Abstract
While research on the actions of ethanol at the GABAergic synapse has focused on postsynaptic mechanisms, recent data have demonstrated that ethanol also facilitates GABA release from presynaptic terminals in many, but not all, brain regions. The ability of ethanol to increase GABA release can be regulated by different G protein-coupled receptors (GPCRs), such as the cannabinoid-1 receptor, corticotropin-releasing factor 1 receptor, GABAB receptor, and the 5-hydroxytryptamine 2C receptor. The intracellular messengers linked to these GPCRs, including the calcium that is released from internal stores, also play a role in ethanol-enhanced GABA release. Hypotheses are proposed to explain how ethanol interacts with the GPCR pathways to increase GABA release and how this interaction contributes to the brain region specificity of ethanol-enhanced GABA release. Defining the mechanism of ethanol-facilitated GABA release will further our understanding of the GABAergic profile of ethanol and increase our knowledge of how GABAergic neurotransmission may contribute to the intoxicating effects of alcohol and to alcohol dependence.
Keywords: Ethanol, Presynaptic, GABA release, G protein-coupled receptor, Internal calcium store
1. Introduction
While previously thought to be just an amino acid, gamma-aminobutyric acid (GABA) was recognized as a major inhibitory neurotransmitter in the central nervous system during the 1960s (Krnjevic and Phillis, 1963; Otsuka et al., 1966; Roberts and Kuriyama, 1968). In the presynaptic terminal, glutamate decar-boxylase converts glutamate into GABA, and GABA is actively transported into the synaptic vesicles by a vacuolar proton pump that establishes an electrochemical gradient across the vesicle (Martin, 1993; McIntire et al., 1997; Sagne et al., 1997). The vesicles dock at the plasma membrane through an interaction between synaptobrevin, which is located on the synaptic vesicles, and syntaxin 1 and SNAP-25, which are located on the plasma membrane (see Sudhof, 1995, 2004). This core complex allows for a number of protein–protein interactions to occur that are necessary for exocytosis, including the calcium sensor synaptotagmin triggering the final stage of vesicle fusion with the membrane. Once the vesicles fuse with the membrane, GABA is released from the presynaptic terminal into the synaptic cleft and binds to the postsynaptic GABAA receptors, allowing chloride to flow into the neuron.
The flow of chloride through the GABAA receptor can be measured in an in vitro slice preparation using whole-cell voltage-clamp recordings. This technique can measure both spontaneous (action potential-independent and dependent) and evoked GABA release. Action potential-independent GABA release is isolated by tetrodotoxin—a drug that blocks action potentials via inhibition of voltage-gated sodium channels. Treatment with tetrodotoxin allows for measurement of a GABAA receptor-mediated chloride flux response known as a miniature inhibitory postsynaptic current (mIPSC). When the number of mIPSC events is increased, the change is interpreted as an increase in spontaneous GABA release. In the absence of tetrodotoxin, there is a mix of action potential-dependent and independent GABA release, and this measured GABAA receptor-mediated chloride flux response is referred to as a spontaneous inhibitory postsynaptic current (sIPSC). Interpreting an increase in sIPSC frequency is not as straightforward as interpreting an increase in mIPSC frequency because an increase in sIPSC frequency can be due to a direct effect on the presynaptic terminal (i.e., increase in terminal release probability), an increase in action potential firing, or activation of a neighboring neuron that affects the presynaptic neuron (i.e., increase in somatic/axonal excitability).
In contrast to spontaneous GABA release, measuring evoked GABA release involves stimulating an action potential to induce GABA release. This measured GABAA receptor-mediated chloride flux response is known as an evoked inhibitory postsynaptic current (eIPSC). Because there is no direct readout for changes in presynaptic release with analysis of eIPSCs, a paired pulse ratio (PPR) is used to make this assessment. The PPR is the ratio of the amplitudes of two eIPSCs (eIPSC2/eIPSC1) that are evoked by stimuli applied 20–200 ms apart (for review see Zucker, 1989). A change in the PPR is inversely related to a change in evoked neurotransmitter release (Siggins et al., 2005); therefore, if ethanol decreases the PPR, it is interpreted as ethanol increasing evoked GABA release. Through the use of whole-cell voltage-clamp recordings and the analysis of mISPCs, sIPSCs, and eIPSCs, it was determined that ethanol increases GABA release in the brain slice preparation, which will be discussed in greater detail below.
2. The history of ethanol-enhanced GABA release
Starting in the late 1970s, there were a number of behavioral studies showing an interaction between ethanol and drugs that affect the GABAA receptor (for reviews see Criswell and Breese, 2005; Siggins et al., 2005; Weiner and Valenzuela, 2006), which led the field to focus on ethanol–GABA interactions. One of the earliest studies on the effect of ethanol on GABA function interpreted an overall decrease in brain GABA content following a 4.3 g/kg ethanol dose as an indication that ethanol releases GABA from neurons (Gordon, 1967). However, several subsequent studies failed to reproduce this effect (Frye and Breese, 1982; Sutton and Simmonds, 1973; Volicer and Klosowicz, 1979). With the development of the dialysis probe, additional studies produced varied but primarily negative results. Systemically administering ethanol did not change GABA dialysate levels in the anterior cingulate cortex (Zuo et al., 2007), hippocampus (Dahchour and De Witte, 1999; Ward et al., 2009), ventral tegmental area (VTA; Kemppainen et al., 2010), or nucleus accumbens (Smith et al., 2004). Systemic ethanol administration decreases GABA dialysate levels in the cerebellar nuclei (Manto et al., 2005) and ventral pallidum (Kemppainen et al., 2010). There was also an ethanol-induced decrease in GABA dialysate in the nucleus accumbens in alcohol-tolerant, but not alcohol-nontolerant, rat lines (Piepponen et al., 2002) and in alcohol-dependent rats (Dahchour and De Witte, 2000). Thus, the preponderance of dialysis data indicated a lack of ethanol effect on GABA release, or even a slight decrease, following systemic ethanol administration.
When intracellular recordings were first used to study the GABAergic effect of ethanol, Carlen et al. (1982) found that ethanol enhanced the size of eIPSCs recorded from the CA1 region of the hippocampus. While analyzing eIPSC size does not indicate whether ethanol is acting pre- or postsynaptically (see Introduction), Carlen et al. (1982) nonetheless postulated that the increase in eIPSC size was due to a presynaptic mechanism. Based upon a later discovery that inhibition of GABAB receptors is necessary for ethanol to consistently increase eIPSC amplitude in the hippocampus (Wan et al., 1996), the authors speculated that these GABAB receptors were located presynaptically (Siggins et al., 1999). An experiment with spinal cord neurons found that ethanol increases sIPSC frequency without having an effect on sIPSC amplitude (Cheng et al., 1999). Together, these data provided the first clues that ethanol might act at the presynaptic terminal to increase the release of GABA.
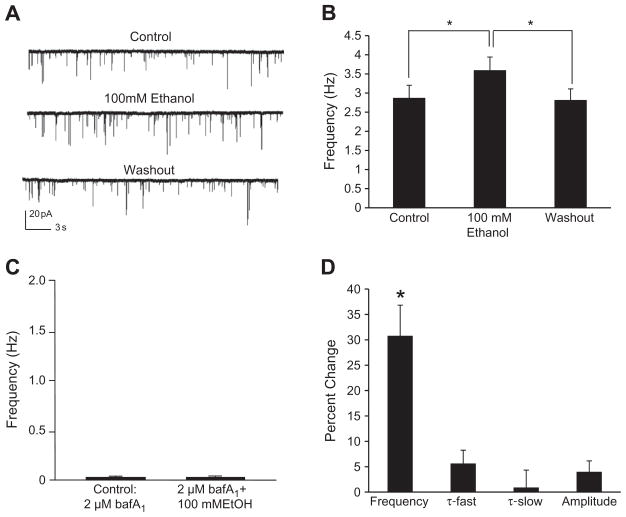
In 2002 Dr. Dennis Twombly organized a National Institute on Alcohol Abuse and Alcoholism workshop to discuss the deficiency in studies exploring the presynaptic effects of ethanol (see Roberto et al., 2006). By determining if ethanol had an effect on mIPSC frequency and the PPR, a number of reports thereafter provided support for ethanol increasing spontaneous and evoked GABA release, respectively, in a number of brain regions (see Table 1 for summary). One of the first studies showed that ethanol increases both spontaneous and evoked GABA release in the central nucleus of the amygdala (CeA; Roberto et al., 2003). In addition to the CeA (Nie et al., 2004; Roberto et al., 2003), ethanol has been shown to increase GABA release in the following brain regions: basolateral amygdala (Silberman et al., 2008; Zhu and Lovinger, 2006), hippocampus (Ariwodola and Weiner, 2004; Li et al., 2006; Sanna et al., 2004), VTA (Melis et al., 2002; Theile et al., 2008), cerebellum (Carta et al., 2004; Criswell et al., 2008; Hirono et al., 2009; Kelm et al., 2007; Kelm et al., 2008; Ming et al., 2006), substantia nigra (Criswell et al., 2008) and brainstem/spinal cord (Sebe et al., 2003; Ziskind-Conhaim et al., 2003). Fig. 1 includes data collected from the cerebellar interneuron-Purkinje cell synapse demonstrating that ethanol increases mIPSC frequency (Fig. 1A and B) by increasing vesicular release of GABA (Fig. 1C) while having little or no effect postsynaptically (Fig. 1D). While ethanol has been shown to increase GABA release in a number of brain regions with the use of slice electrophysiology, ethanol does not increase GABA release in every brain region; specifically, ethanol has no effect on GABA release in the cortex, lateral septum, and thalamus (Criswell et al., 2008; Jia et al., 2008). In the brain regions where ethanol does increase GABA release, significant progress has been made regarding the underlying mechanism responsible for this action of ethanol.
Table 1.
A summary of the slice electrophysiological techniques used in each brain region to measure the effect of ethanol on GABA release.
| Neuronal type recorded | Type of GABA release measured | Ethanol effect | Reference |
|---|---|---|---|
| Basolateral amygdala (MDN) | mIPSCs | Increased mIPSC frequency | Zhu and Lovinger (2006) |
| Basolateral amygdala (MDN) | sIPSCs | Increased sIPSC frequency | Zhu and Lovinger (2006) |
| Basolateral amygdala | PPR | Decreased PPR | Silberman et al. (2008) |
| Brainstem motor | mIPSC | Increased mIPSC frequency | Sebe et al. (2003) |
| Central nucleus of the amygdala | mIPSC | Increased mIPSC frequency | Roberto et al. (2003), Kang-Park et al. (2007), Nie et al. (2009) |
| Central nucleus of the amygdala | PPR | Decreased PPR | Roberto et al. (2003), Nie et al. (2004) |
| Cerebellar Purkinje (MDN and slice) | mIPSCs | Increased mIPSC frequency | Ming et al. (2006), Kelm et al. (2007), Mameli et al. (2008), Hirono et al. (2009) |
| Cerebellar Purkinje (MDN and slice) | sIPSCs | Increased sIPSC frequency | Criswell et al. (2008), Mameli et al. (2008), Hirono et al. (2009) |
| Cerebellar Purkinje | PPR | Decreased PPR | Kelm et al. (2007), Criswell et al. (2008), Mameli et al. (2008) |
| Cerebellar granule | mIPSCs | Increased mIPSC frequency | Carta et al. (2004) |
| Cerebellar granule | sIPSCs | Increased sIPSC frequency | Carta et al. (2004) |
| Cerebrocortical (MDN) | sIPSCs | No effect | Criswell et al. (2008) |
| Hippocampal CA1 pyramidal | mIPSCs | Increased mIPSC frequency | Li et al. (2006) |
| Hippocampal CA1 pyramidal | sIPSCs | Increased sIPSC frequency | Ariwodola and Weiner (2004), Li et al. (2006) |
| Lateral septal (MDN) | sIPSCs | No effect | Criswell et al. (2008) |
| Lateral septal | PPR | No effect | Criswell et al. (2008) |
| Spinal cord motor | mIPSCs | Increased mIPSC frequency | Ziskind-Conhaim et al. (2003) |
| Spinal cord motor | sIPSCs | Increased sIPSC frequency | Cheng et al. (1999) |
| Substantia nigra (MDN) | mIPSCs | Increased mIPSC frequency | Criswell et al. (2008) |
| Thalamocortical relay | sIPSCs | No effect | Jia et al. (2008) |
| VTA | mIPSCs | Increased mIPSC frequency1 and no effect2 | 1: Theile et al. (2008), 2: Xiao and Ye (2008)2 |
| VTA | sIPSCs | Increased sIPSC frequency | Theile et al. (2008), Xiao and Ye (2008)a |
| VTA | PPR | Decreased PPR | Theile et al. (2008) |
Note: The increase in sIPSC frequency was only seen in the presence of a μ-opioid receptor agonist. MDN: mechanically dissociated neurons.
Fig. 1.
Ethanol increases vesicular release of GABA. A: A trace from a representative neuron illustrating the effect of ethanol (100mM) on mIPSC frequency. B: Ethanol significantly increased mIPSC frequency compared to control and washout [*p<.05; repeated measures ANOVA; F(2,42)=18.3]. The mIPSC frequency values for the control and washout groups were not significantly different. C: Incubating the slice with bafilomycin A1 (baf A1), which eliminates the pH and electrical gradients necessary for GABA transporters to fill the vesicles (Maycox et al., 1990), greatly reduced mIPSC frequency. In the presence of bafilomycin A1, 100 mM ethanol did not significantly increase mIPSC frequency compared to control. D: Ethanol (100 mM) significantly increased mIPSC frequency (see B) in contrast to weak and non-significant effects on mIPSC fast decay time (τ-fast), slow decay time (τ-slow) and amplitude. Overall, these results support that the ethanol-induced increase in mIPSC frequency should be interpreted as an increase in spontaneous GABA release from the presynaptic terminal. This figure was reproduced from Kelm et al. (2010).
The dichotomy between the effects of locally applied ethanol, which usually increases GABA release when measured with intracellular recordings, and systemic ethanol, which usually has no effect on GABA release when measured with dialysis, suggests that the net effect of ethanol on intact neural circuits may differ from the effect of ethanol on individual neurons. This difference may be due to ethanol increasing GABA release onto inhibitory interneurons and thereby decreasing the subsequent release of GABA from the interneurons (see Celada et al., 1999; Tan et al., 2010). Thus, local application of ethanol may be necessary to study the immediate mechanism of ethanol-enhanced GABA release while the indirect effect of ethanol on GABA function may be modeled with dialysis experiments. Interestingly, when ethanol was applied locally by dialysis into the CeA, ethanol increased GABA release, which verified the effect of bath-applied ethanol on CeA neurons in the slice preparation (Roberto et al., 2004, 2010).
3. G protein-coupled receptors influence ethanol-enhanced GABA release
A number of laboratories have demonstrated that the ability of ethanol to increase GABA release can be regulated by different G protein-coupled receptors (GPCRs, see Table 2 for summary). For example, the functionality of a Gαs- or Gαq-coupled GPCR is necessary for ethanol to increase GABA release in certain brain regions. Inhibition of the corticotropin-releasing factor (CRF)1 receptor, a GPCR coupled to Gαs, blocks ethanol from increasing GABA release in the CeA (Nie et al., 2004; Roberto et al., 2010). This finding suggests that activation of the CRF1 receptor is essential for ethanol to increase the release of GABA in this brain region. Inhibition of the Gαq-coupled 5-hydroxytryptamine-2C (5-HT2C) receptor blocks ethanol-enhanced GABA release in the VTA, which suggests that activation of the 5-HT2C receptor is essential for ethanol to increase GABA release at this site (Theile et al., 2009). More studies should be conducted to determine if activation of Gαs- or Gαq-coupled GPCRs is necessary for ethanol to induce GABA release in different brain regions.
Table 2.
A summary of the role of GPCRs in ethanol-enhanced GABA release.
| GPCR | Neuronal type recorded | G Protein | Effect on ethanol-enhanced GABA release* | Effect on baseline GABA release | Reference |
|---|---|---|---|---|---|
| CB1 receptor | Basolateral amygdala | Gαi | Agonist blocked ethanol effect; antagonist facilitated ethanol effect | Not reported | Talani and Lovinger (2008) |
| CB1 receptor | CeA | Gαi | Agonist blocked ethanol effect | Agonist decreased GABA release | Roberto et al. (2008) |
| CB1 receptor | Cerebellar Purkinje | Gαi | Agonist blocked the ethanol effect1, while the antagonist had no effect2 | Agonist decreased GABA release1 | 1: Kelm et al., 2008; 2: Kelm et al., 2007 |
| CRF1 receptor | CeA | Gαs | Antagonist blocked the ethanol effect | Not reported | Nie et al. (2004) |
| δ-Opioid receptor | CeA | Gαi | Antagonist enhanced the ethanol effect | Antagonist had no effect on GABA release | Kang-Park et al. (2007) |
| GABAB receptor | Basolateral amygdala | Gαi | Antagonist enhanced the ethanol effect1,2, and agonist blocked the ethanol effect2 | Antagonist had no effect on spontaneous GABA release1,2; antagonist decreased evoked GABA release at local synapses2 | 1: Zhu and Lovinger, 2006; 2: Silberman et al., 2009 |
| GABAB receptor | Cerebellar Purkinje | Gαi | Agonist blocked the ethanol effect, while the antagonist had no effect | Agonist decreased GABA release; antagonist increased GABA release | Kelm et al. (2007), Kelm et al. (2008) |
| GABAB receptor | Hippocampal CA1 pyramidal | Gαi | Antagonist enhanced the ethanol effect | Antagonist had no effect on GABA release | Ariwodola and Weiner (2004) |
| GABAB receptor | VTA | Gαi | Agonist and antagonist did not affect the ethanol effect | Agonist decreased GABA release; antagonist increased GABA release | Theile et al. (2008) |
| μ-Opioid receptor | CeA | Gαi | Antagonist and null mice did not affect the ethanol effect | Antagonist increased GABA release; GABA release greater in null mice | Kang-Park et al. (2009) |
| μ-Opioid receptor | VTA | Gαi | Ethanol only increased GABA release in the presence of agonist | Agonist decreased GABA release | Xiao and Ye (2008) |
| Nociceptin/orphani FQ peptide receptor | CeA | Gαi | Agonist blocked ethanol effect | Agonist decreased GABA release | Roberto and Siggins (2006) |
| Serotonin 5-HT2C receptor | VTA | Gαq | Antagonist blocked ethanol effect | Antagonist had no effect on GABA release | Theile et al. (2009) |
For each experiment, the antagonist or agonist was applied before the application of ethanol and remained present through the ethanol application to determine the effect of the agonist/antagonist on ethanol-enhanced GABA release.
Agonists for Gαi-coupled GPCRs have the same inhibitory effect on ethanol-enhanced GABA release as antagonists for the Gαs/Gαq-coupled GPCRs. In the basolateral amygdala, activation of the Gαi-coupled cannabinoid-1 (CB1) receptor (Talani and Lovinger, 2008) or activation of the Gαi-linked GABAB receptor (Silberman et al., 2009; Zhu and Lovinger, 2006) inhibits ethanol-enhanced GABA release. At the cerebellar interneuron-Purkinje cell synapse, the ability of ethanol to increase GABA release is likewise blocked by activation of CB1 receptors or GABAB receptors (Kelm et al., 2007, 2008). In the CeA, activation of either the nociceptin/orphanin FQ peptide receptor (Roberto and Siggins, 2006), a Gαi-coupled GPCR, or the CB1 receptor (Roberto et al., 2008) blocks ethanol from enhancing GABA release. Consistent with activation of Gαi-coupled GPCRs inhibiting ethanol-enhanced GABA release, inhibition of Gαi-coupled GPCRs enhances the ability of ethanol to increase GABA release. In the basolateral amygdala (Ariwodola and Weiner, 2004; Silberman et al., 2009) and hippocampus (Zhu and Lovinger, 2006), an antagonist for Gαi-linked GABAB receptors enhances the ability of ethanol to increase GABA release, as does an antagonist for the Gαi-linked CB1 receptors in the basolateral amygdala (Talani and Lovinger, 2008). In the CeA, an antagonist at the Gαi-linked δ-opioid receptor augments the ability of ethanol to increase the release of GABA (Kang-Park et al., 2007).
Interestingly, the presence of a Gαi-coupled GPCR does not guarantee that activation or inhibition of the receptor will affect ethanol-enhanced GABA release. Both μ-opioid receptor null mice and a μ-opioid receptor antagonist increase baseline GABA release in the CeA but have no effect on the ability of ethanol to increase GABA release in the CeA (Kang-Park et al., 2009). A GABAB receptor antagonist increases baseline GABA release in the cerebellum, but inhibition of GABAB receptors does not affect ethanol-enhanced GABA release in this brain region (Kelm et al., 2008). In the VTA neither a GABAB receptor agonist nor antagonist had an effect on the ability of ethanol to increase GABA release, despite the fact that both the agonist and antagonist affected baseline release of GABA (Theile et al., 2008). Given that agonists and antagonists for the GPCRs mentioned above affect baseline spontaneous GABA release, it is likely that these GPCRs are expressed at the presynaptic terminals. Because the presence of a functional GPCR at a presynaptic terminal does not guarantee that an agonist/antagonist for that GPCR will affect ethanol-enhanced GABA release, it is unlikely that ethanol acts directly on a GPCR to regulate release of GABA.
Instead of binding directly to a GPCR to influence GABA release, ethanol could act upstream of the GPCR to change the amount of ligand reaching the GPCR, or ethanol could indirectly alter the affinity of the GPCR for its ligand or the constitutive activity of the GPCR. The appropriate endogenous ligands are present in the CeA and the VTA, which are sites where activation of the CRF1 receptor (Nie et al., 2004) and 5-HT2C receptor (Theile et al., 2009), respectively, is required for ethanol-enhanced GABA release. Specifically, GABAergic interneurons synthesize CRF in the CeA (Veinante et al., 1997), and serotonergic afferents innervate neurons in the VTA (Herve et al., 1987). However, ethanol could also act downstream from a GPCR to increase GABA release by binding to adenylate cyclase (Yoshimura et al., 2006) or protein kinase Cε (Das et al., 2009), and these possibilities have not been tested.
To test whether ethanol is acting upstream or downstream of the 5-HT2C receptor to induce GABA release in the VTA, an illuminating experiment would be to block synthesis of 5-HT with parachlorophenylalanine (PCPA) and determine if ethanol can still increase GABA release. Because activation of the 5-HT2C receptor is required for ethanol to increase GABA release in the VTA (Theile et al., 2009), a lack of effect of ethanol in the presence of PCPA would indicate that endogenous 5-HT is essential for this ethanol action. If PCPA blocks ethanol-enhanced GABA release, administration of a 5-HT2C agonist (Ro-60-0175) to the slice in the absence of ethanol in the 5-HT-depleted tissue would be expected to increase GABA release. If ethanol does not induce a further increase in GABA release in the presence of the 5-HT2C agonist, this outcome would suggest that ethanol influences levels of serotonin to increase GABA release. On the other hand, if ethanol induces a further increase in GABA release in the presence of both PCPA and the 5-HT2C receptor agonist, this outcome would suggest that ethanol can act downstream of the GPCR. Described next is evidence that downstream GPCR-linked second messenger pathways are also involved in the ability of ethanol to increase GABA release.
4. Evidence that GPCR-linked intracellular messengers influence ethanol-enhanced GABA release
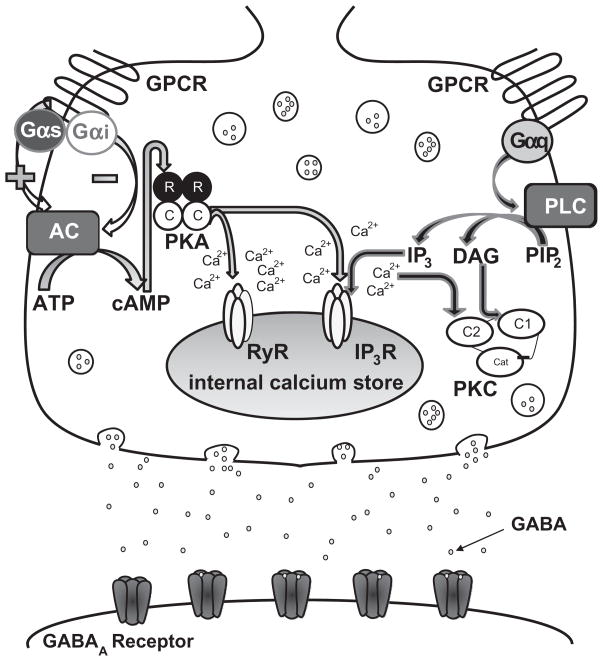
Activation of Gαs-, Gαq-, and Gαi-coupled GPCRs affects signaling through different intracellular messenger pathways (see Fig. 2). Both the Gαi and Gαs subunits modulate adenylate cyclase with Gαs stimulating adenylate cyclase and Gαi inhibiting it. When adenylate cyclase is activated, it converts adenosine-5′-triphosphate (ATP) into 3′-5′-cyclic adenosine monophosphate (cAMP), which can bind to protein kinase A (PKA) regulatory subunits (Hanoune and Defer, 2001). The binding of cAMP to PKA frees the PKA catalytic subunits from the regulatory subunits, allowing the catalytic subunits to phosphorylate nearby targets. The Gαq subunit activates phospholipase C (PLC), which catalyzes the conversion of phosphoinositol 4,5-bisphosphate into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3; Kiselyov et al., 2003). The released calcium from activation of the IP3R and the presence of DAG both activate conventional protein kinase C (PKC) isoforms (α, βI, βII, γ). Given the involvement of GPCRs in ethanol-enhanced GABA release, it seems logical that the intracellular messengers linked to the GPCRs would influence the ethanol-induced increase in GABA release.
Fig. 2.
Ethanol, GABAergic neurotransmission, and GPCRs. Ethanol has been shown to act directly at the postsynaptic GABAA receptor, but ethanol can also increase the amount of GABA released from the presynaptic terminal. While the mechanism mediating this ethanol effect is not fully understood, evidence suggests that the GPCRs and their respective pathways are involved. It is unlikely that ethanol is binding directly to the GPCR and instead could be acting upstream of the receptor by increasing the amount of ligand reaching the receptor or changing the receptor properties. Ethanol could also interact directly with one of the intracellular messengers (see the text for a detailed description of the GPCR pathways).
In the presence of adenylate cyclase antagonists (dideoxya-denosine and SQ 22,536) or PKA antagonists (H-89 and Rp-cAMP), ethanol was not able to significantly increase GABA release at the interneuron-Purkinje cell synapse (Hirono et al., 2009; Kelm et al., 2008). Additionally, a PLC antagonist (edelfosine) and two general PKC antagonists (chelerythrine and calphostin C) significantly blocked ethanol from increasing spontaneous GABA release at the interneuron-Purkinje cell synapse (Kelm et al., 2010). Studies with a PKCε antagonist and PKCε null mice found that PKCε is required for ethanol to increase GABA release in the CeA (Bajo et al., 2008). Importantly, Bajo et al. (2008) also found that PKCε is required for CRF to increase GABA release through activation of the CRF1 receptor, but it is unknown if PKCε is facilitating activation of the CRF1 receptor or if activation of the CRF1 receptor leads to activation of PKCε. Overall, these results suggest that the Gαq- and Gαs-coupled GPCR pathways play a critical role in the ability of ethanol to increase GABA release.
While the Gαq-coupled (i.e. PLC/PKC) and Gαs-coupled (i.e. adenylate cyclase/PKA) pathways are both necessary for ethanol to increase spontaneous GABA release at the interneu-ron-Purkinje cell synapse, it is unknown if ethanol activates each pathway separately or if there is cross-talk occurring between the pathways. Cross-talk occurs between PKA and PKC at the GABAergic nucleus basalis of Meynert synapse (Kubota et al., 2003), and there are effects of ethanol that involve cross-talk between these protein kinases. For example, ethanol increases adenylate cyclase isoform 7 activity through a PKCδ-mediated mechanism, which leads to activation of PKA (Tabakoff et al., 2001). Ethanol also induces PKCε translocation to the cytosol through a PKA-dependent mechanism (Yao et al., 2008); specifically, this translocation of PKCε is thought to involve PKA activation of PLCβ. If ethanol is activating the pathways separately, there could be no convergence of the pathways, and PKA and PKC could act directly at the release machinery (Boczan et al., 2004; Lou et al., 2008). However, the pathways could converge at the internal calcium stores (see Fig. 2) because phosphorylation of the IP3R and ryanodine receptor (RyR) by PKA and PKC increases the amount of calcium release from the internal stores (Bardo et al., 2006; Bugrim, 1999; Mignery et al., 1990; Patterson et al., 2004; Sobie et al., 2006). As discussed below, calcium release from internal stores plays an important role in ethanol-enhanced GABA release.
5. The role of calcium in ethanol-enhanced GABA release
It is well established that changes in presynaptic intracellular calcium levels can alter spontaneous and evoked GABA release (Bardo et al., 2002, 2006; Glitsch, 2008; Yamasaki et al., 2006). Incubating a cerebellar slice with a calcium chelator blocks the ability of ethanol to increase GABA release at the interneuron-Purkinje cell synapse (Kelm et al., 2010). Because limiting exposure of a membrane impermeable calcium chelator to the postsynaptic neuron had no effect on this ethanol mechanism (Kelm et al., 2007), these results suggest that a presynaptic, calcium-dependent mechanism is mediating ethanol-enhanced GABA release at this synapse. To investigate the role of extracellular calcium in this ethanol mechanism, a mechanically dissociated neuron preparation was used instead of a slice to allow for instantaneous access of the calcium-free extracellular solution to the synapse. This approach reduces the likelihood of simultaneously decreasing both intracellular and extracellular calcium levels. Ethanol was still able to increase spontaneous GABA release at the mechanically dissociated Purkinje cell synapse in a calcium-free extracellular solution or in the presence of a voltage-dependent calcium channel inhibitor (Kelm et al., 2007). These results demonstrate that, while calcium signaling does play a role in this ethanol mechanism, this calcium is not coming from an extracellular source, a finding which suggests that this calcium signaling is occurring intracellularly.
One potential source of intracellular calcium is the internal calcium store, which allows for increases in calcium release through activation of IP3Rs and RyRs. Therefore, it is likely that IP3Rs play a central role in ethanol-enhanced GABA release because an IP3R antagonist blocks ethanol-enhanced GABA release in the cerebellum (Kelm et al., 2007) and VTA (Theile et al., 2009). Similarly, blocking calcium release from the RyRs also inhibits ethanol-enhanced GABA release (Kelm et al., 2007). After the internal stores are depleted of calcium, a sarco (endo)plasmic reticulum Ca2+ ATPase (SERCA) pump senses this depletion and replenishes the stores (Kiselyov et al., 2003). After using a SERCA pump antagonist in a protocol that depletes the internal stores of calcium, the ability of ethanol to increase GABA release is blocked in the cerebellum (Kelm et al., 2007) and VTA (Theile et al., 2009). Theile et al. (2009) also found that the ability of a 5-HT2C receptor agonist to increase GABA release in the VTA is blocked by a SERCA pump antagonist. This finding is consistent with 5-HT2C receptors activating a Gαq-protein that results in a subsequent release of calcium through activation of the IP3Rs (Kiselyov et al., 2003), a process which supports the internal calcium stores being necessary for a 5-HT2C receptor agonist to increase release of GABA. As noted earlier, the ability of ethanol to increase GABA release in the VTA is dependent on activation of the 5-HT2C receptor (Theile et al., 2009). Overall, these data suggest that calcium release from internal stores plays an important role in ethanol-enhanced GABA release.
6. The brain region-specificity of ethanol-enhanced GABA release
As noted above, while ethanol enhances GABA release in several brain regions, it is ineffective at increasing GABA release in the cortex, lateral septum and thalamus (Criswell et al., 2008; Jia et al., 2008). However, the mechanism mediating this brain region-specificity is unknown. One explanation for the lack of ethanol effect could be the absence of the relevant GPCRs at these presynaptic terminals. If ethanol acts upstream of the GPCRs to release ligand, ethanol may not be able to increase the ligand concentration near the appropriate GPCRs. Further, other factors could act indirectly to decrease the affinity or constitutive activity of the GPCR. Alternatively, if both a Gαi- and Gαs-coupled GPCR are activated by ethanol on the same terminal, the effect of one GPCR could negate the effect of the other GPCR, leading to an overall lack of effect of ethanol on GABA release.
The regional specificity of ethanol-enhanced GABA release could also be mediated downstream from the GPCRs, with a likely source being the internal calcium stores. Blockade of RyRs or IP3Rs or depletion of internal calcium stores prevents ethanol-enhanced GABA release while leaving a large portion of spontaneous GABA release intact (Kelm et al., 2007; Theile et al., 2009). Thus, ethanol appears selective for the subtype of GABA release that is dependent on calcium release from internal stores. If the proportion of GABA release dependent upon internal calcium stores differs between brain regions, as has been suggested (Yamasaki et al., 2006), brain regions with a low dependence on internal stores would be less sensitive to the effect of ethanol on GABA release than regions with a high dependence. Finally, the regional differences could be due to a differential function of one or more of the multiple intracellular messenger pathways involved in ethanol-enhanced GABA release.
Therefore, much work is needed to understand this regionally specific effect of ethanol on GABA release. While it is possible that this lack of ethanol effect on GABA release in certain brain regions could be due to an artifact introduced by the techniques used to prepare the tissue or record from the neurons, this possibility seems unlikely because Criswell et al. (2008) used the same preparation to show that ethanol increases GABA release in one brain region while having no effect in another. Defining the basis of this regional specificity of ethanol should be a priority because this new information will likely facilitate a broader understanding of the means by which ethanol alters brain function via GABAergic mechanisms.
7. Functional relevance of ethanol-enhanced GABA release
While there is clear evidence that ethanol increases GABA release, the behavioral and functional relevance of this ethanol-induced increase in GABA release is less clear. To date, all of the manipulations that influence the ability of ethanol to release GABA have other effects that are independent from ethanol-enhanced GABA release; therefore, we cannot directly link any behavioral consequences to ethanol-induced GABA release. For example, a CRF1 receptor antagonist prevents ethanol-enhanced GABA release in the CeA (Nie et al., 2004) and also blocks the increased anxiety associated with repeated ethanol withdrawals (Huang et al., 2010; Overstreet et al., 2004; Wills et al., 2009). While the correlation between the prevention of ethanol-enhanced GABA release and the decrease in withdrawal-induced anxiety is solid, the causal link is unclear. Furthermore, ethanol concentrations below 20 mM have been ineffective at enhancing GABA release (Zhu and Lovinger, 2006). A 20 mM ethanol concentration is equivalent to a blood ethanol level of almost 0.1%, which is a concentration that is seldom reached by social drinkers, suggesting that many of the effects of mild intoxication are not mediated by ethanol increasing GABA release.
Because binge drinkers often exceed a 20-mM ethanol concentration, one possibility is that this deleterious drinking pattern selectively initiates ethanol-induced GABA release to contribute to the long-term consequences of alcohol abuse. Further, chronic ethanol intake does not appear to result in tolerance to the effect of ethanol on GABA release (Roberto et al., 2004, 2006). Thus, the increased ethanol intake required to produce the same inebriating effect in a tolerant individual would result in an increase in any deleterious effects associated with the elevated release of GABA. In ethanol-dependent rats who are in withdrawal, there is an increase in GABA dialysate and mIPSC frequency in the CeA (Roberto et al., 2004), and administration of a CRF1 receptor antagonist blocks this increase in GABA release (Roberto et al., 2010). This study suggests that there is a homeostatic effect of GABA release during withdrawal from chronic ethanol, and that the relationship between acute ethanol exposure and the GPCRs in the mechanism of ethanol-enhanced GABA release could extend to chronic ethanol. Therefore, while more work is needed to determine the behavioral relevance of ethanol-enhanced GABA release, future efforts should focus on the role of chronic ethanol in ethanol-enhanced GABA release.
If future pharmacotherapy options for alcohol use disorders are based upon a change in GABA release, the strategy will likely involve modulation of a GPCR, as do approximately 30% of all marketed prescription drugs (Hopkins and Groom, 2002). Naltrexone, which is one of the drugs approved for the treatment of alcohol dependence, acts at a GPCR. Naltrexone reduces alcohol intake and craving by blocking the rewarding effects of alcohol (Volpicelli et al., 1995), and there is evidence that this mechanism involves modulation of GABA release. Specifically, naltrexone reverses the ethanol-induced increase in dopamine signaling in the mesolimbic pathway, suggesting that opioids are a mediator in the ethanol–dopamine interaction (Benjamin et al., 1993). Importantly, opioids do not to affect VTA dopamine neurons directly but instead hyperpolarize GABAergic interneurons (i.e. disinhibition) that provide inhibitory signals to the VTA dopamine neurons (Johnson and North, 1992). Overall, these data suggest that increasing the amount of GABA released onto the VTA dopamine neurons can reduce the rewarding properties associated with dopamine signaling in the mesolimbic pathway.
In addition to naltrexone, there are other drugs targeting GPCRs that have the potential to be new treatment options. In alcohol-dependent individuals, prazosin, an antagonist at the Gαq-linked α1 adrenergic receptor, reduces the number of drinking days (Simpson et al., 2009). Likewise, LY686017, an antagonist at the Gαq-linked neurokinin 1 receptor, reduces alcohol craving in alcohol-dependent individuals (George et al., 2008). Baclofen, a GABAB receptor agonist, promotes abstinence, prevents relapse, and reduces withdrawal in individuals with an alcohol use disorder (Addolorato et al., 2002, 2007), but these results have not always been reproducible (Garbutt et al., 2007). Baclofen can be taken by alcohol-dependent individuals who also have cirrhosis of the liver, which is a population without many treatment options due to extensive liver metabolism of most drugs (Addolorato et al., 2007). Overall, these data demonstrate that pharmacological manipulation of GPCR signaling is a promising strategy for treating alcohol use disorders, and different GPCR agonists and antagonists should continue to be pursued as treatment options, especially the drugs that target the GPCRs that affect GABA release. While the intracellular messengers located downstream from the GPCRs could possibly be targeted in certain circumstances, the promiscuity of these second messengers will likely limit their use.
8. Summary
In addition to any postsynaptic actions, evidence is provided that ethanol increases GABA release in multiple brain regions. The ability of ethanol to increase GABA release can be influenced by GPCRs and the intracellular messengers located downstream from the GPCRs. In the CeA and the VTA, activation of a GPCR is required for ethanol to increase GABA release; therefore, future work should focus on determining if activation of other GPCRs is required for ethanol to increase GABA release in other brain regions. The way in which ethanol interacts with the GPCR to increase GABA release is uncertain, but it likely does not involve ethanol binding directly to the GPCR. Ethanol does not increase GABA release in every brain region, and this circumstance could potentially be explained by whether or not there is activation of a GPCR pathway at a GABAergic synapse or if the GABA released at a synapse is dependent on calcium release from internal stores. Overall, significant progress has been made in exploring the mechanism of ethanol-enhanced GABA release, and it has been clearly established that GPCRs play an important role in this ethanol mechanism and in the treatment of alcohol use disorders. To expand upon the potential relevance of this ethanol effect on GABA release to alcohol dependence, future work should focus on whether chronic ethanol exposure influences ethanol-enhanced GABA release and if GPCRs contribute to any of the observed differences.
Abbreviations
- cAMP
3′-5′-cyclic adenosine monophosphate
- 5-HT2C
5-hydroxytryptamine 2C
- ATP
adenosine-5′-triphosphate
- baf A1
bafilomycin A1
- CB1
cannabinoid-1
- CeA
central nucleus of the amygdala
- CRF
corticotropin-releasing factor
- DAG
diacylglycerol
- eIPSC
evoked inhibitory postsynaptic current
- GABA
gamma-aminobutyric acid
- GPCRs
G protein-coupled receptors
- IP3
inositol 1,4,5-trisphosphate
- mIPSC
miniature inhibitory postsynaptic current
- PPR
paired pulse ratio
- PCPA
parachlorophenylalanine
- PLC
phospholipase C
- PKA
protein kinase A
- PKC
protein kinase C
- RyR
ryanodine receptor
- SERCA
sarco(endo)plasmic reticulum Ca2+ ATPase
- sIPSC
spontaneous inhibitory postsynaptic current
- VTA
ventral tegmental area
References
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D’Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci USA. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo S, Robertson B, Stephens GJ. Presynaptic internal Ca2+ stores contribute to inhibitory neurotransmitter release onto mouse cerebellar Purkinje cells. Br J Pharmacol. 2002;137:529–537. doi: 10.1038/sj.bjp.0704901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci. 2006;27:78–84. doi: 10.1016/j.tips.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Benjamin D, Grant ER, Pohorecky LA. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res. 1993;621:137–140. doi: 10.1016/0006-8993(93)90309-b. [DOI] [PubMed] [Google Scholar]
- Boczan J, Leenders AG, Sheng ZH. Phosphorylation of syntaphilin by cAMP-dependent protein kinase modulates its interaction with syntaxin-1 and annuls its inhibitory effect on vesicle exocytosis. J Biol Chem. 2004;279:18911–18919. doi: 10.1074/jbc.M400496200. [DOI] [PubMed] [Google Scholar]
- Bugrim AE. Regulation of Ca2+ release by cAMP-dependent protein kinase. A mechanism for agonist-specific calcium signaling? Cell Calcium. 1999;25:219–226. doi: 10.1054/ceca.1999.0027. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Gurevich N, Durand D. Ethanol in low doses augments calcium-mediated mechanisms measured intracellularly in hippocampal neurons. Science. 1982;215:306–309. doi: 10.1126/science.7053581. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Paladini CA, Tepper JM. GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata. Neuroscience. 1999;89:813–825. doi: 10.1016/s0306-4522(98)00356-x. [DOI] [PubMed] [Google Scholar]
- Cheng G, Gao B, Verbny Y, Ziskind-Conhaim L. Ethanol reduces neuronal excitability and excitatory synaptic transmission in the developing rat spinal cord. Brain Res. 1999;845:224–231. doi: 10.1016/s0006-8993(99)01968-x. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Kelm MK, Breese GR. Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. J Pharmacol Exp Ther. 2008;326:596–603. doi: 10.1124/jpet.107.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res. 1999;23:1698–1703. doi: 10.1111/j.1530-0277.1999.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Taurine blocks the glutamate increase in the nucleus accumbens microdialysate of ethanol-dependent rats. Pharmacol Biochem Behav. 2000;65:345–350. doi: 10.1016/s0091-3057(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Das J, Pany S, Rahman GM, Slater SJ. PKC epsilon has an alcohol-binding site in its second cysteine-rich regulatory domain. Biochem J. 2009;421:405–413. doi: 10.1042/BJ20082271. [DOI] [PubMed] [Google Scholar]
- Frye GD, Breese GR. GABAergic modulation of ethanol-induced motor impairment. J Pharmacol Exp Ther. 1982;223:750–756. [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Kampov-Polevoy A, Flannery B, Kalka-Juhl L, Gallop R. Placebo-controlled trial of baclofen in alcohol dependence. Abstract Alcohol Clin Exp Res. 2007;31:209. doi: 10.1111/j.1530-0277.2010.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Glitsch MD. Spontaneous neurotransmitter release and Ca2+—how spontaneous is spontaneous neurotransmitter release? Cell Calcium. 2008;43:9–15. doi: 10.1016/j.ceca.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Gordon ER. The effect of ethanol on the concentration of gamma-aminobutyric acid in the rat brain. Can J Physiol Pharmacol. 1967;45:915–918. doi: 10.1139/y67-107. [DOI] [PubMed] [Google Scholar]
- Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- Hirono M, Yamada M, Obata K. Ethanol enhances both action potential-dependent and action potential-independent GABAergic transmission onto cerebellar Purkinje cells. Neuro-pharmacology. 2009;57:109–120. doi: 10.1016/j.neuropharm.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Chandra D, Homanics GE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;326:475–482. doi: 10.1124/jpet.108.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park MH, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Presynaptic delta opioid receptors regulate ethanol actions in central amygdala. J Pharmacol Exp Ther. 2007;320:917–925. doi: 10.1124/jpet.106.112722. [DOI] [PubMed] [Google Scholar]
- Kang-Park MH, Kieffer BL, Roberts AJ, Roberto M, Madamba SG, Siggins GR, Moore SD. Mu-opioid receptors selectively regulate basal inhibitory transmission in the central amygdala: lack of ethanol interactions. J Pharmacol Exp Ther. 2009;328:284–293. doi: 10.1124/jpet.108.140749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323:356–364. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. The role of protein kinase A in the ethanol-induced increase in spontaneous GABA release onto cerebellar Purkinje neurons. J Neurophysiol. 2008;100:3417–3428. doi: 10.1152/jn.90970.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Weinberg RJ, Criswell H, Breese G. The PLC/IP3R/PKC pathway is required for ethanol-enhanced GABA release. Neuropharmacology. 2010;58:1179–1186. doi: 10.1016/j.neuropharm.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen H, Raivio N, Nurmi H, Kiianmaa K. GABA and glutamate overflow in the VTA and ventral pallidum of alcohol-preferring AA and alcohol-avoiding ANA rats after ethanol. Alcohol Alcohol. 2010;45:111–118. doi: 10.1093/alcalc/agp086. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Shin DM, Muallem S. Signalling specificity in GPCR-dependent Ca2+ signalling. Cell Signal. 2003;15:243–253. doi: 10.1016/s0898-6568(02)00074-8. [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Phillis JW. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Katsurabayashi S, Moorhouse AJ, Murakami N, Koga H, Akaike N. GABAB receptor transduction mechanisms, and cross-talk between protein kinases A and C, in GABAergic terminals synapsing onto neurons of the rat nucleus basalis of Meynert. J Physiol. 2003;551:263–276. doi: 10.1113/jphysiol.2003.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of spontaneous and miniature IPSCs to ethanol. Alcohol Clin Exp Res. 2006;30:119–126. doi: 10.1111/j.1530-0277.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- Lou X, Korogod N, Brose N, Schneggenburger R. Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J Neurosci. 2008;28:8257–8267. doi: 10.1523/JNEUROSCI.0550-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Botta P, Zamudio PA, Zucca S, Valenzuela CF. Ethanol decreases Purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther. 2008;327:910–917. doi: 10.1124/jpet.108.144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto M, Laute MA, Pandolfo M. Depression of extra-cellular GABA and increase of NMDA-induced nitric oxide following acute intra-nuclear administration of alcohol in the cerebellar nuclei of the rat. Cerebellum. 2005;4:230–238. doi: 10.1080/14734220500243835. [DOI] [PubMed] [Google Scholar]
- Martin DL. Short-term control of GABA synthesis in brain. Prog Biophys Mol Biol. 1993;60:17–28. doi: 10.1016/0079-6107(93)90010-h. [DOI] [PubMed] [Google Scholar]
- Maycox PR, Hell JW, Jahn R. Amino acid neurotransmission: spotlight on synaptic vesicles. Trends Neurosci. 1990;13:83–87. doi: 10.1016/0166-2236(90)90178-d. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery GA, Newton CL, Archer BT, 3rd, Sudhof TC. Structure and expression of the rat inositol 1, 4, 5-trisphosphate receptor. J Biol Chem. 1990;265:12679–12685. [PubMed] [Google Scholar]
- Ming Z, Criswell HE, Yu G, Breese GR. Competing presynaptic and postsynaptic effects of ethanol on cerebellar purkinje neurons. Alcohol Clin Exp Res. 2006;30:1400–1407. doi: 10.1111/j.1530-0277.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR. Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. ScientificWorldJournal. 2009;9:68–85. doi: 10.1100/tsw.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Iversen LL, Hall ZW, Kravitz EA. Release of gamma-aminobutyric acid from inhibitory nerves of lobster. Proc Natl Acad Sci USA. 1966;56:1110–1115. doi: 10.1073/pnas.56.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RL, Boehning D, Snyder SH. Inositol 1, 4, 5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- Piepponen TP, Kiianmaa K, Ahtee L. Effects of ethanol on the accumbal output of dopamine, GABA and glutamate in alcohol-tolerant and alcohol-nontolerant rats. Pharmacol Biochem Behav. 2002;74:21–30. doi: 10.1016/s0091-3057(02)00937-1. [DOI] [PubMed] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci USA. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Treistman SN, Pietrzykowski AZ, Weiner J, Galindo R, Mameli M, Valenzuela F, Zhu PJ, Lovinger D, Zhang TA, Hendricson AH, Morrisett R, Siggins GR. Actions of acute and chronic ethanol on presynaptic terminals. Alcohol Clin Exp Res. 2006;30:222–232. doi: 10.1111/j.1530-0277.2006.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Schweitzer P. Interactions of ethanol and cannabinoids on synaptic transmission in central amygdala. Abstract Alcohol Clin Exp Res. 2008;32:48. [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E, Kuriyama K. Biochemical–physiological correlations in studies of the gamma-aminobutyric acid system. Brain Res. 1968;8:1–35. doi: 10.1016/0006-8993(68)90170-4. [DOI] [PubMed] [Google Scholar]
- Sagne C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe JY, Eggers ED, Berger AJ. Differential effects of ethanol on GABA(A) and glycine receptor-mediated synaptic currents in brain stem motoneurons. J Neurophysiol. 2003;90:870–875. doi: 10.1152/jn.00119.2003. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Nie Z, Madamba S. A metabotropic hypothesis for ethanol sensitivity of GABAergic and glutamatergic central synapses. In: Liu Y, Hunt W, editors. The ‘drunken’ Synapse: Studies of Alcohol-Related Disorders. Kluwer Academic/Plenum Publishers; New York: 1999. p. 219. [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology. 2009;56:886–895. doi: 10.1016/j.neuropharm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Smith A, Watson CJ, Frantz KJ, Eppler B, Kennedy RT, Peris J. Differential increase in taurine levels by low-dose ethanol in the dorsal and ventral striatum revealed by microdialysis with on-line capillary electrophoresis. Alcohol Clin Exp Res. 2004;28:1028–1038. doi: 10.1097/01.alc.0000131979.78003.34. [DOI] [PubMed] [Google Scholar]
- Sobie EA, Guatimosim S, Gomez-Viquez L, Song LS, Hartmann H, Saleet Jafri M, Lederer WJ. The Ca 2+ leak paradox and rogue ryanodine receptors: SR Ca 2+ efflux theory and practice. Prog Biophys Mol Biol. 2006;90:172–185. doi: 10.1016/j.pbiomolbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle: a cascade of protein–protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sutton U, Simmonds MA. Effects of acute and chronic ethanol on the gamma-amino-butyric acid system in rat brain. Biochem Pharmacol. 1973;22:1685–1692. doi: 10.1016/0006-2952(73)90381-x. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Nelson E, Yoshimura M, Hellevuo K, Hoffman PL. Phosphorylation cascades control the actions of ethanol on cell cAMP signalling. J Biomed Sci. 2001;8:44–51. doi: 10.1007/BF02255970. [DOI] [PubMed] [Google Scholar]
- Talani G, Lovinger D. Ethanol and endocannabinoid interactions in the control of GABA release in rat basolateral amygdala. Abstract Alcohol Clin Exp Res. 2008;32:808. [Google Scholar]
- Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Luscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res. 2008;32:1040–1048. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 2009;329:625–633. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Stoeckel ME, Freund-Mercier MJ. GABA- and peptide-immunoreactivities co-localize in the rat central extended amygdala. NeuroReport. 1997;8:2985–2989. doi: 10.1097/00001756-199709080-00035. [DOI] [PubMed] [Google Scholar]
- Volicer L, Klosowicz BA. Effect of ethanol and stress on gamma-aminobutyric acid and guanosine 3′, 5′-monophosphate levels in the rat brain. Biochem Pharmacol. 1979;28:2677–2679. doi: 10.1016/0006-2952(79)90048-0. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Watson NT, King AC, Sherman CE, O’Brien CP. Effect of naltrexone on alcohol “high” in alcoholics. Am J Psychiatry. 1995;152:613–615. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc Natl Acad Sci USA. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, Ballini C, Corte LD, Dexter D. Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. J Neurochem. 2009;111:1119–1128. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2009;33:455–463. doi: 10.1111/j.1530-0277.2008.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Ye JH. Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: role of mu-opioid receptors. Neuroscience. 2008;153:240–248. doi: 10.1016/j.neuroscience.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Hashimoto K, Kano M. Miniature synaptic events elicited by presynaptic Ca2+ rise are selectively suppressed by cannabinoid receptor activation in cerebellar Purkinje cells. J Neurosci. 2006;26:86–95. doi: 10.1523/JNEUROSCI.2258-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Gordon A, Mochly-Rosen D, Diamond I. Dopamine and ethanol cause translocation of epsilonPKC associated with epsilonRACK: cross-talk between cAMP-dependent protein kinase A and protein kinase C signaling pathways. Mol Pharmacol. 2008;73:1105–1112. doi: 10.1124/mol.107.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Pearson S, Kadota Y, Gonzalez CE. Identification of ethanol responsive domains of adenylyl cyclase. Alcohol Clin Exp Res. 2006;30:1824–1832. doi: 10.1111/j.1530-0277.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Gao BX, Hinckley C. Ethanol dual modulatory actions on spontaneous postsynaptic currents in spinal motoneurons. J Neurophysiol. 2003;89:806–813. doi: 10.1152/jn.00614.2002. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- Zuo GC, Yang JY, Hao Y, Dong YX, Wu CF. Ethanol and acetaldehyde induce similar changes in extracellular levels of glutamate, taurine and GABA in rat anterior cingulate cortex. Toxicol Lett. 2007;169:253–258. doi: 10.1016/j.toxlet.2006.09.016. [DOI] [PubMed] [Google Scholar]