskeleton is also associated with the expression of extracellular matrix proteins 8–10, another key event related to intimal hyperplasia. Therefore, because HSP27 expression and phosphorylation largely influence the cytoskeleton and consequently, events associated with intimal hyperplasia, modulation of its phosphorylation may be a target to prevent intimal hyperplasia.
The goal of the present study was to develop a cell-permeant peptide inhibitor (named MK2i) of the kinase that phosphorylates and activates HSP27, and to evaluate its potential as a new strategy to prevent intimal hyperplasia. HSP27 is phosphorylated by a kinase cascade that involves p38 MAP kinase, which phosphorylates and activates MAPKAP kinase II, which in turn, phosphorylates HSP27. To date, HSP27 is the only heat shock protein known to be phosphorylated by MAPKAP kinase II 11. While small molecule inhibitors of p38 MAP kinase have been developed, toxicity has limited the clinical use of these inhibitors 4. We developed MK2i using a protein transduction domain and a modification of a peptide designed by Hayess and Benndorf that binds to and inhibits the catalytic site of MAPKAP kinase II 12. Protein transduction domains (PTDs) were used because of their ability to carry other peptides, proteins and even small particles, across cell membranes13. In this study, we evaluated the effect of MK2i on the phosphorylation of HSP27, cell migration, global protein expression, and intimal thickening. Our results demonstrate the potential of MK2i to prevent intimal hyperplasia.
MATERIAL AND METHODS
Materials
MK2i (WLRRIKAWLRRIKALNRQLGVAA) was synthesized and purified using standard FMOC chemistry and high pressure liquid chromatography. All chemicals were purchased from Sigma Chemical Co (St Louis, MO) unless specified otherwise.
Cell culture
A7R5 cells (embryonic rat aortic smooth muscle cells) were purchased from ATCC (Manassas, VA, USA) and were grown at 37°C and 5% CO2 in Dulbecco’s modification of Eagle’s medium (DMEM) containing 10% fetal bovine serum, penicillin and streptomycin (1%), in 60 mm2 dishes. When the cells reached 70% confluence, they were growth-arrested by using a media containing 1% BSA for 24 h prior to each experiment. A7R5 cells were chosen because this is a well characterized smooth muscle cell line, often used in cell migration assays, and sensitive to TGF and LPA stimulations, mediators related to the development of intimal hyperplasia 14.
Cell treatment
On the day of each experiment, fresh media (containing 1% BSA) was added to the dishes, and cells were either untreated (control) or pre-treated with MK2i (5, 10 or 20 μM) or with 20 μM SB203580 (SB, a p38 MAP kinase inhibitor, Cal Biochem, San Diego, CA) for 2h. For comparison, cells were also treated with the commercial peptide inhibitor of HSP27 phosphorylation that is not cell permeant (BN, 10 μM) 12. The cells were then stimulated with LPA (25 μM) for 1 h or sodium arsenite (ARS, 500 μM) for 0.5 h as described earlier18. Stimulation with LPA or ARS has been demonstrated to increase the phosphorylation of HSP27 15,16. At the end of the experiments, cells were rinsed with PBS, quick frozen, and protein extracted using Urea-DTT-CHAPS buffer (8M urea, 10 mM Dithiothreitol (DTT) 4% CHAPS).
Cell migration
Migration was studied using a scratch wound motility assay. The scratch-wound assay has been used for nearly half a century as an in vitro model of wound healing and as a tool to discover factors important for cell migration 17. For this assay, A7R5 cells were cultured in a 6-well dish and allowed to reach confluence; a linear scratch (~2 mm wide) was performed with a 10 μL pipette tip across the diameter of the well and rinsed with PBS. Cells were kept in serum-free medium for 24 hours, pre-treated with MK2i at 10 μM for 2 h, and stimulated with LPA, which stimulates migration of smooth muscle cells14. Pictures were taken on a Zeiss Axiovert 200 M epifluorescence microscope, at a magnification of 20 and 40 X at 0 and 48 h, and the number of cells that invaded the scratch was determined.
Stable Isotopic Labeling of Cells in Culture
To determine the effect of MK2i on global protein expression, model system human dermal keloid fibroblasts were used. The choice of keloid fibroblasts was based on the fact that TGF-β effects are better characterized in these cells compared to endothelial or smooth muscle cells. Since TGF-β is an important mediator of intimal hyperplasia (causing matrix production and deposition, smooth muscle α-actin expression and myofibroblast differentiation 18), we opted to use a cell model in which TGF-β effects are well described. Also, our earlier investigation had characterized the effect of MK2i peptide on the TGF-β-induced phosphorylation of HSP27 and expression of connective tissue growth factor and collagen I in keloid fibroblasts, a wound healing model 19. In addition, keloid fibroblasts express robust stress fibers and α-smooth muscle actin, characteristics that confer some similarity to smooth muscle cells.
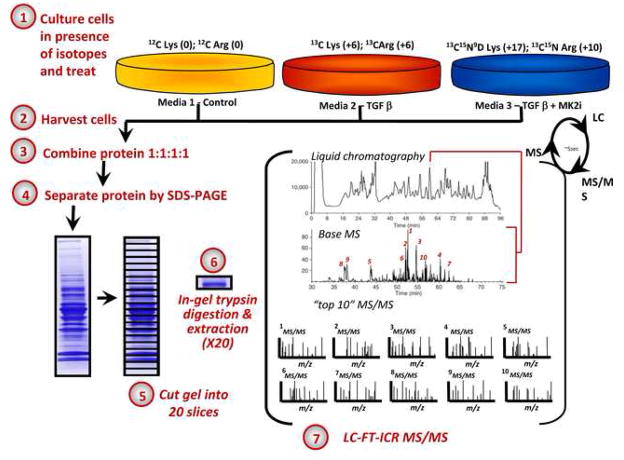
Fibroblasts were cultured in 60 mm dishes in the presence of one of the isotopic medias (with labeled aminoacid, arginine, lysine or cysteine) containing 10% dialyzed FBS. Cells cultured in these media for 6 passages achieved a 98.9% incorporation of isotopic amino acids (data not shown). The workflow of cell manipulation is shown in Figure 1. Cells potentiated in 0.5% dialyzed FBS for 48 h prior to treatment were treated for 24 h with 1) a control – untreated; 2) TGF-β1 (1.25 ng/mL) or 3) TGF-β1 (1.25 ng/mL) + 10 μM MK2i. After treatment, cells were washed in PBS then harvested by scraping in Urea/DTT/CHAPS buffer. Protein extracts from each media (25 μg each) were combined 1:1:1 based on the Coomassie Plus protein assay. The pooled protein extracts were separated on 4–20% pre-cast gradient SDS-polyacrylamide gels and visualized with Coomassie blue. The resulting gel lane from each experiment was cut into 20 slices of approximately equal size. HPLC-ESI-MSn was performed on a linear ion trap (LTQ)-mass spectrometer LTQ-Fourier Transform Ion Cyclotron Resonance mass spectrometer (LTQ FT; Thermo Fisher; San Jose, CA) fitted with a PicoView™ nanospray source (New Objective, Woburn, MA) as described20. The search parameters used for both RAW and DAT file searches were: 0.5 Da mass tolerance for precursor ion masses and 10 ppm for product ion masses; digestion with trypsin/p; a maximum of two missed tryptic cleavages; variable modifications of oxidation of methionine and phosphorylation of serine, threonine, and tyrosine, +6 on lysine (13C6 label), +6 on arginine (13C6 label), +10 on arginine (U-13C6, U-15N4 label), +17 on lysine (U-2H9, U-13C6, U-15N2), +57 on cysteine (carbamidomethylation). Probability assessment of peptide assignments and protein identifications within “DAT” files were made through use of Scaffold (version Scaffold-01_07_17, Proteome Software Inc., Portland, OR). Only peptides with ≥95% probability as considered by the ProteinProphet algorithm were included in the final table. Criteria for protein identification included detection of at least 2 unique identified peptides and a probability score of ≥99%. Proteins were quantified using the MSQuant Suite of software as described 21.
Figure 1.
Schematic of workflow used for a three-way SILAC-MS experiment. Three cell populations are isotopically labeled with normal and stable isotope-substituted arginine (R) and lysine (K) amino acids, creating three cell populations distinguishable by mass. Each population is stimulated as indicated yielding 3 samples which are combined in equal amounts (25 μg each), trypsin-digested, and analyzed using mass spectrometry.
Construction of cell permeant HSP27 fusion protein
The cDNA encoding human HSP27 was PCR amplified from an I.M.A.G.E. clone (clone ID 6083486; Clontech, Palo Alto, CA) using a forward primer (5′-GATCGAGCTCATGACCGAGCGCCGCGTC-3′) and a reverse mutagenic primer (5′-gatcggtaccttacttggcggcagtctcatcgg-3′) then cloned into pCDNA3.1 (Invitrogen, Carlsbad, CA), yielding pCDNA3.1-HSP27. Complementary oligonucleotides (5′-TATGGGTGGTTATGCTAGAGCTGCTGCTAGACAAGCTAGAGCTGGTACCGAGCTCCT CGAGG-3′ and 5′-GATCCCTCGAGGAGCTCGGTACCAGCTCTAGCTTGTCTAGCAGCAGCTCTAGCATAA CCACCCA-3′) encoding a PTD were annealed, phosphorylated and ligated into NdeI-BamHI-digested pET14b (Novagen, Madison, WI) producing pET14b-PTD. The cDNA encoding HSP27 was liberated from pCDNA3.1-HSP27 with SacI-XhoI digestion and ligated into SacI/XhoI-digested pET14b-PTD yielding pET14b-PTD-HSP27. Base sequences for all DNAs were confirmed by nucleotide sequence analysis and protein was expressed in Escherichia coli. Briefly, single colonies of BL21 (DE3) (Novagen) containing recombinant pET14b-PTD-HSP27 was used to inoculate 3 liters of Luria Broth (LB) containing 50mg/L of ampicillin. Cultures were induced with 2 mM isopropyl-1-thio-β-D-galactopyranoside when the optical density at 600-nm wavelength reached 0.6–1. After 5 h, cells were harvested by centrifugation (6,000 g, 10 min), resuspended in 1× TNE buffer (50 mM NaCl, 1 mM EDTA, and 500 mM Tris, pH 8.0) and sonicated on ice. After sonication, the inclusion bodies were harvested by centrifugation (19,000 g, 10 min) and resuspended in binding buffer (20 mM Na2HPO4, 0.5 M NaCl, 50 mM imidazole, pH 7.4, and 8 M urea). The sample was then added to Ni2+-charged Chelating Sepharose Fast Flow (Pharmacia Biotech, Peapack, NJ) and incubated overnight at 4°C. The resin was then loaded into a Poly-Prep column (Bio-Rad, Richmond, CA) and protein was eluted with 2 mL of 500 mM Imidazole and dialyzed with Phosphate Buffer (20mM Na2HPO4, 0.5 M NaCl).
Muscle physiology
De-identified discarded segments of human saphenous vein (HSV) were collected after the approval of the Institutional Review Board of the Arizona Heart Institute (Phoenix, AZ) from consented patients undergoing coronary artery bypass or peripheral vascular bypass surgery. To test the viability, rings (1.0 mm in width) were cut from segments of saphenous vein, dissected free of fat and connective tissue, stripped of the endothelium (to focus on smooth muscle responses) and contracted as described earlier15,38. To test the effect of MK2i on the relaxation of HSV, rings were washed in bicarbonate buffer and incubated with either buffer (control), 25 μM SB or 30 μM MK2i for 2 hr. Rings were contracted with norepinephrine (0.5μM) and relaxed with cumulative log doses of sodium nitroprusside (0.01–1μM), a nitric oxide donor.
Organ culture
After viability was determined in the muscle bath, additional rings (3 mm in length) were cut, placed in 8-well chamber slides, and maintained in RPMI 1640with 30% FBS, 1% L-glutamine and 1% penicillin/streptomycin for 14 days at 37°C/5% CO2 as described 38. The rings were either untreated, or treated with MK2i (5 or 10 μM), SB (25 μM), or recombinant HSP27 protein attached to a protein transduction domain for cell penetration (19 μM). The experiments were conducted in duplicate for every vein segment: one set of rings was used for histological evaluation, while the other set was used for evaluation of HSP27 phosphorylation and CTGF expression by Western blot. To study the effect of MK2i on collagen accumulation in cultured vein rings, control rings and those treated with MK2i at 10 μM were also stained for collagen using the Masson’s trichrome stain.
The second set of rings was snap frozen with liquid nitrogen, pulverized and homogenized using Urea-DTT-CHAPS buffer. Lysates were centrifuged (6000 g, 20 min), and the supernatant was collected for subsequent evaluation of HSP27 phosphorylation and CTGF expression.
Immunoblot analysis
Protein from cells or HSV ring lysates were electrophoretically separated, transferred to Immobilon membranes and analyzed by western blotting as described earlier 10 with the following primary antibodies: rabbit anti-CTGF (Torrey Pines Biolabs, Houston, TX), mouse anti-HSP27 (gift from Dr. Michael Welsh, University of Michigan), rabbit anti-phosphorylated HSP27 22, and rabbit anti-β-actin (Sigma, St Louis, MO).
Statistical analysis
All protein expression data are presented as means ± standard deviation. The Western blot bands were quantified by densitometry, and proteins expression normalized from the amount of the loading control (β-actin) expression. One-way ANOVA followed by Tukey test was used to compare experimental groups, with a significance level of P <.05.
RESULTS
The effect of MK2i on HSP27 phosphorylation in A7R5 cells
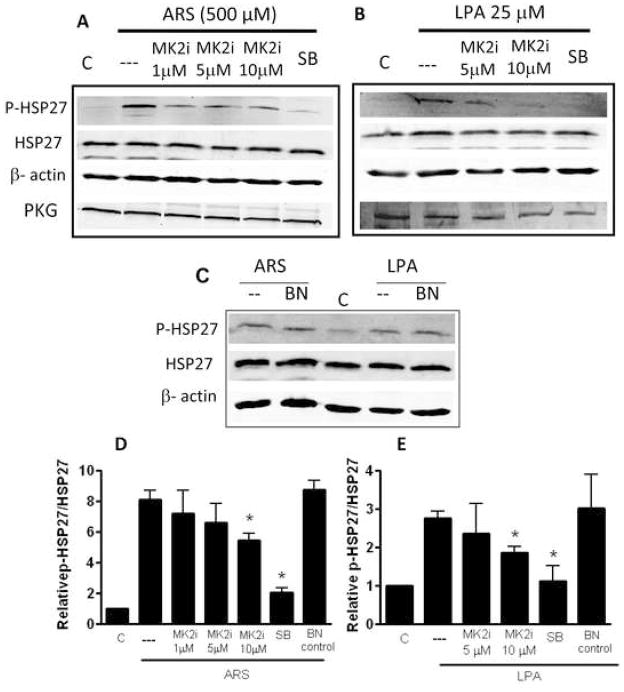
Treatment with arsenite (0.5 mM, ARS) or lysophosphatidic acid (25 μM, LPA) increased the phosphorylation of HSP27 in A7R5 cells (Figure 2). Pre-treatment with 10 μM MK2i decreased ARS and LPA-induced HSP27 phosphorylation by 36±9% and 33±10%, respectively (P < .05, Figure 2). Since rat aortic smooth muscle cells undergo changes in culture from the contractile to a synthetic phenotype, the level of PKG (cGMP-dependent protein kinase, a phenotypic marker of smooth muscle cells) was monitored, and experiments were conducted only in cells expressing PKG (data not shown). Several investigations have demonstrated that expression of cyclic GMP dependent protein kinase results in the expression of smooth muscle specific myosin heavy chains, calponin, and alpha-smooth muscle actin, all markers considered to be associated with the contractile phenotype 23, 24.
Figure 2.
Effect of MK2i on the phosphorylation of HSP27 in A7R5 cells: The cells were serum starved for 24 h and treated with arsenite (ARS, 500μM) or lysophosphatidic acid (LPA, 25 μM) for 0.5h or 1h. Cells were either untreated or pre-treated with MK2i (1, 5 or 10 μM), SB202580 (20 μM) or the non-cell permeant peptide inhibitor of MK2i (BN10 μM) for 2 h prior stimulation with ARS or LPA. The cells were homogenized, proteins separated by SDS PAGE, transferred to Immobilon membranes and bands were quantified by densitometry. Phosphorylated HSP27 or non-phosphorylated HSP27 expression were quantified relative to actin expression. The ratio of phosphorylated HSP27/total HSP27 (relative p-HSP27/HSP27) in untreated cells was set to 1 for comparison of different blots. Data are expressed as mean ± standard deviation for a set of 4 separate experiments. *: P < .05 compared to the untreated rings (C). A: cells stimulated with ARS and treated with MK2i or SB; B: cells stimulated with LPA and treated with MK2i or SB; C: Cells stimulated with either ARS or LPA and treated with BN; D and E: ratios of relative phosphorylated/non-phosphorylated HSP27 normalized to actin expression. The level of PKG was monitored as a marker for the contractile phenotype of rat aortic smooth muscle cells.
As a control, cells were also treated with the p38 MAP kinase inhibitor SB. P38 MAP kinase acts upstream to MAPKAP kinase II, phosphorylating and activating it. SB pre-treatment led to decreases in the phosphorylation of HSP27 after stimulation with either ARS or LPA (71 ±7% or 52±23% respectively compared to no SB, P <.05). Additionally, cells treated with the peptide inhibitor of MAPKAP kinase II that does not contain a protein transduction domain (BN) did not lead to decreases in LPA or ARS-induced increases in the phosphorylation of HSP27 (Figure 2C). This is likely due to the inability of this peptide to penetrate into cells.
Effect of MK2i on cell migration
A scratch assay was performed to determine the effect of MK2i on smooth muscle cell migration stimulated by LPA14. MK2i (10 μM) pre-treatment decreased LPA-induced migration by 52±16% (P < .05) compared to cells stimulated with LPA but not pre-treated with MK2i (Figure 3).
Figure 3.
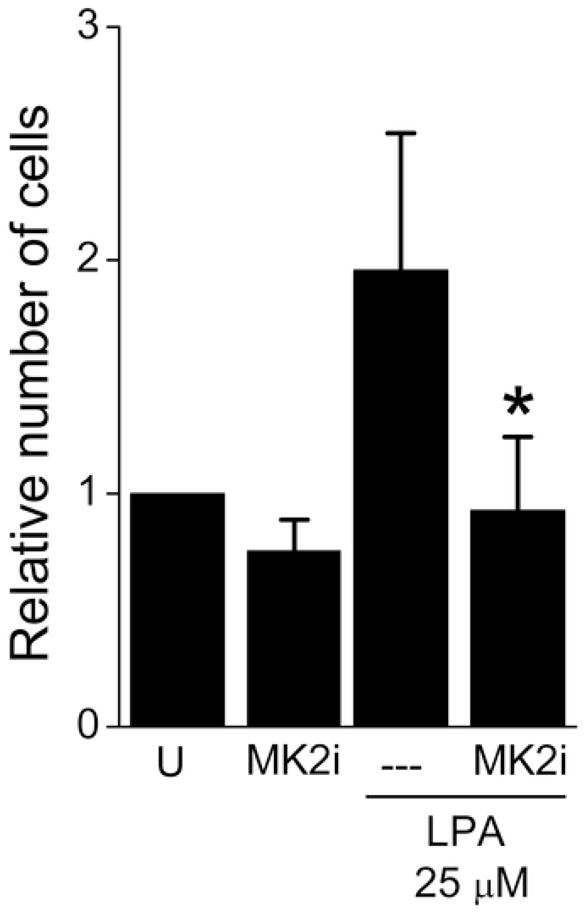
Effect of MK2i treatment on A7R5 cell migration induced by LPA. Migration assays were performed by scratch wound motility assay. Cells were kept in serum-free medium for 24 hours, pre-treated with MK2i at 10 μM for 2 h, and stimulated with LPA. For each well, pictures were taken on a microscope at a magnification of 20 and 40 X at 0 and 48 h, and the number of cells that invaded the scratch was determined. U: untreated cells.
Proteomic profiling of MK2i mediated protein change
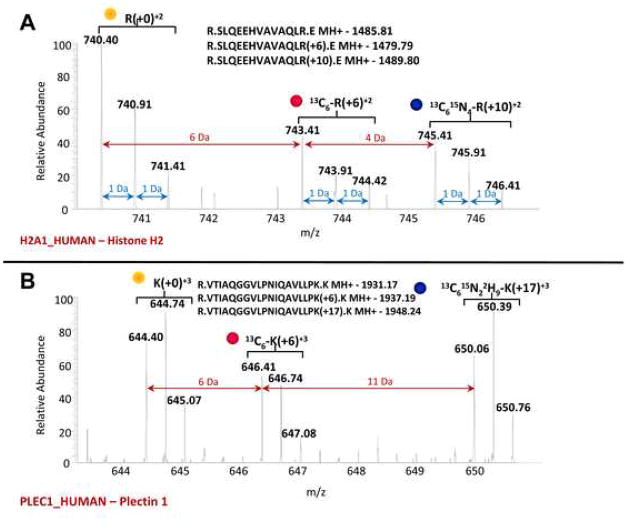
Stable Isotopic Labeling using Amino acids in Cell culture (SILAC) in combination with liquid chromatography (LC) Mass Spectrometryn (MS/MS) was used to characterize cellular responses in human dermal keloid fibroblasts upon stimulation with TGF-β that has demonstrated to induce HSP27 phosphorylation 10. Figure 4 shows a typical MS/MS mass spectra of an arginine-containing peptide triplet (that is the same peptide derived from each of the three different media/treatments) and that of a lysine-containing peptide triplet. The isotopic envelope of a +2 charged peptide SLQEEHVAVAQLR, derived from the protein histone H2 in Media 1, has a monoisotopic protonated mass of 1485.81 (yellow dot), a +6R amino acid from Media 2 (1479.79, red dot), and the +10R labeled peptide from Media 3 (1489.80, blue dot). The envelope of each peptide reflects a typical distribution and is a consequence of naturally-abundant carbon, nitrogen, oxygen and hydrogen atoms. The spacing of the monoisotopic mass peaks from each +2 charged peptide envelope is indicated by the red arrows. The relative intensities of each monoisotopic mass peak are proportional to the abundance of that peptide in the media. Figure 4B shows a similar mass spectra for a lysine-containing +3-charged peptide triplet derived from the protein plectin 1. Table 1 lists those proteins most up-regulated upon TGF-β stimulation and those most down-regulated upon TGF-β + MK2i treatment. Of note, two extracellular matrix proteins implicated in hyperplastic lesions, fibronectin and collagen were down regulated after MK2i treatment. MK2i not only decreased the phosphorylation of HSP27 but also decreased its expression.
Figure 4.
Mass spectra of eluting SILAC peptide triplets (the same peptide from the three cell populations) measured for accurate mass determination. The masses of the singly protonated peptide sequences derived from each media are listed above each spectra. Brackets above each peptide isotope cluster denote the isotopic amino acid incorporated in that peptide. A) Mass spectra of an arginine-containing tryptic peptide (SLQEEHVAVAQLR) triplet (+2 charge state) derived from the protein histone H2 (H2A1). Red arrows indicate the mass difference between the monoisotopic masses from each peptide of the triplet. For example, the m/z difference between the doubly-charged (z=2) peptide derived from media one (740.40 Da) and media 2 (743.41 Da) = 3. Blue arrows delineate the m/z difference between each species of a peptide isotopic envelope as a consequence of naturally occurring isotopes of carbon, nitrogen and oxygen. In this example, the distribution of each peptide occurs at a 1:0.5:0.5 m/z ratio (0:+6:+10). B) Mass spectra of a lysine-containing tryptic peptide (VTIAQGGVLPNIQAVLLPK) triplet (+3 charge state) derived from the plectin 1 (PLEC1). In this example, the distribution of each peptide occurs at a 1:0.6:1 m/z ratio (0:+6:+17).
Table 1.
Protein expression results identified through SILAC-based proteomic profiling. Human dermal keloid fibroblasts treated with TGF-β (1.25 ng/ml) and TGF-β + MK2i (10 μM) for 24 h were analyzed using SILAC-labeling and MSQuant-mediated protein quantification.
| Proteins most up-regulated with TGFβ | Log2 TGF β: control | Log2: TGFβ +MK2i: control | |
|---|---|---|---|
| Gene Name | Protein Name | ratio | ratio |
| CO1A1_HUMAN | Collagen alpha-1(I) chain precursor | 0.91 | −2.53 |
| BLVRB_HUMAN | Biliverdin reductase B | 0.81 | 0.44 |
| MYO1C_HUMAN | Myosin-Ic | 0.55 | −0.67 |
| CKAP4_HUMAN | Cytoskeleton-associated protein 4 | 0.51 | 0.35 |
| SPTA2_HUMAN | Spectrin alpha chain, brain | 0.39 | −0.08 |
| HSP71_HUMAN | Heat shock 70 kDa protein 1 | 0.38 | −0.36 |
| ANXA2_HUMAN | Annexin A2 (Lipocortin II) | 0.37 | −0.6 |
| FINC_HUMAN | Fibronectin precursor | 0.3 | −0.81 |
| LIMA1_HUMAN | LIM domain and actin-binding protein 1 | 0.29 | 2.49 |
| ARP2_HUMAN | Actin-like protein 2 | 0.25 | 0.02 |
| CH60_HUMAN | 60 kDa heat shock protein, mitochondrial precursor | 0.22 | 0.92 |
| LDHA_HUMAN | L-lactate dehydrogenase A chain | 0.21 | −0.44 |
| PEA15_HUMAN | Astrocytic phosphoprotein PEA-15 | 0.2 | −0.56 |
| EZRI_HUMAN | Ezrin | 0.19 | 0.23 |
| TPM4_HUMAN | Tropomyosin alpha-4 chain | 0.17 | −1.83 |
| MOES_HUMAN | Moesin | 0.05 | −0.02 |
| DUS3_HUMAN | Dual specificity protein phosphatase 3 | 0.01 | −0.47 |
| CO6A1_HUMAN | Collagen alpha-1(VI) chain precursor | −0.01 | −0.13 |
| ANXA4_HUMAN | Annexin A4 (Lipocortin IV) | −0.09 | −0.8 |
| RS2_HUMAN | 40S ribosomal protein S2 (S4) | −0.08 | 0.24 |
| Proteins most down-regulated with TGFβ + inhibitor | |||
| RL23_HUMAN | 60S ribosomal protein L23 | −4.32 | −2.98 |
| VDAC1_HUMAN | Voltage-dependent anion-selective channel protein 1 | −0.14 | −2.72 |
| NDKA_HUMAN | Nucleoside diphosphate kinase A | −2.15 | −2.6 |
| CO1A1_HUMAN | Collagen alpha-1(I) chain precursor | 0.91 | −2.53 |
| RL27_HUMAN | 60S ribosomal protein L27 | −2.85 | −2.47 |
| CALR_HUMAN | Calreticulin precursor | −2.02 | −2.47 |
| GSTO1_HUMAN | Glutathione transferase omega-1 | −2.42 | −2.23 |
| H4_HUMAN | Histone H4 | −0.35 | −2.2 |
| GLRX1_HUMAN | Glutaredoxin-1 | −1.93 | −2.1 |
| HSPB1_HUMAN | Heat shock 27 kDa protein | −1.51 | −2.02 |
| CALD1_HUMAN | Caldesmon | −1.58 | −1.93 |
| FKB1A_HUMAN | FK506-binding protein 1A | −1.61 | −1.94 |
| ROA2_HUMAN | Heterogeneous nuclear ribonucleoproteins A2/B1 | −1.19 | −1.87 |
| TPM4_HUMAN | Tropomyosin alpha-4 chain | 0.17 | −1.83 |
| HNRPD_HUMAN | Heterogeneous nuclear ribonucleoprotein D0 | −2.07 | −1.78 |
| GSTM3_HUMAN | Glutathione S-transferase Mu 3 | −1.54 | −1.81 |
| ATP5L_HUMAN | ATP synthase subunit g, mitochondrial | −0.95 | −1.72 |
| CRYAB_HUMAN | Alpha crystallin B chain (Heat-shock protein beta-5) | −0.93 | −1.72 |
| BASP_HUMAN | Brain acid soluble protein 1 | −1.43 | −1.68 |
| ZYX_HUMAN | Zyxin | −1.28 | −1.7 |
Effect of MK2i on the phosphorylation of HSP27 and expression of CTGF in cultured HSV rings
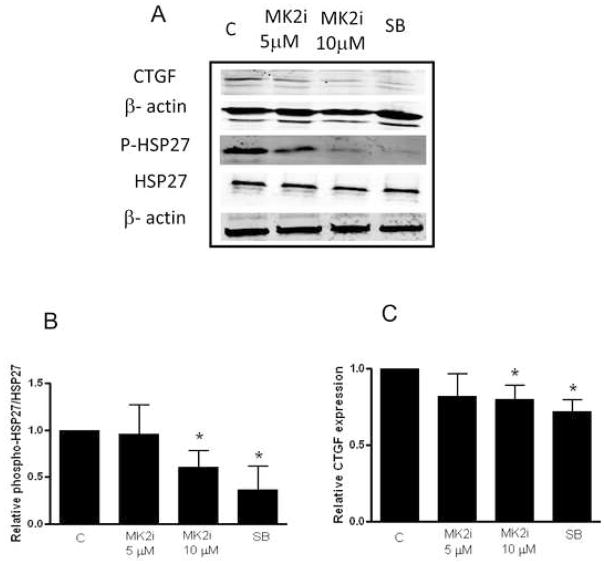
We evaluated the effect of MK2i on the expression/phosphorylation of HSP27 and CTGF in cultured HSV rings used as an ex vivo model of intimal hyperplasia. MK2i (10 μM) decreased the phosphorylation of HSP27 by 40±17% (P < .05, Figure 5A, B) while SB decreased the phosphorylation of HSP27 by 58±22% (P < .05) compared to untreated controls.
Figure 5.
Effect of MK2i or SB treatment on CTGF expression and phosphorylation of HSP27 in HSV rings. The vein rings were cultured in RPMI medium supplemented with L-glutamine (1%), penicillin/streptomycin (1%) and FBS (30%) at 5% CO2 and 37°C for 14 days. The rings were either untreated (C) or treated with MK2i (5 or 10 μM) or SB (20 μM). The Western blot bands were quantified by densitometry (panel A), and expression of phosphorylated HSP27/non-phosphorylated HSP27 (panel B) or CTGF (panel C) were related to actin to correct for loading differences. The expression of CTGF, HSP27 and phospho-HSP27 in untreated rings was set to 1 for comparison of different blots. Data are expressed as mean ± standard deviation of 6 experiments. *: P < .05 compared to the untreated rings (C).
CTGF has been proposed to mediate some of the pro-fibrotic effects of TGF-β (and other pro-fibrotic mediators) including production of extracellular matrix proteins. An intact cytoskeleton is required for CTGF expression 8. Since HSP27 phosphorylation is associated with actin cytoskeleton stabilization, we hypothesized that reduction of HSP27 phosphorylation would reduce CTGF expression. The expression of CTGF was decreased by 17±5% with MK2i (10 μM), and by 28±7% with SB compared to untreated controls (P < .05, Figure 5A,C). MK2i also decreased TGF-β-induced CTGF expression in A7R5 cells (29±11%, data not shown).
Effect of MK2i on the development of intimal hyperplasia
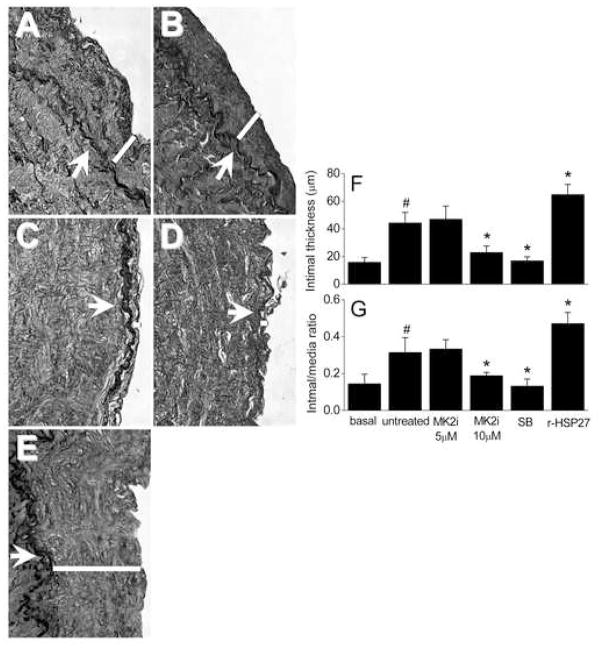
Finally, we determined the effect of MK2i on intimal thickening in HSV. The average intimal thickness of the pre-cultured HSV was 15.8±3.4 μm. After 14 days in culture, the average intimal thickness of the untreated (control) rings was 44.3±9.4 μm, an increase of over 100% in the thickness of the intimal layer (Figure 6A). Treatment with MK2i (10 μM) and SB significantly reduced the intimal thickness by 48.2±10.5% and 61.9±5.4%, respectively compared to untreated controls (P < .05). To further support the hypothesis that HSP27 is involved in the development of intimal hyperplasia, we generated a fusion protein containing recombinant HSP27 attached to a protein transduction domain to allow cell penetration of the protein (r-HSP27). The rings treated with r-HSP27 (19 μM) developed an increase of 48±14% (P <.05) in intimal thickening compared to control rings.
Figure 6.
Effect of MK2i, SB or r-HSP27 treatment on the intimal layer thickening. The white bars show the intimal thickness. Vein rings (n=4–6 for each group) were cultured in RPMI medium supplemented with L-glutamine (1%), penicillin/streptomycin (1%) and FBS (30%) at 5% CO2 and 37°C for 14 days. The rings were either untreated (C) or treated with MK2i (5 or 10 μM), SB (20 μM) or r-HSP27 (19 μM). After 14 days, the rings were fixed in formalin, sectioned at 10 μm and stained using Weigert’s resorcin-fucsin. A: control rings cultured for 14 days; B: rings treated with MK2i at 5 μM; C: rings treated with MK2i at 10 μM; D: rings treated with SB at 20 μM; E: rings treated with r-HSP27; F: intimal layer thickness, G: intimal/media layer ratio: Magnification: 40X. The white arrows in panels A–E indicate the internal elastic lamina, whereas the white bars indicate the thickness of the intimal layer.
The ratio intimal/media layer for each treatment is shown in Figure 6G. Again, a significant (P < .05) decrease in this ratio was observed when the rings were treated with MK2i (at 10 μM) or SB compared to the untreated rings, whereas r-HSP27 increased this ratio.
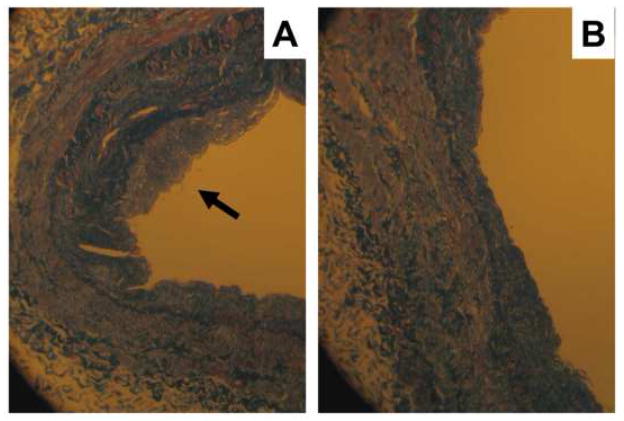
Since deposition of extracellular matrix proteins is a key feature of intimal hyperplasia, we evaluated how MK2i treatment affected collagen deposition in the vein rings. Control rings and those treated with MK2i at 10 μM were stained for collagen using the Masson’s trichrome stain. As can be seen in Figure 7, deposition of collagen was observed in the neointima of untreated rings cultured for 14 days (Figure 7A). Treatment with MK2i reduced collagen accumulation (Figure 7B).
Figure 7.
Accumulation of collagen in HSV rings. Rings were either untreated (A) or treated with MK2i at 10 μM (B) for 14 days. They were then stained for collagen using Masson’s trichrome stain and observed under halogen light (magnification = 100x).
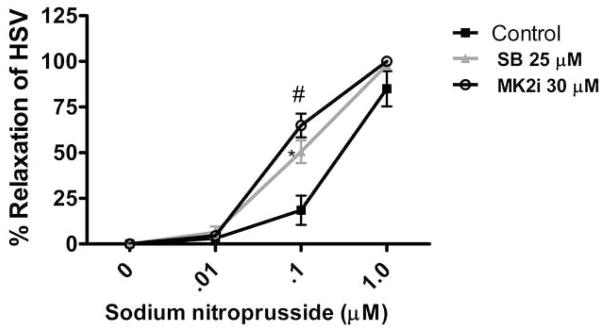
MK2i enhances relaxation of human saphenous vein
We examined the effect of inhibiting the phosphorylation of HSP27 on nitric oxide-induced relaxation of saphenous vein. Human saphenous vein (HSV) rings were contracted with norepinephrine (0.5 μM) and relaxed with sodium nitroprusside (a nitric oxide donor 0.01–1 μM), with or without preincubation with MK2i or SB and the force generated was recorded. Preincubation of HSV with 30 μM MK2i increased sodium nitroprusside (0.1 μM) induced relaxation from 18.47±8 % to 64.93 ± 6.52 % (Figure 8) while SB (25 μM) increased relaxation from 18.47±8 % to 50.5±6.2%. MK2i did not affect the contraction of HSV significantly at the concentration used (data not shown).
Figure 8.
The p38 MAP kinase inhibitor, SB 203580 and HSP27 kinase inhibitor, MK2i, enhances nitroprusside induced relaxation in saphenous vein: Saphenous veins were cut into rings, suspended in a muscle bath and equilibrated in Krebs Ringer bicarbonate buffer for 2 h. The rings were contracted with KCl (110 mM) and force generated was measured to check for viability. The rings were then pretreated with buffer (control), 25μM SB203580 (SB) or 30 μM MK2i for 2 hrs and then contracted with norepinephrine (NE, 0.5 μM) and relaxed with increasing doses of sodium nitroprusside (SNP, 0–1μM). SB or MK2i treatment led to significant increases in the relaxation of saphenous vein. * P =0 .0335, # P= 0.0108 at 0.1μM SNP compared to control, n=3.
DISCUSSION
The role of HSP27 in stabilizing the actin cytoskeleton (which leads to several key events associated with intimal hyperplasia) has been supported by several lines of evidence. Transfection of cells with dominant active phosphorylated mutants of HSP27 leads to abundant stress fiber formation 6,7. The presence of central actin stress fibers in cultured cells is associated with the “synthetic” or “myofibroblast” phenotype 25. HSP27 has also been shown to be induced by stress, particularly in the vasculature 22,26. Increases in the phosphorylation of HSP27 are also associated with smooth muscle migration 4. Migration of smooth muscle cells from the media to the hyperplastic lesion has been implicated as one of the inciting events in intimal hyperplasia 27. The expression and phosphorylation of HSP27 doubles after exposure of human saphenous vein segments to arterial flow ex vivo 28. Vein graft arterialization also leads to activation of p38 MAPK 29. Hence, molecular strategies that inhibit HSP27 phosphorylation would prevent smooth muscle cell migration, phenotypic modulation to a myofibroblast, extracellular matrix production, and intimal hyperplasia.
In this study we examined the effect of a cell-permeant peptide inhibitor (MK2i) of the kinase that phosphorylates and activates HSP27, and evaluated its potential to inhibit the phosphorylation of HSP27 and prevent intimal hyperplasia. We used HSV cultured in the presence of high serum as an ex vivo model of intimal hyperplasia which has been reported as a representative ex vivo model of the changes following vein graft stenosis in man 30,31 and had been used previously in our laboratory32. Accordingly, marked similarities were previously observed in the cellular and extracellular matrix composition in the intimal layer of the cultured vein and the pathological lesion, with both showing abundance of collagen and smooth muscle cells of secretory phenotype 33. Using bromodeoxyuridine as proliferation marker, it was observed that early cell proliferation in the cultured vein intima gave rise to the neointima. Both proliferation and neointimal thickness were maximal by day 14 in culture, hence justifying the culture of rings for 14 days. Cell proliferation and increases in the cross-sectional area of the medial layer have also been observed in this model 34.
The fact that the pre-treatment with MK2i decreased LPA- and ARS-induced phosphorylation of HSP27 in a smooth muscle cell line suggests the potential of this peptide to penetrate into cells and target MAPKAP kinase II. As expected, this effect was not observed when the peptide inhibitor of MAPKAP kinase II was not attached to a protein transduction domain (and therefore, not cell permeant), suggesting that the transduction domain is necessary for adequate cell penetration of the peptide.
During the course of the intimal hyperplasia the tissue transitions from highly cellularized and proliferative to a neointima dominated by matrix components, with fibrosis becoming the prevailing event 35. Recent studies have implicated TGF-β1 and CTGF as canonical mediators of both phases of intimal hyperplasia since these growth factors are involved in matrix production and deposition, smooth muscle α-actin expression and myofibroblast differentiation 18. While an increased expression of TGF-β1 was observed in hyperplastic tissue, TGF-β1 antisense treatment reduced intimal hyperplasia and stenosis in animal models 36. This and previous studies from our group demonstrate that TGF-β1 treatment increases phosphorylation of HSP27 and production of connective tissue growth factor (CTGF) and collagen 19 (Table 1). Since several of the TGF-β and CTGF-mediated effects are dependent on MAPKAP kinase II 37, MK2i has the potential to counteract such effects 19. As demonstrated by Stable Isotopic Labeling by Aminoacid in Culture (SILAC) method, a tool that can determine the effect of agonists and drugs on global protein expression 21, TGF-β1 treatment increased the expression of two of the major extracellular matrix proteins found in intimal hyperplastic lesions: collagen I and fibronectin (Table 1). MK2i treatment decreased the expression of both proteins. Interestingly, HSP27 expression was also regulated by MK2i. Recently, our group have evaluated the effect of MK2i on TGF-β-induced phosphorylation of HSP27 in keloid cells and demonstrated that, compared with the TGF-β stimulation, MK2i (10 μM) significantly diminished HSP27 phosphorylation to levels similar to that of the untreated control cells 19. Therefore, MK2i seems to be involved in decreasing expression and phosphorylation of HSP27. Upregulation of HSP27 in the smooth muscles cells of saphenous vein was previously observed as a result of vein exposure to simulated venous or arterial flow 28, further supporting a role for HSP27 in tissue remodeling and intimal hyperplasia.
Intimal injury is believed to be followed by release of inflammatory mediators (such as growth factors and cytokines) that induce phenotypic changes of vascular smooth muscle cells from the quiescent “contractile” state to the active “synthetic” state and their migration from the media to the intimal layer. Once in the intima, these cells proliferate (becoming the major cell type) and synthesize extracellular matrix proteins, the predominant component of intimal hyperplasia. Collagen is the most abundant matrix protein, but hyaluronic acid and fibronectin are also present 38. As can be seen in Figure 7, deposition of collagen can be found in the neointima of rings cultured for 14 days in high serum, whereas MK2i treatment prevented such accumulation.
Treatment of the rings with MK2i significantly decreased the thickness of intimal layer compared to untreated HSV, while the opposite was observed for rings treated with a recombinant fusion protein of HSP27 (r-HSP27). The reduction of intimal thickening was associated with decreases in HSP27 phosphorylation, CTGF expression and collagen deposition. The fact that MK2i decreased HSP27 expression in cells, phosphorylation and intimal thickening in cultured vein segments provide evidences for the involvement of HSP27 in the actin cytoskeleton reorganization and development of intimal hyperplasia. Because expression of CTGF is believed to depend on an intact actin cytoskeleton, agents that compromise this integrity most likely have an effect in modulating CTGF expression 9,10,39. CTGF has been implicated as an important mediator of intimal hyperplasia due to its involvement in collagen production, smooth muscle α-actin expression and myofibroblast differentiation. Further investigation on signaling cascades affected by MK2i and their link to HSP27 phosphorylation and CTGF expression are still necessary, but because increases in the phosphorylation of HSP27 are associated with stabilization of the actin cytoskeleton, it is reasonable to expect that by decreasing HSP27 phosphorylation, MK2i affects CTGF expression and, in turn, collagen deposition.
Taken together, these results demonstrate the potential of using cell permeant peptide-based therapeutics to target MAPKAP kinase II and reduce HSP27 phosphorylation as a strategy to inhibit the development of intimal hyperplasia and enhance the patency of vein grafts.
Clinical relevance.
Considering that intimal hyperplasia represents the leading cause of prosthetic and autologous vascular graft failure and that, in spite of the many recent technological advances in vascular interventions, it remains an expensive, morbid, and unsolved problem, this study presents a new strategy in the battle against such disorder. A cell permeant peptide-based therapeutics was able to target MAPKAP kinase II and reduce HSP27 phosphorylation, and as a result, prevent the development of intimal hyperplasia. This peptide acts downstream in the signaling cascade compared to other compounds, and therefore may generate less adverse effects. Vein graft represent an ideal target for cell permeant peptide therapeutics in that the grafts can be treated ex vivo at the time of surgery. Recent data suggest that the peptides are present intracellularly for up to 7 days in cultured fibroblasts. 40
Acknowledgments
This study was supported by NIH RO1HL70715 and a VA Merit Review to C.M. Brophy.
Footnotes
DISCLOSURE: The authors of this paper do not have any competing interests or affiliations or financial involvement with any organization or entity with a financial interest in, or in financial competition with, the subject matter or materials discussed in the manuscript. All financial and material support for this research and work are clearly identified in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clowes AW, Reidy MA. Prevention of stenosis after vascular reconstruction: pharmacologic control of intimal hyperplasia--a review. J Vasc Surg. 1991;13:885–891. doi: 10.1067/mva.1991.27929. [DOI] [PubMed] [Google Scholar]
- 2.Conte MS. Technical factors in lower-extremity vein bypass surgery: how can we improve outcomes? Semin Vasc Surg. 2009;22:227–233. doi: 10.1053/j.semvascsurg.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Fu M, Zhang J, Tseng YH, Cui T, Zhu X, Xiao Y, et al. Rad GTPase attenuates vascular lesion formation by inhibition of vascular smooth muscle cell migration. Circulation. 2005;111:1071–1077. doi: 10.1161/01.CIR.0000156439.55349.AD. [DOI] [PubMed] [Google Scholar]
- 4.Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, et al. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem. 1999;274:24211–24219. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- 5.Hirano S, Shelden EA, Gilmont RR. HSP27 regulates fibroblast adhesion, motility, and matrix contraction. Cell Stress Chaperones. 2004;9:29–37. doi: 10.1379/471.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 8.Heusinger-Ribeiro J, Eberlein M, Wahab NA, Goppelt-Struebe M. Expression of connective tissue growth factor in human renal fibroblasts: regulatory roles of RhoA and cAMP. J Am Soc Nephrol. 2001;12:1853–1861. doi: 10.1681/ASN.V1291853. [DOI] [PubMed] [Google Scholar]
- 9.Ott C, Iwanciw D, Graness A, Giehl K, Goppelt-Struebe M. Modulation of the expression of connective tissue growth factor by alterations of the cytoskeleton. J Biol Chem. 2003;278:44305–44311. doi: 10.1074/jbc.M309140200. [DOI] [PubMed] [Google Scholar]
- 10.Lopes LB, Furnish EJ, Komalavilas P, Flynn CR, Ashby P, Hansen A, et al. Cell permeant peptide analogues of the small heat shock protein, HSP20, reduce TGF-beta1-induced CTGF expression in keloid fibroblasts. J Invest Dermatol. 2009;129:590–598. doi: 10.1038/jid.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaestel M. MAPKAP kinases - MKs - two’s company, three’s a crowd. Nat Rev Mol Cell Biol. 2006;7:120–130. doi: 10.1038/nrm1834. [DOI] [PubMed] [Google Scholar]
- 12.Hayess K, Benndorf R. Effect of protein kinase inhibitors on activity of mammalian small heat-shock protein (HSP25) kinase. Biochem Pharmacol. 1997;53:1239–1247. doi: 10.1016/s0006-2952(96)00877-5. [DOI] [PubMed] [Google Scholar]
- 13.Flynn CR, Komalavilas P, Tessier D, Thresher J, Niederkofler EE, Dreiza CM, et al. Transduction of biologically active motifs of the small heat shock- related protein, HSP20, leads to relaxation of vascular smooth muscle. Faseb J. 2003;10:1358–1360. doi: 10.1096/fj.02-1028fje. [DOI] [PubMed] [Google Scholar]
- 14.Ai S, Kuzuya M, Koike T, Asai T, Kanda S, Maeda K, et al. Rho-Rho kinase is involved in smooth muscle cell migration through myosin light chain phosphorylation-dependent and independent pathways. Atherosclerosis. 2001;155:321–327. doi: 10.1016/s0021-9150(00)00585-2. [DOI] [PubMed] [Google Scholar]
- 15.Komalavilas P, DaCosta L, et al. Lysophosphatidic acid induces phosphorylation of heat shock protein 27. Medimond International Proceedings. 2005:133–138. [Google Scholar]
- 16.Rossi MR, Somji S, Garrett SH, Sens MA, Nath J, Sens DA. Expression of hsp 27, hsp 60, hsc 70, and hsp 70 stress response genes in cultured human urothelial cells (UROtsa) exposed to lethal and sublethal concentrations of sodium arsenite. Environ Health Perspect. 2002;110:1225–1232. doi: 10.1289/ehp.021101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soderholm J, Heald R. Scratch n′ screen for inhibitors of cell migration. Chem Biol. 2005;12:263–265. doi: 10.1016/j.chembiol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, Yu P, Tao M, Fernandez C, Ifantides C, Moloye O, et al. TGF-beta- and CTGF-mediated fibroblast recruitment influences early outward vein graft remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H482–488. doi: 10.1152/ajpheart.01372.2006. [DOI] [PubMed] [Google Scholar]
- 19.Lopes LB, Flynn C, Komalavilas P, Panitch A, Brophy CM, Seal BL. Inhibition of HSP27 phosphorylation by a cell-permeant MAPKAP Kinase 2 inhibitor. Biochem Biophys Res Commun. 2009;382:535–539. doi: 10.1016/j.bbrc.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hojlund K, Yi Z, Hwang H, Bowen B, Lefort N, Flynn CR, et al. Characterization of the human skeletal muscle proteome by one-dimensional gel electrophoresis and HPLC-ESI-MS/MS. Mol Cell Proteomics. 2008;7:257–267. doi: 10.1074/mcp.M700304-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster LJ, Rudich A, Talior I, Patel N, Huang X, Furtado LM, et al. Insulin-dependent interactions of proteins with GLUT4 revealed through stable isotope labeling by amino acids in cell culture (SILAC) J Proteome Res. 2006;5:64–75. doi: 10.1021/pr0502626. [DOI] [PubMed] [Google Scholar]
- 22.Knoepp L, Beall A, Woodrum D, Mondy JS, Shaver E, Dickinson M, et al. Cellular stress inhibits vascular smooth muscle relaxation. J Vasc Surg. 2000;31:343–353. doi: 10.1016/s0741-5214(00)90164-2. [DOI] [PubMed] [Google Scholar]
- 23.Boerth NJ, Dey NB, Cornwell TL, Lincoln TM. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J Vasc Res. 1997;34:245–259. doi: 10.1159/000159231. [DOI] [PubMed] [Google Scholar]
- 24.Brophy CM, Woodrum DA, Pollock J, Dickinson M, Komalavilas P, Cornwell TL, et al. cGMP-dependent protein kinase expression restores contractile function in cultured vascular smooth muscle cells. J Vasc Res. 2002;39:95–103. doi: 10.1159/000057758. [DOI] [PubMed] [Google Scholar]
- 25.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. Faseb J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- 26.Udelsman R, Blake MJ, Stagg CA, Ding-gang L, Putney DJ, Holbrook NJ. Vascular heat shock protein expression in response to stress. J Clin Invest. 1993;91:465–473. doi: 10.1172/JCI116224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y, O’Brien JE, Jr, Mannion JD, Morrison RC, Chung W, Fard A, et al. Remodeling of autologous saphenous vein grafts. The role of perivascular myofibroblasts. Circulation. 1997;95:2684–2693. doi: 10.1161/01.cir.95.12.2684. [DOI] [PubMed] [Google Scholar]
- 28.McGregor E, Kempster L, Wait R, Gosling M, Dunn MJ, Powell JT. F-actin capping (CapZ) and other contractile saphenous vein smooth muscle proteins are altered by hemodynamic stress: a proteonomic approach. Mol Cell Proteomics. 2004;3:115–124. doi: 10.1074/mcp.M300046-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Saunders PC, Pintucci G, Bizekis CS, Sharony R, Hyman KM, Saponara F, et al. Vein graft arterialization causes differential activation of mitogen-activated protein kinases. J Thorac Cardiovasc Surg. 2004;127:1276–1284. doi: 10.1016/j.jtcvs.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Porter KE, Dickinson T, London NJ. Inhibition of neointima formation in an organ culture of human saphenous vein: a comparison of dual endothelin-converting enzymeneutral endopeptidase and selective neutral endopeptidase inhibition. J Vasc Surg. 2001;34:548–554. doi: 10.1067/mva.2001.115960. [DOI] [PubMed] [Google Scholar]
- 31.Colotti C, Vittorini S, Ottaviano V, Maltinti M, Angeli V, Del Ry S, et al. Nitric oxide treatment reduces neo-intimal formation and modulates osteopontin expression in an ex-vivo human model of intimal hyperplasia. Cytokine. 2009;46:228–235. doi: 10.1016/j.cyto.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Tessier DJ, Komalavilas P, Liu B, Kent CK, Thresher JS, Dreiza CM, et al. Transduction of peptide analogs of the small heat shock-related protein HSP20 inhibits intimal hyperplasia. J Vasc Surg. 2004;40:106–114. doi: 10.1016/j.jvs.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 33.Porter KE, Varty K, Jones L, Bell PR, London NJ. Human saphenous vein organ culture: a useful model of intimal hyperplasia? Eur J Vasc Endovasc Surg. 1996;11:48–58. doi: 10.1016/s1078-5884(96)80134-1. [DOI] [PubMed] [Google Scholar]
- 34.Castronuovo JJ, Jr, Smith TJ, Price RM. Validation of an in vitro model of human saphenous vein hyperplasia. J Vasc Surg. 2002;35:152–157. [PubMed] [Google Scholar]
- 35.Jiang Z, Tao M, Omalley KA, Wang D, Ozaki CK, Berceli SA. Established neointimal hyperplasia in vein grafts expands via TGF-beta-mediated progressive fibrosis. Am J Physiol Heart Circ Physiol. 2009;297:H1200–1207. doi: 10.1152/ajpheart.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedl R, Li J, Schumacher B, Hanke H, Waltenberger J, Hannekum A, et al. Intimal hyperplasia and expression of transforming growth factor-beta1 in saphenous veins and internal mammary arteries before coronary artery surgery. Ann Thorac Surg. 2004;78:1312–1318. doi: 10.1016/j.athoracsur.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 37.Sousa AM, Liu T, Guevara O, Stevens J, Fanburg BL, Gaestel M, et al. Smooth muscle alpha-actin expression and myofibroblast differentiation by TGFbeta are dependent upon MK2. J Cell Biochem. 2006 doi: 10.1002/jcb.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willis AI, Pierre-Paul D, Sumpio BE, Gahtan V. Vascular smooth muscle cell migration: current research and clinical implications. Vasc Endovascular Surg. 2004;38:11–23. doi: 10.1177/153857440403800102. [DOI] [PubMed] [Google Scholar]
- 39.Muehlich S, Cicha I, Garlichs CD, Krueger B, Posern G, Goppelt-Struebe M. Actin-dependent regulation of connective tissue growth factor. Am J Physiol Cell Physiol. 2007;292:C1732–1738. doi: 10.1152/ajpcell.00552.2006. [DOI] [PubMed] [Google Scholar]
- 40.Flynn CR, Cheung-Flynn J, Smoke CC, Lowry D, Roberson R, Sheller MR, et al. Internalization and intracellular trafficking of a PTD-conjugated anti-fibrotic peptide, AZX100, in human dermal keloid fibroblasts. J Pharm Sci. 99:3100–3121. doi: 10.1002/jps.22087. [DOI] [PubMed] [Google Scholar]