Abstract
Purpose
To apply a magnetic resonance (MR) arterial spin labeling (ASL) technique to evaluate kidney perfusion in native and transplanted kidneys.
Materials and Methods
This study was compliant with the Health Insurance Portability and Accountability Act (HIPAA) and approved by the institutional review board. Informed consent was obtained from all subjects. Renal perfusion exams were performed at 1.5 T in a total of 25 subjects: 10 with native and 15 with transplanted kidneys. A flow-sensitive alternating inversion recovery (FAIR) ASL sequence was performed with respiratory triggering in all subjects and under free-breathing conditions in five transplant subjects. Thirty-two control/tag pairs were acquired and processed using a single-compartment model. Perfusion in native and transplanted kidneys was compared above and below an estimated glomerular filtration rate (eGFR) threshold of 60 ml/min/1.73m2 and correlations with eGFR were determined.
Results
In many of the transplanted kidneys, major feeding vessels in the coronal plane required a slice orientation sagittal to the kidney. Renal motion during the examination was observed in native and transplant subjects and was corrected with registration. Cortical perfusion correlated with eGFR in native (r=0.85, p=0.002) and transplant subjects (r=0.61, p=0.02). For subjects with eGFR≥60 ml/min/1.73m2, native kidneys demonstrated greater cortical (p=0.01) and medullary (p=0.04) perfusion than transplanted kidneys. For subjects with eGFR<60 ml/min/1.73m2, native kidneys demonstrated greater medullary perfusion (p=0.04) compared to transplanted kidneys. Free-breathing acquisitions provided renal perfusion measurements that were slightly lower compared to the coached/triggered technique, although no statistical differences were observed.
Conclusion
In conclusion, FAIR-ASL was able to measure renal perfusion in subjects with native and transplanted kidneys, potentially providing a clinically viable technique for monitoring kidney function.
Introduction
Current diagnostic measurements used to assess severity of kidney failure, such as serum creatinine levels, are relatively insensitive to small but potentially significant functional change. They are also non-specific, requiring a biopsy to characterize the underlying cause of renal dysfunction. Early characterization of dysfunction is crucial for transplant patients because a delay in treatment can lead to irreversible nephron loss and accelerate graft failure [1, 2]. Transplant patients are often subjected to multiple biopsies to assess dysfunction longitudinally and guide treatment decisions. Biopsies are not optimal for longitudinal assessment because they are painful and can result in bleeding, infection, and even graft loss [3]. Moreover, they are expensive and cannot quantify changes in renal function associated with the identified pathology. These limitations have motivated research to find non-invasive diagnostic tools that can both characterize renal disease and provide earlier detection of functional change.
Functional magnetic resonance imaging (MRI) offers a variety of methods to assess blood flow that are especially useful in the kidney. Not only is renal blood flow essential for kidney viability, but it also plays an integral role in blood filtration and regulation. Nuclear medicine and X-ray computed tomography techniques can measure perfusion, but both require intravenous injection and radiation exposure. MR imaging offers a less invasive method of measuring kidney perfusion and has demonstrated potential in assessing renal disease in both native and transplanted kidneys [4-9]. For example, MR perfusion measurements have correlated with histology in transplanted rat kidneys [7], and human studies indicate that perfusion may help differentiate cyclosporine toxicity, acute rejection, and acute tubular necrosis following transplantation [4, 5, 9]. While MRI can measure renal perfusion with or without exogenous contrast agents, non-contrast techniques may be preferable due to the risk of nephrogenic systemic fibrosis associated with gadolinium-based contrast agents in patients with renal insufficiency and in the setting of longitudinal monitoring. Nephrogenic systemic fibrosis is an irreversible systemic disease, which visibly affects the skin and joints, causing skin thickening and loss of joint range of motion. Although rare, this is a devastating disease with no effective treatment [10].
Arterial spin labeling (ASL) uses the blood as an endogenous contrast agent, allowing perfusion measurements without the administration of gadolinium. Inflowing blood is selectively labeled to have an opposite magnetization compared to the destination tissue. The difference between a labeled image (tag) and a non-labeled image (control) can be used to calculate tissue perfusion. ASL has been used extensively for brain perfusion [11] and more recently has been applied to native and transplanted kidneys with both normal and altered function [6, 8, 9, 12-16]. ASL studies using a flow sensitive alternating inversion recovery (FAIR-ASL) scheme, first demonstrated in the kidneys by Martirosian et al. [15], appear particularly promising, correlating kidney perfusion with renal artery stenosis grade [6] and renal plasma flow [6, 16].
The goal of this study was to develop methodology to examine perfusion in both native and transplanted kidneys over a broad range of function. Perfusion was measured using a FAIR-ASL technique with respiratory triggering. In a subset of transplant subjects, results using respiratory triggering were compared to measurements obtained under free breathing to assess feasibility of the technique in sick patients unable to maintain a consistent respiratory rate.
Materials and Methods
Subjects
This study was compliant with the Health Insurance Portability and Accountability Act (HIPAA) and approved by our institutional human subjects review committee. Written informed consent was obtained from all subjects. Subjects with normal kidney function were recruited from a pool of healthy volunteers who expressed interest in participating in MRI research studies. Subjects with chronic kidney disease (CKD) and kidney transplant recipients were recruited by referring nephrologists, in a consecutive fashion, when they presented to their routine clinic appointments if they met the study's inclusion criteria. Subjects were included in the study if they were adults (>18 yrs old), MRI compatible, and clinically stable. In this study estimated glomerular filtration rate (eGFR) was used to stratify patients according to their renal function and to assess the viability of ASL perfusion in transplant and native kidneys over a broad range of function.
Between February 2008 and February 2009, MR renal perfusion exams were performed on a total of 25 subjects, including ten native subjects (six men, four women; mean age ± standard deviation (SD), 55 ± 13 years; age range, 33-79 years) and fifteen transplant subjects (twelve men, three women; mean age ± SD, 49 ± 14 years; age range, 21-71 years). Serum creatinine was measured immediately prior to the MRI examination and eGFR was calculated using the Modification of Diet in Renal Disease formula [17]. In order to minimize the effect of recent fluid consumption on renal perfusion, all subjects refrained from fluids for four hours before MR imaging.
Scan Protocol
Scans were performed on a 1.5 T MR scanner (Signa HDx, GE Healthcare, Milwaukee, WI, USA) with an eight-element phased array cardiac coil (GE Healthcare, Milwaukee, WI, USA). ASL images were acquired using a FAIR-balanced steady state free procession (b-SSFP) acquisition scheme [15] with a 20 ms hyperbolic secant adiabatic inversion pulse and the following readout parameters: TR/TE/flip = 4.6ms/2.3ms/70°, BW = 83.33 kHz, FOV = 34-36 cm, matrix = 128 × 128, and slice thickness = 8 mm. In a few cases, larger subjects required an increase of the FOV, negating the use of zoom gradients and lengthening the TR/TE to 5.8/2.9 ms. A single imaging slice was positioned central to a 20 mm thick slice selective inversion slab. The inversion slab was carefully chosen not to include the feeding vessels. For native kidneys the oblique-coronal orientation allowed for proper placement, however in the transplant kidney, the oblique-sagittal orientation was needed in eight of the fifteen subjects to avoid major feeding arteries to the transplanted kidney. The inversion pulse was respiratory triggered by a breathing belt post-exhalation, and following an inversion time (TI) of 1.2 s, a centric phase encoded b-SSFP image was acquired. The subjects were coached, prior to scanning, to breathe after completion of the image readout. Respiratory rates were 12 breaths/min or lower to allow sufficient magnetization recovery between inversions (≥ 5 s between breaths). Control and tag images were alternated until 64 total images (32 control-tag pairs) were acquired. Proton density images (to measure M0) were obtained with a NEX = 4 using the b-SSFP readout with no inversion preceding it. Scanning was completed in 6-9 min, depending on the respiratory rate.
Free-Breathing Acquisition
ASL was performed without coaching or triggering for five transplant subjects, directly after the standard coached and triggered acquisition. Subjects were instructed to breathe freely for this scan, and data were acquired using a fixed 5 s delay between each inversion (6 min for the entire exam). The slice selective inversion thickness was increased in the free-breathing acquisitions from 20 mm to between 24-28 mm to accommodate greater expected slice motion.
Segmentation and Processing
Data were analyzed using custom scripts written in MATLAB (version 7.5, The MathWorks Inc., Cambridge, MA, USA). Rectangular regions of interest (“ROIs”) were drawn around each kidney and were registered independently through the image series by using automated rigid registration based on normalized mutual information (NMI). ROIs that could not be registered, due to either through-plane motion or deformation, were excluded from the averaged data set. In one native kidney, the M0 image could not be aligned to the FAIR images because of a difference in kidney orientation. A single, average M0 value was used in this instance. The mean of the registered tag images, MT, was then subtracted from the mean of the registered control images, MC, to obtain a difference image, ΔM, of the kidney. After manually segmenting out the kidney from the T1-weighted, MC image, the kidney cortex, which had higher signal intensity than the medulla, was readily differentiated with thresholding. For a limited number of cases where the coil sensitivity varied across the kidney or the segmental vessels were visible, additional manual segmentation was necessary.
Perfusion was determined on a pixel-by-pixel basis using a one compartment model:
where f ≡ perfusion, λ ≡ partition coefficient = 80 ml/100g[13], α ≡ inversion efficiency =1.0 (assumed), TI ≡ inversion time = 1.2 s, and T1 ≡ longitudinal relaxation time = 966 ms for cortex and 1,410 ms for medulla [18]. The measured ΔM and M0 from each pixel were used to generate a perfusion map for the imaged slice of the kidney. All pixels in a specific tissue (e.g. cortex) were averaged together to provide mean perfusion. Cortical pixels with f > 1000 ml/min/100g and medullary pixels with f > 300 ml/min/100g were excluded from the mean perfusion calculation as non-physiologic outliers.
Statistical Analysis
A Wilcoxon signed rank test was used to determine if there were perfusion differences between left and right native kidneys. An intra-class correlation coefficient (ICC) was also calculated to determine agreement between left and right native kidneys. For each native subject, left and right kidney perfusion measurements were averaged for a specific tissue (e.g. cortex) and used in the remaining statistical analyses. The correlations between the perfusion values and eGFR for both native and transplant subjects were examined using Pearson (r) and Spearman (rs) correlation coefficients. Additionally, all subjects were divided into two groups based on healthy normal vs poor function, with healthy normal function denoted by an eGFR above 60 ml/min/1.73m2 and poor function below 60 ml/min/1.73m2. This threshold was selected since CKD is defined as kidney damage or a GFR below 60 ml/min/1.73m2 for three months or more [19]. Cortical and medullary results for these groups are shown with dotplots. Statistical differences between native and transplant subjects in the same eGFR group were determined using the Wilcoxon rank sum test. Lastly, the Wilcoxon signed rank test was again used to test for statistical differences between the free-breathing and coached/triggered ASL measurements. All statistical analyses were performed using SAS 9.1 for windows (SAS Institute Inc. Cary, NC). Statistical significance was defined as a two-tailed p-value < 0.05.
Results
Sagittal slices representing the control, tag, difference (ΔM), and perfusion map images are displayed in Figure 1 for a transplant subject who exhibited negligible motion. In four of the kidneys (two native and two transplanted), banding artifacts due to B0 inhomogeneity affected perfusion measurements in a small portion of the cortex (Figure 2a). The affected area was then excluded from the mean perfusion calculation. In many of the transplant subjects, major feeding vessels prevented a coronal acquisition (Figure 2b), providing reason to place the slice orientation sagittal to the kidney.
Figure 1.
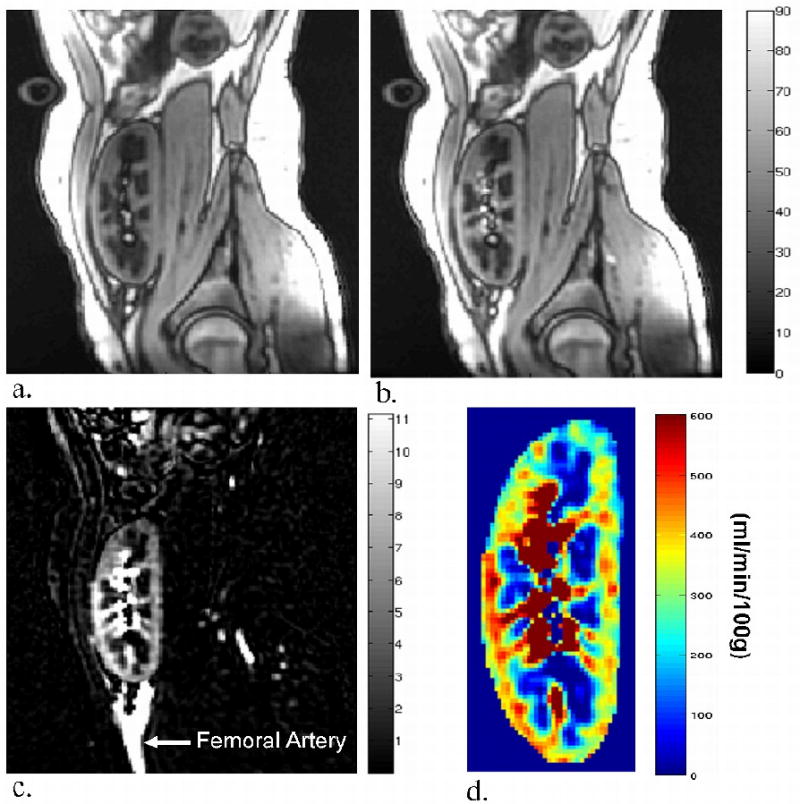
Tag (a), control (b), and difference, (c) images acquired in a sagittal plane for a healthy transplant kidney subject (no. 1 in Table 2) with negligible motion (eGFR = 74 ml/min/1.73m2) along with the resulting perfusion map (d) shown in units of ml/min/100g.
Figure 2.
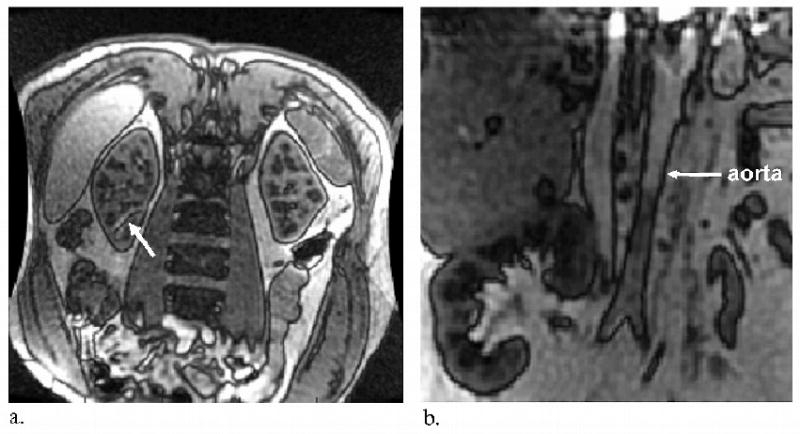
(a) Worst case example of b-SSFP banding artifact inside the kidney for a healthy native kidney subject. The nulled banding area (white arrow) is excluded from mean cortical perfusion calculations. (b) Intersection of the imaging slice with major feeding vessels, such as the aorta in this example (white arrow), prevented a coronal acquisition in many of the transplant subjects. In these cases, a coronal acquisition would have caused the slice selective inversion to invert the inflowing blood spins which are presumed to be at equilibrium magnetization upon entrance to the kidney.
Although respiratory coaching and triggering were used in the acquisition, both native and transplanted kidneys frequently shifted position throughout the exams. Figure 3 demonstrates motion in two transplanted kidneys which manifests as blurring after averaging (Figure 3c,h). Registration significantly reduced the motion artifacts (Figure 3d,i). Perfusion measurement was not obtained in one of the 34 kidneys examined in this study because retrospective image registration could not sufficiently align the images.
Figure 3.
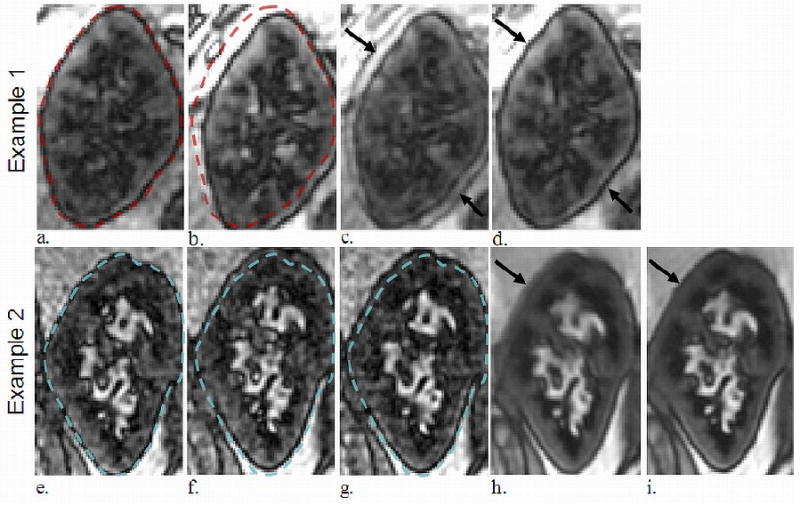
Two different transplanted kidneys which demonstrated significant motion during the ASL exam. Example 1 displays translation due to respiratory motion (dotted outline in a-b). The average of the two images pre-registration demonstrated motion artifact at the boundary of the kidney body and blurring (arrow in c), while averaging post-registration mitigated these (arrow in d). Example 2 displays another transplanted kidney in three different positions (e-g). The average of all 64 FAIR images pre-registration demonstrated blurring (arrow in h) which the post-registration average reduced (arrow in i).
Quantitative perfusion results obtained from a triggered and coached acquisition are listed in Table 1 for the native subjects and Table 2 for the transplant subjects. Perfusion values for healthy native subjects in this study ranged from 407-456 ml/min/100g with mean 427 (±20) ml/min/100g in the cortex and 47-121 ml/min/100g with mean 85 (±33) ml/min/100g in the medulla. These measurements agree with other renal perfusion studies performed using ASL [6, 12-14] and other contrast techniques [20]. For native subjects with CKD (native subjects 6-10, Table 1), the underlying etiologies varied and included: lupus nephritis, glomerulonephritis, hypertension, and diabetes. Perfusion values measured for native right and left kidneys (i.e., right kidney perfusion – left kidney perfusion) were not statistically different for cortical (17 ± 49.37 ml/min/100g; mean ± SD; p = 0.43) or medullary perfusion (-2.67 ± 16.74 ml/min/100g; p = 0.76). Intraclass correlation coefficients also indicated a high level of agreement between right and left kidney perfusions (cortical ICC = 0.93; medullary ICC = 0.87) which provided reason to average the right and left kidney measurements for each native subject in these analyses.
Table 1. Age, sex, eGFR and respiratory triggered/coached perfusion results for the ten subjects with native kidneys.
| Native Subject | Sex | Age | eGFR* | Mean Perfusion† | |
|---|---|---|---|---|---|
| Cortical‡ | Medullary‡ | ||||
| 1H | F | 33 | 88 | 426 | 110 |
| 2 H | F | 52 | 80 | 410 | 47 |
| 3 H | M | 41 | 78 | 456 | 93 |
| 4 H | F | 65 | 77 | 407 | 56 |
| 5 H | M | 53 | 67 | 435 | 121 |
| 6 | M | 45 | 50 | 236 | 62 |
| 7 | M | 79 | 42 | 233 | 52 |
| 8 | M | 60 | 30 | 276 | 59 |
| 9 | F | 60 | 23 | 298§ | 96§ |
| 10 | M | 64 | 19 | 81 | 33 |
eGFR cited in ml/min/1.73m2.
data represents mean of the right and left kidney.
cortical and medullary perfusion values are listed in ml/min/100g.
denotes a healthy subject with no renal disease per their medical history.
measured only for left kidney due to a shift in the slice position of the right kidney during the exam.
Table 2. Age, sex, eGFR and respiratory triggered/coached perfusion results for the fifteen subjects with transplanted kidneys.
| Transplant Subject | Sex | Age | Calcineurin Inhibitors | eGFR* | Mean Perfusion† | |
|---|---|---|---|---|---|---|
| Cortical | Medullary | |||||
| 1 | M | 21 | Yes | 74 | 360 | 70 |
| 2 | M | 34 | Yes | 67 | 262 | 39 |
| 3 | M | 38 | No | 66 | 291 | 31 |
| 4 | M | 55 | Yes | 61 | 307 | 34 |
| 5 | F | 61 | Yes | 60 | 352 | 11 |
| 6 | M | 66 | No | 54 | 276 | 22 |
| 7 | M | 64 | Yes | 54 | 194 | 49 |
| 8 | M | 56 | Yes | 51 | 247 | 44 |
| 9 | F | 54 | No | 50 | 409 | 42 |
| 10 | M | 54 | Yes | 48 | 287 | 56 |
| 11 | M | 71 | No | 46 | 134 | 38 |
| 12 | F | 41 | Yes | 41 | 299 | 40 |
| 13 | M | 30 | No | 22 | 254 | 37 |
| 14 | M | 41 | Yes | 21 | 125 | 14 |
| 15 | M | 44 | No | 18 | 128 | 17 |
eGFR cited in ml/min/1.73m2.
perfusion values are listed in ml/min/100g.
Perfusion values for the transplanted kidneys with good function (eGFR ≥ 60 ml/min/100g) ranged from 262-360 ml/min/100g with mean 314 (±41) ml/min/100g in the cortex and 11-70 ml/min/100g with mean 37 (±21) ml/min/100g in the medulla. Four of these five healthy transplant subjects were taking calcineurin inhibitors which may account for lower perfusion values compared to the healthy native subjects in this study. These cortical perfusion values agree with a recently published study by Lanzman et al. using a similar FAIR-ASL approach in the transplanted renal cortex [9].
Cortical perfusion correlated with eGFR in both native (r = 0.85, p = 0.002; rs = 0.76, p = 0.01) and transplant kidneys (r = 0.61, p = 0.015; rs = 0.57, p = 0.03), while medullary perfusion did not show significant correlation with eGFR in either group. Groupwise comparison of perfusion separated by renal type (native vs transplant) and function based on eGFR is displayed for cortical and medullary perfusion in tables 3 and 4 respectively. Cortical perfusion was greater in native vs transplant subjects with eGFR ≥ 60 ml/min/1.73m2 (p < 0.01; Figure 4a). Medullary perfusion was greater in native vs transplant subjects for both eGFR < 60 ml/min/1.73m2 (p = 0.04) and eGFR ≥ 60 ml/min/1.73m2 (p = 0.04; Figure 4b).
Table 3. Groupwise Comparison of Cortical Perfusion.
| eGFR* | Group | N | Mean | Median | Std Dev | Minimum | Maximum | P-value |
|---|---|---|---|---|---|---|---|---|
| <60 | Native | 5 | 225 | 236 | 85 | 81 | 298 | 0.81 |
| Transplant | 10 | 235 | 251 | 91 | 125 | 409 | ||
| ≥60 | Native | 5 | 427 | 426 | 20 | 407 | 456 | 0.01 |
| Transplant | 5 | 314 | 307 | 41 | 262 | 360 | ||
Note – perfusion data listed in Mean, Median, Std Dev, Minimum, and Maximum are all reported in ml/mm/100g.
eGFR cited in ml/min/1.73m2.
P-value < .05 indicates a significant difference.
Table 4. Groupwise Comparison for Medullary Perfusion.
| eGFR* | Group | N | Mean | Median | Std Dev | Minimum | Maximum | P-value |
|---|---|---|---|---|---|---|---|---|
| <60 | Native | 5 | 60 | 59 | 23 | 33 | 96 | 0.04 |
| Transplant | 10 | 36 | 39 | 14 | 14 | 56 | ||
| ≥60 | Native | 5 | 85 | 93 | 33 | 47 | 121 | 0.04 |
| Transplant | 5 | 37 | 34 | 21 | 11 | 70 | ||
Note – perfusion data listed in Mean, Median, Std Dev, Minimum, and Maximum are all reported in ml/min/100g.
eGFR cited in ml/min/1.73m2.
P-value < .05 indicates a significant difference.
Figure 4.
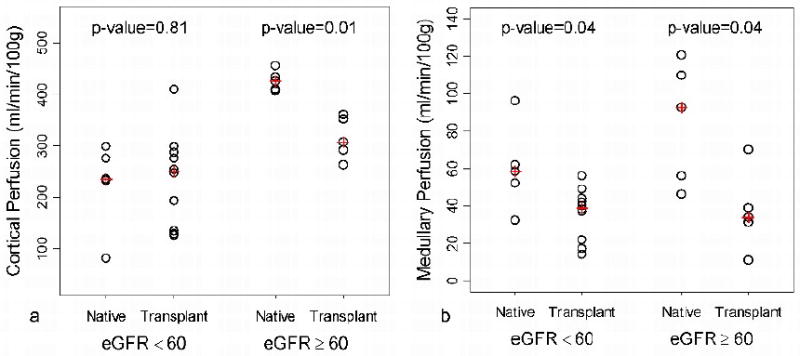
Groupwise comparison of native and transplant cortical (a) and medullary (b) perfusion separated into two groups based on an eGFR threshold of 60 ml/min/1.73m2. For subjects with eGFR ≥ 60 ml/min/1.73m2, a statistical difference between native and transplant is observed in cortical (p =0.01) and medullary (p = 0.04) perfusion. A statistical difference in medullary perfusion (p = 0.04) was also observed in subjects with eGFR < 60 ml/min/1.73m2. (+: median)
Results from the substudy comparing free-breathing and the standard respiratory triggered/coached acquisition methods are displayed in Figures 5 and 6. Figure 5 demonstrates two similar perfusion maps for the same transplanted kidney. Data in 5a were acquired with the standard respiratory coaching and triggering while those in 5b were obtained while the subject breathed freely. A comparison of all available free-breathing and triggered/coached data can be seen in Figure 6 for both cortical (a) and medullary flow (b). Although no statistical differences were observed, ASL values obtained with a free-breathing acquisition were lower on average than the triggered and coached values with a mean difference of (mean ± SD) -37 ± 51 ml/min/100g in the cortex and -17 ± 18 ml/min/100g in the medulla.
Figure 5.
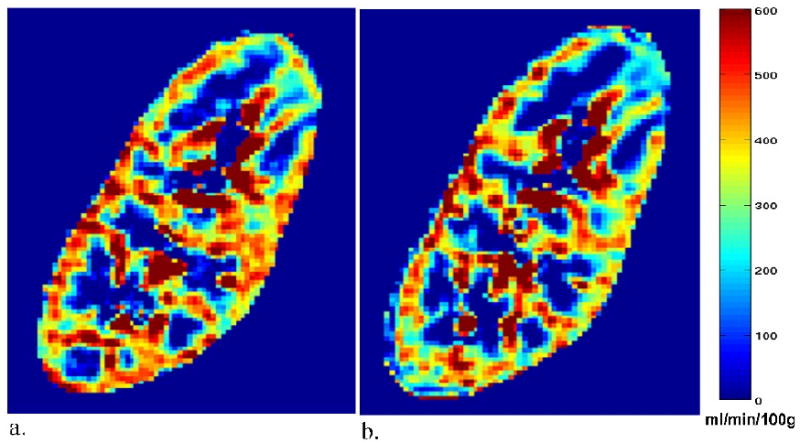
Sagittal perfusion maps for a transplant subject (no. 5 in Table 2) with an eGFR of 60 ml/min/1.73m2 acquired with the standard respiratory triggering and coaching (a) and acquired during free-breathing (no respiratory triggering or coaching) (b). Mean cortical perfusion under respiratory triggering/coaching and free-breathing was a) 382 ml/min/100g and b) 344 ml/min/100g, respectively. Mean medullary perfusion was a) 36 ml/min/100g and b) 13 ml/min/100g, respectively.
Figure 6.
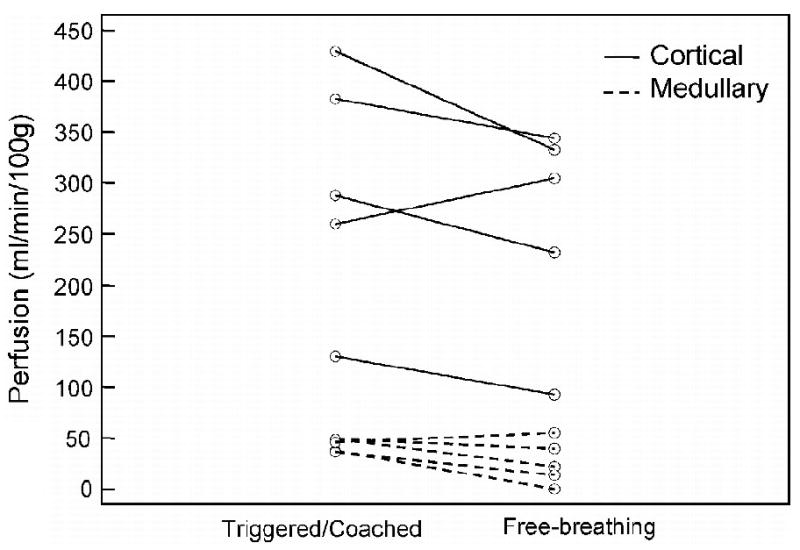
Comparison of respiratory triggered/coached and free-breathing perfusion measurements in five transplant subjects. All exams were performed with an oblique-sagittal orientation. Data trend toward lower perfusion values under free-breathing.
Discussion
Functional MR imaging for the native and transplanted kidney can be performed without injecting a contrast agent and may be helpful in the longitudinal assessment of kidney function in patients with renal disease. Before these methods will be clinically useful, applicable techniques must be developed. Our group has implemented a FAIR-ASL technique to measure perfusion in both native and transplanted kidneys over a broad range of function. Specifically we chose a sagittal orientation in many of the transplanted kidneys to avoid major feeding vessels, utilized a respiratory triggered/coached breathing acquisition, and performed automatic image registration using NMI. We were able to measure the cortical and medullary perfusion in all but 1 of 35 kidneys with this technique and found correlations between cortical perfusion and eGFR. Groupwise comparisons indicate cortical perfusion differences between native and transplant subjects with eGFR > 60 ml/min/1.73m2 and medullary perfusion differences for both eGFR > 60 and eGFR < 60 ml/min/1.73m2. We also successfully implemented a free breathing protocol in a subset of transplanted kidneys to assess robustness of the technique in patients who may not be able to breathe at a consistent rate or follow respiratory coaching (i.e. very ill patients).
While perfusion values in this study are similar to other renal ASL work [6, 12-14], cortical perfusion values for healthy native subjects in this study are slightly higher than values reported by Fenchel et al. using a similar pulse sequence [6]. This may partly be due to variation in acquisition and processing. As for acquisition differences, the present study allowed two additional seconds (for a total of 5 s) for magnetization recovery between inversions. This is more representative of the compartment modeling, which assumes complete recovery and should provide a larger difference between tag and control, increasing the perfusion value. As for processing differences that would lead to higher perfusion values, a more inclusive threshold for cortical perfusion of 1000 ml/min/100g (only < 2% of pixels were rejected) was used compared to 600 ml/min/100g used in the Fenchel et al. study.
Prospective respiratory coaching and triggering was applied to minimize motion artifacts, but it proved necessary to use post-acquisition image registration in most cases and this was critical in certain native and transplant subjects. Registering the MC and MT images eliminated blurring, allowing better tissue segmentation and perfusion measurement. Without registration, the perfusion-weighted difference image would be blurred throughout the kidney as well at the kidney border, where blurring could lead to artificially low cortical perfusion values. Retrospective respiratory sorting was an alternative, as it has been shown to reduce motion artifacts in renal ASL imaging compared to a simple coached acquisition (without respiratory triggering) [14]. With a FAIR acquisition, the inversion slab must encompass the imaging slice so prospective respiratory triggering was used in addition to coaching for this study.
The oblique-sagittal orientation was found to be more practical for transplant subjects whose feeding vessel trajectories precluded the possibility of a coronal acquisition. For example, in subjects with native kidneys, an oblique slice could be positioned both coronal to the kidneys and posterior to the renal arteries allowing coverage of both kidneys with one FOV. However, in many transplanted kidneys, a coronal slice orientation placed the feeding vasculature in-plane. In these cases, a coronal acquisition would have led to artificially low perfusion measurements by slice-selectively inverting the inflowing blood spins which are presumed to be at equilibrium magnetization. An oblique-sagittal slice omitted major feeding vessels in these transplant subjects and allowed for sufficient coverage of the transplanted kidney (Figure 1). Slice orientation would not be restricted for other ASL techniques that label the blood upstream, such as proximal inversion with a control for off-resonance effects (PICORE) or pulsed-continuous methods. Unfortunately, labeling via these methods would lengthen the transit time of the tagged blood and increase the variability in arrival time from subject to subject. Since diseased kidneys are likely to demonstrate more variable flow, adjusting the delay time is also a source of potential error that is avoided by use of the FAIR technique.
The respiratory triggered and coached perfusion measurements in this investigation were comparable to a study by Lanzman et al. [9] which used a free-breathing FAIR-ASL approach in transplanted kidneys. The free-breathing, sagittal perfusion measurements in this study were also similar to respiratory triggered and coached measurements, although slightly lower. Thus free-breathing may be a reasonable option for patients who are unable to follow the regimented breathing pattern. The free-breathing perfusion measurements may be lower because the slice selective inversion thickness was larger than the standard 20 mm to increase the tolerance for through-plane motion. Consequently, more blood spins outside of the imaging slice were inverted when they flowed into the imaging plane.
As expected there was a positive correlation between cortical perfusion measurements and eGFR in both transplant and native kidneys supporting the regulation of glomerular filtration rate by renal blood flow. However, perfusion values were significantly reduced in transplanted kidneys compared to native kidneys for subjects with eGFR > 60 ml/min/1.73m2 (Figure 4). This may result from differential regulation of blood flow in transplanted kidneys or the vasoconstrictive effects of calcineurin inhibitors that are commonly used in kidney transplantation to prevent rejection [21-25] and is an area of future study.
Several limitations apply to the current study. No gold-standard was available to compare the perfusion results, however other studies using FAIR-ASL in the kidneys have shown good correlation with standard references [6, 16, 26]. The results in this work were obtained from a limited number of native and transplant subjects and must be extended to a larger population before drawing firm conclusions regarding practicality and clinical significance. This study was performed on a 1.5T system which has limited SNR compared to a 3T system but should have fewer banding artifacts. Banding was visible in four of the kidneys, although only a small portion of the kidney body was affected, and unaffected measurements were obtained for the majority of the kidney. Higher order shimming or adjusting the center frequency of the radiofrequency pulse would likely shift these artifacts outside of the kidney [27]. This FAIR ASL sequence did not apply bi-polar gradients before readout to suppress signal from tagged spins in the arterioles. This may have introduced a bias towards higher perfusion measurements as well.
Perfusion values presented in this study were determined using a one compartment model which requires many assumptions, such as rapid water exchange between the intravascular and extravascular space. A two compartment model [28] would reduce those assumptions and allow more accurate perfusion quantification, however it requires measurements at multiple delay times which is not possible to achieve in a clinically feasible scan time. Further assumptions used in this study for perfusion measurement include a constant tissue T1 and inversion efficiency, α. In reality, the α can vary depending on rf-amplifier performance, stability and pulse design, while the T1 is a patient-specific parameter that may vary regionally and with kidney disease and setting. The cortical T1 assumed in this study may have introduced a bias towards higher perfusion for lower functioning kidneys because cortical T1 can increase with renal insufficiency [29]. Incorporating a patient-specific measured T1 and pulse-specific α into the model should increase the quantitative accuracy of these measurements with the trade-off of slightly increased scanning time.
In conclusion, FAIR-ASL was able to measure renal perfusion in subjects with native and transplanted kidneys, potentially providing a clinically viable technique for monitoring kidney function. Results from this study demonstrate that medullary perfusion was systematically lower in transplant vs native kidneys, and that cortical perfusion was lower for transplanted kidneys as well for kidneys with higher function, eGFR > 60 ml/min/1.73m2. Cortical perfusion correlated with eGFR in both native and transplanted kidneys. Future work will compare FAIR-ASL with microsphere perfusion in a swine model, assess reproducibility in human subjects, and measure the ability of this technique to characterize renal disease and provide earlier detection of functional change.
Acknowledgments
Funding: This research was supported by the National Institute of Health (NIH grants R01 DK 073680, R21 DK070243)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nathan S. Artz, Department of Medical Physics, University of Wisconsin – Madison, Wisconsin Institute for Medical Research, 1111 Highland Avenue, Madison, WI 53705-2275.
Elizabeth A. Sadowski, Department of Radiology, University of Wisconsin – Madison, Wisconsin Institute for Medical Research, 1111 Highland Avenue, Madison, WI 53705-2275.
Andrew L. Wentland, Department of Medical Physics, University of Wisconsin – Madison, Wisconsin Institute for Medical Research, 1111 Highland Avenue, Madison, WI 53705-2275.
Thomas M. Grist, Department of Radiology and Department of Medical Physics, University of Wisconsin – Madison, Wisconsin Institute for Medical Research, 1111 Highland Avenue, Madison, WI 53705-2275.
Songwon Seo, Department of Biostatistics and Medical Informatics, University of Wisconsin – Madison, Wisconsin Institute for Medical Research, 1111 Highland Avenue, Madison, WI 53705-2275.
Arjang Djamali, Department of Nephrology, University of Wisconsin – Madison, Wisconsin Institute for Medical Research, 1111 Highland Avenue, Madison, WI 53705-2275.
Sean B. Fain, Department of Medical Physics and Department of Radiology, University of Wisconsin – Madison, Wisconsin Institute for Medical Research, 1111 Highland Avenue, Madison, WI 53705-2275.
References
- 1.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–974. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 2.Wu O, Levy AR, Briggs A, Lewis G, Jardine A. Acute rejection and chronic nephropathy: a systematic review of the literature. Transplantation. 2009;87(9):1330–1339. doi: 10.1097/TP.0b013e3181a236e0. [DOI] [PubMed] [Google Scholar]
- 3.Irshad A, Ackerman SJ, Campbell AS, Anis M. An overview of renal transplantation: current practice and use of ultrasound. Semin Ultrasound CT MR. 2009;30(4):298–314. doi: 10.1053/j.sult.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Szolar DH, Preidler K, Ebner F, Kammerhuber F, Horn S, Ratschek M, Ranner G, Petritsch P, Horina JH. Functional magnetic resonance imaging of human renal allografts during the post-transplant period: preliminary observations. Magn Reson Imaging. 1997;15(7):727–735. doi: 10.1016/s0730-725x(97)00088-x. [DOI] [PubMed] [Google Scholar]
- 5.Sharma RK, Gupta RK, Poptani H, Pandey CM, Gujral RB, Bhandari M. The magnetic resonance renogram in renal transplant evaluation using dynamic contrast-enhanced MR imaging. Transplantation. 1995;59(10):1405–1409. doi: 10.1097/00007890-199505270-00008. [DOI] [PubMed] [Google Scholar]
- 6.Fenchel M, Martirosian P, Langanke J, Giersch J, Miller S, Stauder NI, Kramer U, Claussen CD, Schick F. Perfusion MR imaging with FAIR true FISP spin labeling in patients with and without renal artery stenosis: initial experience. Radiology. 2006;238(3):1013–1021. doi: 10.1148/radiol.2382041623. [DOI] [PubMed] [Google Scholar]
- 7.Wang JJ, Hendrich KS, Jackson EK, Ildstad ST, Williams DS, Ho C. Perfusion quantitation in transplanted rat kidney by MRI with arterial spin labeling. Kidney Int. 1998;53(6):1783–1791. doi: 10.1046/j.1523-1755.1998.00945.x. [DOI] [PubMed] [Google Scholar]
- 8.Michaely HJ, Schoenberg SO, Ittrich C, Dikow R, Bock M, Guenther M. Renal disease: value of functional magnetic resonance imaging with flow and perfusion measurements. Invest Radiol. 2004;39(11):698–705. doi: 10.1097/00004424-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Lanzman RS, Wittsack HJ, Martirosian P, Zgoura P, Bilk P, Kropil P, Schick F, Voiculescu A, Blondin D. Quantification of renal allograft perfusion using arterial spin labeling MRI: initial results. Eur Radiol 2009. 20(6):1485–1491. doi: 10.1007/s00330-009-1675-0. [DOI] [PubMed] [Google Scholar]
- 10.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242(3):647–649. doi: 10.1148/radiol.2423061640. [DOI] [PubMed] [Google Scholar]
- 11.Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Top Magn Reson Imaging. 2004;15(1):10–27. doi: 10.1097/00002142-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Karger N, Biederer J, Lusse S, Grimm J, Steffens J, Heller M, Gluer C. Quantitation of renal perfusion using arterial spin labeling with FAIR-UFLARE. Magn Reson Imaging. 2000;18(6):641–647. doi: 10.1016/s0730-725x(00)00155-7. [DOI] [PubMed] [Google Scholar]
- 13.Roberts DA, Detre JA, Bolinger L, Insko EK, Lenkinski RE, Pentecost MJ, Leigh JS., Jr Renal perfusion in humans: MR imaging with spin tagging of arterial water. Radiology. 1995;196(1):281–286. doi: 10.1148/radiology.196.1.7784582. [DOI] [PubMed] [Google Scholar]
- 14.Robson PM, Madhuranthakam AJ, Dai W, Pedrosa I, Rofsky NM, Alsop DC. Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling. Magn Reson Med. 2009;61(6):1374–1387. doi: 10.1002/mrm.21960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martirosian P, Klose U, Mader I, Schick F. FAIR true-FISP perfusion imaging of the kidneys. Magn Reson Med. 2004;51(2):353–361. doi: 10.1002/mrm.10709. [DOI] [PubMed] [Google Scholar]
- 16.Ritt M, Janka R, Schneider MP, Martirosian P, Hornegger J, Bautz W, Uder M, Schmieder RE. Measurement of kidney perfusion by magnetic resonance imaging: comparison of MRI with arterial spin labeling to para-aminohippuric acid plasma clearance in male subjects with metabolic syndrome. Nephrol Dial Transplant. 2009;25(4):1126–1133. doi: 10.1093/ndt/gfp639. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004;230(3):652–659. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]
- 19.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 20.Lerman LO, Flickinger AL, Sheedy PF, 2nd, Turner ST. Reproducibility of human kidney perfusion and volume determinations with electron beam computed tomography. Invest Radiol. 1996;31(4):204–210. doi: 10.1097/00004424-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Amuchastegui SC, Azzollini N, Mister M, Pezzotta A, Perico N, Remuzzi G. Chronic allograft nephropathy in the rat is improved by angiotensin II receptor blockade but not by calcium channel antagonism. J Am Soc Nephrol. 1998;9(10):1948–1955. doi: 10.1681/ASN.V9101948. [DOI] [PubMed] [Google Scholar]
- 22.Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol. 2003;285(3):R619–631. doi: 10.1152/ajpregu.00766.2002. [DOI] [PubMed] [Google Scholar]
- 23.Kompanowska-Jezierska E, Walkowska A, Johns EJ, Sadowski J. Early effects of renal denervation in the anaesthetised rat: natriuresis and increased cortical blood flow. J Physiol. 2001;531(Pt 2):527–534. doi: 10.1111/j.1469-7793.2001.0527i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard BL, Malpas SC, Denton KM, Madden AC, Evans RG. Differential control of intrarenal blood flow during reflex increases in sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol. 2001;280(1):R62–68. doi: 10.1152/ajpregu.2001.280.1.R62. [DOI] [PubMed] [Google Scholar]
- 25.Mervaala E, Lassila M, Vaskonen T, Krogerus L, Lahteenmaki T, Vapaatalo H, Karppanen H. Effects of ACE inhibition on cyclosporine A-induced hypertension and nephrotoxicity in spontaneously hypertensive rats on a high-sodium diet. Blood Press. 1999;8(1):49–56. doi: 10.1080/080370599438392. [DOI] [PubMed] [Google Scholar]
- 26.Warmuth C, Nagel S, Hegemann O, Wlodarczyk W, Ludemann L. Accuracy of blood flow values determined by arterial spin labeling: a validation study in isolated porcine kidneys. J Magn Reson Imaging 2007. 26(2):353–358. doi: 10.1002/jmri.21011. [DOI] [PubMed] [Google Scholar]
- 27.Boss A, Martirosian P, Graf H, Claussen CD, Schlemmer HP, Schick F. High resolution MR perfusion imaging of the kidneys at 3 Tesla without administration of contrast media. Rofo. 2005;177(12):1625–1630. doi: 10.1055/s-2005-858761. [DOI] [PubMed] [Google Scholar]
- 28.Parkes LM. Quantification of cerebral perfusion using arterial spin labeling: two-compartment models. J Magn Reson Imaging 2005. 22(6):732–736. doi: 10.1002/jmri.20456. [DOI] [PubMed] [Google Scholar]
- 29.Lee VS, Kaur M, Bokacheva L, Chen Q, Rusinek H, Thakur R, Moses D, Nazzaro C, Kramer EL. What causes diminished corticomedullary differentiation in renal insufficiency? J Magn Reson Imaging. 2007;25(4):790–795. doi: 10.1002/jmri.20878. [DOI] [PubMed] [Google Scholar]


