Abstract
In a previous study, we demonstrated that the anticancer synthetic retinoid N-(4-hydroxyphenyl)retinamide (4HPR) redox cycles at the mitochondrial enzyme dihydroorotate dehydrogenase to trigger anomalous reactive oxygen species (ROS) production and attendant apoptosis in transformed human epithelial cells. Furthermore, we speculated that the hydroxyl functional group of 4HPR was required for this prooxidant property. In this study, we investigated the role of the hydroxyl functional group in 4HPR's in vitro cytotoxicity. Using 4HPR, its primary in vivo metabolite N-(4-methoxyphenyl)retinamide (4MPR), and the synthetic derivative N-(4-trifluromethylphenyl)retinamide (4TPR), we examined the prooxidant and apoptotic effects, as well as the cellular uptake, of these three N-(4-substituted-phenyl)retinamides in premalignant and malignant human skin, prostate, and breast epithelial cells. Compared to 4HPR, both 4MPR and 4TPR were ineffective in promoting conspicuous cellular ROS production, mitochondrial disruption, or DNA fragmentation in these transformed cells. Interestingly, both 4MPR and 4TPR were not particularly cell permeant relative to 4HPR in skin or breast epithelial cells, which implicated an additional role for the hydroxyl functional group in 4HPR's cellular uptake. Moreover, the short-term uptake of 4HPR was directly proportional to cell size, but this characteristic, in obvious contrast to cellular bioenergetic status and/or dihydroorotate dehydrogenase expression, was not fundamentally influential in the overall sensitivity to the promotion of cellular ROS production and apoptosis induction by this agent. Together, these results strongly implicate the hydroxyl functional group in the cytotoxic effects of 4HPR.
Keywords: N-(4-hydroxyphenyl)retinamide, 4HPR, fenretinide, N-(4-methoxyphenyl)retinamide, cellular uptake, alkylphenol, reactive oxygen species, dihydroorotate dehydrogenase, apoptosis
Introduction
Natural retinoids like all-trans retinoic acid (ATRA, Fig. 1) and 9-cis retinoic acid can suppress tumorigenesis by inducing differentiation and/or cytostasis in transformed cells. This is accomplished through the retinoid receptor-mediated transactivation of genes that ultimately encourage the aforementioned effects in target cells [1]. The atypical retinoid N-(4-hydroxyphenyl)retinamide (4HPR, also known as fenretinide, Fig. 1) is an analog of ATRA that was first synthesized by R. W. Johnson Pharmaceuticals in the late 1960's. Like natural retinoids, 4HPR exhibits anticancer activity in vivo, yet it is a potent apoptogenic agent in transformed cells in vitro (reviewed in [2]).
Fig. 1.
The chemical structures of ATRA, 4HPR, 4MPR, and 4TPR.
The primary long-term side effect of systemic 4HPR use is nyctalopia, and adverse side effects have been minimal in trial participants who have taken relatively high doses of this agent for cancer chemoprevention or therapy. Furthermore, 4HPR's side effects are considered inconsequential compared to those (e.g., liver toxicity and dyspnea) commonly observed in subjects having similar systemic exposures to the parent compound ATRA. The diminution of 4HPR's adverse side effects, relative to those caused by ATRA, is believed to be a result of the substitution of an amide-linked 4-hydroxyphenyl moiety for the carboxyl functional group of ATRA. This chemical modification markedly diminishes 4HPR's binding affinity for, and transactivation of, nuclear retinoid receptors [3], and, as a result, 4HPR's anticancer effects are not believed to be mediated via this mechanism [2, 4].
4HPR's controlling apoptogenic feature is the anomalous production of reactive oxygen species (ROS), which has been reported in various types of transformed cells in vitro [2]. Recently, we demonstrated the mitochondrial enzyme dihydroorotate dehydrogenase (DHODH) was essential for the 4HPR-induced ROS production and attendant apoptosis induction in premalignant and malignant human epithelial cells [5]. In this previous study, we also speculated that the hydroxyl functional group of 4HPR was required for this agent's prooxidant effect. Very little is known about 4HPR's chemical biology, especially the chemical biology that could directly influence the prooxidant and cytotoxic effects of this agent in transformed cells. However, much evidence exists to implicate alkylphenols like 4HPR as potential intracellular redox cycling agents [6-8]. Interestingly, several studies have shown alkyl phenol analogues of 4HPR like p-dodecylaminophenol [9, 10] and 4-hydroxybenzylretinone [11, 12] exhibit anticancer activity comparable to that of 4HPR, but these studies did not investigate the possible prooxidant effects of these 4HPR analogues in cultured cells. Nonetheless, in a cell-free system p-dodecylaminophenol was shown to prevent lipid peroxidation and scavenge superoxide [10]. Hence, this study was conducted to investigate the likelihood that 4HPR's hydroxyl functional group is required for ROS production and apoptosis induction [5]. Here, we present evidence that strongly implicates this functional group in 4HPR-induced cellular ROS production and apoptosis in transformed human epithelial cells in vitro. Moreover, the hydroxyl functional group appears to be essential for mediating the cellular uptake of 4HPR in these cells. Consequently, these findings would further suggest that the hydroxyl functional group of 4HPR is necessary for the anticancer effects of this agent observed in vivo.
Experimental procedures
Cell culture and reagents
The COLO 16 and SRB-12 human skin carcinoma cells, the premalignant HaCaT human cutaneous keratinocytes, the LNCaP and DU-145 human prostate carcinoma cells, and the MCF-7 breast carcinoma cells (kindly provided by Dr. David Ross University of Colorado School of Pharmacy, Aurora, CO) were cultured in 1:1 Dulbecco's Modified Eagle's medium/Ham's F12 (DMEM/F12) medium supplemented with 2% fetal bovine serum (both purchased from Invitrogen Corporation, Carlsbad, CA). The respiration-deficient derivatives (i.e., ρ0 cells lacking mitochondrial DNA) of COLO 16, SRB-12, and DU-145 cells were isolated and characterized as described previously [13, 14]. These cells were cultured in DMEM containing 4.5 mg/ml D-glucose and 110 μg/ml pyruvate supplemented with 2% FBS (both purchased from Invitrogen) and 50 μM uridine (purchased from Sigma-Aldrich Chemical Company, St Louis, MO). Approximately 1.5 × 106 cells were seeded/plate in 10 cm tissue culture plates 24 h prior to treatment with the with the indicated N-(4-substituted-phenyl)retinamides or the vehicle dimethyl sulfoxide (Me2SO, control). The N-(4-substituted-phenyl)retinamides were administered to the cell cultures under subdued lighting. All of the cell cultures were incubated at 37°C in humidified air containing 5% CO2. Where indicated, cell counts were determined using an automated hemacytometer (Countess Automated Cell Counter) that was purchased from Invitrogen.
4HPR and N-(4-methoxyphenyl)retinamide (4MPR, Fig. 1) were kindly provided by the Division of Cancer Prevention, National Cancer Institute, Bethesda, MD. Me2SO, 2’,7’-dichlorofluorescin diacetate, dihydroethidium, 3,3’-dihexyloxacarbocyanine iodide [DiOC6(3)], and propidium iodide (PI) were purchased from Sigma-Aldrich Chemical Company.
Synthesis of N-(4-trifluromethylphenyl)retinamide (4TPR)
4TPR (Fig. 1) was synthesized by converting ATRA to the corresponding acyl chloride and reacting this compound with 4-(trifluromethy)aniline. This procedure was adapted from a method described previously [15]. All of the reagents used in the 4TPR synthesis were purchased from Sigma-Aldrich Chemical Co. Under subdued lighting, ATRA (307 mg) was mixed for 30 min with N,N-dimethylformamide (7 ml) in a 25-ml, round-bottom flask suspended in an ice bath. Next, oxalyl chloride was added dropwise until the solution turned a bright-red color. This solution was warmed to room temperature and stirred for an additional 2 h. A solution containing triethylamine (284 μl) and 4-(trifluoromethyl)aniline (127 μl) was added to the flask, and the contents were stirred overnight (i.e., ~15 h). For storage, the flask was sealed, covered with aluminum foil, and placed in a freezer set at -20°C. The reaction mixture was purified on a silica gel using a hexanes and ethyl acetate (19:1) solution. The concentrated 4TPR consisted of a bright-orange powder. Following two independent isolations, the purified 4TPR accounted for roughly 12% of the mass of the total reaction mixture.
Continuous, short-term determination of cellular ROS generation
Approximately 1.8 × 105 cells/well were seeded in 6-well tissue culture plates for 24 h. The wells were washed twice with 2 ml of Krebs-Ringer buffer (Sigma-Aldrich) and covered with 2 ml of Krebs-Ringer buffer containing 10 μg/ml 2’,7’-dichlorofluorescin diacetate and 10 μM of the indicated N-(4-substituted-phenyl)retinamide or vehicle Me2SO (control). Fluorescence emission at 538 nm (representing 2’,7’-dichlorofluorescein (DCF) production) was measured immediately following mixing (time zero) and subsequently at 30-min intervals over a 150-min period using a SpectraMax Gemini EM dual-scanning microplate spectrofluorimeter (Molecular Devices, Sunnyvale, CA) [16].
Assays for determining mitochondrial ROS production and/or inner transmembrane potential (ΔΨm)
Following a 6-h exposure to the vehicle Me2SO or 10 μM of the indicated N-(4-substituted-phenyl)retinamide, MCF-7 cells were stained for 20 min with 40 nM DiOC6(3) and 5 μM dihydroethidium. Concurrent determinations of mitochondrial ΔΨm dissipation [measured by the cationic dye DiOC6(3)], and ROS production (measured by the oxidation of dihydroethidium to ethidium) were accomplished by cytofluorometric analysis as described previously [17]. A similar flow cytometry procedure to assess ΔΨm alone in the COLO 16, HaCaT, LNCaP, and MCF-7 cells exposed to Me2SO or 10 μM of the indicated N-(4-substituted-phenyl)retinamide for 6 h was accomplished by cell staining for 20 min with 40 nM DiOC6(3) as described previously [18]. The flow cytometry procedures were performed by a Beckman Coulter FC500 flow cytometer with CXP software (Beckman Coulter, Inc., Fullerton, CA).
Assessment of cytosolic cytochrome c
Extra-mitochondrial cytochrome c was determined as described previously [16]. Briefly, the COLO 16 and MCF-7 cells were treated for 6 h with 10 μM of the indicated N-(4-substituted-phenyl)retinamide or an equal volume of the vehicle Me2SO. The cells were harvested, washed once with 1 ml of phosphate-buffered saline, and gently votrexed for 30 s in 80 μl of ice-cold cell permeabilization buffer. The cell suspensions were centrifuged at 12,000 × g for 5 min at 4°C in order to separate the soluble protein fraction from the permeabilized cells. The soluble proteins contained in ~50 μl of the supernatants were subjected to electrophoresis in a SDS-polyacrylamide slab gel and evaluated using immunoblot analysis.
Cell cycle evaluation, apoptosis assay, and cell size determination
The cells were treated for 24 or 48 h with 10 μM of the indicated N-(4-substituted-phenyl)retinamide or the vehicle Me2SO. Cellular DNA fragmentation was ascertained by flow cytometry using a hypotonic solution of PI [16]. This procedure was also used to examine the cellular DNA content of viable cells relative to their cell cycle progression. The PI histograms generated by flow cytometry were analyzed using ModFit LT version 3.2 software (Verity Software House, Inc., Topsham, ME), which modeled the cell cycle and provided the percentage of G0/G1, S, and G2/M phase cells in each sample. Cell size was assessed via microscopic morphometry and flow cytometry. For the flow cytometry procedure, the cells were gated using their forward scatter peak height versus their forward scatter peak area.
Immunoblot analyses
Cellular proteins were characterized as described previously [17]. The membranes were probed with the antibodies for the human proteins DHODH (purchased from Sigma-Aldrich), cytochrome c oxidase (i.e., complex IV) subunit 2 (purchased from Invitrogen), Mn superoxide dismutase (purchased from Upstate Cell Signaling Solutions, Lake Placid, NY), cytochrome c, or β-actin (both purchased from Santa Cruz Biotechnology, Santa Cruz, CA). The binding of the primary antibody was detected with a horseradish peroxidase-linked secondary antibody using an enhanced chemiluminescence kit (Amersham Biosciences Corp., Piscataway, NJ). The immunoblots were subjected to densitometric analysis using ImageJ software (National Institutes of Health, Bethesda, MD). Where indicated, the band intensities of DHODH, cytochrome c oxidase subunit 2 and, Mn superoxide dismutase were normalized as a percent of the loading control β-actin.
Liquid chromatography-mass spectrometry procedure for detecting N-(4-substituted-phenyl)retinamides in cultured cells
The COLO 16, COLO 16 ρ0, and MCF-7 cells were seeded at 2 × 106 cells/10 cm tissue culture dish and allowed to attach for 6 h. 4HPR, 4MPR, or 4TPR (a final concentration of 10 μM), or the vehicle Me2SO was added to the culture medium for a 4-h (4HPR, 4MPR, and 4TPR) or 24-h (4MPR and 4TPR) exposure. The cells were harvested and washed with 3 ml of phosphate-buffered saline. The cells were pelleted via centrifugation and the supernatant was removed. The resulting cell pellet was resuspended in 500 μl of an ice-cold 70% methanol/deionized water solution (v/v) and the cell debris was removed via filtration centrifugation.
The supernatants from 2 × 106 cells/sample were subjected to liquid chromatography-mass spectrometry analysis using an AB SCIEX 4000 mass spectrometer (AB SCIEX, Foster City, CA) equipped with a Shimadzu high-performance liquid chromatograph (Shimadzu Scientific Instruments, Inc.; Columbia, MD) and a LEAP auto sampler (LEAP Technologies; Carrboro, NC). The high-performance liquid chromatography employed a Zorbax C18 5 m particle size 50 × 4.6 mm column (Agilent Technologies, Santa Clara, CA) at 40°C with an isocratic flow rate of 0.4 ml/min. The mobile phases consisted of a solution of 10 mM ammonium acetate in aqueous 0.1% formic acid (A), and a 50:50 acetonitrile and methanol solution (B).
Stock solutions (i.e., 1 mM) of each N-(4-substituted-phenyl)retinamide were prepared in Me2SO. These stock solutions were used to prepare working solutions (i.e., 10 μM in a 1:1 methanol and acetonitrile solution) that were used to generate the standard curves for 4HPR, 4MPR, and 4TPR. A total of 10 μl of the standard samples or the diluted (i.e., in a 1:1 methanol and acetonitrile solution) cell supernatant samples was injected on column. The high-performance liquid chromatography 12-min run time used for the 4HPR and 4MPR samples started with mobile phase (A) for 4.5 min and was switched to mobile phase (B) for 6 min. The mobile phase was switched back to (A) at 10.5 min, and held for an additional 1.5 min. The 18-min run time used for 4TPR samples started with mobile phase (A) for 6 min and switched to mobile phase (B) for 10.5 min. The mobile phase was switched back to (A) at 16.5 min, and held for an additional 1.5 min.
For the mass spectrometry, 4HPR and 4MPR were monitored via electrospray ionization positive ion mode using the following instrument settings: 1) an ion-spray voltage of 5000V; 2) a temperature of 450°C; 3) the curtain gas (i.e., nitrogen) was set at 10 and the collisionally activated dissociation was set at 5; 4) the ion source gas 1 and ion source gas 2 were set at 25; 5) the entrance potential was set at 10V; 6) quadruple 1 and quadruple 3 were set on unit resolution; and 7) the dwell time was set at 200 ms. The detector voltage settings used for the 4HPR (392.3 → 283.3 m/z) samples were 56V for the declustering potential, 17V for the collision energy, and 18V for the collision cell exit potential. The detector voltage settings used for the 4MPR (406.3 → 283.2 m/z) samples were 71V for the declustering potential, 17V for the collision energy, and 20V for the collision cell exit potential. 4TPR was monitored via electrospray ionization negative ion mode using the instrument settings listed above, except the instrument temperature was set at 400°C and the 4TPR (442.2 → 159.8 m/z) detector voltage settings were –115V for the declustering potential, -38V for the collision energy, and –9V for the collision cell exit potential.
Representative liquid chromatography-mass spectrometry chromatograms for 4HPR, 4MPR, and 4TPR standards are presented in supplementary Fig. S1. The 4HPR standard was a mixture of three isomers with retention times of ~5.8, 6, and 6.2 min. Similarly, the 4MPR standard contained three isomers with retention times of ~6.5, 6.8, and 7 min. The 4TPR standard contained two isomers with retention times of roughly 9.7 and 10.5 min. We limited exposure of the retinoids to light during all of our experimental manipulations to minimize photoisomerization [19], and the ATRA we used to synthesize 4TPR produced one peak in its liquid chromatography-mass spectrometry chromatogram (data not shown). Nonetheless, the multiple peaks in our chromatograms for the 4HPR, 4MPR, and 4TPR standards indicated that these retinoids had undergone isomerization to comprise a mixture potentially containing 11-cis, 9-cis, and all-trans isomers (listed in order of increasing retention time [19]). This type of chemical isomerization is inevitable when manipulating retinoids in vitro (e.g., during synthesis processes or even when these agents are diluted in an organic solvent for delivery to cells in culture) [19, 20].
For each N-(4-substituted-phenyl)retinamide, the area under each curve was calculated, and the sum of the total area for all curves (AUC) was used to prepare a standard curve and assess the 4HPR, 4MPR, and 4TPR concentrations in cultured cells. Supplementary Fig. S2 illustrates the standard curves derived for 4HPR, 4MPR, and 4TPR. The limit of detection for all three of the N-(4-substituted-phenyl)retinamides was ~0.4 ng/ml, and each compound displayed a linear analytical response (i.e., 1/x2 weighted linear regression for 0.4 to 550 ng/ml). Analyst version 1.4.1 enhanced validation software (AB SCIEX) was used for data acquisition and analysis.
Statistical analyses
The statistical significance between the means of two groups or more was determined using a two-sided, unpaired t test or a one-way ANOVA with Dunnett's post test, respectively (GraphPad InStat version 3.0 software, GraphPad Software, Inc., San Diego, CA). Where indicated, the results are expressed as the mean value of triplicate samples ± SD (error bars). All means ± S.D. for triplicate samples were calculated with Microsoft Excel 2003 SP2 software (Microsoft Corporation, Seattle, WA). In all statistical analyses, the results were considered significant for P<0.05.
Results and discussion
4MPR and 4TPR do not trigger rapid ROS production in transformed human skin, prostate, or breast epithelial cells
The primary in vivo metabolite for 4HPR is 4MPR, both in animals [21] and in humans [22]. We elected to compare the in vitro cytotoxic effects of 4MPR and 4HPR because the transformed skin, prostate, and breast epithelial cells chosen for this study are all sensitive to 4HPR-induced ROS production and apoptosis induction [5, 23-26], and several studies have reported that 4MPR is equally, or more, effective as 4HPR in triggering cell death in several normal and transformed epithelial cell types, including keratinocytes [27-29]. However, the role of ROS production in the 4HPR- and/or 4MPR-induced apoptosis was not investigated in the latter studies. We also chose to examine the in vitro cytotoxicity of 4TPR in this study because this N-(4-substituted-phenyl)retinamide is presumably inert to potential biotransformation processes that could conceivably act on 4MPR. For example, 4MPR is susceptible to conversion to 4HPR by cells in vivo [21] and in vitro [30], presumably due to enzymatic demethylation.
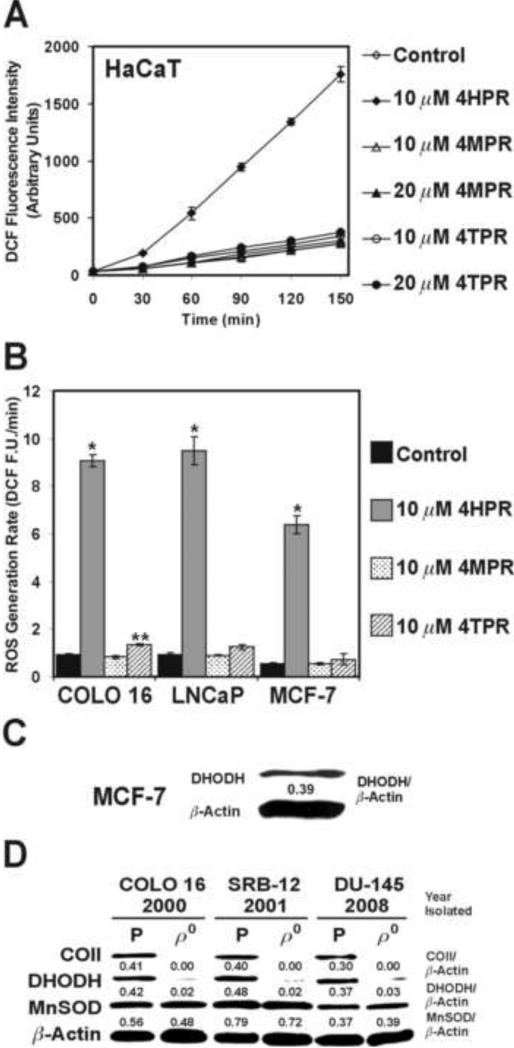
We examined the short-term oxidation of 2’,7’-dichlorofluorescin in the premalignant HaCaT human keratinocytes exposed to various concentrations of 4HPR, 4MPR, or 4TPR. An increase in DCF fluorescence intensity (representing ROS production via the oxidation of 2’,7’-dichlorofluorescin) could be detected as soon as 30 min after exposure to 10 μM 4HPR in the HaCaT cells, and this escalation was linear during the subsequent 120-min. Conversely, a 150-min exposure to 10 or 20 μM 4MPR or 4TPR produced DCF fluorescence intensities that were essentially the same as the Me2SO-treated control cells (Fig. 2A). We also observed a marked increase in the rates of DCF fluorescence generation in the COLO 16, LNCaP, and MCF-7 cells exposed to 10 μM 4HPR relative to similar exposures to 4MPR or 4TPR (Fig. 2B). Interestingly, 4MPR reportedly did not promote ROS production in human cervical carcinoma cells [31], and we have illustrated previously that ATRA and other retinoids that bind to nuclear retinoic acid receptors fail to enhance ROS generation in human skin carcinoma cells [23].
Fig. 2.
Evaluation of N-(4-substituted-phenyl)retinamide-induced ROS production and DHODH expression in premalignant and malignant human epithelial cells. (A) The HaCaT cells were exposed to the indicated N-(4-substituted-phenyl)retinamide and assessed for DCF production over a 150-min period. (B) The ROS generation rates (DCF fluorescence units/min, F.U./min) in the COLO 16, LNCaP, and MCF-7 cells exposed to the indicated N-(4-substituted-phenyl)retinamide or Me2SO (control) were derived from the slopes of lines obtained between 30 and 150 min for triplicate wells in a 6-well tissue culture plate. The 6-well tissue culture plates were incubated at 37°C between the 30-min intervals for fluorescence determination. *P<0.01 compared to the respective controls, **P<0.05 compared to the respective control. (C) An immunoblot assessment of the DHODH expression in the MCF-7 cells. (D) An immunoblot assessment of the cytochrome c oxidase subunit II (COII), DHODH, and manganese superoxide dismutase (MnSOD) expression in the parental (P) COLO 16, SRB-12, and DU-145 cells and their ρ0 derivatives. The ρ0 clones were isolated in the year indicated below the individual cell lines.
The mitochondrial bioenergetic enzyme DHODH is an intermediate in de novo pyrimidine biosynthesis and an established target for 4HPR-induced ROS production [5]. The MCF-7 human breast carcinoma cells express this protein (Fig. 2C) at a level comparable to that expressed by premalignant and malignant human skin and prostate epithelial cells [5]. DHODH is required for rapid cell proliferation in transformed human skin and prostate epithelial cells [5, 16, 17], and the de novo pyrimidine biosynthesis pathway is constitutively active in MCF-7 cells [32]. Furthermore, the COLO 16, HaCaT, LNCaP, and MCF-7 cells double in roughly 24 h (give or take 3 h, data not shown). Thus, we would contend that DHODH is the target for 4HPR-induded ROS production in the MCF-7 cells, and de novo pyrimidine biosynthesis is required for the rapid proliferation of these cells.
Interestingly, when we knock out mitochondrial DNA-encoded proteins like cytochrome c oxidase subunit II in malignant skin and prostate epithelial cells by selectively depleting their mitochondrial DNA, the expression of the nuclear DNA-encoded protein DHODH is also greatly attenuated in the ρ0 cells. The expression of the nuclear DNA-encoded antioxidant enzyme Mn superoxide dismutase was not markedly changed by this process (Fig. 2D), despite the fact that the redox tone in the ρ0 cells is considerably less oxidative than that detected in their parental counterparts [14, 18, 33, 34]. This would indicate that pyrimidine biochemistry is potentially more important than redox biochemistry in these epithelial cells, particularly during the promotion phase of tumorigenesis. We draw this conclusion because the ρ0 cells are also pyrimidine auxotrophs, and potentially represent a metabolic phenotype of malignant cells adapted to survive in a hypoxic in vivo environment (e.g., a solid tumor mass or the bone marrow) [14].
The time required for the ρ0 cells to double their cell number is roughly three-fold longer than their respective parental counterparts, and the ρ0 cells are also resistant to 4HPR-induced ROS and apoptosis [13, 14]. Furthermore, the slow-growing SW480 human colon carcinoma cells, which are also pyrimidine auxotrophs because they lack DHODH expression, are similarly resistant to 4HPR-induced ROS production and apoptosis [5]. Together, these observations further implicate DHODH expression, a dependence on de novo pyrimidine biosynthesis to support rapid cell proliferation, and/or cellular bioenergetic status as dictating the overall sensitivity to 4HPR-induced ROS production in transformed human epithelial cells.
4HPR, but not 4MPR or 4TPR, causes morphological changes suggestive of early cell demise and mitochondrial disruption in transformed human epithelial cells
Features associated with the mitochondrial permeability transition (e.g., the dissipation of ΔΨm and the mitochondrial release of cytochrome c) are caused by 4HPR-induced ROS generation, and these features precede the terminal characteristics of apoptosis (i.e., annexin V reactivity, caspase activation, and DNA fragmentation) in cultured epithelial cells [13, 14, 23]. These mitochondrial changes are also closely coupled with early morphological changes observed in the dying cells [14]. Indeed, compared to their respective control cells, we could detect progressive cell shrinkage and the development of pearly cytoplasmic vacuolizations in the COLO 16 and MCF-7 cells imaged 6 h after treatment with 4HPR (Fig. 3A). These effects were not present in the COLO 16 or MCF-7 cells exposed to either 4MPR or 4TPR for 6 h (data not shown).
Fig. 3.
Assessment of pre-apoptotic events in N-(4-substituted-phenyl)retinamide-treated transformed human epithelial cells. (A) Micrographs of COLO 16 and MCF-7 cells exposed to Me2SO (control) or 10 μM 4HPR for 6 h. The scale bars equal 18 μm. (B) The COLO 16, HaCaT, LNCaP, and MCF-7 cells were treated with 10 μM 4HPR, 4MPR, or 4TPR, or the vehicle Me2SO (control) for 6 h, stained with DiOC6(3), and assessed for the retention of this dye via flow cytometry. *P<0.01 compared to the respective controls and **P<0.05 compared to the respective control. (C) The MCF-7 cells were treated with the indicated N-(4-substituted-phenyl)retinamide, stained with DiOC6(3) and dihydroethidium, and assayed for the retention of DiOC6(3) and the oxidation of dihydroethidium to ethidium by flow cytometry. (D) An immunoblot analysis of cytosolic cytochrome c obtained from cells exposed to the indicated N-(4-substituted-phenyl)retinamide or vehicle Me2SO (control) for 6 h. The band intensities below the individual immunoblots were determined using ImageJ software.
The cellular retention of a cationic dye can be used as a surrogate indicator of ΔΨm and mitochondrial function in intact cells [23]. We examined the retention of the cationic dye DiOC6(3) in the COLO 16, HaCaT, LNCaP, and MCF-7 cells exposed for 6 h to 4HPR, 4MPR, or 4TPR. As illustrated in Fig. 3B, the exposure to 4HPR caused a loss of DiOC6(3) fluorescence in >50% of the COLO 16, HaCaT, LNCaP, and MCF-7 cells. A similar exposure to 4MPR was ineffective in this regard, while 4TPR caused a slight, but discernable and statistically significant, increase in the number of DiOC6(3) low cells in the COLO 16, HaCaT, and MCF-7 treatment populations. This would indicate that 4TPR had a moderate effect on the ΔΨm and mitochondrial function in these cells.
We repeated the 6-h exposures to 4HPR, 4MPR, and 4TPR using the MCF-7 cells. In addition to staining these cells with DiOC6(3), we also added the oxidation-sensitive dye dihydroethidium to determine if the decrease in DiOC6(3) fluorescence caused by 4HPR and 4TPR (Fig. 3B) was accompanied by enhanced oxidative stress in these cells. The exposure to 4HPR triggered a loss of DiOC6(3) fluorescence in roughly 60% of the MCF-7 cells, and a similar percentage of these cells displayed elevated ethidium fluorescence. We did not detect this unique pattern of cell staining in the MCF-7 cells exposed to Me2SO, 4MPR, or 4TPR (Fig. 3C). These results illustrate that ROS production is associated with 4HPR-induced mitochondrial disruption in the MCF-7 cells.
We next assessed extra-mitochondrial cytochrome c in the COLO 16 and MCF-7 cells exposed to 4HPR, 4MPR, or 4TPR for 6 h to further substantiate the possibility of mitochondrial disruption in these cells. We were unable to detect a distinct signal for cytosolic cytochrome c in the cells exposed to Me2SO, 4MPR, or 4TPR. Conversely, the exposure to 4HPR caused a marked increase in the cytosolic cytochrome c signal in the both the COLO 16 and MCF-7 cells (Fig. 3D). The results presented in Fig. 2 and Fig. 3 clearly demonstrate that the hydroxyl functional group of 4HPR is not only responsible for the acute ROS production triggered by this agent, but also the mitochondrial disruption observed in the COLO 16, HaCaT, LNCaP, and MCF-7 cells.
24- and 48-h exposures to 4MPR and 4TPR do not cause DNA fragmentation in transformed human epithelial cells
The anticancer effect of 4HPR is believed to be dependent on this agent's ability to engage apoptosis pathways in transformed cells [2, 4]. Anomalous ROS production and mitochondrial disruption are two of the many mechanisms that can regulate the process of apoptosis [35], including 4HPR-induced apoptosis [2]. However, the appropriate mechanisms for initiating apoptosis by 4MPR and 4TPR many not have been accounted for in our short-term assessments of cytotoxicity thus far in this study. Consequently, we next focused on a terminal outcome of apoptosis, specifically DNA fragmentation, as an absolute illustrator of in vitro cytotoxicity.
A 24-h exposure to 4HPR caused a conspicuous increase in hypoploid apoptotic COLO 16 cells as illustrated by the PI histogram in Fig. 4A, which shows roughly 80% of the treatment population exhibited DNA content below 300 fluorescence units of PI. The PI histograms for the 24-h exposures to 4MPR and 4TPR were no different than the PI histogram observed for the control COLO 16 cells. In fact, we were unable to detect a notable increase in hypoploid COLO 16 cells after 24-h exposures to 4MPR or 4TPR that were up to four-fold higher than the concentration of 4HPR. This was also the case for 48-h exposures to 10 and 20 μM 4MPR or 4TPR. These results mirrored the response of the HaCaT, LNCaP, and MCF-7 cells exposed to 4HPR, 4MPR, or 4TPR for 24 and 48 h (Fig. 4B).
Fig. 4.
Appraisal of apoptotic DNA fragmentation in N-(4-substituted-phenyl)retinamide-treated COLO 16, HaCaT, LNCaP, and MCF-7 human epithelial cells. (A) Representative PI DNA histograms for COLO 16 cells exposed for 24 h to the indicated N-(4-substituted-phenyl)retinamide or Me2SO (control). The hypoploid apoptotic cells are the gated population detected below ~300 fluorescence units of PI on the linear x-axis of the representative histograms. (B) A summary of the hypoploid cells detected in the COLO 16, HaCaT, LNCaP, and MCF-7 cells exposed to the indicated N-(4-substituted-phenyl)retinamide or Me2SO (control) for 24 or 48 h. *P<0.001 compared to the respective control cells.
While both 4MPR and 4TPR were not acutely cytotoxic to the COLO 16, HaCaT, LNCaP, or MCF-7 cells, we did detect a slight cytostatic effect (i.e., a decrease in cell number compared to the control cells) for these agents at 24 and 48 h, which was statistically significant for the exposures to 10 and 20 μM 4TPR (supplementary Fig. S3A). Furthermore, for the 24- and 48-h exposures to 10 and 20 μM 4TPR there was a time-dependent decrease in the S and G2/M cells in the cell cycle of all of the transformed cells we examined. Representative PI histograms for the HaCaT and MCF-7 cells treated for 48 h with 10 μM 4TPR or the vehicle control Me2SO are presented in supplementary Fig. S3B.
4HPR exhibits greater cell permeability than either 4MPR or 4TPR in human skin and breast epithelial cells
4MPR has a longer plasma half-life than 4HPR [21, 22], indicating that the tissue uptake and metabolism of 4HPR and 4MPR are distinct [21]. A recent study showed the short-term cellular uptake of 4HPR peaked at 4 h in A2780 human ovarian carcinoma cells, and then declined substantially within 24 h. This was also the case for A2780 cells made resistant to 4HPR. However, the 4-h 4HPR uptake in these resistant cells was only ~60% of that observed for their parental counterparts [36]. We examined the 4-h uptake of 4HPR, 4MPR, and 4TPR in the COLO 16 and MCF-7 cells. Surprisingly, 4HPR was detected at much higher concentrations (e.g., as much as 30-fold higher in the MCF-7 cells) than those measured for either 4MPR or 4TPR in these cells. Moreover, the concentration of 4HPR that partitioned in to the COLO 16 cells was roughly two-fold higher than the concentration of 4HPR retained by the MCF-7 cells, and the ρ0 COLO 16 derivatives displayed almost 40% more uptake of 4HPR than their parental counterparts (Fig. 5A). Representative chromatograms for the COLO 16 cells exposed 4 h to 10 μM 4HPR, 4MPR, or 4TPR are presented in Fig. 5B. The relative peak heights and peak distributions in these chromatograms were markedly similar to those observed in the chromatograms for the 4HPR, 4MPR, and 4TPR standards (Fig. S1). This was also the case for the MCF-7 cells exposed 4 h to 4HPR, 4MPR, or 4TPR (data not shown). These results clearly confirm that the hydroxyl functional group of 4HPR, and not its possible isomeric state, mediated the cellular uptake of this synthetic retinoid.
Fig. 5.
Characterizing N-(4-substituted-phenyl)retinamide uptake in human skin and breast epithelial cells. (A) The COLO 16, COLO 16 ρ0, and MCF-7 cells were exposed for 4 h to 10 μM of the indicated N-(4-substituted-phenyl)retinamide. The cells were harvested, washed, and lysed using a 70% methanol/deionized water solution. The cell supernatants were assayed for 4HPR, 4MPR or 4TPR using a liquid chromatography-mass spectrometry technique. *P<0.001 compared to the 4MPR or 4TPR treatment. (B) Representative chromatograms for the COLO 16 cells exposed to the indicated N-(4-substituted-phenyl)retinamide as described in (A). (C) The light scattering characteristics of the cells described in (A) were determined by flow cytometry. (D and E) The COLO 16 and MCF-7 cells were exposed to 10 M 4MPR or 4TPR for 4 or 24 h. The cells were then processed as described in (A) and the abundance of 4MPR and 4TPR (D) or 4HPR (E) in the cell supernatants was determined using a liquid chromatography-mass spectrometry technique. *P<0.001 compared to 4HPR detected in the COLO 16 cells.
In the 4HPR resistant A2780 cells, a decrease in drug uptake was attributed to the diminished cytotoxicity of 4HPR. These resistant cells were also smaller than their parental counterparts [36]. The micrographs for the Me2SO-treated COLO 16 and MCF-7 cells presented in Fig. 3A reveal that the COLO 16 cells are larger than the MCF-7 cells. The nuclei are roughly the same size in the COLO 16 and MCF-7 cells. However, the nuclear to cytoplasmic ratio for the COLO 16 cell is estimated to be ~0.33, while the nuclear to cytoplasmic ratio for the MCF-7 cells is roughly 1.
All of the ρ0 epithelial cells we have generated are obviously larger than their parental counterparts as evidenced by microscopic morphometry [14, 18]. We also illustrated this feature via the light-scattering properties of these cells. As shown in Fig. 5C, the COLO 16 ρ0 cells were ~40% larger than their parental counterparts based on the light-scattering characteristics of these cells. Furthermore, the light-scattering characteristics of the COLO 16 cells suggested they were roughly twice the size of the MCF-7 cells.
Compared to the 4-h exposure, a 24-h exposure to 4TPR caused a modest increase in the uptake of this N-(4-substituted-phenyl)retinamide in both the COLO 16 and MCF-7 cells. There was also a time-dependent decrease in the 4MPR retained by the COLO 16 cells and a time-dependent increase in 4MPR retention in the MCF-7 cells after 24 h relative to their respective 4-h exposures (Fig. 5D). The resulting time-dependent increases in 4MPR and/or 4TPR were still only a fraction of the 4HPR detected in these cells at 4 h (Fig. 5A). Furthermore, the relative peak heights and peak distributions in the 4MPR and 4TPR chromatograms were equivalent at 4 and 24 h (data not shown), which would indicate that the extended 24-h cell exposure to these N-(4-substituted-phenyl)retinamides did not markedly influence their isomer composition.
It is certainly possible that the lack of ROS production and apoptosis-inducing activity of 4MPR and 4TPR could be largely due to their poor cellular uptake. However, given the fact that the short-term uptake of 4HPR in the COLO 16 ρ0 cells was notably more than that retained by either their parental counterparts or the MCF-7 cells (Fig. 5A), ρ0 cells are resistant to the prooxidant and apoptosis-inducing activity of 4HPR [13, 14], and the ρ0 derivatives have markedly diminished DHODH expression compared to their parental counterparts (Fig. 2D) or the MCF-7 cells (Fig. 2C), it seems far more accurate to propose that 4HPR's hydroxyl functional group as well as DHODH expression are required for the in vitro prooxidant and apoptogenic effects of this agent in transformed human epithelial cells.
Interestingly, in the 24-h 4MPR samples from both the COLO 16 and MCF-7 cells we were able to detected 4HPR, and this 4HPR was as high as 20% of the concentration of 4MPR that was present in the MCF-7 cells (Fig. 5E). The generation of 4HPR from 4MPR could conceivably represent the activity of a cytochrome P450 demethylase (e.g., 14-α demethylase or aromatase) in these cells. Nevertheless, this 4MPR-derived 4HPR was apparently insufficient to cause any acute cytotoxicity in these cells. Furthermore, we did not detect 4HPR or 4MPR in the COLO 16 or MCF-7 cells exposed to 4TPR for 24 h.
Given these results, we conclude that the uptake of 4HPR is primarily commensurate with cell size, and 4HPR is evidently more lipophilic than either 4MPR or 4TPR. Moreover, the short-term uptake of 4HPR does not appear to directly dictate the cytotoxicity of this agent. For instance, the COLO 16 ρ0 cells displayed a greater short-term uptake of 4HPR than their parental counterparts as well as the MCF-7 cells, yet, as we mentioned previously, ρ0 cells are resistant to the prooxidant and apoptotic effects of this agent [13, 14]. In addition, the MCF-7 cells were equally susceptible to the cytotoxic effects of 4HPR as the COLO 16 cells (Fig. 2, 3, and 4), and their short-term uptake of this agent was roughly half that detected in the COLO 16 cells.
The atypical retinoid 4HPR was identified almost 3 decades ago as “promising” because of the consistent anticancer activity it demonstrated in animal carcinogenesis models [37]. Nevertheless, a consistent benefit from 4HPR against the development or treatment of cancer in humans has not been observed in the limited number of clinical trials that have been conducted to date. The discrepancy between the aforementioned animal and human data may be related to an insufficient dose of 4HPR used in the human studies. Extrapolations from recent in vitro studies indicate that a daily 4HPR dose of 200 mg (considered a “safe” dose for many of the previous cancer chemoprevention and therapy trials [38]) likely does not achieve apoptogenic concentrations of the drug in most target tissues in vivo; perhaps with the exception of tissues with a high fat content such as breast tissue. It is believed that 4HPR concentrations ≥5 μM are needed at target tissues to achieve an apoptogenic response in transformed cells [2, 4]. This concentration is about five-fold higher than the peak plasma level typically attained with a 200 mg daily dose of 4HPR [38], which would suggest that we are way off the mark for potentially obtaining a consistent anticancer effect for this agent in humans.
Children with neuroblastoma have received oral doses of 4HPR as high as 4,000 mg/m2/d for 4 wk with no dose-limiting adverse side effects. This dose resulted in roughly a 13 μM sustained plasma concentration of 4HPR [39]. Dose escalation similar to that attained in children with neuroblastoma as well as novel formulations of 4HPR (e.g., the recently described organized lipid matrix LYM-X-SORB [40], encapsulated in polymeric micelles [41], and complexation with amphiphilic dextrins [42]) are promising to deliver even higher levels of this agent to target tissues in the body. Our in vitro data strongly implicates the hydroxyl functional group of 4HPR in this agent's prooxidant and apoptogenic effects in transformed human epithelial cells. This functional group also appears to mediate the cellular uptake of 4HPR. Thus, we would also predict that the alkyphenolic quality of 4HPR facilitates the anticancer effects of this agent observed in vivo, provided the 4HPR is delivered at a sufficient quantity to the target tissue. Continued in vitro examinations of 4HPR are warranted. These investigations will certainly elucidate additional indispensable features about the chemical biology and the anticancer effects of 4HPR, which, in turn, should lead to innovations that will ultimately increase its overall effectiveness in preventing or treating cancers in humans.
Supplementary Material
Fig. S1 Representative chromatograms for 4HPR, 4MPR, and 4TPR. Please see the experimental procedures section for additional details.
Fig. S2 The liquid chromatography-mass spectrometry standard curves for 4HPR (A), 4MPR (B), and 4TPR (C). The results are expressed as the mean value of triplicate samples ± SD (error bars). Please see the experimental procedures section for additional details.
Fig. S3 Examination of the cytostatic effects of 4MPR and 4TPR in premalignant and malignant human epithelial cells. (A) The COLO 16, HaCaT, LNCaP, and MCF-7 cells were cultured in the presence of 10 or 20 μM 4MPR or 4TPR for 24 and 48 h. The cells were counted and the cell number is presented as a percent of the respective Me2SO-treated control cells. #P<0.05 compared to the respective controls and *P<0.01 compared to the respective controls. (B) Representative PI DNA histograms for HaCaT and MCF-7 cells exposed for 48 h to 10 μM 4TPR or Me2SO (control). The gated cells detected between ~500 to 800 fluorescence units of PI on the linear x-axis of the representative histograms are designated as the S and G2/M phases of the treatment population as determined by ModFit LT analysis.
Acknowledgments
this work was supported by funds provided by the University of Colorado School of Pharmacy, and a University of Colorado School of Pharmacy Medicinal Chemistry Core grant provided by the National Center for Research Resources at the National Institutes of Health (grant # 5UL1RR025780 to M. F. Wempe). We thank Christine Childs with the University of Colorado Cancer Center Flow Cytometry Core for her assistance with the acquisition of the flow cytometry data presented in this study.
Abbreviations used
- ATRA
all-trans retinoic acid
- 4HPR
N-(4-hydroxyphenyl)retinamide
- 4MPR
N-(4-methoxyphenyl)retinamide
- 4TPR
N-(4-trifluoromethlyphenyl)retinamide
- DCF
2’,7’-dichlorofluorescein
- ΔΨm
mitochondrial inner transmembrane potential
- DHODH
dihydroorotate dehydrogenase
- DiOC6(3)
3,3’-dihexyloxacarbocyanine iodide
- Me2SO
dimethyl sulfoxide
- PI
propidium iodide
- ρ0
respiration deficient cells lacking mitochondrial DNA ROS, reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lotan R. Retinoids and apoptosis: implications for chemoprevention and therapy. J. Natl. Cancer Inst. 1995;87:1655–1657. doi: 10.1093/jnci/87.22.1655. [DOI] [PubMed] [Google Scholar]
- 2.Hail N, Jr., Kim HJ, Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis. 2006;11:1677–1694. doi: 10.1007/s10495-006-9289-3. [DOI] [PubMed] [Google Scholar]
- 3.Formelli F, Barua AB, Olson JA. Bioactivities of N-(4-hydroxyphenyl)-retinamide and retinoyl β-glucuronide. FASEB J. 1996;10:1014–1024. doi: 10.1096/fasebj.10.9.8801162. [DOI] [PubMed] [Google Scholar]
- 4.Meyskens FL., Jr. Another negative chemoprevention trial: what can we learn? Clin. Cancer Res. 2008;14:2–3. doi: 10.1158/1078-0432.CCR-07-2215. [DOI] [PubMed] [Google Scholar]
- 5.Hail N, Jr., Chen P, Kepa JJ, Bushman LR, Shearn C. Dihydroorotate dehydrogenase is required for N-(4-hydroxyphenyl)retinamide-induced reactive oxygen species production and apoptosis. Free Radic. Biol. Med. 2010;49:109–116. doi: 10.1016/j.freeradbiomed.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galati G, Sabzevari O, Wilson JX, O'Brien PJ. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology. 2002;177:91–104. doi: 10.1016/s0300-483x(02)00198-1. [DOI] [PubMed] [Google Scholar]
- 7.Hail N, Jr, Lotan R. Cancer chemoprevention and mitochondria: targeting apoptosis in transformed cells via the disruption of mitochondrial bioenergetics/redox state. Mol. Nutr. Food Res. 2009;53:49–67. doi: 10.1002/mnfr.200700527. [DOI] [PubMed] [Google Scholar]
- 8.Kagan VE, Tyurina YY. Recycling and redox cycling of phenolic antioxidants. Ann. N. Y. Acad. Sci. 1998;854:425–434. doi: 10.1111/j.1749-6632.1998.tb09921.x. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi N, Watanabe Y, Maitani Y, Yamauchi T, Higashiyama K, Ohba T. p-Dodecylaminophenol derived from the synthetic retinoid, fenretinide: antitumor efficacy in vitro and in vivo against human prostate cancer and mechanism of action. Int. J. Cancer. 2008;122:689–698. doi: 10.1002/ijc.23154. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi N, Ohba T, Yamauchi T, Higashiyama K. Antioxidant and anticancer activities of novel p-alkylaminophenols and p-acylaminophenols (aminophenol analogues). Bioorg. Med. Chem. 2006;14:6089–6096. doi: 10.1016/j.bmc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Chapman JS, Weiss KL, Curley RW, Jr., Highland MA, M C-D. Hydrolysis of 4-HPR to atRA occurs in vivo but is not required for retinamide-induced apoptosis. Arch. Biochem. Biophys. 2003;419:234–243. doi: 10.1016/j.abb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Abou-Issa H, Curley RW, Jr., Alshafie GA, Weiss KL, Clagett-Dame M, Chapman JS, Mershon SM. Chemotherapeutic evaluation of 4-hydroxybenzylretinone (4-HBR), a nonhydrolyzable C-linked analog of N-(4-hydroxyphenyl) retinamide (4-HPR) against mammary carcinogenesis. Anticancer Res. 2001;21:3839–3844. [PubMed] [Google Scholar]
- 13.Hail N, Jr., Lotan R. Mitochondrial respiration is uniquely associated with the prooxidant and apoptotic effects of N-(4-hydroxyphenyl)retinamide. J. Biol. Chem. 2001;276:45614–45621. doi: 10.1074/jbc.M106559200. [DOI] [PubMed] [Google Scholar]
- 14.Hail N, Jr., Chen P, Kepa JJ. Selective apoptosis induction by the cancer chemopreventive agent N-(4-hydroxyphenyl)retinamide is achieved by modulating mitochondrial bioenergetics in premalignant and malignant human prostate epithelial cells. Apoptosis. 2009;14:449–863. doi: 10.1007/s10495-009-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das BC, Smith ME, Kalpana GV. Design and synthesis of 4-HPR derivatives for rhabdoid tumors. Bioorg. Med. Chem. Lett. 2008;18:3805–3808. doi: 10.1016/j.bmcl.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Hail N, Jr., Chen P, Bushman LR. Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: evidence supporting a role for teriflunomide in prostate cancer chemoprevention. Neoplasia. 2010;12:464–475. doi: 10.1593/neo.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hail N, Jr., Chen P, Rower J, Bushman LR. Teriflunomide encourages cytostatic and apoptotic effects in premalignant and malignant cutaneous keratinocytes. Apoptosis. 2010 doi: 10.1007/s10495-010-0518-4. in press. [DOI] [PubMed] [Google Scholar]
- 18.Hail N., Jr. Mitochondrial reactive oxygen species affect sensitivity to curcumin-induced apoptosis. Free Radic. Biol. Med. 2008;44:1382–1393. doi: 10.1016/j.freeradbiomed.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Murayama A, Suzuki T, Matsui M. Photoisomerization of retinoic acids in ethanol under room light: a warning for cell biological study of geometrical isomers of retinoids. J. Nutr. Sci. Vitaminol. (Tokyo) 1997;43:167–176. doi: 10.3177/jnsv.43.167. [DOI] [PubMed] [Google Scholar]
- 20.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim. Biophys. Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 21.Hultin TA, Filla MS, McCormick DL. Distribution and metabolism of the retinoid, N-(4-methoxyphenyl)-all-trans-retinamide, the major metabolite of N-(4-hydroxyphenyl)-all-trans-retinamide, in female mice. Drug Metab. Dispos. 1990;18:175–179. [PubMed] [Google Scholar]
- 22.Formelli F, Clerici M, Campa T, Di Mauro MG, Magni A, Mascotti G, Moglia D, De Palo G, Costa A, Veronesi U. Five-year administration of fenretinide: pharmacokinetics and effects on plasma retinol concentrations. J. Clin. Oncol. 1993;11:2036–2042. doi: 10.1200/JCO.1993.11.10.2036. [DOI] [PubMed] [Google Scholar]
- 23.Hail N, Jr., Lotan R. Mitochondrial permeability transition is a central coordinating event in N-(4-hydroxyphenyl)retinamide-induced apoptosis. Cancer Epidemiol. Biomarkers Prev. 2000;9:1293–1301. [PubMed] [Google Scholar]
- 24.Davies M, Paterson IC, Ganapathy A, Prime SS. Cell death induced by N-(4-hydroxyphenyl)retinamide in human epidermal keratinocytes is modulated by TGF-beta and diminishes during the progression of squamous cell carcinoma. Int. J. Cancer. 2006;119:2803–2811. doi: 10.1002/ijc.22263. [DOI] [PubMed] [Google Scholar]
- 25.Hursting SD, Shen JC, Sun XY, Wang TT, Phang JM, Perkins SN. Modulation of cyclophilin gene expression by N-4-(hydroxyphenyl)retinamide: association with reactive oxygen species generation and apoptosis. Mol. Carcinog. 2002;33:16–24. doi: 10.1002/mc.10020. [DOI] [PubMed] [Google Scholar]
- 26.Sun S-Y, Yue P, Lotan R. Induction of apoptosis by N-(4-hydroxyphenyl)retinamide and its association with reactive oxygen species, nuclear retinoic acid receptors, and apoptosis related genes in human prostate carcinoma cells. Mol. Pharmacol. 1999;55:403–410. [PubMed] [Google Scholar]
- 27.Sabichi AL, Xu H, Fischer S, Zou C, Yang X, Steele VE, Kelloff GJ, Lotan R, L CJ. Retinoid receptor-dependent and independent biological activities of novel fenretinide analogues and metabolites. Clin. Cancer Res. 2003;9:4606–4613. [PubMed] [Google Scholar]
- 28.Xu H, Cheepala S, McCauley E, Coombes K, Xiao L, Fischer SM, Clifford JL. Chemoprevention of skin carcinogenesis by phenylretinamides: retinoid receptor-independent tumor suppression. Clin. Cancer Res. 2006;12:969–979. doi: 10.1158/1078-0432.CCR-05-1648. [DOI] [PubMed] [Google Scholar]
- 29.Clifford JL, Sabichi AL, Zou C, Yang X, Steele VE, Kelloff GJ, Lotan R, Lippman SM. Effects of novel phenylretinamides on cell growth and apoptosis in bladder cancer. Cancer Epidemiol. Biomarkers Prev. 2001;10:391–395. [PubMed] [Google Scholar]
- 30.Mehta RG, Hultin TA, Moon RC. Metabolism of the chemopreventive retinoid N-(4-hydroxyphenyl)retinamide by mammary gland in organ culture. Biochem. J. 1988;256:579–584. doi: 10.1042/bj2560579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oridate N, Suzuki S, Higuchi M, Mitchell MF, Hong WK, Lotan R. Involvement of reactive oxygen species in N-(4-hydroxyphenyl)retinamide-induced apoptosis in cervical carcinoma cells. J. Natl. Cancer Inst. 1997;89:1191–1198. doi: 10.1093/jnci/89.16.1191. [DOI] [PubMed] [Google Scholar]
- 32.Sigoillot FD, Sigoillot SM, Guy HI. Breakdown of the regulatory control of pyrimidine biosynthesis in human breast cancer cells. Int. J. Cancer. 2004;109:491–498. doi: 10.1002/ijc.11717. [DOI] [PubMed] [Google Scholar]
- 33.Hail N, Jr., Lotan R. Examining the role of mitochondrial respiration in vanilloid-induced apoptosis. J. Natl. Cancer Inst. 2002;94:1281–1292. doi: 10.1093/jnci/94.17.1281. [DOI] [PubMed] [Google Scholar]
- 34.Hail N, Jr., Lotan R. Apoptosis induction by the natural product cancer chemopreventive agent deguelin is mediated through the inhibition of mitochondrial respiration. Apoptosis. 2004;9:437–447. doi: 10.1023/B:APPT.0000031449.57551.e1. [DOI] [PubMed] [Google Scholar]
- 35.Hail N, Jr., Carter BZ, Konopleva M, Andreeff M. Apoptosis effectors mechanisms: a requiem performed in different keys. Apoptosis. 2006;11:889–904. doi: 10.1007/s10495-006-6712-8. [DOI] [PubMed] [Google Scholar]
- 36.Appierto V, Cavadini E, Pergolizzi R, Cleris L, Lotan R, Canevari S, Formelli F. Decrease in drug accumulation and in tumour aggressiveness marker expression in a fenretinide-induced resistant ovarian tumour cell line. Br. J. Cancer. 2001;84:1528–1534. doi: 10.1054/bjoc.2001.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon RC, McCormick DL, Becci PJ, Shealy YF, Frickel F, Paust J, Sporn MB. Influence of 15 retinoic acid amides on urinary bladder carcinogenesis in the mouse. Carcinogenesis. 1982;3:1469–1472. doi: 10.1093/carcin/3.12.1469. [DOI] [PubMed] [Google Scholar]
- 38.Serrano D, Baglietto L, Johansson H, Mariette F, Torrisi R, Onetto M, Paganuzzi M, Decensi A. Effect of the synthetic retinoid fenretinide on circulating free prostate-specific antigen, insulin-like growth factor-I, and insulin-like growth factor binding protein-3 levels in men with superficial bladder cancer. Clin. Cancer Res. 2005:2083–2088. doi: 10.1158/1078-0432.CCR-04-1549. [DOI] [PubMed] [Google Scholar]
- 39.Garaventa A, Luksch R, Lo Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR, Boni L, Ponzoni M, Decensi A, De Bernardi B, Bellani FF, Formelli F. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin. Cancer Res. 2003;9:2032–2039. [PubMed] [Google Scholar]
- 40.Maurer BJ, Kalous O, Yesair DW, Wu X, Janeba J, Maldonado V, Khankaldyyan V, Frgala T, Sun BC, McKee RT, Burgess SW, Shaw WA, Reynolds CP. Improved oral delivery of N-(4-hydroxyphenyl)retinamide with a novel LYM-X-SORB organized lipid complex. Clin. Cancer Res. 2007;13:3079–3086. doi: 10.1158/1078-0432.CCR-06-1889. [DOI] [PubMed] [Google Scholar]
- 41.Okuda T, Kawakami S, Higuchi Y, Satoh T, Oka Y, Yokoyama M, Yamashita F, Hashida M. Enhanced in vivo antitumor efficacy of fenretinide encapsulated in polymeric micelles. Int. J. Pharm. 2009;373:100–106. doi: 10.1016/j.ijpharm.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Orienti I, Zuccari G, Carosio R, Montaldo P. Improvement of aqueous solubility of fenretinide and other hydrophobic anti-tumor drugs by complexation with amphiphilic dextrins. Drug Deliv. 2009;16:389–398. doi: 10.1080/10717540903101655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Representative chromatograms for 4HPR, 4MPR, and 4TPR. Please see the experimental procedures section for additional details.
Fig. S2 The liquid chromatography-mass spectrometry standard curves for 4HPR (A), 4MPR (B), and 4TPR (C). The results are expressed as the mean value of triplicate samples ± SD (error bars). Please see the experimental procedures section for additional details.
Fig. S3 Examination of the cytostatic effects of 4MPR and 4TPR in premalignant and malignant human epithelial cells. (A) The COLO 16, HaCaT, LNCaP, and MCF-7 cells were cultured in the presence of 10 or 20 μM 4MPR or 4TPR for 24 and 48 h. The cells were counted and the cell number is presented as a percent of the respective Me2SO-treated control cells. #P<0.05 compared to the respective controls and *P<0.01 compared to the respective controls. (B) Representative PI DNA histograms for HaCaT and MCF-7 cells exposed for 48 h to 10 μM 4TPR or Me2SO (control). The gated cells detected between ~500 to 800 fluorescence units of PI on the linear x-axis of the representative histograms are designated as the S and G2/M phases of the treatment population as determined by ModFit LT analysis.