Abstract
Lysophosphatidic acid (LPA) is produced by tumor cells and is present in the ascites fluid of ovarian cancer patients. To determine the role of endogenous LPA in the ovarian cancer cell line SKOV3, we treated cells with the LPA receptor antagonist VPC32183 and found that it inhibited cell growth and induced apoptosis. Exogenous LPA further stimulated ERK and Akt phosphorylation and NF-κB activity. To determine if reactive oxygen species (ROS), which have been implicated as second messengers in cell signaling, were also involved in LPA signaling, we treated cells with the NADPH oxidase inhibitor diphenyleneiodonium (DPI), and antioxidants N-acetyl cysteine, EUK-134 and curcumin, and showed that all blocked LPA-dependent NF-κB activity and cell proliferation. DPI and EUK-134 also inhibited Akt and ERK phosphorylation. LPA was shown to stimulate dichlorofluorescein fluorescence, though not in the presence of DPI, apocynin (a specific inhibitor of NADPH oxidase), VPC32183, or PEG-catalase. Akt phosphorylation was also inhibited by PEG-catalase and apocynin. These data indicate that NADPH oxidase is a major source of ROS and H2O2 is critical for LPA-mediated signaling. Thus, LPA acts as a growth factor and prevents apoptosis in SKOV3 cells by signaling through redox-dependent activation of ERK, Akt, and NF-κB-dependent signaling pathways.
Keywords: Ovarian cancer, Lysophosphatidic acid (LPA), Reactive oxygen species (ROS), H2O2, NADPH oxidase
INTRODUCTION
Ovarian cancer is the fifth leading cause of death in women because of the lack of early detection and frequent development of resistance to chemotherapy. Moreover, because early stages of the disease are asymptomatic, more than two thirds of patients with ovarian cancer present with advanced and essentially unmanageable metastatic disease. Such patients are commonly characterized by symptoms involving the peritoneum, bowel and omentum, the most significant being accumulation of ascites fluid in the patients’ abdominal cavities. This fluid stimulates ovarian cancer cell proliferation in vitro as well as in vivo [1], which is related to the increased amounts of lysophosphatidic acid (LPA) in the ascites fluid (1 – 80 µM) [2]. LPA in ascites fluid is produced by the ovarian tumors, and ovarian cancer cells in culture constitutively produce and release this lysophospholipid [3].
Both 1-O-alkyl-2-acyl-sn-glycero-3-phosphocholine (alkyl-PC) and 1,2-diacyl-sn-glycero-3-phosphocholine (acyl-PC) are present in many cell types and can serve as precursors of LPA. Alkyl and acyl LPAs have been identified in ascites fluids and shown to stimulate ovarian cancer cell proliferation, survival, invasiveness, and resistance to chemotherapy [2]. They elicit these responses through G protein-coupled receptors belonging to the endothelial-cell-differentiation gene family (Edg, now referred to as LPA receptors). LPA receptors are expressed in ovarian cancer cells [4]. LPA receptors variably activate heterotrimeric G proteins Gαi Gαq Gα12/13 to initiate a variety of cell signaling pathways, regulating the expression of multiple genes, including early response genes (c-fos, c-myc, and egr-1, and cox-2) [5] that induce the production of diverse factors and cytokines (e.g. interleukin-6 and interleukin-8). Expression of these genes is associated with a poor initial response to chemotherapy and a poor prognosis in ovarian cancer. These clinical findings are associated with the function of LPA receptor-regulated genes in promoting cell motility and migration, chemotaxis, vascular remodeling, angiogenesis, proliferation, survival, and metastasis [3].
LPA also induces proliferative signaling through the generation of second messengers. Recently reactive oxygen species (ROS) have become recognized as intermediate signaling molecules in non-phagocytic cells [6, 7]. Superoxide stimulates proliferation in a range of human cell lines, including smooth muscle cells [8]. Low concentrations of hydrogen peroxide are also mitogenic [9]. Furthermore, human tumor lines have been found to produce increased amounts of ROS [10], suggesting that these molecules contribute to the promotion of cancer phenotypes. ROS promote cell survival through the reversible activation or inhibition of pro-proliferative and regulatory signaling proteins [7].
The sources of the reactive species in non-phagocytic cells include the NADPH oxidases (NOXs), multimeric complexes that produce superoxide through the transfer of electrons from NADPH to oxygen molecules [6]. While superoxide is the immediate product of NADPH oxidase action, H2O2 is also inevitably generated through superoxide disumutase-catalyzed or uncatalyzed dismutation of superoxide to form H2O2 and O2. NOX isoforms are known to play roles in agonist-induced signaling, and overexpression of certain NOX isoforms is linked to increased basal and stimulus-induced activation of proliferative signaling pathways that are frequently altered in progression of carcinogenesis [4]. Importantly, NOX expression is critical for the aggressiveness of prostate cancer cells. Chemical inhibition of NOX proteins in several prostate cancer cell lines inhibits their ROS generation, proliferation, and invasiveness [11]. In addition, inhibition of NOX5 expression induces apoptosis in DU145 prostate cancer cells, indicating the necessity of NOX generated reactive oxygen species for survival of various cancers [12].
One important downstream effector stimulated by both LPA receptor activation and ROS activity is the serine/ threonine kinase, Akt [2]. LPA also stimulates the extracellular signal regulated/ mitogen activated protein kinase (ERK/ MAPK) cascade, and it is thought to be important in the proliferation and survival of ovarian cancers [2]. Stimulation of this pathway occurs through the activation of the G-protein Ras, which causes sequential activation of the Raf, MEK and ERK kinases. Activated ERK translocates into the cell nucleus, where it phosphorylates the transcription factor ELK-1 to initiate the transcription of survival, proliferation, and differentiation genes [13]. Studies in a murine model indicate the importance of ERK 1/2 signaling in the cellular survival response, as knockouts of upstream activators of ERK prove to be fatal [14]. ERK signaling is also important to chemoresistance in cancer, as inhibition of ERK signaling sensitizes ovarian cancer cell lines to cisplatin [15, 16].
Another major signaling molecule affected by ROS is the pro-survival transcription factor NF-κB, a molecule associated with proliferation, angiogenesis, and apoptosis suppression [17]. NF-κB has been identified as a redox-sensitive transcription factor, as it can be activated by hydrogen peroxide [18]. Also, decreasing the levels of the intracellular antioxidant glutathione causes IκB phosphorylation, leading to NF-κB activation [18, 19]. IκB phosphorylation triggers NF-κB activity, as the transcription factor is retained in the cytoplasm by this inhibitory protein in its unphosphorylated state. Various stimuli trigger upstream activation of IKK proteins, serine kinases that induce ubiquitination and proteosomal degradation of IκB, allowing NF-κB to translocate to the nucleus for transcription of anti-apoptotic genes. Aberrant NF-κB expression and activity have been identified with multiple cancers [20], and links have already been established between increased NF-κB activity and chemotherapy resistance in some ovarian cancer models [21]. ROS are suggested to have a role in NF-κB function in ovarian cancer, as treatment with the antioxidant curcumin caused a decline in NF-κB activity in ovarian cancer cell lines and subsequent reductions in cell viability and resistance to chemotherapy [22]. ROS control of NF-κB is thought to occur through direct mechanisms, specifically targeting subunits of NF-κB [23], as well as indirect mechanisms through oxidant modification of upstream kinases such as IKK [24] and Akt, an activator of IKK [25].
The relationship between LPA- and ROS-mediated signaling has been suggested in other cell types such as HeLa cells [26] and smooth muscle cells [27], but this interaction is not well documented in ovarian cancer cells. Akt and ERK represent potential signaling junctions between LPA and ROS activation. This is supported by the concept that Akt and ERK pathways are upregulated during oxidative insult [28], and that agents that prevent upstream receptor activation also prevent oxidant activation of Akt and ERK [29–31]. Because ROS seemingly modulate proliferative pathways that are LPA-activated in ovarian cancers, we questioned whether ROS and LPA were linked in the promotion of proliferation and survival of ovarian cancer cells. This study examined the role of ROS as intermediates in LPA-induced Akt and ERK signaling in SKOV3 ovarian cancer cells. The findings suggest that ROS are essential to SKOV3 cell survival and that NOX may be a critical source of ROS in these cells. The data also implicate NF-κB as an important component in the LPA and ROS-dependent signaling which regulates survival and proliferation.
MATERIALS AND METHODS
Reagents and Antibodies
Poly-ADP-Ribose polymerase (PARP), p44/42 MAP Kinase (ERK), Akt, phospho-p44/42 MAP Kinase (p-T202, p-Y204) (p-ERK), and phospho-Akt (p-T308) specific antibodies were purchased from Cell Signaling Technology. Avanti Polar Lipids, Inc was the supplier of VPC32183 and 18:1 lysophosphatidic acid (LPA, alkyl- and acyl-). PEG-catalase was purchased from Sigma, and Apocynin was supplied by Calbiochem. DMEM, RPMI 1640 medium, and Opti-MEM I + Glutamax media were from Invitrogen. The pNiFty-SEAP (secreted alkaline phosphatase) reporter construct plasmid was from Invivogen. The Great EscAPe SEAP detection kit was purchased from Clontech. Diphenyleneiodonium chloride (DPI) was from Calbiochem. Euk-134 was from Cayman Chemical. Curcumin and N-acetyl-cysteine (NAC) were purchased from Sigma-Aldrich. DCF-DA was from Invitrogen. Sulforhodamine B was from Sigma-Aldrich.
Cell Culture and Transfection
SKOV3 (ATCC) epithelial ovarian carcinoma cells were grown and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), L-glutamine, penicillin and streptomycin. Cells were maintained at 37°C incubation with 5% CO2. SKOV3 cells were transfected with the pNiFty-SEAP reporter construct to measure in vitro stimulus-mediated NF-κB activity. Briefly, cells were plated at 60% confluency. The following day, 2.0 µg of pNiFty-SEAP plasmid was added to cells using Lipofectamine transfection reagent (Invitrogen) according to the manufacturer’s instructions. The plates were incubated overnight at 37°C, 5% CO2.
LPA (alkyl- and acyl-) supplied and stored in chloroform was dried under a stream of nitrogen, resuspended at a concentration of 1 mM in phosphate buffered saline (PBS) containing 1% fatty acid free bovine serum albumin (BSA), then diluted in serum free medium to indicated concentrations. VPC32183 was resuspended and stored at a concentration of 10 mM in PBS containing 3% fatty acid free BSA, and diluted to indicated concentrations in serum free medium.
NF-κB Activity Assay
The activity of NF-κB was evaluated by a chemiluminescent method using the Great EscAPe SEAP detection kit (BD Biosciences) according to the manufacturer’s instructions. Cells were transfected with the pNiFty-SEAP NF-κB activity reporter plasmid which contains five copies of the consensus DNA binding sequence coupled to genes encoding a secretable form of alkaline phosphatase. Transfected cells were plated at 2 × 105 cells per 35 mm plate for each experimental condition. Media samples containing secreted alkaline phosphatase were collected in 96 well plates and reacted with a chemiluminescent substrate. Chemiluminescence was measured using a MicroLumatPlus LB 96 V luminometer from Berthold Technologies, Oak Ridge, TN.
Western Blotting
SKOV3 cells were plated at 1×106 cells per dish in 60-mm dishes. Cultures were then incubated in RPMI 1640 medium without serum for 18 h prior to challenge. Cells were harvested by washing with cold, Ca2+ free PBS and scraping into lysis buffer containing 50 mM Tris-HCl, 100 mM NaCl, 2mM EDTA, 0.1% SDS, 0.5% sodium deoxycholate, 1mM PMSF, 10 µg/ mL aprotinin, 10 µg/ mL leupeptin, 50 mM NaF, and 1mM sodium vanadate. Samples were sonicated with 10 × 1 second bursts and centrifuged for 10 min at 16,000 × g to remove cellular debris. The protein concentration of the supernatant was determined using Pierce BCA protein assay. Proteins (10 – 60 µg) prepared by boiling in sample buffer were loaded onto 10 or 12% SDS polyacrylamide gels, resolved by electrophoresis, and transferred to nitrocellulose membranes (Schleicher and Schuell). Blots were probed with protein specific antibodies and visualized using Western Lightning chemiluminescence reagent (Perkin Elmer).
Proliferation Assay
Cells were plated at 1.5 × 103 cells per well to a final volume of 200 µL media per well. Cells were incubated at 37°C and 5% CO2 overnight and challenged as indicated in serum free media. Proliferation was assessed at the indicated time points using MTS-based Cell Titer 96 AQueous One solution reagent (ProMega Corporation) per the manufacturer’s instructions. Absorbance was measured at 450 nm using a Molecular Devices VersaMax tunable microplate reader.
Alternatively, the sulforhodamine B (SRB) assay was used to determine cell proliferation based on the measurement of cellular protein content. SKOV3 cells were plated in 96 well plates at 1.5 × 103 cells per well and incubated overnight at 37°C, 5% CO2. The cells were then deprived of serum for 18 h before challenge. Cellular reactions were stopped by removing the culture media and fixing the cells with 10% (w/v) trichloroacetic acid, followed by staining with sulforhodamine B (0.4% w/v in 1% acetic acid) for 10 min. The excess dye was removed by washing repeatedly with 1% (vol/vol) acetic acid. The protein-bound dye was finally dissolved in 10 mM Tris base solution for OD determination at 564 nm using a Molecular Devices VersaMax tunable microplate reader.
ROS Measurement by DCF Fluorescence
Confocal Imaging. SKOV3 cells (2.5 × 104) were plated in 3 mL RPMI supplemented with 10% FBS in 1 well Lab-Tek II Chambered Coverglasses and incubated for 24 h before overnight incubation in serum free medium. Cells were incubated with 50 µM 2',7'-dichloro-fluorescein diacetate (DCF-DA) for 15 minutes, washed with serum free medium, and then control cells or cells challenged with 10 nM alkyl LPA were visualized for DCF fluorescence using a Zeiss LSM510 laser scanning confocal microscope with a C-Apo 63X water-corrected lens with an N.a. of 1.2, and an Argon-Ion laser excitation wavelength of 488 nanometers. The FITC filter set consisted of a 505–530 nm bandpass filter. The software is Zeiss software. Where indicated, DPI (1 µM) was added 1 min prior to stimulation with 10 nM 18:1 alkyl LPA. Fluorescent Microscopy. SKOV3 cells (5 × 104) were plated in 2 ml RPMI supplemented with 10% FBS in 35 mm dishes. Where indicated, cells were pre-incubated with PEG-catalase overnight, or with VPC32183 or apocynin for 30 minutes prior to stimulation without or with 100 nM alkyl LPA for 30 minutes. Cells were incubated with DCF-DA for the final 10 minutes of LPA treatment, then washed, and visualized using an Olympus inverted epi-fluorescent microscope with FITC filters.
RESULTS
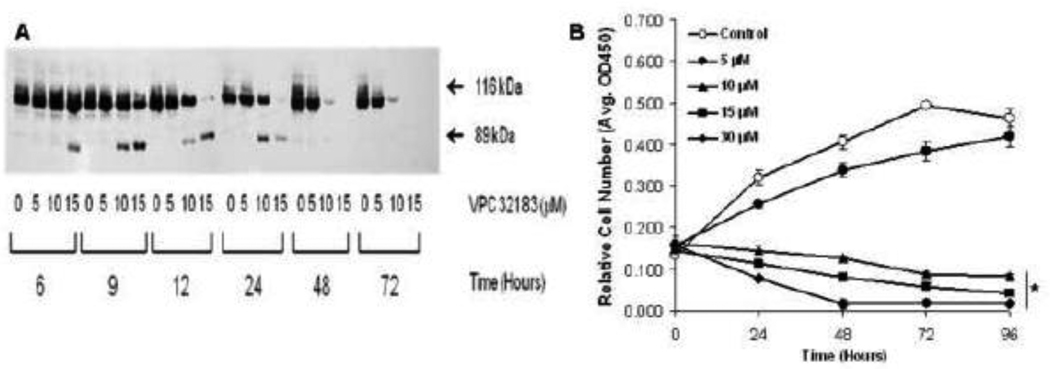
Our initial experiments were designed to test the hypothesis that in SKOV3 cells the endogenous production of LPA can support cell growth and survival. We first investigated the ability of SKOV3 cells to grow in the absence of fetal bovine serum. The SKOV3 cells without serum grew somewhat slower than the cells in 10% FBS (data not shown). However, there was a significant amount of growth without serum indicating that the cells produced endogenous growth factors. Since SKOV3 cells have been shown to make LPA [32], we used VPC32183, an LPA receptor antagonist, to determine if LPA was acting as an endogenous growth factor in the SKOV3 cells. We found that VPC32183 caused a rapid time- and dose-dependent increase in apoptosis in SKOV3 cells (Fig.1A). At the highest dose tested (15 µM), there was evidence of apoptosis as indicated by PARP cleavage as early as six hours after treatment. At 9 hours after treatment, PARP cleavage was evident with 10 µM VPC32183. After 12 hours, PARP was totally degraded after exposure to 15 µM VPC32183. In control cells, or with the lowest dose of VPC32183 (5 µM), no PARP cleavage was detected at 72 hours. Thus, endogenously produced LPA appears to be critical to cell survival of the SKOV3 cell line. In addition, we determined the effect of VPC32183 on the proliferation of SKOV3 cells. At 5 µM, VPC32183 had little effect on cell growth (Fig.1B) or apoptosis (Fig.1A). However, at the higher doses tested (>5 µM) VPC32183 inhibited proliferation.
Figure 1. Blocking LPA-dependent signaling induces SKOV3 cell apoptosis and inhibits proliferation.
(A) SKOV3 cells were incubated with VPC32183 (0, 5, 10, or 15 µM) for the indicated times and harvested by detergent lysis. The cell lysates were assayed for apoptosis by Western blotting using antibodies specific for PARP. The appearance of the 89 kDa band of cleaved PARP is indicative of apoptosis. The blot presented is representative of at least four repetitions. (B) SKOV3 cells were incubated in serum free culture medium without or with the indicated concentrations of VPC32183. At the indicated time points cell viability was determined using CellTiter 96 AQueous One solution assay. The data presented are the mean ± SEM from a representative one of three experiments. The data were analyzed using Student’s t-test and values were significantly different from untreated cells as indicated. The asterisk indicates that all data points for treatments greater than 10 µM VPC32183 are statistically significant from control (p<0.00006).
To determine potential protective pathways in LPA-stimulated SKOV3 cells, we treated cells with exogenous LPA containing either an alkyl- or acyl- linkage in the sn-1 position. These were of interest since both linkages exist in choline phospholipids, the precursors of LPA, and are both observed in ascites fluids [2]. We found that both alkyl-and acyl-LPA caused a time- and concentration-dependent increase in Akt phosphorylation and ERK phosphorylation without changing the total amounts of either enzyme (Fig.2A–B).
Figure 2. LPA stimulates phosphorylation of Akt and ERK in SKOV3 cells.
Cells were plated as described in Materials and Methods. (A) SKOV3 cells were treated for the indicated times with 1 µM acyl-LPA. (B) Cells were stimulated with the indicated concentrations of alkyl(alk)- or acyl- 18:1 LPA for 30 minutes. After stimulation, cells were harvested, and lysates were resolved by electrophoresis, and immunoblots of transferred proteins were probed with phospho-Akt (p-T308), total Akt, phospho-ERK 1/2 (p-T202, p-Y204), or total ERK 1/2 specific antibodies. The blots are representative of greater than ten repetitions. Blots were analyzed by densitometry and fold change is presented.
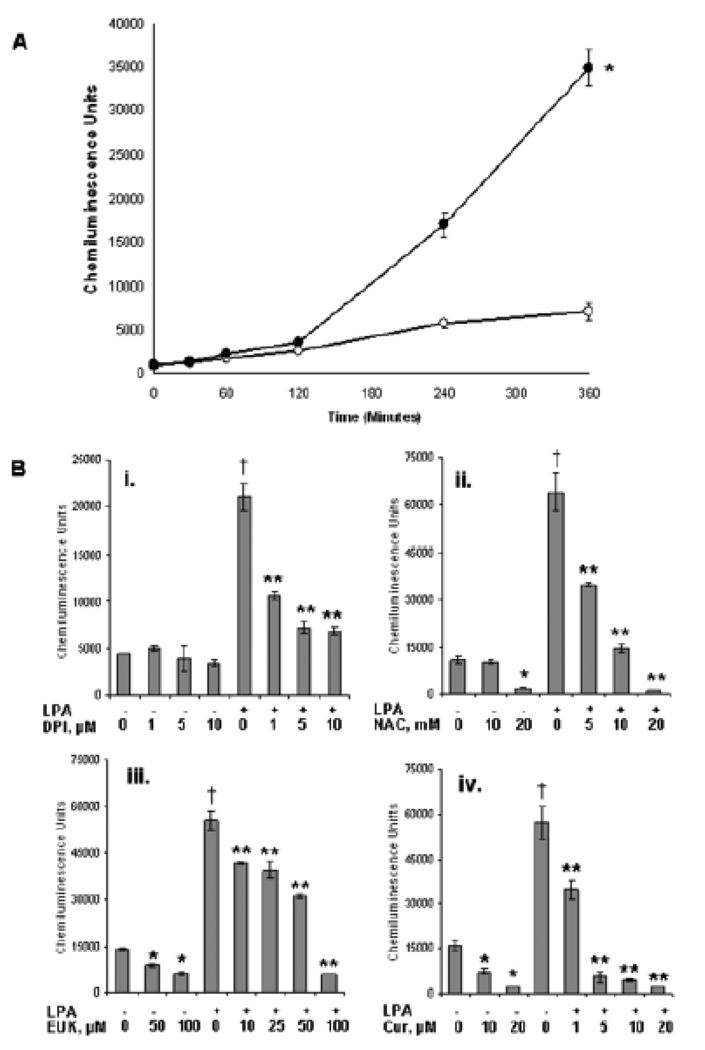
NF-κB is a transcription factor that is linked to growth control and resistance to apoptosis in many cancer cells. We found that LPA causes a marked increase in NF-κB-dependent transcription in SKOV3 cells (Fig.3A). NF-κB is thought to be controlled by redox signaling, but the specific targets for redox regulation and the mechanism of increased ROS generation have not been determined. One potential source of ROS is through activation of the NOX complex. DPI is an inhibitor of flavin-containing enzymes including NOX. We used DPI in unstimulated SKOV3 cells and found that it decreased the constitutive levels of NF-κB (Fig.3B panel (i)). Also, there was a marked inhibition of LPA-stimulated NF-κB induction. Similarly, NAC has been used to inhibit redox-dependent signaling and we found that NAC inhibited NF-κB activity in both unstimulated and LPA-stimulated SKOV3 cells (Fig.3B panel (ii)). EUK-134 is a synthetic manganese-porphyrin complex that scavenges oxidative species including both superoxide and H2O2. We found that EUK-134 inhibits LPA-stimulated NF-κB activity and unstimulated NF-κB activity (Fig.3B panel (iii)). Also, curcumin caused a dose-dependent inhibition of both LPA-stimulated NF-κB activity and the activity in unstimulated cells (Fig.3B, panel (iv)).
Figure 3. LPA-mediated NF-κB activity in SKOV3 cells is ROS dependent.
(A) SKOV3 cells transfected with the pNiFty-SEAP NF-κB reporter construct were treated without (open circles) or with 1 µM LPA (closed circles). NF-κB activity was monitored in the medium at the indicated times as secreted alkaline phosphatase induced chemiluminescence. The data are representative of the mean of three experiments. * indicates that LPA stimulation is significant at all data points p<0.02. (B) SKOV3 cells transfected with the reporter construct were incubated without or with 1 µM LPA in the presence of the indicated amounts of (i) DPI; (ii) N-acetyl cysteine; (iii) EUK-134; or (iv) curcumin. NF-κB activity was measured at 6 hours. The data are representative of the mean of 12 data points. The data were analyzed using Student’s t-test and values were significantly different from untreated cells as indicated. † indicates that LPA stimulation is statistically significant compared to control p<0.007. * indicates data are statistically significant compared to control p<0.003. ** indicates data are statistically significant compared to LPA stimulated p<0.008.
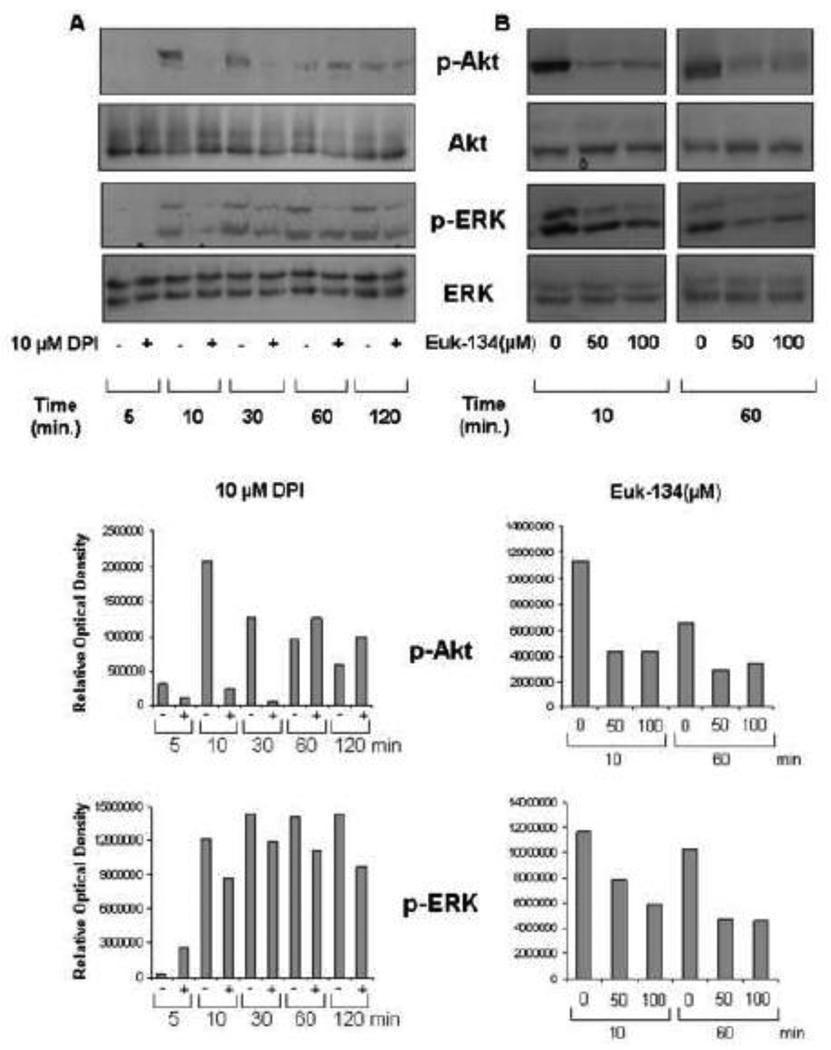
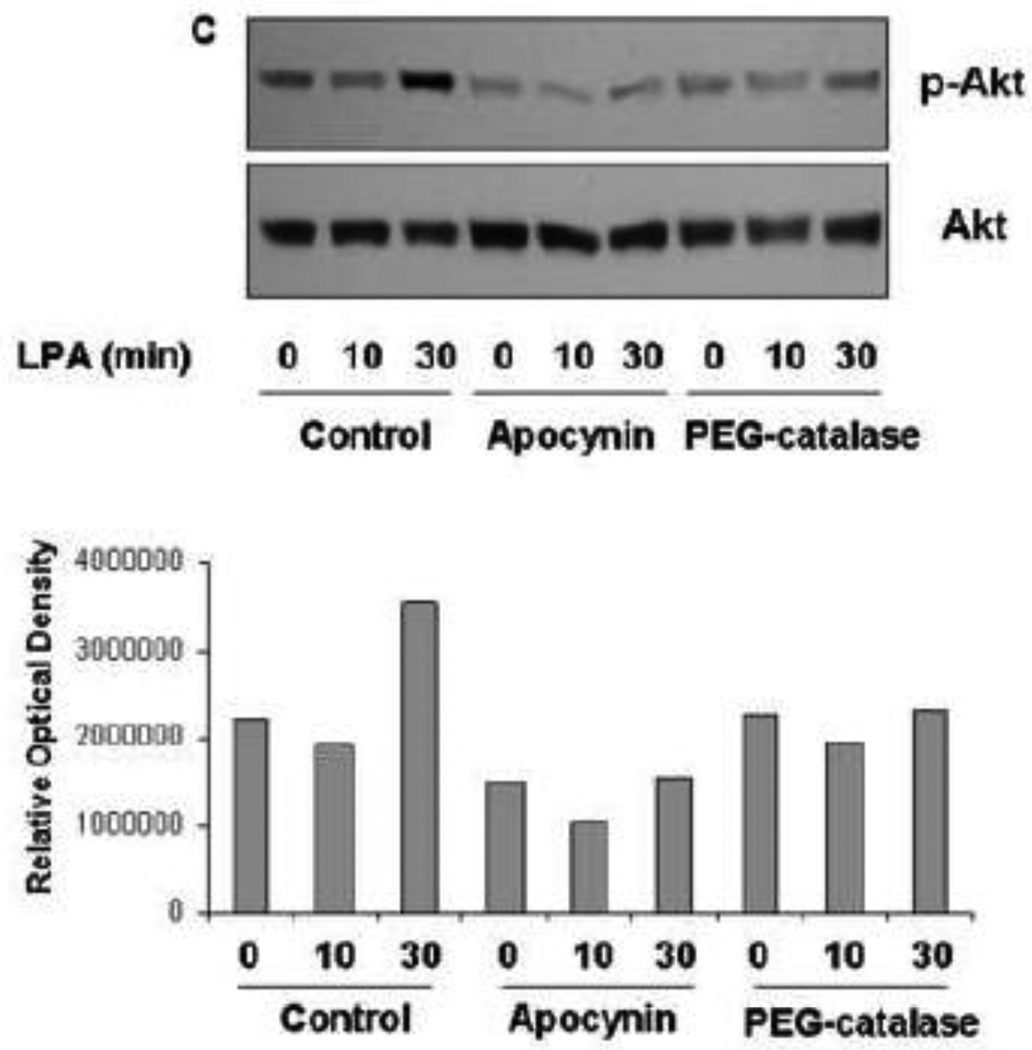
Because the above experiments appear to indicate a requirement for NOX-dependent ROS generation in NF-κB activation, we next determined the effects of DPI and EUK-134 on signaling through the ERK and Akt pathways. First, we find that DPI inhibits Akt phosphorylation without affecting total levels of Akt (Fig.4A). Similarly, DPI inhibits ERK phosphorylation without changing the total levels of ERK. Next, we find that EUK-134 inhibits phosphorylation of Akt without affecting the levels of total Akt (Fig.4B). Similarly, EUK-134 inhibits ERK phosphorylation without causing changes in the total level of ERK. We also used apocynin (1-(4-hydroxy-3-methoxyphenyl) ethanone), an inhibitor with greater specificity toward NOX than DPI, and found that it inhibited LPA-stimulated phosphorylation of Akt without changing the levels of total Akt (Fig. 4C). Polyethylene glycol-conjugated catalase (PEG-catalase), a cell permeable form of catalase was also used and found to inhibit LPA-dependent Akt-phosphorylation (Fig. 4C), demonstrating a critical role for H2O2 in this signaling process.
Figure 4. DPI and EUK-134 decrease LPA induced Akt and ERK activation.
(A) SKOV3 cells in serum free media were pretreated with 10 µM DPI before stimulation with LPA (1µM) for 5, 10, 30, 60, and 120 minutes. (B) SKOV3 cells in serum free media were pretreated with 0, 50, or 100 µM EUK-134 before stimulating with LPA (1µM) for indicated times. (C) SKOV3 cells in serum free media were pretreated with apocynin or PEG-catalase before stimulation with LPA for 10 and 30 minutes respectively. Cells were harvested and lysates were subjected to Western blot analysis using phospho-Akt (p-T308), total Akt, phospho-ERK 1/2 (p-T202, p-Y204), or total ERK specific antibodies. The blots are representative of three separate experiments. Blots were analyzed by densitometry and fold change is presented.
The experiments described above indicate that endogenously produced LPA promotes cell growth and survival through redox-dependent signaling mechanisms and that the Akt, ERK and NF-κB pathways may be involved. We next investigated whether inhibitors of redox signaling inhibited growth of SKOV3 cells. We found that DPI caused a dose-dependent decrease in cell growth with significant inhibition at all concentrations above 50 nM (Fig.5A). Similarly, NAC, curcumin and EUK-134 inhibited proliferation of SKOV3 cells (Fig.5B–D). Taken together, these data indicate that a variety of antioxidant compounds, with different mechanisms of action, can inhibit LPA-dependent signaling pathways and cell growth.
Figure 5. Antioxidant treatment decreases cell proliferation.
SKOV3 cells in serum free media were incubated with DPI (A), NAC (B), curcumin (C), or EUK-134 (D). (A)For DPI, proliferation was measured using ProMega Cell Titer 96 AQueous One solution assay as previously described. (B–D)The reducing effects of the other antioxidants were not compatible with the MTS assay. Therefore, for NAC, EUK-134, and curcumin cellular proteins were stained with 0.4% sulforhodamine B at the indicated times and assayed as described in Materials and Methods. The data are representative of the mean of three experiments. The data were analyzed using Student’s t-test and values were significantly different from untreated cells as indicated. * p<0.01, **p<5×10−6, ***p<2×10−12.
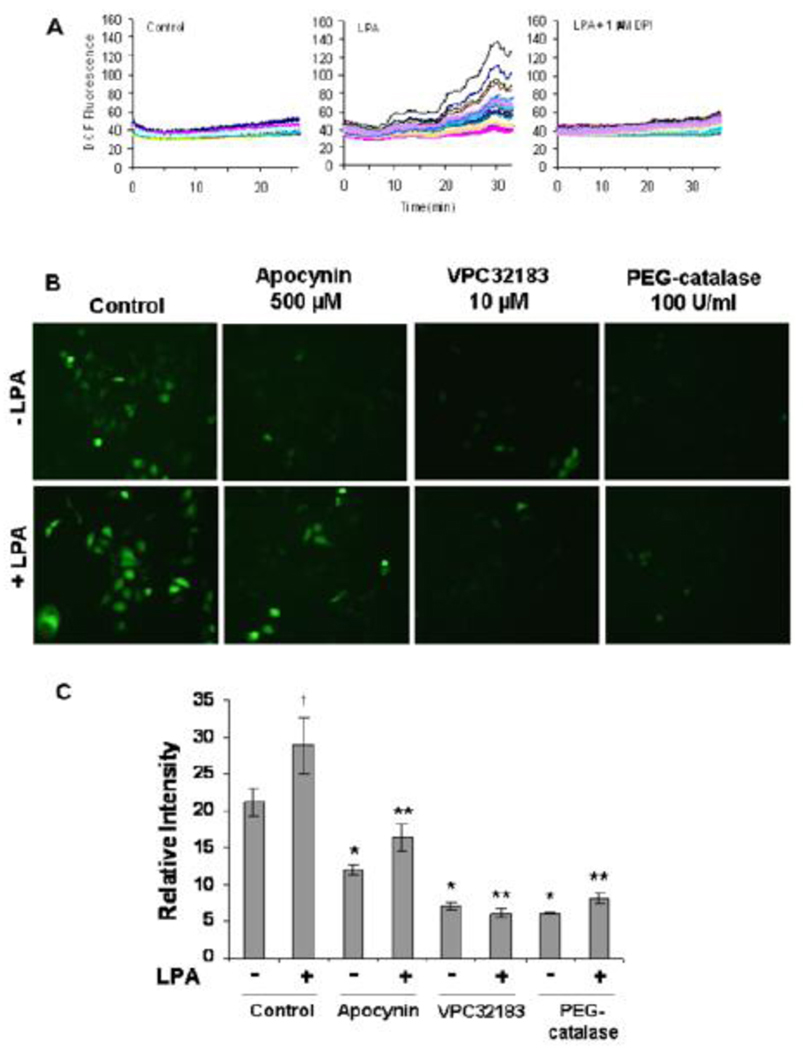
Given the apparent requirement for ROS as second messengers in LPA-dependent signaling, we sought a more direct way to evaluate the ability of LPA to stimulate ROS in SKOV3 cells by using cells loaded with DCF-DA prior to LPA-stimulation. We found that LPA caused an increase in ROS which was evident at 10 minutes and was followed by an increase in ROS formation up to 30 minutes (Fig.6A). When we followed the fluorescence in individual cells by confocal microscopy we found a heterogeneous response among cells with some high and some lower responders. However, increased ROS production was seen in a majority of the cells (Fig.6A). We also observed that DPI at 1 µM completely suppressed the ROS production in response to LPA.
Figure 6. LPA stimulates increased ROS generation through NADPH oxidase in SKOV3 cells.
(A) SKOV3 cells in 1 well Lab-Tek II Chambered Coverglasses were incubated with 50 µM DCF-DA 15 minutes prior to stimulation without or with LPA (10 nM alkyl-). DCF fluorescence was visualized at the specified times using a Zeiss LSM510 laser scanning confocal microscope with a 63X objective. Cells were incubated with 50 µM DCF-DA as described. DCF fluorescence was visualized every 30s for 30–35 minutes after LPA stimulation using the confocal microscope. 1 µM DPI was added 1 minute before stimulation with 10 nM LPA. Individual cells were analyzed for relative fluorescent intensities using LSM Image Browser Software from Zeiss. The response of individual cells are represented by the individual lines on the graphs.
(B) SKOV3 cells were pre-incubated with indicated inhibitors prior to stimulation without or with LPA (10 nM alkyl-). Cells were incubated with 50 µM DCF-DA for the final 10 minutes of LPA stimulation, washed, and visualized for fluorescence using an Olympus inverted epi-fluorescent microscope with FITC filter sets. (C) The relative DCF fluorescence was determined using ImageJ software and averaged from at least 35 cells. The data were then analyzed using two-tailed unpaired Students t-tests with values compared to the respective controls
These data indicated that a major source of ROS was LPA-dependent NOX activation. To gain further evidence for NOX activation we used apocynin, a specific NOX inhibitor, and found that it inhibited LPA-dependent stimulation of ROS (Fig. 6B–C). Apocynin also decreased the endogenous levels of ROS in unstimulated cells indicating that SKOV3 cells have constitutively active NOX that increases ROS in the cells without external stimuli. VPC32183 also decreased ROS in cells stimulated with exogenous LPA and in control cells. These data indicate that the constitutive levels of ROS are due to stimulation of NOX by endogenously produced LPA. The LPA-stimulated ROS was also decreased by preincubating the cells with PEG-catalase. Also, the background levels of ROS in control cells was decreased by PEG-catalase. These data indicate that H2O2 is produced in LPA-stimulated cells and by the control cells without exogenous LPA.
DISCUSSION
Once thought to be solely detrimental to cell viability, reactive oxygen species are gaining recognition as stimulants for survival and proliferation of normal and malignant cells. Importantly, ROS are controlled in a manner that gives them properties as intracellular second messengers [33]. The goal of our studies was to evaluate the role of ROS as second messengers in the LPA-mediated survival signaling in ovarian cancer. We first characterized the relative importance of LPA activity and signaling in the ovarian cancer cell line SKOV3. LPA was an effective stimulus for the activation of Akt and ERK 1/2 kinases, two major constituents of the most potent survival pathways in ovarian cancer. We also found that LPA was able to increase the transcription of critical survival and anti-apoptotic genes through enhancement of the activity of transcription factor NF-κB. Conversely, LPA receptor blockade diminished SKOV3 cell proliferation, decreased survival gene transcription, and ultimately resulted in the induction of apoptosis.
Of particular interest in our study was the functional delineation between alkyl and acyl LPA species. In several instances, alkyl 18:1 LPA was used. We speculated that these lipid molecules could have similar activity, and this concept was supported by the literature. Several studies document that synthetic alkyl LPA analogs have agonist function similar to the acyl lipid classes at LPA receptors 1–3 [34, 35]. Importantly, analysis of ascites fluid from ovarian cancer patients revealed that naturally occurring alkyl LPAs were present. This same study also demonstrated that the alkyl lipid species were able to elicit phosphorylation of ERK and Akt similarly to the ester linked LPA [2]. We found that 18:1 alkyl LPA was even more potent than the acyl species at stimulating both Akt and ERK phosphorylation in SKOV3 cells (Fig.2B). We also observed that this alkyl lysophospholipid caused the robust increase in ROS generation that we determined to be critical for propagating survival signals required for serum-free proliferation. Strikingly, this effect was elicited at a concentration of 10 nM, which is 100 times less than the concentration of acyl LPA that is typically considered to be stimulatory. This effect is likely due to the enhanced stability of the ether linked LPAs. Lu et al. [2] compared the stability of alkyl to acyl LPAs in ascities fluid and found that the alkyl lipids were nearly 80 times more stable than the ester linked LPAs. This stability is possibly due to the resistance of the alkyl LPAs to degradation by phosphatases and lipases that have more activity on acyl LPAs. While alkyl LPA was more potent at triggering intracellular ROS release in SKOV3 cells, this is by no means limited to this lipid species or cell line, as we observed the same results using 1 µM acyl LPA in a highly aggressive prostate cancer cell line (PC-3; data not shown).
LPA directly stimulated ROS generation in our model ovarian cancer cell line (Fig. 6). Interestingly, we noticed that maximal LPA-induced ROS generation occurred approximately at the same time (30 min after stimulation) that we observed maximal phosphorylation of Akt and ERK in the SKOV3 cells. ROS generation included the production of H2O2, which was shown to be critical for the phosphorylation of Akt given the strongly inhibitory effect of PEG-catalase (Fig. 4C). Each of the major pathways that we found to be upregulated by LPA in SKOV3 cells are also functionally enhanced by ROS modulation and candidate targets for therapeutics in the treatment of multiple cancers [36–38]. In light of this, we might consider some ROS-induced oxidative events as “true transformation” events, potentially triggering modifications in protein function that enhance tumorigenicity.
The transient nature of these modulatory oxidative reactions caused us to consider the source of the ROS produced in our model ovarian cancer cell line. We used the flavoprotein inhibitor DPI and the NOX inhibitor apocynin to inhibit generation of NOX-derived ROS in SKOV3 cells. We found that DPI and apocynin were potent inhibitors of ROS production, also DPI inhibited survival signaling and cell proliferation at nanomolar concentrations (Fig. 3–6). These data suggest that NOX enzymes generate necessary ROS that are used to maintain LPA-activated pathways that promote ovarian cancer cell persistence. The data also suggest that NOXs may be useful targets for stand alone cancer therapies or as supplements to current chemotherapeutic drugs. We are particularly interested in understanding the differential expression of NOX isoforms in ovarian cancers. Of the several types, NOX1 and NOX4 have both been described as being overexpressed in ovarian tumors [39, 40]. Thus, NOX may prove to have specificity in ovarian cancer therapy.
In summary, we provide evidence of a potentially novel link between LPA signaling and the most critical pathways for the progression and malignant propagation of ovarian cancer, and the more recently identified and poorly understood function of ROS as intracellular signaling modulators. The oncogenic characteristics of ROS are becoming clearer as new proteins are being identified as having redox regulation potential, along with new associations being made between ROS and well known oncogenic pathways. Ovarian tumors that constitutively produce and respond to LPA may also have chronically elevated levels of ROS, which could confer a proliferative advantage, but could also negatively impact their abilities to escape chemotherapy-induced death.
Because there are multiple strategies for lowering ROS, this novel mechanism is a potential target for the treatment of ovarian cancer. NAC is a clinically approved drug, which could be used as an addition to the current regimen for treating ovarian cancer. It would be beneficial to engage in studies to evaluate the effectiveness of NAC and other antioxidants on tumor cell growth. Potential studies could involve measuring activation of oxidation-dependent survival signaling proteins pre- and post-antioxidant therapy, as well as directly measuring the effect of antioxidant therapies. Alternatively, increasing ROS in tumors may induce cell death by increasing instability of critical genes and proteins, thereby making them more sensitive to chemotherapy or radiation treatments. In fact, both chemotherapy and radiation stimulate ROS and ROS appear to be critical to their mechanism of action [41]. Thus, tumor cells appear to require a critical range of ROS concentrations, a redox signaling window, in which reversible redox signaling can occur. Further studies to determine the critical targets of redox dependent cell signaling are needed to determine the molecular mechanisms involved. As we learn more about the nature of redox-dependent signaling in the tumorigenesis and malignancy of ovarian cancers, new avenues will be opened for future therapies which can exploit our understanding of redox-regulation of cell growth.
ACKNOWLEDGEMENTS
Financial Support
This work was supported in part by NIH grants RO1 CA142838 (L.W. Daniel), R33 CA126659 (L.B. Poole), and F31 CA106199 (J.A. Saunders).
LIST OF ABBREVIATIONS
- Akt
Protein kinase B
- BSA
Bovine serum albumin
- Cox-2
Cyclooxygenase-2
- DCF
Dichloro-fluorescein
- DCF-DA
Dichlorodihydro-fluorescein diacetate
- DPI
Diphenyleneiodonium chloride
- Edg
Endothelial-cell-differentiation gene
- EDTA
Ethylenediaminetetraacetic acid
- ERK
p44/42 MAP Kinase
- FBS
Fetal bovine serum
- FITC
Fluorescein isothiocyanate
- IKK
I kappa kinase
- LPA
Lysophosphatidic acid
- NAC
N-acetyl cysteine
- NF-κB
Nuclear Factor kappa B
- NOX
NADPH oxidase
- PARP
Poly ADP ribose polymerase
- PEG-catalase
Polyethylene glycol conjugated catalase
- PMSF
Phenylmethylsulphonyl fluoride
- ROS
Reactive oxygen species
- SDS
Sodium dodecyl sulfate
- SEAP
Secreted alkaline phosphatase;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, Kennedy AW, Belinson J, Markman M, Casey G. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Xiao Yj YJ, Baudhuin LM, Hong G, Xu Y. Role of ether-linked lysophosphatidic acids in ovarian cancer cells. J Lipid Res. 2002;43:463–476. [PubMed] [Google Scholar]
- 3.Umezu-Goto M, Tanyi J, Lahad J, Liu S, Yu S, Lapushin R, Hasegawa Y, Lu Y, Trost R, Bevers T, Jonasch E, Aldape K, Liu J, James RD, Ferguson CG, Xu Y, Prestwich GD, Mills GB. Lysophosphatidic acid production and action: validated targets in cancer? J Cell Biochem. 2004;92:1115–1140. doi: 10.1002/jcb.20113. [DOI] [PubMed] [Google Scholar]
- 4.Fang X, Gaudette D, Furui T, Mao M, Estrella V, Eder A, Pustilnik T, Sasagawa T, Lapushin R, Yu S, Jaffe RB, Wiener JR, Erickson JR, Mills GB. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann N Y Acad Sci. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 5.Symowicz J, Adley BP, Woo MM, Auersperg N, Hudson LG, Stack MS. Cyclooxygenase-2 functions as a downstream mediator of lysophosphatidic acid to promote aggressive behavior in ovarian carcinoma cells. Cancer Res. 2005;65:2234–2242. doi: 10.1158/0008.5472.CAN-04-2781. [DOI] [PubMed] [Google Scholar]
- 6.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat RevImmunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 7.Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediatedcysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher DL, Betteridge LJ, Patel MK, Schachter M. Effect of oxidants on vascular smooth muscle proliferation. Biochem Soc Trans. 1993;21:98S. doi: 10.1042/bst021098s. [DOI] [PubMed] [Google Scholar]
- 9.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 10.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 11.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 12.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 13.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa J, Ohmichi M, Kurachi H, Ikegami H, Kimura A, Matsuoka T, Jikihara H, Mercola D, Murata Y. Inhibition of extracellular signal-regulated protein kinase or c-Jun N-terminal protein kinase cascade, differentially activated by cisplatin, sensitizes human ovarian cancer cell line. J Biol Chem. 1999;274:31648–31654. doi: 10.1074/jbc.274.44.31648. [DOI] [PubMed] [Google Scholar]
- 16.Persons DL, Yazlovitskaya EM, Cui W, Pelling JC. Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells: inhibition of extracellular signal-regulated kinase activity increases sensitivity to cisplatin. Clin Cancer Res. 1999;5:1007–1014. [PubMed] [Google Scholar]
- 17.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mihm S, Galter D, Droge W. Modulation of transcription factor NF kappa B activity by intracellular glutathione levels and by variations of the extracellular cysteine supply. FASEB J. 1995;9:246–252. doi: 10.1096/fasebj.9.2.7781927. [DOI] [PubMed] [Google Scholar]
- 20.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human ancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 21.Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, Saito M, Kawagoe J, Takahashi K, Yada-Hashimoto N, Sakata M, Motoyama T, Kurachi H, Tasaka K, Murata Y. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro in vivo ovarian cancer models. J Biol Chem. 2004;279:23477–23485. doi: 10.1074/jbc.M313709200. [DOI] [PubMed] [Google Scholar]
- 22.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK, Aggarwal BB, Sood AK. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 23.Pantano C, Reynaert NL, van der, Vliet A, Janssen-Heininger YM. Redox- sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal. 2006;8:1791–1806. doi: 10.1089/ars.2006.8.1791. [DOI] [PubMed] [Google Scholar]
- 24.Kamata H, Manabe T, Oka S, Kamata K, Hirata H. Hydrogen peroxide activates IkappaB kinases through phosphorylation of serine residues in the activation loops. FEBS Lett. 2002;519:231–237. doi: 10.1016/s0014-5793(02)02712-6. [DOI] [PubMed] [Google Scholar]
- 25.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Olashaw N, Wu J. Participation of reactive oxygen species in the lysophosphatidic acid-stimulated mitogen-activated protein kinase kinase activation pathway. J Biol Chem. 1995;270:28499–28502. doi: 10.1074/jbc.270.48.28499. [DOI] [PubMed] [Google Scholar]
- 27.Kaneyuki U, Ueda S, Yamagishi S, Kato S, Fujimura T, Shibata R, Hayashida A, Yoshimura J, Kojiro M, Oshima K, Okuda S. Pitavastatin inhibits lysophosphatidic acid-induced proliferation and monocyte chemoattractant protein-1 expression in aortic smooth muscle cells by suppressing Rac-1-mediated reactive oxygen species generation. Vascul Pharmacol. 2007;46:286–292. doi: 10.1016/j.vph.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 29.Knebel A, Rahmsdorf HJ, Ullrich A, Herrlich P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 1996;15:5314–5325. [PMC free article] [PubMed] [Google Scholar]
- 30.Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf HJ. Involvement of growth factor receptors in the mammalian UVC response. Cell. 1994;78:963–972. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Yu X, Cohen RA, Brecher P. Distinct effects of N-acetylcysteine and nitric oxide on angiotensin II-induced epidermal growth factor receptor phosphorylation and intracellular Ca(2+) levels. J Biol Chem. 2000;275:12223–12230. doi: 10.1074/jbc.275.16.12223. [DOI] [PubMed] [Google Scholar]
- 32.Snider AJ, Zhang Z, Xie Y, Meier KE. Epidermal growth factor increases lysophosphatidic acid production in human ovarian cancer cells: roles for phospholipase D2 and receptor transactivation. Am J Physiol Cell Physiol. 298:C163–C170. doi: 10.1152/ajpcell.00001.2009. [DOI] [PubMed] [Google Scholar]
- 33.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 34.Qian L, Xu Y, Simper T, Jiang G, Aoki J, Umezu-Goto M, Arai H, Yu S, Mills GB, Tsukahara R, Makarova N, Fujiwara Y, Tigyi G, Prestwich GD. Phosphorothioate analogues of alkyl lysophosphatidic acid as LPA3 receptor-selective agonists. ChemMedChem. 2006;1:376–383. doi: 10.1002/cmdc.200500042. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Tanaka M, Arai H, Aoki J, Prestwich GD. Alkyl lysophosphatidic acid and fluoromethylene phosphonate analogs as metabolically-stabilized agonists for LPA receptors. Bioorg Med Chem Lett. 2004;14:5323–5328. doi: 10.1016/j.bmcl.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Carnero A. The PKB/AKT pathway in cancer. Curr Pharm Des. 16:34–44. doi: 10.2174/138161210789941865. [DOI] [PubMed] [Google Scholar]
- 37.Falasca M. PI3K/Akt signalling pathway specific inhibitors: a novel strategy to sensitize cancer cells to anti-cancer drugs. Curr Pharm Des. 16:1410–1416. doi: 10.2174/138161210791033950. [DOI] [PubMed] [Google Scholar]
- 38.McCubrey JA, Steelman LS, Abrams SL, Chappell WH, Russo S, Ove R, Milella M, Tafuri A, Lunghi P, Bonati A, Stivala F, Nicoletti F, Libra M, Martelli AM, Montalto G, Cervello M. Emerging MEK inhibitors. Expert Opin Emerg Drugs. 15:203–223. doi: 10.1517/14728210903282760. [DOI] [PubMed] [Google Scholar]
- 39.Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther. 2005;4:1367–1373. doi: 10.4161/cbt.4.12.2233. [DOI] [PubMed] [Google Scholar]
- 40.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther. 2008;7:1875–1884. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]