Abstract
The HIV-infected population in the U.S. is expanding as patients survive longer and new infections are identified. In many areas, particularly rural/medically-underserved regions, there is a growing shortage of providers with sufficient HIV expertise. HIV services incorporated into community-based, primary care settings may therefore improve the distribution and delivery of HIV treatment. Our objective was to describe/compare patients and treatment outcomes in two settings: a community-located, primary care-based HIV program and a hospital-based specialty center. Community-based providers had on-site access to generalist HIV experts. The hospital center was staffed primarily by infectious disease physicians. This was a retrospective cohort study of 854 HIV-positive adults initiating care between 1/2005 and 12/2007 within an academic medical center network in the Bronx, NY. Treatment outcomes were virologic and immunologic response at 16–32 and 48 weeks, respectively, after combination antiretroviral therapy (cART) initiation. We found that hospital-based subjects presented with a higher prevalence of AIDS (59% vs. 46%, p < 0.01) and lower initial CD4 (385 vs. 437, p < 0.05) than community-based subjects. Among 178 community vs. 237 hospital subjects starting cART, 66% vs. 62% achieved virologic suppression ([95% CI difference −0.14–0.06]) and 49% vs. 59% achieved immunologic success, defined as a 100 cell/mm3 increase in CD4 ([95% CI difference 0.00–0.19]). The multivariate-adjusted likelihoods of achieving viral suppression (OR = 1.24 [95% CI 0.69–2.33]) and immunologic success (OR = 0.76 [95% CI 0.47–1.21]) were not statistically significant for community vs. hospital subjects. Because this was an observational study, propensity scores were used to address potential selection bias when subjects presented to a particular setting. In conclusion, HIV-infected patients initiate care at community-based clinics earlier and with less advanced HIV disease. Treatment outcomes are comparable to those at a hospital-based specialty center, suggesting that HIV care can be delivered effectively in community settings.
Keywords: HIV primary care, community-based health services, HIV treatment outcomes
Introduction
HIV has evolved from an acute, life-threatening illness to a chronic disease, fueling demand for HIV-experienced clinicians. Although over one million people are infected in the U.S., there are only 5000–6000 board-certified infectious disease specialists and approximately 1500 American Academy of HIV Medicine (AAHIVM)-designated HIV Specialists™ (CDC 2008; http://www.aahivm.org). HIV expertise is especially needed for rural/medically-underserved areas and vulnerable populations (Cohn et al., 2001; Napravnik et al., 2006; Rosen et al., 2004). Moreover, several disparities exist: minorities are disproportionately affected by new infections while substance users do not receive the same degree of treatment benefit (Gebo et al., 2005; Lert & Kazatchkine, 2007). Consequently, there is increasing need to improve the distribution and delivery of HIV services to achieve key public health goals—including those outlined in Healthy People 2010 (http://www.healthypeople.gov).
There is no uniquely-defined training standard for acquiring HIV expertise; data suggest HIV care is similar between specialty-trained (eg. infectious disease) providers and HIV-proficient generalists (Landon et al., 2005; Landon et al., 2003). However, little is known regarding HIV-positive patients managed by generalists in non-specialty settings (Rastegar, Fingerhood, & Jasinski, 2003). Collaborative care—which has been successfully applied to other chronic illnesses (Smith et al., 2008; Smith, Allwright, & O’Dowd, 2007) and involves the integration of HIV expertise into primary care practices (or vice versa)—could facilitate appropriate HIV management while preserving accessible, comprehensive, and longitudinal care. To our knowledge, no studies have examined HIV-infected patients and treatment outcomes under a collaborative model in community-based, primary care settings.
The purposes of this study were to (1) compare patient characteristics in two settings of HIV care in the Bronx, NY: a hospital-based HIV/AIDS specialty center and a community-based primary care network; and (2) compare HIV treatment outcomes between these settings. We hypothesized that virologic and immunologic outcomes would be equivalent between the two settings.
Methods
Research subjects
Subjects were non-pregnant HIV-positive adults 18 years and older initiating HIV care between 1 January 2005 and 31 December 2007. Patients seen at a community-based site prior to 1 January 2005 but subsequently testing HIV-positive were eligible. Subjects received HIV care exclusively at either the hospital-based center or within the community-based network during the study period, with at least one visit per year. For subjects starting/switching combination antiretroviral therapy (cART), outcomes data were collected through 31 December 2008.
Study sites
Subjects received care at Montefiore Medical Center, a large tertiary care center and teaching hospital in the Bronx, NY. Montefiore provides outpatient HIV care in many settings, two of which are the hospital-based specialty clinic and a large community-based network (see below).
Study design
This was a retrospective cohort study.
HIV care at the hospital-based specialty clinic
The hospital-based Center for Positive Living, one of the largest HIV/AIDS referral centers in the metropolitan New York area, is staffed by infectious disease-trained clinicians (attendings and fellows) and mid-level providers. Patients are followed longitudinally by one provider; there is rarely co-management/consultation regarding HIV treatment between independently-certified clinicians.
Collaborative care at community-based sites
Montefiore’s community-based HIV/AIDS program involves 10 general internal and family medicine practices (6 are training sites for medical residents); it is affiliated with a local substance use harm reduction program. Non-HIV-expert primary care providers (PCPs) have formal partnerships with on-site New York State/AAHIVM-accredited HIV Specialists (all general internal/family medicine-trained), and HIV collaboration is “mentored” and/or “shared”, usually based on PCP preference. With mentored collaboration, PCPs discuss the case with the HIV Specialist (hereafter referred to as “expert”) but no direct patient-expert interaction occurs. With shared collaboration, patients have medical visits with the expert in addition to routine PCP visits. Expert consultations (required for antiretroviral changes) are documented in the medical record. All providers at CB sites are regularly updated regarding guidelines for cART initiation, and reminded to consult with experts when expected virologic decreases have not occurred after cART initiation.
Identification of HIV-positive patients at community-based clinics
An HIV case-finding algorithm was developed by the authors (GU, RB) to identify HIV-positive patients at community sites; it was based on coding/billing data and HIV-related laboratory results extracted from the hospital’s electronic clinical information system. Patients were considered [potentially] HIV-positive if they had at least one outpatient visit with an ICD9 code of “AIDS” (042) or “asymptomatic HIV infection” (V08), and at least one of following laboratory result ever: 1) positive Western Blot after HIV-1/2 antibody test, 2) CD4 and concurrent detectable HIV viral load (VL), 3) CD4 and concurrent undetectable VL, 4) detectable VL alone, or 5) CD4 alone. HIV status was then confirmed by medical chart review.
Outcome measures
The primary treatment outcome, virologic success, was defined as attaining an undetectable HIV VL within 16–32 weeks of initiating cART. The secondary treatment outcome was immunologic success (achieving a 100 cell/mm3 CD4 increase within 48 weeks). [Regimens which qualified as highly-active combinations were based on U.S. Department of Health and Human Services guidelines (October 2004 through 2007) (http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf).]
Data collection
One author (CC) collected data from inpatient/outpatient records and the hospital’s electronic clinical information system (which contains longitudinal laboratory and medication records). cART adherence was calculated from patient self-report as documented by clinicians. A standardized chart abstraction tool, adapted from established quality improvement and research protocols, was developed and translated into Microsoft Access.
Statistical analyses
Statistical analyses were performed using Stata/IC v. 10.1 (StataCorp LP, College Station, TX). After inspecting data for accuracy, categorical data were compared with Pearson’s Chi-square/Fisher’s exact test and continuous data were compared with student’s t-test/Wilcoxon rank-sum test as appropriate. Non-normal continuous data were tested using parametric and non-parametric methods; when there was no difference in significance parametric testing results are given (otherwise the more conservative non-parametric estimate is reported). A multivariate logistic regression model using age, gender, ethnicity, risk factor, time since diagnosis, antiretroviral exposure history, illicit drug use, pre-treatment CD4/VL, type of cART initiated, cART adherence, and visit frequency was constructed to analyze treatment outcomes. Hosmer and Lemeshow goodness-of-fit testing yielded a χ2 test statistic = 7.30 and p = 0.29 (indicating a good fit). Model variables were selected based on theoretical/clinical relevance and empirical evidence found on bivariate testing. Differences were considered statistically significant at α = 0.05; reported confidence intervals are two-sided.
Multivariate-adjusted odds ratios were evaluated using two-sided equivalence testing: specifically, the likelihood of community-based subjects achieving virologic/immunologic success was considered equivalent to that of hospital-based subjects if the 95% CI was contained entirely within the interval 0.85–1.15 (corresponding to a 15% margin of acceptable difference). Sample size calculations demonstrated that 380 subjects (per group) would need to initiate cART to detect this margin with 80% power (assuming a 60% virologic success rate among hospital-based subjects).
Because this was a non-randomized/observational study, we incorporated propensity scores (Shadish, Clark, & Steiner 2008) in an attempt to account for selection bias that potentially affected where individuals sought care. Prior to assessing outcomes, we considered relevant variables and found that the measured covariates captured a reasonably inclusive set of factors which may have influenced where a patient presented. Using logistic regression, propensity scores were created based on age, gender, ethnicity, Montefiore hospitalization in the 3 months prior, insurance, risk factor, time since diagnosis, prior HIV care, antiretroviral exposure, AIDS at presentation, hepatitis C co-infection, illicit drug use, co-morbid medical/psychiatric diagnosis, initial CD4/VL, and nadir CD4. Using these covariates, the balancing property was confirmed. Subjects were then stratified into propensity score-based quintiles and logistic regression was carried out after adjustment for quintile.
This study, including waiver of informed consent, was approved by the Institutional Review Board at Montefiore Medical Center and was supported through the NYS Department of Health’s Empire Clinical Research Investigator Program. It was also made possible by CTSA Grants UL1 RR025750, KL2 RR025749, and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessary represent the official view of the NCRR or NIH.
Results
Subject identification
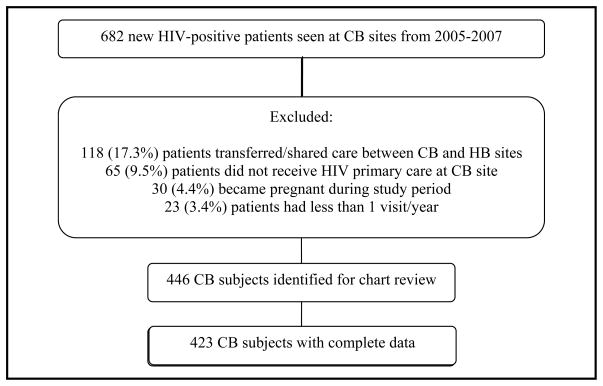
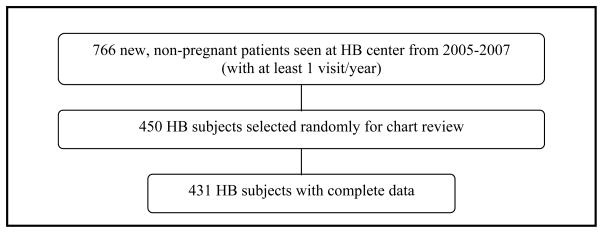
Using the HIV case-finding algorithm, we identified 682 new HIV-infected patients initiating care at community-based (CB) sites from 2005–2007, of which 236 were excluded (Figure 1). Complete data were obtained for 423 of 446 (95%) CB subjects. 766 new patients initiated care at the hospital-based (HB) center during 2005–2007 who met inclusion criteria; these patients were identified from the center’s administrative records (Figure 2). 450 HB subjects were randomly selected for data collection to create similar sizes between the two groups; complete data were obtained for 431 (96%) HB subjects.
Figure 1.
Community-based subject selection
[abbreviations: CB = community-based, HB = hospital-based]
Figure 2.
Hospital-based subject selection
Study site clinicians
10 experts participated in the care of CB subjects during the study period, with a range of HIV experience from less than five to over 20 years. Subject volume ranged from 10 to 60+ subjects (managed directly or indirectly) per expert. Although the experts were also the PCP for 53% of CB subjects, more than 75 non-HIV expert PCPs managed at least one CB subject over the entire study period with expert collaboration. At the specialty center, 15 attending physicians, three nurse practitioners, two physician assistants, and six fellows cared for HB subjects; range of years providing HIV care and subject volume were similar. Overall, 17% of CB and 42% of HB subjects had a non-attending physician (i.e. resident/fellow/mid-level provider) as their PCP.
Baseline subject characteristics
Table 1 compares baseline characteristics by care setting. Among the predominantly minority sample, there were no significant differences in age, gender, time since diagnosis, antiretroviral exposure, hepatitis C co-infection, or illicit substance use. Compared to CB subjects, there was a higher rate of heterosexual HIV acquisition (57.7% vs. 48.7%) and lower rate of infection via intravenous drug use (19.7% vs. 23.6%) among HB subjects (p < 0.01). 22% and 21% of CB and HB subjects were newly diagnosed; initial CD4 was 449 CB vs. 350 HB cells/mm3 (p = 0.05) for these subjects.
Table 1.
Subject characteristics at presentation to community- and hospital-based clinics
| Mean (SD), median [IQR], or frequency (%) | CB (n = 423) | HB (n = 431) | p-value* |
|---|---|---|---|
| Age in years | 43.7 (9.97) | 44.4 (9.95) | 0.25 |
| Gender | |||
| Male | 242 (57.2%) | 244 (56.6%) | 0.74 |
| MTF transgender | 5 (1.2%) | 3 (0.7%) | |
| Female | 176 (41.6%) | 184 (42.7%) | |
| Ethnicity† | |||
| African American | 194 (45.9%) | 195 (45.2%) | < 0.01 |
| Latino | 166 (39.2%) | 207 (48.0%) | |
| Other | 63 (14.9%) | 29 (6.7%) | |
| Insurance | |||
| Public (e.g. Medicaid) | 329 (77.8%) | 386 (89.6%) | < 0.01 |
| Commercial/private | 51 (12.1%) | 40 (9.3%) | |
| Other/self-pay | 43 (10.2%) | 5 (1.2%) | |
| Hospitalization within 3 months of first visit | |||
| None | 301 (71.2%) | 281 (65.2%) | < 0.01 |
| Montefiore Medical Center | 86 (20.3%) | 145 (33.6%) | |
| Other hospital | 36 (8.5%) | 5 (1.2%) | |
| HIV risk factor | |||
| MSM | 57 (13.5%) | 64 (14.9%) | < 0.01 |
| HSP | 206 (48.7%) | 248 (57.5%) | |
| IDU | 100 (23.6%) | 85 (19.7%) | |
| Other (incl. dual risk) or un- identified | 60 (14.2%) | 34 (7.9%) | |
| Years since HIV diagnosis | 9 [3–15] | 10 [3–16] | 0.28 |
| History of AIDS-defining condition | 195 (46.1%) | 251 (58.6%) | < 0.01 |
| Initial CD4 [cells/mm3] | 437 (302.07) | 385 (357.23) | 0.02 |
| Nadir CD4 [cells/mm3] | 311 (252.69) | 236 (236.12) | < 0.01 |
| Hepatitis C co-infection | 144 (34.0%) | 129 (29.9%) | 0.20 |
| Antiretroviral history | |||
| Current | 165 (39.6%) | 176 (40.8%) | 0.75 |
| Ever | 113 (27.1%) | 107 (24.8%) | |
| None | 139 (33.3%) | 148 (34.3%) | |
| Current illicit substance use | |||
| Yes | 183 (43.3%) | 177 (41.1%) | 0.52 |
| No/unknown | 240 (56.7%) | 254 (58.9%) | |
| Current mental health co- morbidity | 201 (47.5%) | 198 (45.9%) | 0.64 |
Note:
p-values based on student’s t-test, Wilcoxon rank-sum test, or Pearson χ2
based on hospital administrative data
[abbreviations: SD = standard deviation; IQR = interquartile range; CB = community-based; HB = hospital-based; MTF = male-to-female; MSM = men who have sex with men; HSP = high-risk sexual partner; IDU = intravenous drug use]
Approximately 50% of the entire study population presented with AIDS, although HB subjects generally presented with more advanced illness as indicated by a higher prevalence of AIDS-defining condition (58.6% vs. 46.1%), lower initial CD4 (385 vs. 437 cells/mm3), and lower nadir CD4 (236 vs. 311 cells/mm3) (all p < 0.05). Among all newly-diagnosed subjects, approximately 40% presented with AIDS.
Virologic outcomes
178 (42%) CB and 237 (55%) HB subjects initiated new cART during the review period. cART was indicated for 11 CB and 9 HB subjects but not started for various reasons (subjects declined treatment, were actively using illicit drugs, had a history of non-adherence, or were lost to follow-up). Overall, pretreatment CD4 and VL were 247 vs. 202 cells/mm3 and 4.9 vs. 5.1 log10 copies/mL for CB and HB subjects, respectively (both p < 0.05). For subjects whose initial CD4 was ≤ 350 cells/mm3, pretreatment CD4 was higher for the CB group (163 vs. 134 cells/mm3, p < 0.05). For individuals with initial CD4 > 350 cells/mm3, pretreatment CD4 was similar between CB and HB subjects (406 vs. 398 cells/mm3, p = 0.84). One subject, followed at the hospital, died within 48 weeks of starting cART; all others had treatment follow-up through at least 48 weeks (a total of ~772 person-years of follow up was accrued). There were no significant differences in regimen selection by setting (57% of combinations were PI- and 38% were NNRTI-based).
Overall, 251 (60%) subjects achieved an undetectable VL within 16–32 weeks. Unadjusted viral suppression rates between CB and HB subjects were similar, 66% vs. 62% ([95% CI difference −0.14–0.06]). Bivariate testing demonstrated that age ≥ 50 years, no current illicit substance use, ≥ 95% cART adherence, duration of HIV diagnosis < 5 years, higher nadir CD4, lower pre-treatment VL, no previous antiretroviral exposure, NNRTI initiation, and higher visit frequency all significantly increased the likelihood of virologic success. Subjects starting NNRTI-based regimens were more likely to maintain ≥ 95% adherence compared to those starting non-NNRTI-based regimens (OR = 1.75 [95% CI 1.23–2.50]).
Multivariate analyses
Multivariate-adjusted estimates of the likelihood of virologic success at community versus hospital-based sites confirm that virologic outcomes were comparable (adjusted OR = 1.24, [95% CI 0.69–2.33]). Table 2 shows odds ratios after accounting for multiple covariates including site of care and propensity scores. cART adherence and pre-treatment VL were the only consistently significant predictors for virologic outcome: individuals with ≥ 95% adherence and pre-treatment VL < 100k copies/mL were approximately 22 and 2 times likelier to achieve virologic success. Furthermore, there was no significant difference between CB subjects whose PCP was an HIV expert and CB subjects whose PCP was not an expert (adjusted OR = 1.22 [95% CI 0.41–3.63]), suggesting that consultation/collaboration was occurring appropriately at community sites.
Table 2.
Adjusted odds of virologic success for patient, disease, and treatment-related predictors
| Adjusted OR for virologic success‡ [95% CI] | p-value | |
|---|---|---|
| CB care [n = 178] (vs. HB [n=237]) | 1.24 [0.69–2.33] | |
| Patient characteristics | ||
| Age ≥ 50 years | 1.22 [0.61–2.44] | 0.57 |
| Male gender | 1.18 [0.64–2.19] | 0.60 |
| Ethnicity | ||
| African-American | 1.54 [0.44–5.41] | 0.50 |
| Latino | 1.19 [0.33–4.33] | 0.79 |
| HIV risk factor | ||
| IDU | 1.73 [0.76–3.91] | 0.19 |
| Current illicit substance use | 0.96 [0.50–1.84] | 0.89 |
| ≥ 95% cART adherence | 21.5 [11.4–40.6] | < 0.01 |
| Disease characteristics | ||
| HIV diagnosis ≥ 5 years | 0.78 [0.34–1.80] | 0.57 |
| Pre-treatment CD4 > 200 cells/mm3 | 1.13 [0.60–2.14] | 0.71 |
| Pre-treatment VL > 100k copies/mL | 0.47 [0.24–0.93] | 0.03 |
| Treatment characteristics | ||
| Current/ever antiretroviral use | 0.48 [0.20–1.17] | 0.11 |
| NNRTI-based regimen | 1.59 [0.81–3.11] | 0.18 |
| Visit frequency (vs. 1–3 visits/year) | ||
| 4–5 visits/year | 1.56 [0.65–3.75] | 0.32 |
| ≥ 6 visits/year | 2.89 [1.22–6.84] | 0.02 |
Note:
including adjustment for propensity score
[abbreviations: CB = community-based; HB = hospital-based; NNRTI = non-nucleoside reverse transcriptase inhibitor]
Secondary outcomes: immunologic success
Overall, 266 (54%) subjects starting cART achieved a CD4 increase of at least 100 cells/mm3 within 48 weeks. Unadjusted success rates were significantly different between CB and HB subjects: 49% vs. 59% ([95% CI difference 0.00–0.19]). Bivariate analyses demonstrated other significant predictors for immunologic success: non-IDU risk factor, no illicit substance use, ≥95% cART adherence, duration of HIV diagnosis < 5 years, higher pre-treatment VL, no previous antiretroviral exposure, and increased visit frequency. However, most of these predictors (including site of care) were not statistically significant after multivariate adjustment (Table 3). The adjusted OR for community vs. hospital-based subjects achieving immunologic success was 0.76 [95% CI 0.47–1.21]. Only treatment adherence retained a significant association: subjects who maintained ≥95% adherence were five times likelier to achieve immunologic success. We found no difference in immunologic response by type of cART, and the increase in CD4 among subjects starting cART was 119 vs. 136 cells/mm3 at CB vs. HB sites (p = 0.40).
Table 3.
Adjusted odds of immunologic success for patient, disease, and treatment-related predictors
| Adjusted OR for immunologic success‡ [95% CI] | p-value | |
|---|---|---|
| CB care [n = 178] (vs. HB [n=237]) | 0.76 [0.47–1.21] | |
| Patient characteristics | ||
| Age ≥ 50 years | 1.02 [0.60–1.73] | 0.96 |
| Male gender | 0.97 [0.60–1.57] | 0.90 |
| Ethnicity | ||
| African-American | 1.43 [0.54–3.81] | 0.47 |
| Latino | 0.99 [0.36–2.70] | 0.98 |
| HIV risk factor | ||
| IDU | 0.66 [0.34–1.27] | 0.21 |
| Current illicit substance use | 0.79 [0.48–1.31] | 0.36 |
| ≥ 95% cART adherence | 5.30 [3.20–8.77] | < 0.01 |
| Disease characteristics | ||
| HIV diagnosis ≥ 5 years | 0.74 [0.39–1.42] | 0.37 |
| Pre-treatment CD4 > 200 cells/mm3 | 0.87 [0.52–1.44] | 0.58 |
| Pre-treatment VL > 100k copies/mL | 1.67 [0.96–2.91] | 0.07 |
| Treatment characteristics | ||
| Current/ever antiretroviral use | 0.63 [0.31–1.25] | 0.19 |
| NNRTI-based regimen | 0.96 [0.56–1.63] | 0.87 |
| Visit frequency (vs. 1–3 visits/year) | ||
| 4–5 visits/year | 1.68 [0.84–3.34] | 0.14 |
| ≥ 6 visits/year | 2.42 [1.22–4.81] | 0.01 |
Note:
including adjustment for propensity score
[abbreviations: CB = community-based, HB = hospital-based]
Discussion
Our results demonstrate that antiretroviral outcomes in community-located, primary care settings are comparable to those at a hospital specialty center. Collaborative HIV care in these settings may be valuable for 1) regions lacking HIV providers, 2) patients unable to frequently access specialty centers or reluctant to attend “HIV-identified” clinics, and 3) patients with multiple co-morbidities who would particularly benefit from comprehensive primary care.
There were some notable clinical differences between our “cohorts”. Hospital subjects presented with more advanced HIV, and almost 1/3 had been referred from a recent hospitalization. Approximately 20% of CB subjects were identified through testing at a CB clinic or outreach program; this possibly contributed to their higher baseline CD4. Together, such findings may partially explain differences in “timeliness” of cART initiation and treatment outcomes: even though most (75%) subjects with initial CD4 ≤ 350 cells/mm3 started cART within 1 month at all sites, HB subjects did so with lower CD4 counts/higher viral loads (both of which impact antiretroviral response (Demeter et al., 2001; Robbins et al., 2007; Wolbers et al., 2007)).
Despite many subjects starting cART at suboptimal CD4 levels, we observed relatively high rates of virologic success in both settings: ~60% of all subjects achieved virologic success, a higher rate than what has been published (40–50%) from similar urban areas (Blanchard, Klibanov, Axelrod, Palermo, & Samuel 2008; Deeks et al., 1999; Lucas, Chaisson, & Moore, 1999). This may be due to the fact that study sites were academically-affiliated clinics with resources for adherence support and frequent patient monitoring. All sites were also federally-funded and participated in large research protocols; mandated reporting could have led toward a general trend in improved performance. Also, ~95% of subjects initiating cART had consistent antiretroviral access through insurance programs which potentially prevented lapses in adherence.
This study had a number of limitations. (1) Subjects were not randomized to treatment setting. Because there were clinically-relevant baseline differences between the two groups, we believe propensity analyses were appropriate for this work. However, there may have been unmeasured covariates that resulted in hidden bias affecting our estimates: propensity scores cannot correct this. (2) We had fewer patients initiating cART than needed (based on a priori calculations) to demonstrate statistical equivalence between community- and hospital-based outcomes. Although a larger study is necessary, our findings do not suggest any compelling differences [in antiretroviral outcomes] between the two settings. (3) Although our treatment settings were distinct, delivery of care may have been less so—particularly regarding mid-level providers (who saw 2% and 38% of CB and HB subjects, respectively) and patterns of collaboration. However, all mid-level providers had considerable HIV experience and generally managed patients independently—with necessary oversight from attending physicians. Secondly, it was difficult to create a fixed, dichotomous variable for the type of collaboration, as management was not always exclusively “shared” or “mentored” (ie. subjects who switched regimens multiple times may have had expert appointments, and thus “shared” collaboration, for one switch but not for others). This was a largely unavoidable limitation—as often occurs with observational studies of real-world practice. We do not believe this limitation weakens our overall conclusion as we sought to compare care settings, not collaboration types. Even though HIV experts were all generalists in this study, other models may include collaboration between infectious-disease/other specialists and community providers. Finally, (4) outcome measures were short-term; it will be important to consider other clinically-meaningful/long-term outcomes to determine the broader effectiveness of HIV treatment in community settings.
In conclusion, HIV-infected patients starting antiretroviral therapy in community-based primary care settings achieve favorable treatment outcomes through collaborative care. Collaboration offers a novel approach to HIV as a chronic disease where HIV experts are formally linked to primary care providers as well as to patients. Community-based services may reduce HIV health disparities by facilitating access to treatment and continuity of care (Korthuis et al., 2008). With the aging HIV-positive population and a growing prevalence of co-morbidities (e.g. diabetes) in these patients (Brown et al., 2005; Glass et al., 2006), involvement of primary care-trained generalists may improve health outcomes in the current treatment era. Finally, with recommendations for expanded HIV screening and community-based outreach (Branson et al., 2006), our findings underscore the need for HIV services to be distributed so that individuals can be identified early and linked quickly to appropriate care. This study suggests that community-located, primary care-based HIV programs may be an effective strategy to provide such care.
Acknowledgments
The authors gratefully acknowledge the support and helpful contributions of Jason Fletcher, Mimi Kim, Matt Anderson, Albert Einstein College of Medicine’s Clinical Research Training Program, and the MMG/Bronx Community Health Network’s CICERO program.
Financial support: This work was funded through the New York State Department of Health’s Empire Clinical Research Investigator Program. In addition, it was made possible by CTSA Grants UL1 RR025750, KL2 RR025749, and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessary represent the official view of the NCRR or NIH.
Footnotes
Previous presentations: Parts of data were presented at the IAPAC 4th International Conference on HIV Treatment Adherence in Miami, Florida (April 5–7, 2009), as well as the 137th Annual Meeting and Exposition of the American Public Health Association in Philadelphia, Pennsylvania (November 7–11, 2009).
References
- Blanchard E, Klibanov OM, Axelrod P, Palermo B, Samuel R. Virologic success in an urban HIV clinic: outcome at 12 months in patients who were HAART naïve. HIV Clin Trials. 2008;9:186–191. doi: 10.1310/hct0903-186. [DOI] [PubMed] [Google Scholar]
- Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss CB, et al. CDC. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR. 2006;55(RR–14):1–17. [PubMed] [Google Scholar]
- Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV Prevalence Estimates—United States, 2006. MMWR. 2008;57:1073–76. [PubMed] [Google Scholar]
- Cohn SE, Berk ML, Berry SH, Duan N, Frankel MR, Klein JD, et al. The care of HIV-infected adults in rural areas of the United States. J Acquir Immune Defic Syndr. 2001;28:385–92. doi: 10.1097/00126334-200112010-00013. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Hecht FM, Swanson M, Elbeik T, Loftus R, Cohen PT, et al. HIV RNA and CD4 cell count response to protease inhibitor therapy in an urban AIDS clinic: response to both initial and salvage therapy. AIDS. 1999;13:F35–43. doi: 10.1097/00002030-199904160-00001. [DOI] [PubMed] [Google Scholar]
- Demeter LM, Hughes MD, Coombs RW, Jackson JB, Grimes JM, Bosch RJ, et al. Predictors of virologic and clinical outcomes in HIV-1-infected patients receiving concurrent treatment with indinavir, zidovudine, and lamivudine. Ann Int Med. 2001;135:954–64. doi: 10.7326/0003-4819-135-11-200112040-00007. [DOI] [PubMed] [Google Scholar]
- Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, Moore RD, et al. HIV Research Network. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- Glass TR, Ungsedhapand C, Wolbers M, Weber R, Vernazza PL, Rickenbach M, et al. Prevalence of risk factors for cardiovascular disease in HIV-infected patients over time: the Swiss HIV Cohort Study. HIV Med. 2006;7:404–10. doi: 10.1111/j.1468-1293.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- http://www.aahivm.org
- http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- http://www.healthypeople.gov/Document/HTML/Volume1/13HIV.htm
- Korthuis PT, Saha S, Fleishman JA, McGrath MM, Josephs JS, Moore RD, et al. HIV Research Network. Impact of patient race on patient experiences of access and communication in HIV care. J Gen Intern Med. 2008;23:2046–52. doi: 10.1007/s11606-008-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon BE, Wilson IB, Cohn SE, Fichtenbaum CJ, Wong MD, Wenger NS, et al. Physician specialization and antiretroviral therapy for HIV: adoption and use in a national probability sample of persons infected with HIV. J Gen Intern Med. 2003;18:233–241. doi: 10.1046/j.1525-1497.2003.20705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon BE, Wilson IB, McInnes K, Landrum MB, Hirschhorn LR, Marsden PV, et al. Physician specialization and the quality of care for human immunodeficiency virus infection. Arch Intern Med. 2005;165:1133–9. doi: 10.1001/archinte.165.10.1133. [DOI] [PubMed] [Google Scholar]
- Lert F, Kazatchkine MD. Antiretroviral HIV treatment and care for injecting drug users: an evidence-based overview. Int J Drug Policy. 2007;18:255–61. doi: 10.1016/j.drugpo.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Int Med. 1999;131:81–7. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- Napravnik S, Eron JJ, Jr, McKaig RG, Heine AD, Menezes P, Quinlivan EB. Factors associated with fewer visits for HIV primary care at a tertiary care center in the Southeastern U.S. AIDS Care. 2006;18(Suppl 1):S45–50. doi: 10.1080/09540120600838928. [DOI] [PubMed] [Google Scholar]
- Rastegar DA, Fingerhood MI, Jasinski DR. Highly active antiretroviral therapy outcomes in a primary care clinic. AIDS Care. 2003;15:231–7. doi: 10.1080/0954012031000068371. [DOI] [PubMed] [Google Scholar]
- Robbins GK, Daniels B, Zheng H, Chueh H, Meigs JB, Freedberg KA. Predictors of antiretroviral treatment failure in an urban HIV clinic. J Acquir Immune Defic Syndr. 2007;44:30–7. doi: 10.1097/01.qai.0000248351.10383.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DL, Golin CE, Schoenbach VJ, Stephenson BL, Wohl DA, Gurkin B, et al. Availability or and access to medical services among HIV-infected inmates incarcerated in North Carolina county jails. J Health Care Poor Underserved. 2004;15:413–25. doi: 10.1353/hpu.2004.0047. [DOI] [PubMed] [Google Scholar]
- Shadish WR, Clark MH, Steiner PM. Can nonrandomized experiments yield accurate answers? A randomized experiment comparing random and nonrandom assignments. J Am Stat Assoc. 2008;103:1334–1343. [Google Scholar]
- Smith SM, Allwright S, O'Dowd T. Effectiveness of shared care across the interface between primary and specialty care in chronic disease management. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD004910.pub2. Art. No.: CD004910. [DOI] [PubMed] [Google Scholar]
- Smith SA, Shah ND, Bryant SC, Christianson TJ, Bjornsen SS, Giesler PD, et al. Chronic care model and shared care in diabetes: randomized trial of an electronic decision support system. Mayo Clin Proc. 2008;83:747–57. doi: 10.4065/83.7.747. [DOI] [PubMed] [Google Scholar]
- Wolbers M, Opravil M, von Wyl V, Hirschel B, Furrer H, Cavassini M, et al. Predictors of optimal viral suppression in patients switched to abacavir, lamivudine, and zidovudine: the Swiss HIV Cohort Study. AIDS. 2007;21:2201–8. doi: 10.1097/QAD.0b013e3282efacb1. [DOI] [PubMed] [Google Scholar]