Abstract
Background
Cognitive deficits in schizophrenia may be related to glutamatergic dysfunction, but in-vivo measurement of glutamate metabolism has been challenging. We examined the relationship between glutamate metabolism and cognitive function in schizophrenia.
Methods
Thirty subjects with DSM-IV schizophrenia and 28 healthy volunteers, were studied using 4 Tesla proton echo planar spectroscopic imaging. Glutamate plus glutamine (Glx), N-acetyl-aspartate compounds (NAAc) and Inositol (Ins) concentrations in gray and white matter and broad neuropsychological function were assessed in all subjects.
Results
Glx was positively correlated with overall cognitive performance in the schizophrenia group (p=0.0006), accounting for about 36% of the variance. No correlation was found in controls. Group-averaged Glx levels were similar in schizophrenia and controls. NAAc was reduced in cortical gray matter in the younger schizophrenia subjects (age <30; p=0.04) compared to age-matched controls. Ins was increased in cortical gray (p=0.002) and white matter (p=0.02) in the older schizophrenia subjects (age>30) compared to age-matched controls.
Conclusions
Although not reduced in schizophrenia as a group, lower Glx levels correlates with impaired cognition in the illness. This suggests heterogeneity in mechanisms that regulate glutamate function in schizophrenia. Patients with reduced glutamatergic reserves may be rendered into a more severe hypoglutamatergic state with cognitive consequences. Reduced cortical gray matter NAAc concentration early in the illness with normalization in older subjects, is consistent with a process of early dendritic retraction with subsequent increased neuronal packing. Later in the illness, Ins elevation suggests glial involvement.
Keywords: glutamate, 1H-MRS, schizophrenia, cognition, N-acetylaspartate, inositol
Introduction
Schizophrenia is characterized by psychosis and functional deterioration. Cognitive impairments are also common, broad and persistent and account for most of the variance in psychosocial deficits (1). Understanding the neurobiological underpinnings of functional deterioration in schizophrenia is crucial for the development of therapeutic strategies that go beyond the resolution of psychotic symptoms. It has been postulated that a glutamate-related process accounts for cognitive impairment in schizophrenia (2). However, in-vivo measurement of glutamate metabolism has been challenging.
Proton magnetic resonance spectroscopy (1H-MRS) has been used to measure glutamate (Glu) and its glial metabolite glutamine (Gln), in the brain of schizophrenia patients (3–10). These studies used the single voxel method and results were largely inconsistent. In schizophrenia, a disease with subtle but broad gray and white matter involvement (11) proton MR spectroscopic imaging (1H-MRSI) is potentially a more powerful tool, since it enables measurement in a much larger brain region, thereby reducing the bias of voxel selection that is intrinsic to single voxel studies. We used MRSI to examine Glx, myoinositol (Ins) as well as traditional peaks (N-acetylaspartate compounds- NAAc, creatine- Cre and choline- Cho) in schizophrenia and healthy controls. Since the disease may be more active (i.e. more frequent psychotic episodes and more pronounced social deterioration), during the initial 10 yrs (12, 13), younger (<30 yrs) and older (≥30 yrs) subjects were studied. We hypothesized Glx abnormalities in schizophrenia as well as reduced NAAc. We also hypothesized that Glx, the measurable metabolite most clearly related to neuronal function (14), would relate to cognitive performance.
Methods
Subjects
Patients were from the University of New Mexico Hospitals. Inclusion criteria were: 1) DSM-IV schizophrenia using the SCID-DSM-IV; 2) clinically stable on the same antipsychotic medications >4 weeks. Exclusion criteria were neurological disorder or active substance use disorder. Healthy controls were excluded if they had: 1) any DSM-IV axis I disorder, determined by SCID-DSM-IV; 2) first-degree relatives with any psychotic disorder; 3) history of neurological disorder. The study was approved by the local IRB and subjects gave informed consent.
Magnetic Resonance Studies
Acquisition
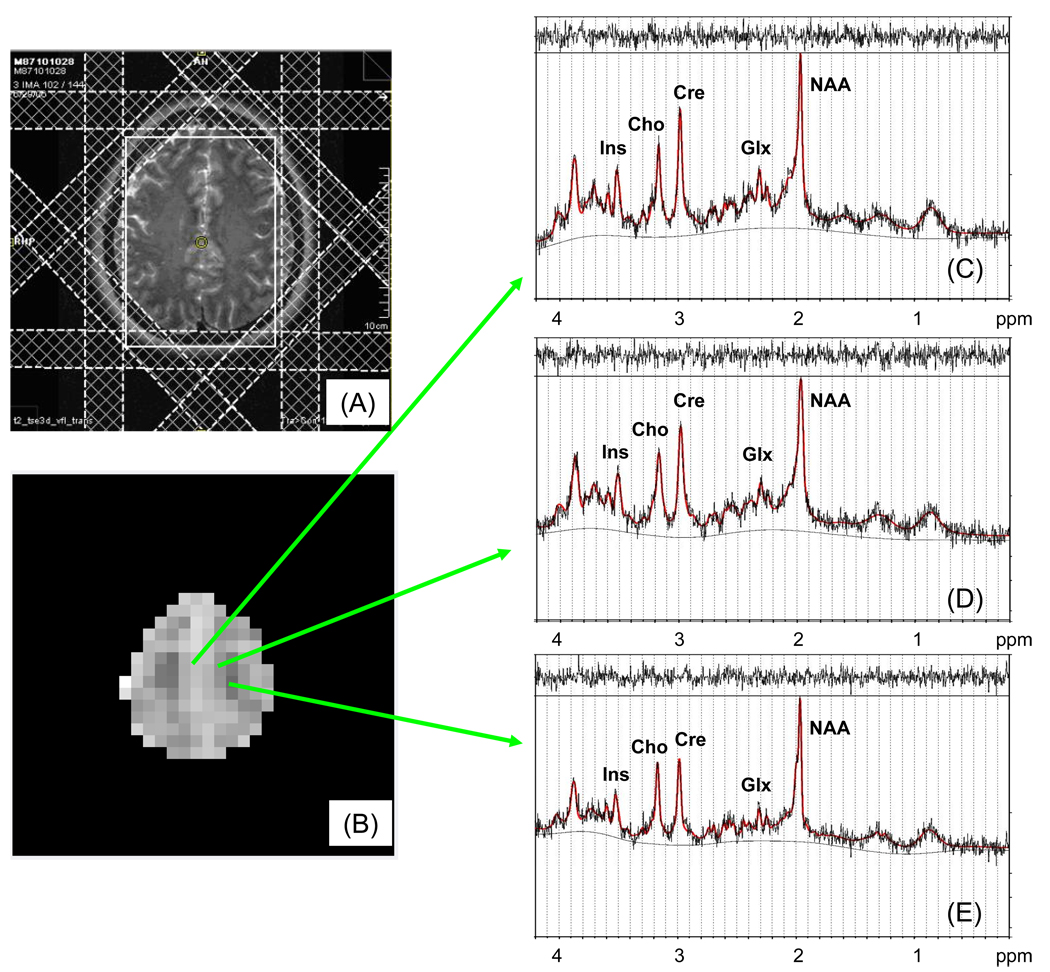
Studies were performed using a 4T scanner (Bruker, MedSpec) with proton-echo-planar-spectroscopic-imaging (PEPSI) methods previously described (15). Head position was fixed with a head mold. Axial T1 MPRAGE images were acquired for PEPSI prescription and for tissue segmentation. PEPSI data was acquired from a slice parallel to AC-PC immediately superior to the lateral ventricles. This location has broad coverage of gray/white matter in bilateral frontal and parietal regions, where metabolic (16), structural (11) and functional abnormalities (17) have been reported in schizophrenia. PEPSI used these parameters: TE=15ms, TR=2s, 32×32 matrix, FOV=256mm, slice thickness=15 mm, resulting in a nominal voxel size of 1 cc. Outer volume suppression consisted of 8 manually prescribed 25 mm thick presaturation slices positioned octagonally along the contours of the brain (figure 1A). A water suppressed data set (WS) with 8 signal averages and a non-water-suppressed reference data set (NWS) with single signal average were collected in 10min. Raw spectral data was automatically processed with an IDL-based in-house developed reconstruction software (15).
Figure 1.
Slice selection for PEPSI placement on axial T2-weighted MR image (A), with corresponding image composed from Glx LC model fitted concentrations (B), and examples of spectral resolution and fitting from voxels of mainly gray (C), mixed gray/white (D) and white matter (E).
Spectral fitting
Localized spectra were quantified using LCModel fitting (Version 6.1;18). Simulated basis sets for sequence parameters included the following metabolites: aspartate (Asp), total choline (Cho), creatine (Cr), gamma-amino-butyric acid, glutamine (Gln), glutathione, glutamate (Glu), myo-inositol (Ins), glucose, lactate, N-acetyl-aspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphocreatine (PCr), phosphoethanolamine, scyllo-inositol and taurine. The following sums were also reported by the fitting program: Cr+PCr (Cre), Gln+Glu (Glx) and NAA+NAAG (NAAc). Spectra were fitted in reference to the NWS data using “water-scaling”.
We automatically selected spectra with the following quality control parameters: full width half mean (FWHM; ppm) <0.06 and signal-to-noise (S/N) > 5, and also restricted to individual metabolite spectra with goodness of fit, as measured by the Cramer Rao Lower Bound (CRLB), of <20 in all selected voxels. Because reliable fits for NAAG and Gln were not generally achieved, all the analyses presented below focus on NAAc and Glx (for exploratory analyses that attempt to separate Gln from Glu see Supplement). Hence, we obtained a contiguous area of about 130 voxels per subject with consistent fit quality for NAAc, Ins, Cre, Cho, and Glx (figure 1B–E; no group differences between the number of voxels selected).
Partial volume correction
T1 images were segmented with SPM2. The cerebrospinal fluid (CSF), gray matter (GM), and white matter (WM) maps were re-sampled and filtered using SPM to match the point spread function, slice thickness and FOV of the PEPSI data. The resulting low resolution maps of GM, WM and CSF were converted into water concentration maps and corrected for the concentration of MR visible water in the three compartments using literature values (19). Relaxation and partial volume correction were performed to obtain millimolal concentrations (mM. see Supplement; 20). Regression, thresholding, and region-of-interest (ROI) approaches were pursued.
1) Regression
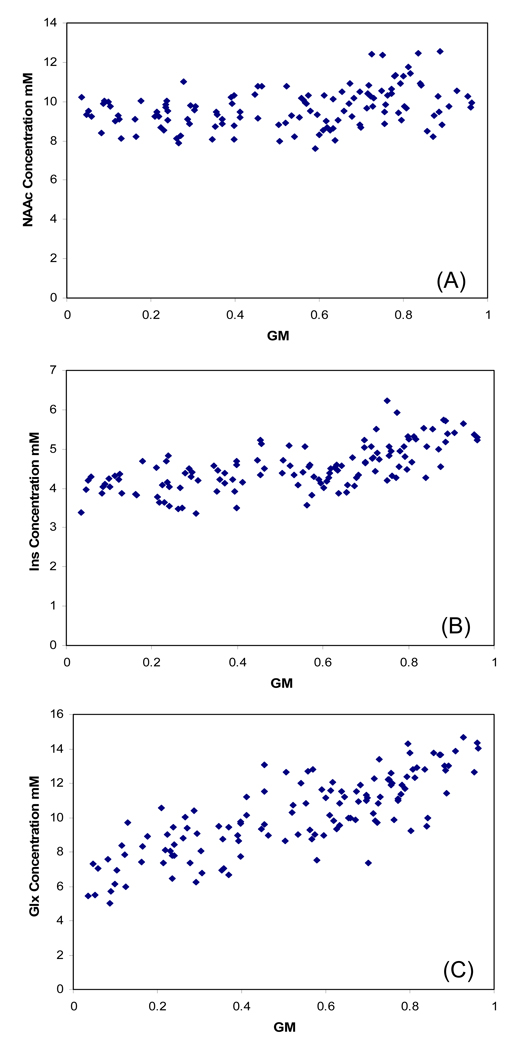
scatterplots of each corrected metabolite concentrations (y axis) and voxel proportion of GM/GM+WM (fractional GM; x axis) were composed, with the corresponding regression lines (21) (figure 2) This generated metabolite concentration vs GM slopes and “pure” gray and white matter metabolite values for each subject. This method provides greater sensitivity to detect GM and WM group differences. However, because segmentation errors of CSF may particularly bias “pure” GM values (20), we also used voxel selection approaches.
Figure 2.
Example from one subject of partial volume corrected concentrations (y axis) plotted against gray matter fraction (x axis), for (A) NAAc, (B) Ins and (C) Glx. All supraventricular voxels that met spectral quality criteria are included.
2) Thresholding
voxels were automatically classified as “predominantly” gray (>66% GM/GM+WM *100), “predominantly” white (<33% GM/GM+WM *100) or mixed (GM/GM+WM *100 between 33–66%). Although this approach has less sensitivity because we exclude mixed voxels (about half of the data), it is less likely to be biased by small errors of CSF segmentation.
3) Regions-of interest
Finally, because of previous reports of cingulate (3), prefrontal (6) and frontal WM (7) glutamatergic abnormalities, we implemented exploratory ROI analyses. ROIs were selected from “predominantly” GM and WM voxels in each hemisphere, anterior (frontal) and posterior (parietal) to the central sulcus. This resulted in 10 ROI’s: left and right frontal lateral GM, frontal medial GM, left and right frontal WM, left and right parietal lateral GM, parietal medial GM, left and right parietal WM.
Neuropsychological and clinical assessments
Patients and controls completed a broad neuropsychological battery which included: Vocabulary, Similarities, Block-Design, Letter-Number (from the WAIS-III (22); Wechsler-Memory-Scale-3 (23) Recall-1 and 2, Hopkins-Verbal-Immediate and Delayed-Recall, Face-Recognition and Delay, Controlled-Oral-Word-Association, Category-Fluency, Trails-A and B, Grooved-Pegboard (dominant/non-dominant) Benton-Visual-Retention, Tower-of-London, California-CalCap (24), and the Wide-Range-Achievement-Test (WRAT-3 (25)). Tests were administered by a psychologist in single session (~2 hours). Order was not counterbalanced.
Patients were assessed for psychopathology with the Schedule-for-Assessment-of-Positive-Symptoms (SAPS;26), the Schedule-for-Assessment-of-Negative-Symptoms (SANS;27) and the Calgary-Depression-Scale (28). They were also rated with the Simpson-Angus-Scale (SAS;29)), the Barnes-Akathisia-Rating-Scale (BARS;30)) and the Abnormal-Involuntary-Movements-Scale (AIMS;31)). Finally, patients were assessed with the social function items of the Psychiatric-Symptoms-You-Currently-Have (PSYCH;32), which measures Employment, Household-duties, Relationships and Recreation.
Statistical analyses
Dependent variables were Glx, NAAc, Ins (main metabolites of interest), Cho and Cre concentrations (mM). For each metabolite the overall analyses used PROC-MIXED (SAS version-8), with diagnosis as the grouping factor and voxel fractional GM as repeated factor. The interaction of diagnosis by fractional GM described potential differences in slopes (of metabolite mM per fractional GM). Age effects (33, 34) were examined with a dichotomous splitting (age-group <30 years or ≥30), to assess interactions between age-group and slopes in four subgroups (young schizophrenia-YouSz, young controls-YCon, old schizophrenia-OldSz and old controls-OldCon). Also, duration of illness was examined as a continuous co-variate within the schizophrenia group.
Results
Demographic and Clinical
Thirty patients and 28 controls participated. There were no significant differences between the groups in: age, gender, ethnic composition, educational or occupational level of the head of household of family of origin (all p’s between 0.2–0.9). The schizophrenia group had less education (t(48)=3.03, p=0.004) and lower occupational history (t(54)=3.13, p=0.003). Table 1 presents these variables divided across the four diagnostic and age groups. The YouSz and OldSz groups did not differ in any clinical variables.
Table 1.
Demographic and clinical characteristics of the subject sample.
| Young Control (n=10) |
Young Schizophrenia (n=12) |
Old Control (n=18) |
Old Schizophrenia (n=18) |
|
|---|---|---|---|---|
| Age years | 22.2±4.4 a | 23.5±3.2 a | 49.5±9.2 b | 49.4±9.6 b |
|
Gender (male/female) |
8/2 a | 9/3 a | 10/8 a | 15/3 a |
|
Ethnicity (NonWhite/White) |
1/9 a | 0/12 a | 4/14 a | 4/14 a |
| Education | 4.1±1.4 a,b | 3.4±0.8 b | 5.2±1.7 a | 3.9±1.4 b |
| Occupation | 4.9±1.6 a | 5.8±1.4 a | 3.4±0.8 b | 4.8±1.9 a |
|
Parental Education |
5.2±1.9 a | 4.3±1.9 a,b | 3.5±1.6 b | 3.3±1.0 b |
|
Parental Occupation |
2.6±0.8 b | 3.9±1.8 a,b | 4.3±1.5 a | 4.4±1.5 a |
| Factor 1 score | 23.9±9.2 a | 18.1±5.9 a,b | 21.9±5.9 a,b | 13.4±7.1 c |
|
Age Onset Psychosis years |
n/a | 20.6±3.6 a | n/a | 21.6±4.8 a |
|
Positive symptoms |
n/a | 5.4±3.0 a | n/a | 8.0±4.1 a |
|
Negative symptoms |
n/a | 7.5±2.9 a | n/a | 8.9±2.1 a |
In Fisher’s Least Significant Difference post-hoc comparisons, groups which do not have the same superscript letter are significantly different (p<0.05).
Group differences in neurometabolites in “pure” gray and white matter
NAAc
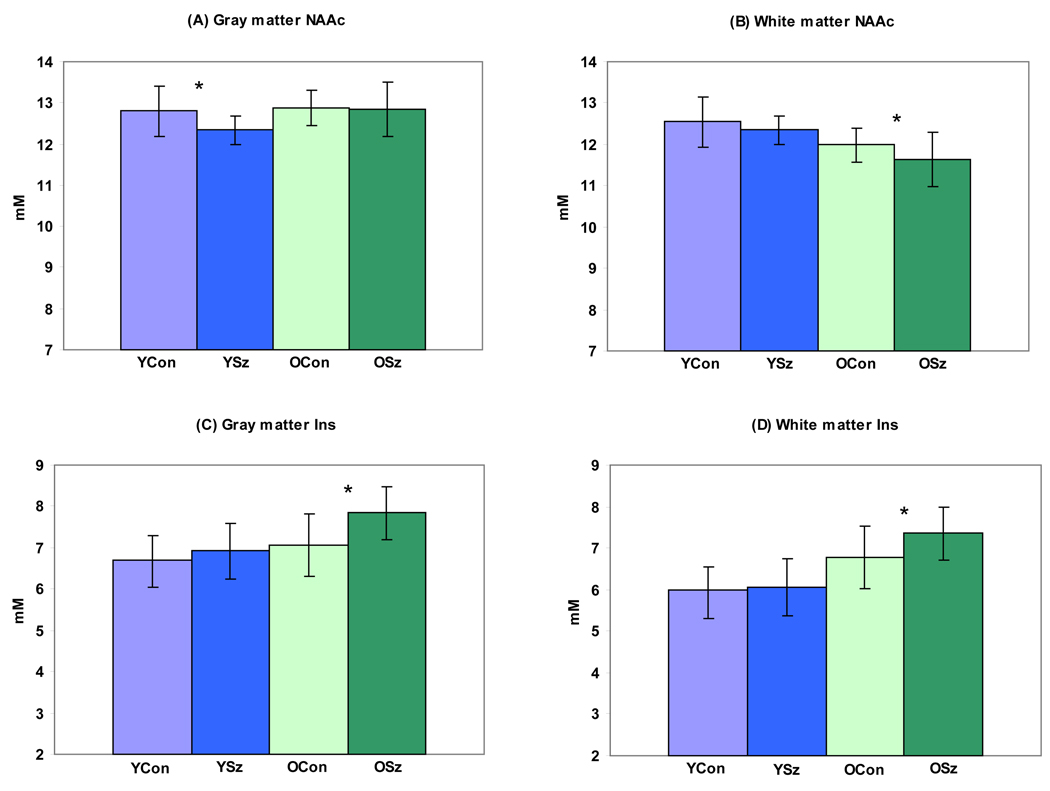
For NAAc, PROC-MIXED found a significant diagnosis by age-group by fractional GM interaction (F(1, 7384)=3.87, p=0.049). The interaction suggested differences in slopes between the four subgroups. Hence, we regressed each voxel metabolite value along its age-group slope to get estimates of “pure” GM and WM concentrations for each subject. Follow-up of the interaction confirmed lower NAAc in “pure” GM in the YouSz compared to the YouCon group (F(1, 20)=4.9, p=0.04; figure 3A). There was also a trend for lower NAAc in WM in the OldSz group (F(1, 34)=3.65, p=0.06; figure 3B).
Figure 3.
Differences in “pure” gray (A) and “pure” white (B) matter N-acetylaspartate (NAAc) concentrations between YouSz (YSz), YouCon (YCon), OldSz (O Sz) and older control (O Con) groups. (YSz < Y Con (F(1, 20)=4.9, p=0.04) in “pure” gray matter). Also differences in “pure” gray (C) and “pure” white (D) matter myoinositol (Ins) concentrations among the subgroups (O Sz > O Con in “pure” gray (F(1, 34)=11.4, p=0.002) and in “pure” white matter (F(1, 34)=6.47, p=0.02)).
The metabolite concentrations presented are corrected for partial volume effects. However, in order to further understand NAAc differences in “pure” GM we measured the % GM, WM and CSF in the whole axial slab volume from which PEPSI was acquired, for each subject (19). There were no diagnoses by age-group interactions for either of the three tissue types (p’s 0.2–0.99). However, for GM% there were clear main effects for diagnosis (F(1, 55)=10.5, p=0.002) and age-group (F(1, 55)=62, p<0.001). For %CSF, there were also effects of diagnosis (F(1, 55)=13.2, p=0.0006) and age-group (F(1, 55)=40.5, p<0.001). Post-hoc pairwise comparisons confirmed 2% lower GM percentage and a 3% higher CSF percentage in schizophrenia (p’s<0.05), regardless of age-group. Older subjects had 4.5% lower GM percentage and 7% higher CSF percentages than the younger group (p’s<0.05).
Ins
For Ins, PROC-MIXED found a diagnosis by fractional GM by age-group interaction (F(1, 7336)=6.36, p=0.012). The interaction suggests differences in slopes between the four subgroups. Follow-up of the interaction confirmed increased Ins in the OldSz compared to the older control group in “pure” GM (F(1, 34)=11.4, p=0.002) and in “pure” white matter GM (F(1, 34)=6.47, p=0.02; figures 3C–D).
Adjusting for education and occupational history did not eliminate the differences between schizophrenia and controls for NAAc (p=0.05) or for Ins (p=0.012). Also, we found a larger proportion of fractional GM in the OldSz compared with the other three groups (mean= 0.84 vs 0.82, respectively, p=0.05). However, the findings in predominantly GM when co-varying (ANCOVA) for GM fraction remained: OldSz having higher Ins (F(1, 33)=4.8, p=0.006) and higher Cre (F(1, 33)=4.0, p=0.05) than the OldCon. The other metabolites still did not differ between these groups (p’s between 0.3–0.9). Also, regarding NAAc in GM, co-varying for dose across all antipsychotics did not eliminate the lower NAAc effect in the YouSz group (F(1, 26)=6.6, p=0.01). However, inclusion of dose did render the reported GM Ins effects in OldSz no longer significant (F(1,26)=0.6, p=0.44). Finally, in the schizophrenia group duration of illness, symptom measures and neuromotoric side-effects were not related to the levels in NAAc or Ins.
Other metabolites
For Cre, PROC-MIXED found a diagnosis by age-group by fractional GM interaction (F(1, 7407)=9.58, p=0.002). Follow-up found increased Cre in “pure” GM in OldSz subjects (F(1, 34)=10.9, p=0.002). For Cho there was a diagnosis by age-group by fractional GM interaction (F(1, 7133)=11.42, p=0.007). However, follow-ups for diagnostic and age-group differences in “pure” GM and WM where non-significant (p=0.22 and p=0.5, respectively). For Glx there was no diagnosis by age-group by fractional GM interaction (p=0.95) and no main effect of diagnosis (p=0.42).
Predominantly gray or white matter
We confirmed a similar pattern of group differences as described with the “pure” GM and WM approach (see Supplement).
ROI analyses
PROC-MIXED found no significant interactions involving ROIs and diagnosis for NAAc, Ins, Glx, Cre or Cho (data not shown). However, we explored glutamine (Gln) and glutamate (Glu) group differences in GM regions of interest with voxels that allowed separation of these peaks with less stringent spectral fitting parameters (CRLB<30). The ratio of Gln/Glu was higher in medial-frontal/parietal GM in the YouSz compared to the YouCon group (F(1, 18)=4.7, p=0.04), but not in the OldSz group (F(1, 30)=0.77, p=0.4; see Supplement).
Cognitive- metabolite relationships
Neuropsychological data from all subjects underwent a factor analysis with VARIMAX rotation. Three factors were retained that explained 69% (factor 1), 27% (factor 2) and 2% (factor 3) of the variance (Table 2). The schizophrenia group performed significantly worse in factors 1 (t(1, 53)=3.9, p=0.0003) and 3 (t(1, 53)=3.03, p=0.004) and marginally worse in factor 2 (t(1, 29)=1.76, p=0.09), than controls.
Table 2.
Neurometabolite means (±SD) in “pure” gray and white matter in young (<30 years old) and older (≥ 30) schizophrenia and age-matched healthy control groups
| Young Control (n=10) |
Young Schizophrenia (n=12) |
Old Control (n=18) |
Old Schizophrenia (n=18) |
|||||
|---|---|---|---|---|---|---|---|---|
| GM | WM | GM | WM | GM | WM | GM | WM | |
| NAAc | 12.80±0.62 | 12.54±0.62 | 12.34±0.34 | 12.35±0.34 | 12.88±0.42 | 11.98±0.42 | 12.84±0.66 | 11.63±0.66 |
| Ins | 6.71±0.56 | 5.99±0.56 | 6.92±0.67 | 6.06±0.67 | 7.06±0.74 | 6.77±0.74 | 7.84±0.64 | 7.36±0.64 |
| Glx | 16.48±1.33 | 9.50±1.33 | 16.15±1.12 | 8.97±1.12 | 15.35±0.74 | 8.02±0.74 | 15.28±0.93 | 7.92±0.93 |
| Cre | 11.48±0.52 | 8.68±0.52 | 11.26±0.46 | 8.50±0.46 | 11.90±0.37 | 8.97±0.37 | 12.54±0.56 | 9.17±0.56 |
| Cho | 1.34±0.20 | 2.00±0.20 | 1.37±0.24 | 2.09±0.24 | 1.46±0.22 | 2.37±0.22 | 1.63±0.20 | 2.38±0.20 |
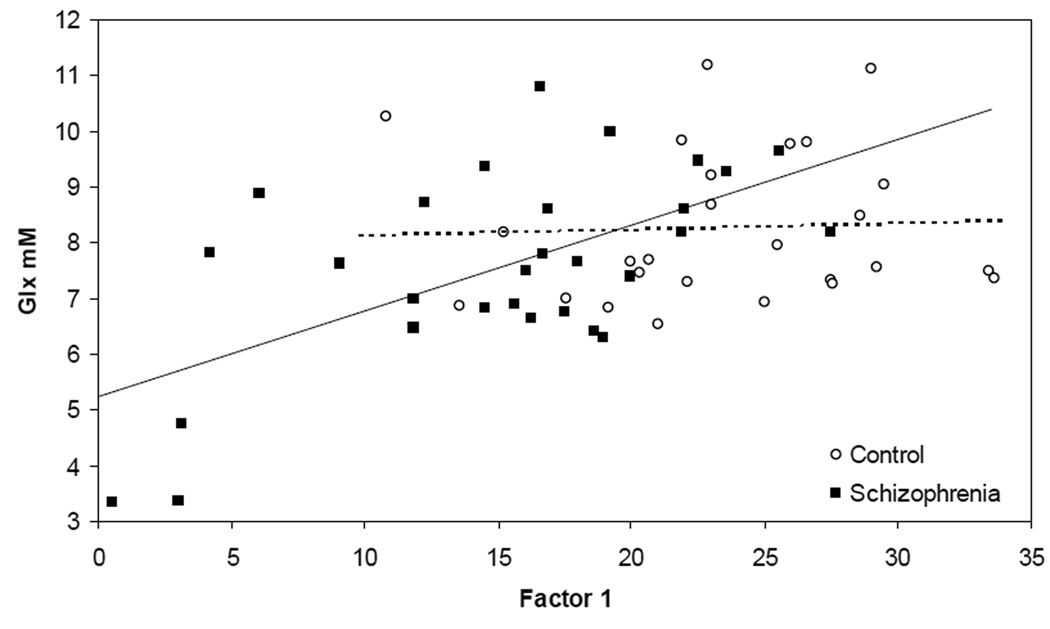
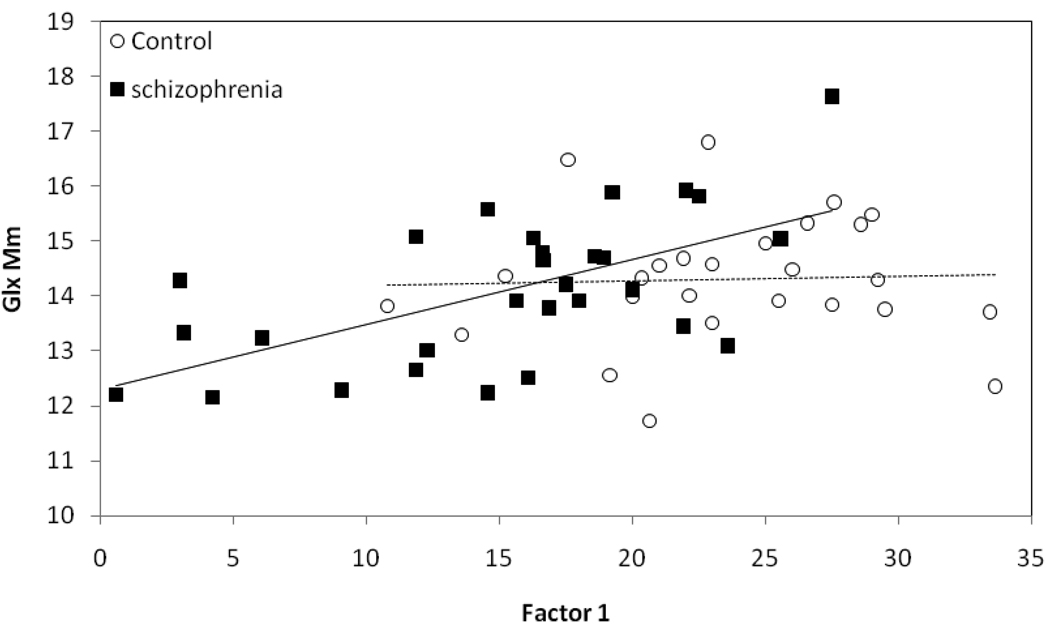
Because of our interest in understanding the neurobiological underpinnings of cognitive dysfunction in schizophrenia, we next implemented “all possible regressions”(35).This approach calculates multiple R-squares for all regression models and is used to verify the "best" model obtained by stepwise selection of variables. We selected each cognitive factor as the dependent variable and the various metabolite concentrations, diagnosis and age-group and their interactions as independent variables. Analyses were performed separately for “pure” GM and WM matter values. For factor 1 and WM metabolites, the best model (R2=0.46) included the diagnosis by Glx interaction (F(1, 51)=4.43, p=0.04) and main effects of Glx (F(1, 51)=10.6, p=0.002) and diagnosis (F(1, 51)= 28.6, p=0.0001). Follow-up of this interaction documented a positive correlation between factor 1 and Glx in the schizophrenia group (r(28)=0.6, p=0.0005) but not in the controls (r(26)= 0.04, p=0.83; figure 4). This suggests that general cognitive function is more dependent on global WM Glx in schizophrenia, than in controls. (A less conservative approach found that performance in 5/7 cognitive domains correlated with global WM Glx in schizophrenia, but none in controls; see Supplement).
Figure 4.
White matter Glx correlates with cognition in schizophrenia. “Pure” white matter glutamate plus glutamine (Glx) concentration correlates with general cognition (Factor 1) in schizophrenia (r(28)=0.6, p=0.0005) but not in the healthy controls (r(26)= 0.04, p=0.83).
Alternative explanations were examined: a) A “floor” effect on Glx levels for controls: however, no difference in Glx variance was found between the groups (F(29, 27)=1.59, p=0.23). b) Impact of age and length of illness on the Glx and factor 1 correlation: ANCOVA’s confirmed that partial correlations for Glx and factor 1 remained significant with inclusion of age (p=0.01) and length of illness (p=0.008) into the model. c) CSF segmentation errors with the projection approach: analyses with predominantly WM Glx voxels and factor 1, supported differential correlations between schizophrenia (r(28)=0.55, p=0.002) and control groups (r(26)= −0.01, p=0.95). d) Possibility that controls are more “efficient” and use more restricted regions to process information comprised by factor 1: we did separate correlations for Glx in frontal or parietal GM and WM regions and factor 1, in schizophrenia and controls. Controls still had no significant correlations in frontal GM (r(26)=0.08, p=0.68) or WM (r(26)= −0.16, p=0.4), or parietal GM (r(26)= −0.1, p=0.61) or WM (r(26)= −0.25, p=0.2). In contrast, schizophrenia patients had significant, positive Glx /factor 1 correlations in frontal GM (r(28)=0.41, p=0.03) and WM (r(28)=0.43, p=0.02), and in parietal GM (r(28)=0.6, p=0.0006; figure 5) and WM (r(28)=0.54, p=0.003). e) Confound of antipsychotic medication. However, the Glx/factor 1 correlation in schizophrenia persisted (F(1, 28)=13.8, p=0.001) when co-varying for antipsychotic dose (36). f) We examined the 3 potential outliers and compared them to the remaining patients (Glx<5mM, figure 4). They had lower AIMS (0 vs 0.46, p=0.04) and marginally higher SANS scores (10.6 vs 8.0, p=0.07) but did not differ in SAPS, akathisia, parkinsonism or depressive symptoms. The higher SANS scores are consistent with the significant correlation between these and Factor 1 performance (see below- Clinical measures). The lower AIMS, as a measure of tardive dyskinesia, would suggest less of this neurological insult in these subjects and hence is unlikely to account for their particularly low cognitive performance.
Figure 5.
Parietal gray matter Glx correlates with cognition in schizophrenia. “Pure” parietal gray matter glutamate plus glutamine (Glx) concentration correlate with general cognition (Factor 1) in schizophrenia (r(28)=0.6, p=0.0005) but not in the healthy controls (r(26)= −0.1, p=0.61)
The “all-possible-regressions” analyses for factor 1 and “pure” GM metabolites were not suggestive of a particular model. Furthermore, “pure” GM Glx did not correlate with factor 1 in schizophrenia (r(28)=0.14, p=0.47) or control (r(28)=0.08, p=0.7) groups. However, analyses with predominant GM Glx resulted in significant correlations in schizophrenia (r(28)=0.49, p=0.006), but not control (r(26)=0.19, p=0.36), groups. Finally, analyses for factors 2 and 3 failed to support any effects of interactions between diagnosis and specific metabolite in GM or WM.
Clinical measures
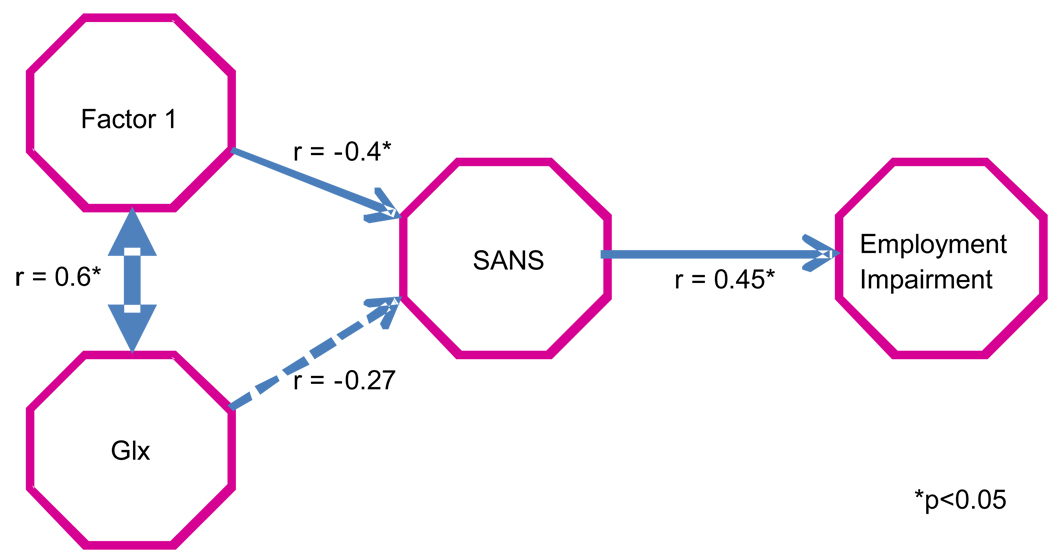
To understand the clinical significance of the relationship between “pure” WM Glx and factor 1 in schizophrenia, we performed path-analyses with analysis-of-covariance (ANCOVA). We informed these analyses by the theoretical model that a relationship between Glx and social dysfunction would be mediated first by cognitive impairment and symptom severity. With regard to all the symptoms (SAPS, SANS, Calgary-Depression), side-effects (SAS, BAS, AIMS) and PSYCH function (Employment, Household-duties, Relationships, Recreation) ratings, factor 1 only correlated with two measures: SANS (r(29)= −0.4, p=0.03) and Employment impairment (r(29)= −0.4, p=0.04). To clarify the direction of these relationships, we adjusted the correlations for each of the other variables included in the model (figure 6). When adjusting for factor 1, Glx was not related to SANS (r(29)= −0.05, p=0.79) or Employment (r(29)= −0.06, p=0.77). After adjusting for Glx, the relationship between factor 1 and SANS became marginal (r(29)= −0.30, p=0.1), but SANS remained correlated with Employment (r(29)= 0.41, p=0.03). Finally, with adjustment for SANS, factor 1 remained correlated with Glx (r(29)= 0.56, p=0.003), but factor 1 no longer correlated with Employment (r(29)= −0.26, p=0.19). Hence, these results suggest that Glx and factor 1 share variance as they relate to SANS. Still, the path goes from factor 1 to SANS to Employment.
Figure 6.
Path Analyses depicting the direction of relationships between Glx (in white matter), global cognition (factor 1), global negative symptoms (SANS) and Employment impairment (PSYCH-up) in schizophrenia subjects. Values with an * have p ≤0.05.
Discussion
In schizophrenia we found a positive correlation between Glx concentrations and cognitive performance, despite group-averaged Glx values being similar to controls. Glx accounted for about 36% of the variance in factor 1 in patients. This is, to our knowledge, the first study to document such a relationship. The potential clinical significance of this finding is highlighted by the complementary inverse relationships between factor 1 and negative symptoms, and between negative symptoms and employment. In addition, we found reductions in GM NAAc in the YouSz but not the OldSz subjects (≥30 y/o), whom tended to have reduced WM NAAc. Elevations of Ins were apparent in GM and WM as well as increased GM Cre, in the OldSz group.
Only a few studies have measured glutamate in schizophrenia, all with single-voxel 1H-MRS and higher field strengths. Theberge et.al (3) at 4T, found increased Gln in anterior cingulate (AC) and thalamus in antipsychotic-naive schizophrenia. In chronically-ill patients Gln was elevated in the thalamus, but Glx was reduced in AC (4). Tibbo et.al (5) at 3T, found increased AC Glx in adolescents at risk for schizophrenia. Tebartz et.al (6) at 2T, found increased Glu in prefrontal and medial-temporal regions in acutely ill subjects. Wood et.al (8) at 3T, found no differences in hippocampal Glx between early schizophrenia and control subjects. Shin’Ya et.al (9) at 3T, 2008, found reduced Glu in AC in chronically ill patients. The above studies all found normal NAAc in the few voxels (1 or 2) examined. Chang et.al (7) studied WM at 4T in older patients. They found elevated Glx in bilateral prefrontal and left occipital WM and reduced NAAc in bilateral prefrontal and temporal regions. Finally, Ongur et.al (10) 4T (TE averaged technique) reported reduced NAA in AC and normal glutamatergic indices in chronic patients during an acute exacerbation. Inconsistencies across this literature are likely due to differences in populations, regions of interest studied as well as spectroscopic technique. Our subjects were clinically stable outpatients, with comparably few active symptoms. Finally, no studies have assessed cognition and glutamateric function in schizophrenia.
Many investigations have measured NAAc in schizophrenia/healthy control comparisons, mostly in large single-voxels at 1.5T(summarized in a meta-analysis; 16). Findings included NAAc reductions, better documented in combined GM and WM prefrontal and medial-temporal regions.
Few studies have used 1H-MRSI at 1.5T, to assess NAAc differences between GM and WM (37–39). Bertolino et.al (38) found reduced NAAc/Cre in prefrontal and hippocampal GM. However, Kegeles et.al (39) using the same methodology, failed to replicate these findings. Lim et.al (37) found reduced WM NAAc in prefrontal, parietal and occipital regions, but normal GM NAAc, in middle aged subjects. However, these patients had reduced cortical GM volumes. These results suggested diffuse axonal abnormalities as well as cortical pathology, with normal overall density of GM neuronal components (37). Consistently, the one study with 1H-MRSI at higher field (3T), found reduced NAAc in temporal WM (40). Glutamate was not examined. Our results of reduced NAAc in WM in OldSz and lower NAAc in cortical GM in YouSz, are consistent with and extend Lim et.al (37) (their patients had a mean age of 43, similar to our OldSz). To our knowledge, there have been no other studies at high field, with 1H-MRSI in YouSz subjects that have examined NAAc in cortical GM.
Very few studies have measured Ins in schizophrenia. Chang et.al (7) found reduced Ins in frontal and temporal WM in elderly patients. Shin’Ya et.al (9) found reduced Ins in AC in chronically-ill adults (mean age 35). Tang et.al (40) reported no differences in WM Ins in adult schizophrenia. However, they did not describe criteria for spectral quality selection or Ins fitting parameters (40).
Several technical strengths in our study may account for the novel results. First, we benefited from superior S/N at 4T. Second, the very short TE, further improved S/N especially for Glx and Ins. Third, PEPSI allowed broad spatial coverage of many small voxels. In schizophrenia, an illness with widespread, subtle GM/WM involvement, broader coverage and higher spatial resolution is desirable. Fourth, fast whole-slice acquisition and head mold application, minimized movement. Finally, sufficient high quality spectra allowed strict automated data screening (FWHM<0.06, S/N>5 and CRLB<20; see Table S2 in the Supplement for CRLB statistics).
The positive relationship between Glx and cognition in schizophrenia with normal metabolite range, suggests that some other factor makes glutamate be less efficient in supporting normal cognitive function in schizophrenia. Hence, the patients with the lower Glx values are particularly vulnerable to cognitive impairment. However, this interpretation would be more coherent with a Glx/cognition relationship specific to GM. Still, the potential clinical relevance of this relationship is highlighted by our path analyses: glutamate→cognition→negative symptoms→employment (consistent with a metanalysis of predictors of social functioning in schizophrenia; 41). Furthermore, strategies focused on dopamine blockade, have been ineffective to improve cognition in schizophrenia (42). Some glutamate-related strategies have been tried with limited success (43). However, the ability to select more “glutamate-deficient” patients may accelerate the systematic investigation of promising compounds.
Lower cortical NAAc in the YouSz subgroup, with no differences in the OldSz is an unexpected finding. NAA, produced in neuronal mitochondria, is localized in the soma, dendrites and axons. NAA reductions suggest lower concentrations per unit of tissue volume. This could be due to neuronal death, mitochondrial dysfunction and/or dendritic/axonal atrophy. Postmortem studies have not found cortical neuronal loss in schizophrenia (44) but neuropil reductions (45) and increased neuronal density (46) have been reported (mostly in tissue from older subjects). Early in the illness, progressive reductions in cortical volume have been documented in several prospective studies (47). In our YouSz, cortical NAAc was reduced but cortical volume was normal, while in the OldSz subgroup volumes were reduced. Hence, it is possible that an early and limited process of dendritic retraction with normal cortical volume and neuronal density (but with lower cortical NAAc), is followed by volume reduction and increased neuronal density (with relatively normalized NAAc concentration at later age. Regardless of the mechanism, reduced cortical NAAc could be a marker of disease activity, useful to test potential neuroprotective agents.
Myo-inositol, is the most abundant biologically active stereoisomer of inositol in the brain. Myo-inositol is a precursor in the phosphatidylinositol (PI) second messenger system, which is activated by a variety of G-protein coupled receptors. Myo-inositol is also a glial marker (48) and is stored in glial cells before its consumption in the PI cycle of neurons (49). Hence, Ins alterations may be important in several neuro-psychiatric conditions. In dementia, elevated Ins, in conjunction with reduced NAAc, has been repeatedly found (50).
Cre is involved in energy metabolism and our findings suggest cortical elevations in older patients. However, the literature on Cre in schizophrenia is largely inconsistent, with reports of elevation (8), reduction (7) as well as no differences (37).
Limitations of this study should be acknowledged. First, sample size was small, especially for age-related analyses. Also, the age cut-off (<30) is somewhat arbitrary and requires replication with a-priori defined age-groups. Second, macromolecules were not measured, though their contribution was modeled using the default LC model function. Third, spatial coverage was limited to supraventricular tissue. Other regions are likely involved in schizophrenia. Fourth, the cross-sectional design supports descriptive, not causal interpretations. Fifth, Glx does not directly examine synaptic glutamate, the more relevant pathophysiological measure. We separated Gln from Glu in medial GM regions, and found increased Gln/Glu, a measure perhaps closer to synaptic glutamate in the YouSz group, as previously reported (51). However, these analyses do not survive correction for multiple comparisons. Sixth, the regression method for partial volume correction assumes uniform spatial distribution of metabolites, which has been questioned especially for Cho (52). Finally, antipsychotic medication use in the patients could account for metabolite differences, a confound common to most schizophrenia studies.
In summary, we found a positive correlation between cognition and glutamatergic metabolism in schizophrenia. We also found evidence of neuronal cortical impairment early in the illness (reduced NAAc), with subsequent glial activation (increased Ins) in older patients. These results are consistent with a progressive process of cortical dendritic retraction and subsequent increased neuronal packing. Regardless of the pathophysiological mechanism, MRSI may help identify patients more likely to respond to specific glutamatergic-modulating agents.
Supplementary Material
Table 3.
Factor Analysis of neuropsychological performance in combined schizophrenia and healthy control groups.
| TESTS | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| WMS 3 Recall 2 | 73* | −25 | −21 |
| Block Design Total | 71* | −21 | −4 |
| Faces Recognition | 67* | −12 | −7 |
| WMS 3 Recall 1 | 66* | −30 | −10 |
| Vocabulary Total | 65* | −18 | −14 |
| Hopkins Verbal Delay | 64* | −24 | 0 |
| Hopkins Verbal Immediate | 64* | −30 | −18 |
| Total Category Fluency | 62* | −36 | −4 |
| Letter Number Total | 58* | −56* | 4 |
| Similarities Total | 54* | −10 | 1 |
| Total FAS | 49* | −22 | −6 |
| WRAT 3 | 47* | 4 | −9 |
| Faces Delay | 41 | −29 | 13 |
| Trails A | −41 | 30 | 13 |
| Pegboard Dominant | −60* | 39 | 13 |
| Trails B | −70* | 65* | −9 |
| Tower of London | 14 | 96* | 26 |
| Pegboard non-Dominant | −47* | 55* | 17 |
| Benton Visual Retention | 29 | −54* | 12 |
| Cal Cap Reaction Time | −22 | 7 | 97* |
Values >44 are flagged by an ‘*’ and represent loading scores that are distinguishable from zero.
Acknowledgements
Supported by the Mental Illness and Neuroscience Discovery Institute (DE-FG03-99ER62764/A002) and by NIMH R01MH084898 to J. Bustillo, MD.
The authors are grateful to Erica Snyder, Tara Biehl, Heather Hawk for their contributions. Also Pierre-Gilles Henry and Gosia Malgorzata for providing the basis sets, Jeff Alger for SRTP software.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures Section:
In the last 12 months Dr Bustillo has received hourly pay from Novartis Corporation for work as a member of a Data Safety Monitoring Board. Dr Lauriello has done consulting for Eli Lilly and Janssen Pharmaceuticals. Dr Apfeldorf has consulted for Pfizer/Eisai and is on the Speaker’s Bureau for AstraZeneca, Lilly and Pfizer. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcomes in schizophrenia: are we measuring the right stuff? Schizophr Bull. 2000;26:119–126. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 2.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 3.Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, Neufeld RW, Rogers J, Pavlosky W, Schaefer B, Densmore M, Al-Semaan Y, Williamson P. Glutamate and glutamine measured with 4.0T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 4.Theberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RW, Rajakumar N, Schaefer B, Densmore M, Drost DJ. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- 5.Tibbo P, Hanstock C, Valiakalayil A, Allen P. 3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am J Psychiatry. 2004;161:1116–1118. doi: 10.1176/appi.ajp.161.6.1116. [DOI] [PubMed] [Google Scholar]
- 6.Tebartz van Elst L, Valerius G, Buchert M, Thiel T, Rusch N, Bubl E, Hennig J, Ebert D, Olbrich HM. Increased prefrontal and hippocampal glutamate and concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58(9):724–730. doi: 10.1016/j.biopsych.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry. 2007;62:1396–1404. doi: 10.1016/j.biopsych.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood S, Berger, Wellard M, Proffitt T, McConchie M, Velakoulis D, McGorry P, Pantelis C. 1H-MRS investigation of the medial temporal lobe in antipsychotic-naïve and early-treated first episode psychosis. Schizophrenia Research. 2008;102:163–170. doi: 10.1016/j.schres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Shin'Ya T, Satsuki S, Kyoko T, Sumiko S, Shusuke N, Jun-ichi I, Masahito N, Shu-ichi U, Masafumi Harada, Tetsuro Ohmori. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy. Schizophrenia Research. 2008;Volume 108:69–77. doi: 10.1016/j.schres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Ongur D, Jensen JE, Prescot A, Storkc C, Lundyd M, Cohen B, Renshaw P. Abnormal Glutamatergic Neurotransmission and Neuronal-Glial Interactions in Acute Mania. Biol Psych. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46:729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]
- 13.McGlashan TH. Is active psychosis neurotoxic? Schizophr Bull. 2006;32(4):609–613. doi: 10.1093/schbul/sbl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laughlin S. Neural energy consumption and the representation of mental events, Chapter 7. In: Schulman RG, Rothman DL, editors. Brain Energetics and Neuronal Activity. Wiley; 2004. pp. 113–115. [Google Scholar]
- 15.Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry PG, Marjanska M, Gasparovic C, Zuo C, Magnotta V, Mueller B, Mullins P, Renshaw P, Ugurbil K, Lim K, Alger J. Proton Echo Planar Spectroscopic Imaging of j-coupled resonances in human brain at 3 and 4 Tesla. Magn Reson Med. 2007;58:236–244. doi: 10.1002/mrm.21287. [DOI] [PubMed] [Google Scholar]
- 16.Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30(11):1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 17.Buchsbaum MS, Hazlett EA. Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr Bull. 1998;24(3):343–364. doi: 10.1093/oxfordjournals.schbul.a033331. [DOI] [PubMed] [Google Scholar]
- 18.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 19.Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. 1.1 Compartments and water. J. Magn. Reson. Series B. 1993 Aug;v.102(no.1):1–8. [Google Scholar]
- 20.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55(6):1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- 21.Hetherington HP, Pan JW, Mason GF, Adams D, Vaughn MJ, Twieg DB, Pohost GM. Quantitative 1H spectroscopic imaging of human brain at 4.1 T using image segmentation. Magn Reson Med. 1996;36(1):21–29. doi: 10.1002/mrm.1910360106. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Administration and Scoring Manual. Third Edition. San Antonio: The Psychological Corporation and Harcourt Brace; 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- 23.Wechsler D. Wechsler Memory Scale. Third Edition. San Antonio: The Psychological Corporation and Harcourt Brace; 1997. [Google Scholar]
- 24.Lezak MD, Howleson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. p. 1,016. [Google Scholar]
- 25.Wilkinson GS. Wide Range Achievement Test. 3rd ed. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- 26.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; 1984. [Google Scholar]
- 27.Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; 1984. [PubMed] [Google Scholar]
- 28.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophrenia Research. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]; Simpson GM, Angus JW. A rating scale for extrapyramidal side-effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 29.Simpson GM, Angus JW. a rating scale for extrapyramidal side-effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 30.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 31.Guy W. ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76–338. Washington, DC: US Department of Health, Education and Welfare; 1976. pp. 534–537. [Google Scholar]
- 32.Andreasen NC, Flaum M, et al. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49(8):15–23. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 33.Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan E, Lim K. In Vivo Spectroscopic Quantification of the N-acetyl Moiety, Creatine, and Choline From Large Volumes of Brain Gray and White Matter: Effects of Normal Aging. Magnetic Resonance in Medicine. 1999;41:276–284. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Maudsley A, Domenig C, Govind V, Darkazanli A, Studholme C, Arheart K, Bloomer C. Mapping of Brain Metabolite Distributions by Volumetric Proton MR Spectroscopic Imaging (MRSI) Magnetic Resonance in Medicine. 2009;61:548–559. doi: 10.1002/mrm.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neter J, Wasserman W. Applied Linear Statistical Models. Chapter 11. Homewood, Illinois: Richard D. Irwin, Inc; 1974. Section 11.3. [Google Scholar]
- 36.Schooler NR. Anti-psychotic medications and schizophrenia: Their effects in acute and maintenance treatment of the illness. In: Cromwell RL, Snyder CR, editors. Schizophrenia: Origins, Processes, Treatment, and Outcome. New York: Oxford University Press; 1993. pp. 284–295. [Google Scholar]
- 37.Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pferfferbaum A. Proton magnetic resonance imaging of cortical gray and white matter in schizophrenia. Arch Gen Psychiatry. 1998;55:346–352. doi: 10.1001/archpsyc.55.4.346. [DOI] [PubMed] [Google Scholar]
- 38.Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CT, Frank JA, Tedeschi G, Weinberger DR. Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopy imaging. Am J Psychiatry. 1996;153:1554–1563. doi: 10.1176/ajp.153.12.1554. [DOI] [PubMed] [Google Scholar]
- 39.Kegeles LS, Shungu DC, Anjilvel S, Chan S, Ellis SP, Xanthopoulos E, et al. Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatr Res Neuroimaging. 2000;98:163–175. doi: 10.1016/s0925-4927(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 40.Tang CY, Friedman J, Shungu D, Chang L, Ernst T, Stewart D, Hajianpour A, Carpenter D, Ng J, Mao X, Hof PR, Buchsbaum MS, Davis K, Gorman JM. Correlations between Difussion Tensor Imaging (DTI) and Magnetic Resonance Spectroscopy (1H MRS) in schizophrenic patients and normal controls. BMC Psychiatry. 2007;7:25. doi: 10.1186/1471-244X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ventura J, Helleman G, Thames A, Koellner V, Neuchterlein K. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: A meta-analysis. Schiz Res. 2009;113:189–199. doi: 10.1016/j.schres.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA. CATIE Investigators; Neurocognitive Working Group: Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 43.Buchanan R, Javitt D, Marder S, Schooler N, Gold J, McMahon R, Heresco-Levy U, Carpenter W. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): The Efficacy of Glutamatergic Agents for Negative Symptoms and Cognitive Impairments. Am J Psychiatry. 2007 Oct;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 44.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 45.Glantz L, Lewis D. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 46.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. Arch Gen Psychiatry. 1995;52:805–819. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 47.Arango C, Moreno C, Martínez S, Parellada M, I Desco M, Moreno D, Fraguas D, Gogtay N, James A, Rapoport J. Longitudinal Brain Changes in Early-Onset Psychosis. Schizophr Bull. 2008;34:341–353. doi: 10.1093/schbul/sbm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glanville NT, Byers DM, Cook HW, Spence MW, Palmer FB. Differences in the metabolism of inositol and phosphoinositides by cultured cells of neuronal and glial origin. Biochim Biophys Acta. 1989;1004:169–179. doi: 10.1016/0005-2760(89)90265-8. [DOI] [PubMed] [Google Scholar]
- 49.Frey R, Metzler D, Fischer P, et al. Myo-inositol in depressive and healthy subjects determined by frontal 1H-magnetic resonance spectroscopy at 1.5 tesla. J Psychiatr Res. 1998;32:411–420. doi: 10.1016/s0022-3956(98)00033-8. [DOI] [PubMed] [Google Scholar]
- 50.Valenzuela M, Sachdev P. Magnetic resonance spectroscopy in Alzheimer’s Disease. Neurology. 2001;56:592–598. doi: 10.1212/wnl.56.5.592. [DOI] [PubMed] [Google Scholar]
- 51.Bustillo J, Rowland L, Mullins P, Jung R, Chen H, Qualls C, Hammond R, Brooks W, Lauriello J. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Molecular Psychiatry. 2010;15(6):629–636. doi: 10.1038/mp.2009.121. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barker EH, Basso G, Barker PB, Smith MA, Bonekamp D, Horská Regional apparent metabolite concentrations in young adult brain measured by (1)H MR spectroscopy at 3 Tesla. A. J Magn Reson Imaging. 2008;27(3):489–499. doi: 10.1002/jmri.21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.