Abstract
In ischemic retinopathies, underlying hypoxia drives abnormal neovascularization that damages retina and causes blindness. The abnormal neovasculature is tortuous and leaky and fails to alleviate hypoxia, resulting in more pathological neovascularization and retinal damage. With an established model of ischemic retinopathy we found that calpain inhibitors, when administered in moderation, reduced architectural abnormalities, reduced vascular leakage, and most importantly reduced retinal hypoxia. Mechanistically, these calpain inhibitors improved stability and organization of the actin cytoskeleton in retinal endothelial cells undergoing capillary morphogenesis in vitro, and they similarly improved organization of actin cables within new blood vessels in vivo. Hypoxia induced calpain activity in retinal endothelial cells and severely disrupted of the actin cytoskeleton, whereas calpain inhibitors preserved actin cables under hypoxic conditions. Collectively, these findings support the hypothesis that hyper-activation of calpains by hypoxia contributes to disruption of the retinal endothelial cell cytoskeleton, resulting in formation of neovessels that are defective both architecturally and functionally. Modest suppression of calpain activity with calpain inhibitors restores cytoskeletal architecture and promotes formation of a functional neovasculature, thereby reducing underlying hypoxia. In sharp contrast to “anti-angiogenesis” strategies that cannot restore normoxia and may aggravate hypoxia, the therapeutic strategy described here does not inhibit neovascularization. Instead, by improving the function of neovascularization to reduce underlying hypoxia, moderate calpain inhibition offers a method for alleviating retinal ischemia, thereby suggesting a new treatment paradigm based on improvement rather than inhibition of new blood vessel growth.
Keywords: calpain, retinopathy, neovascularization, hypoxia, endothelial cell, cytoskeleton
1. Introduction
Retinopathy is a leading cause of blindness. Retinopathy is initiated by vessel loss resulting in retinal hypoxia that can provoke two responses: revascularization with “normal” vessels that relieve hypoxia, or revascularization with abnormal vessels that leak. The abnormal new blood vessels in retinopathy apparently often persist, - leading to tractional retinal detachment, and blindness [1–4]. This persistence suggests that hypoxia is not relieved, although this has not been shown previously. Recent research in ischemic retinopathies has focused primarily on suppression of new blood vessels, i.e. inhibition of angiogenesis. However, such strategies are at cross-purposes with alleviation of the underlying hypoxia that drives recurring cycles of abnormal neovascularization. Moreover, “anti-angiogenesis” strategies do not distinguish between normal and abnormal neovessels and therefore can aggravate hypoxia. Thus, a more physiological and desirable therapeutic strategy is to improve the function of retinal neovessels that arise during retinopathies and thereby alleviate the retinal hypoxia that drives pathological neovascularization and destroys retina.
To this end, we have sought to identify strategies for reducing retinal hypoxia by improving the architecture and function of new blood vessels. In particular, we have focused on strategies involving pharmacological normalization of calpain activity. Calpains are intracellular, calcium-dependent thiol proteases [5, 6]; and, upon activation, these widely expressed enzymes cleave a broad spectrum of functionally important intracellular protein targets [5] that regulate cytoskeletal organization [7], cell adhesion [8–10], and cell migration [10–12]. Calpain activity is induced in hypoxic retina, and calpain hyper-activation has been implicated in retinal pathology [13, 14]. However, the consequences of calpain inhibition for hypoxia-induced neovascularization have not been investigated previously. As described here, our studies with an established mouse model of ischemic retinopathy [15] illustrate first that neovessels do not relieve hypoxia and that moderation of calpain activity offers a novel strategy for normalizing pathological retinal neovascularization and restoring normal oxygenation. Moreover, these studies identify previously unrecognized mechanistic connections between induction of calpain activity by hypoxia, disruption of the retinal endothelial cell cytoskeleton, defective capillary morphogenesis, and unrelieved hypoxia.
2. Materials and methods
2.1 Oxygen-induced retinopathy
All protocols involving mice were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Retinopathy was induced by exposing seven-day-old (P7) C57BL/6 pups with their nursing mother (Jackson Laboratory) to 75% oxygen for 5 days as previously described [15]. At day 12 (P12), the pups and the mother were returned to normal room air (21% oxygen), resulting in hypoxic retina and re-growth of new blood vessels. The calpain inhibitors (Calbiochem), MDL 28170 (0.25 mg/kg), PD 150606 (1.0 mg/kg), ALLN (10 mg/kg) or control vehicle were administered daily by intraperitoneal injection from days P12 to P16 or from days P12 to P20, as indicated, with animals harvested at day P17 or P21 for evaluation of retinal blood vessel architecture and function (below).
2.2 Analyses of retinal vascular coverage and pericyte association, vascular leakiness, vascular perfusion, and hypoxia
Animals were sacrificed, eyes enucleated, whole mount retinas prepared for analyses as described [16] with the following additions/modifications. Following the fixation for 1 hour in 10 % formalin at room temperature, retinas were dissected, washed in PBS (three times), blocked and permeabilized overnight in PBS buffer containing 0.5 % Triton X-100, 10 % goat serum, and 0.02 % sodium azide. For analyses of vascular coverage, retinas were stained with TRITC-Lectin (from Bandeiraea simplicifolia, Sigma). To assess for pericyte association with the vessels, retinas were stained with NG2 antibody (Chemicon International) followed by FITC-conjugated secondary antibody (Santa Cruz Biotechnology). Analyses of vascular perfusion and leak were performed employing lysine-fixable 70-kDa FITC dextran (10 mg/kg, Invitrogen) injected via the tail vein. Animals were harvested after 10 min perfusion. To assess retinal hypoxia, Hypoxyprobe™-1 (pimonidazole 120 mg/kg, Natural Pharmacia International, Inc.) was used instead of FITC-dextran, and it was administered one hour before harvest. Retinas were co-stained with FITC-Hypoxyprobe antibody and TRITC-Lectin (Bandeiraea simplicifolia) for assessment of hypoxia and vasculature, respectively. For actin staining of whole mount retina, fluorescent Oregon Green-conjugated phalloidin (Invitrogen) was employed at a concentration of 0.5 units/ml. Stained retinas were visualized and photographed with a camera Leica DX-300 microscope using 4×, 10× and 20× objectives.
Vascular parameters were all quantified from digital images of whole retinas. Measurement of retinal neovascularization (vascular coverage and perfused neovasculature) and neovascular tuft formation was performed as described previously [17]. Focal leakage points were identified as “clouds” of 70 kDa FITC-dextran outside of vasculature and counted manually. Hypoxia was quantified by measuring hypoxic area and integrating measured hypoxic area with FITC-Hypoxyprobe signal intensity.
2.3 Immunohistochemical staining of vascular tufts in cross-section
Neovascular tufts were identified and quantified as described previously [15]. Eyes were enucleated, embedded in OCT medium, and snap frozen in liquid nitrogen. Five-micron thick sections were cut and endothelial cells stained with CD31 (PECAM-1) antibody (Pharmingen) followed by secondary antibody conjugated with horseradish peroxidase. Antibody staining was visualized with DAB substrate, and sections were counterstained with hematoxylin solution.
2.4 Analyses of human retinal endothelial cells in 3D collagen-I
Human retinal microvascular endothelial cells (RMVEC, Cell-Systems) were propagated in corresponding CS-C Complete Medium Kit (Cell-systems) and used between passage three to seven. Capillary morphogenesis assays were performed by “sandwiching” and “overlaying” confluent cell monolayers with rat tail collagen-I (BD Biosciences), basically as described previously [18]. The sandwich-type assay was performed in 12-well plates with 1.0 mg/ml collagen-I in full medium. Calpain inhibitors at various concentrations or vehicle were added for overnight incubation, prior to adding the upper layer of collagen-I. Capillary morphogenesis was allowed to proceed for 16h; the assay plates were fixed with 10 % formalin for one hour and stained for F-actin with fluorescent Oregon Green-conjugated phalloidin (Invitrogen, final concentration 0.5 units/ml) and subsequently photographed.
For the “overlay” assay, calpain inhibitors were added and incubated with cells in 24-well plates overnight. The next day, each well was overlaid with 300 microliters of collagen-I at concentration of 0.5 mg/ml in CS-C Medium (minus serum and growth factors, Cell-Systems), together with calpain inhibitors as indicated. Capillary morphogenesis was allowed to proceed for 4h; cells were fixed in 10% formalin for 10 minutes, permeabilized for one minute with 0.02% Triton-X100 in PBS and stained for F-actin (as above) or stained with antibody to α–tubulin (Clone DM 1A, Sigma) followed by fluorescent Texas-Red secondary antibody (Jackson Immunoresearch).
Cord length, blind ends, and polygons were quantified using NIH ImageJ software. Cord length was traced and measured through freehand line selections. Blind ends and polygons were determined with point selections. Measured parameters correspond to actual areas of 0.8 mm2.
2.5 Calpain fluorometric assay
Calpain activity was measured in live retinal MVECs with an established fluorescent calpain substrate 7-amino-4-chloromethylcoumarin, t-BOC-L-leucyl-Lmethionine amide (CMAC, t-BOC-Leu-Met; from Invitrogen) [19]; and calpain inhibitors, as indicated, were used to establish specificity. Cells were incubated with 20 µM for 15 min at 37 degrees C; cleavage product was measured with a SpectraMax M5 fluorescent plate reader (excitation/emission 351/430) with SoftMax Pro5 software (Molecular Devices, Sunnyvale CA).
2.6 Statistical analyses
Findings are presented as mean ± standard error. Statistical analyses were performed with InStat 3 software for Macintosh. For comparisons between two groups, we employed the two-tail Mann-Whitney test, assuming unequal variances between the two groups under comparison. For comparisons among multiple groups, data were analyzed with ANOVA, followed by the Bonferroni post-test.
3. Results
3.1 Hypoxia-induced neovascularization fails to alleviate retinal hypoxia
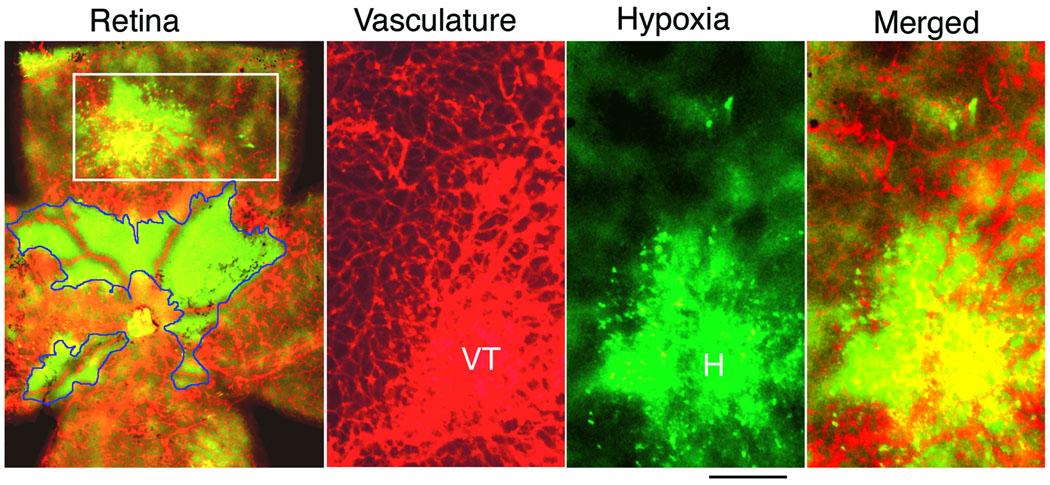
With an established mouse model of retinopathy of prematurity that exhibits pathological neovessels similar to those seen in ischemic retinopathies [15], we first established that neovessels are inefficient at relieving hypoxia in retina. Although long suspected, hypoxia in re-vascularized retina has not been demonstrated previously. In this retinopathy model, regression of immature blood vessels is induced in 7day post-partum (P7) mice with hyperoxia (75% O2) for 5 days. At 12 days post-partum (P12), animals are returned to room air, resulting in retinal hypoxia and induction of HIF-regulated proteins such as vascular endothelial growth factor (VEGF) [20–22] that induce abnormal re-vascularization [23]. After five days of re-vascularization (P17), retinal vasculature was stained with TRITC-lectin (Bandeiraea simplicifolia) and retinas were simultaneously evaluated for hypoxia with an established immuno-histochemical method (Hypoxyprobe™, pimonidazole HCl). Avascular regions of retina were strongly hypoxic as expected (Fig. 1); but hypoxia was equally severe in large neovascularized regions exhibiting abnormal vascular architecture that included vascular tufts, suggesting poor blood flow in these areas. Thus, well-vascularized regions of retina remained hypoxic at P17, establishing that vascular re-growth, in response to hypoxia, failed to restore normal oxygenation (Fig. 1).
Figure 1. Hypoxia-induced neovascularization fails to alleviate retinal hypoxia.
Regression of immature blood vessels was induced in 7 day-old (P7) mice with hyperoxia (75% O2) for 5 days. At P12, animals are returned to room air, resulting in retinal hypoxia and induction of abnormal re-vascularization. After five days of re-vascularization (P17), retinas were evaluated for hypoxia (green color) with Hypoxyprobe™, and retinal vasculature was stained with Bandeiraea simplicifolia TRITC-lectin (red color). Left panel (retina): whole mount retina with green color representing non-vascularized regions with strong hypoxia (encircled with blue lines); yellow color = (red + green) represents vascularized regions that remained hypoxic; red color represents vascularized regions that were not detectably hypoxic. To the right of the whole retina panel are three panels at higher magnification representing the retinal area within the box (note that these images are rotated 90 degrees from the original). Vasculature staining is shown separately in red color (VT = vascular tufts); hypoxia is shown in separately in green color (H = strong hypoxia); and a merged image illustrating overlap between a highly vascularized region with prominent vascular tufts and strong hypoxia (yellow color = overlap between vasculature and hypoxia). Scale bar, 200 µm.
3.2 Compounds that target either the active site or critical calcium-binding domain of calpains improve re-vascularization of retina and reduce retinal hypoxia
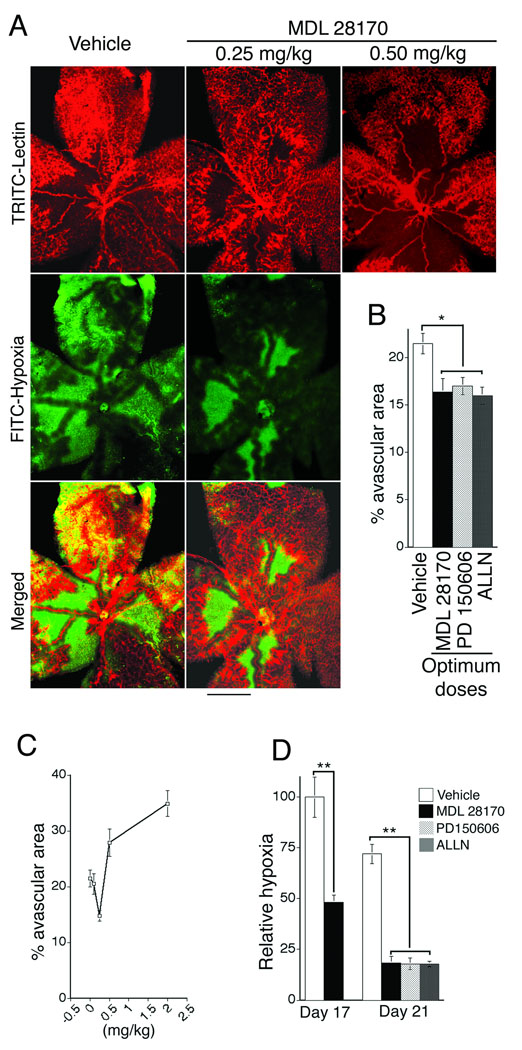
Next, we investigated the consequences of calpain inhibition for hypoxia-induced retinal neovascularization. During the hypoxia-driven re-vascularization phase (P12–P17), animals were treated systemically once daily with calpain inhibitors at doses chosen to normalize rather than strongly inhibit calpain activity (see below). After five days (P17), retinas from treatment and control groups were analyzed and compared. As shown following whole mount staining of retinal vasculature with TRITC-lectin (Bandeiraea simplicifolia), optimum doses of calpain inhibitors MDL 28170 (calpain inhibitor III), PD150606, and ALLN (calpain inhibitor I) each significantly improved vascular re-growth (illustrated in Fig. 2A, top panels – red color; see quantification in Fig. 2B and optimum dose determination in Fig. 2C). Most importantly, each of these calpain inhibitors, at optimum doses, substantially reduced retinal hypoxia (illustrated in Fig. 2A, middle panels – green color; see quantification in Fig. 2D). Retinal hypoxia was reduced by 50% relative to controls at P17 and reduced > 75% at P21 (quantified in Fig.2D; illustrated in supplementary Fig. 1). In control and calpain inhibitor groups, the remaining avascular regions of retina were strongly hypoxic as expected (Fig. 2A, middle panels - green color); but hypoxia was equally severe in large neovascularized regions of controls, consistent with poor perfusion of abnormal blood vessels (Fig. 2A, left bottom panel – yellow color). In sharp contrast, with optimum calpain inhibitor treatment, hypoxia was almost entirely limited to the remaining avascular area that was reduced relative to controls, and nearly all of the vascularized area was non-hypoxic (Fig. 2A, right bottom panel – note absence of yellow color). Thus, in the calpain inhibitor groups, there was greatly reduced hypoxia associated with retinal neovascularization indicating that appropriate doses of calpain inhibitors rendered new blood vessels substantially more effective at reducing hypoxia. These observations, combined with measured improvement in vascular coverage (Fig. 2B), indicate that the marked overall reductions in retinal hypoxia achieved with calpain inhibitors were the result of improved neovascular function together with increased vascular coverage.
Figure 2. Moderate doses of calpain inhibitors improve vascular re-growth and reduce retinal hypoxia associated with ischemic retinopathy.
(A) Animals were treated with vehicle or calpain inhibitor MDL 28170, beginning with the onset of retinopathy for 5 days (P12–17). Whole mount retina vasculature at P17 was stained with Bandeiraea simplicifolia TRITC-lectin (red, upper panels); note that MDL 28170 at 0.25 mg/kg improved vascular re-growth whereas 0.50 mg/kg did not improve re-growth and instead was inhibitory (see Panel C for dose curve). Whole mount retinas were also stained for hypoxia with Hypoxyprobe™ (green, middle panels). Bottom panels are merged images of the upper two panels; green color corresponds to hypoxia in the absence of neovasculature and yellow color (red + green) corresponds to abnormal vascularized area that is still severely hypoxic. Scale bar, 500 µm. (B) Quantification of % avascular area, as determined with Bandeiraea simplicifolia TRITC-lectin staining (Panel “A”, upper panels in red), following treatment with vehicle control or calpain inhibitors MDL 28170, PD150606, and ALLN at optimal doses, - see (C) below (*p<0.05, n≥11 animals for each group). (C) Determination of optimal drug doses for improving vascular re-growth as determined by quantification of avascular area. Maximal improvement in retinal neovascularization was achieved with once daily administration of MDL 28170 at 0.25 mg/kg; higher doses were inhibitory as illustrated in Panel A. Optimal once daily doses for PD150606 (1 mg/kg) and ALLN (10 mg/kg) were determined as illustrated for MDL 28170. (D) Quantification of reduction in hypoxic retinal area achieved with optimal once daily doses of calpain inhibitors MDL 28170 (0.25 mg/kg), PD150606 (1 mg/kg), and ALLN (10 mg/kg). Animals were treated for 5 days and harvested on day 17 (P17) or treated for 9 days and harvested at day 21 (P21), as indicated. Relative to time-matched vehicle controls at P17 and P21, calpain inhibitors substantially reduced the hypoxic retinal area observed in whole mount retinas (**p<0.01, n≥9 animals for each group).
Importantly, apart from optimum dose that is dependent on potency and pharmacokinetics, we observed no differences in effectiveness of the calpain inhibitors used in these experiments despite distinctly different chemistries. ALLN, like MDL 28170, is a cell-permeable peptidometic inhibitor that targets the enzymatic site (reviewed [24]), whereas PD150606 [25] is a cell-permeable α-mercaptoacrylate that targets the calcium-binding domain critical for calpain activity. All three compounds inhibit the principal calpain isoforms found in retina: calpain I (µ-calpain) which is activated by micromolar calcium, and calpain II (m-calpain) which is activated by millimolar calcium [14]. Similar to other peptidyl calpain inhibitors, MDL 28170 and ALLN also inhibit cathepsins due to the similarities in the active sites of these proteases [24]. In contrast, PD150606 targets the calpain calcium domain not found in cathepsins and, therefore, is highly specific (>600-fold) for calpains in comparison with cathepsins [25]. Because PD150606, ALLN, and MDL 28170 all yielded the same outcome in these experiments, we conclude that inhibition of calpain activity is the most likely mechanism through which each of these compounds improved neovascularization and reduced retinal hypoxia.
Notably, while appropriately moderate doses of calpain inhibitors improved vascular coverage, in all cases higher doses were inhibitory. Maximal improvement in vascular re-growth (reduction in avascular area) was observed with MDL 28170 administered i.p. daily at 0.25 mg/kg, but inhibition of neovascularization was observed with daily doses of 0.5 mg/kg and higher (Fig. 2A top right panel, Fig. 2C). With PD150606, optimum improvement in vascular re-growth was observed with daily administrations i.p. of 1 mg/kg, and inhibition of neovascularization was observed with daily administrations of 3.0 mg/kg. For ALLN the dose optimum was 10 mg/kg daily but inhibition was observed at 15 mg/kg daily. No adverse effects on animal health were observed at the optimal or inhibitory doses; therefore, it is likely that inhibition of neovascularization with the higher doses of inhibitors was not simply a consequence of adverse effects on overall animal health. Thus, administration of calpain inhibitors in moderation was essential to achieve improvement rather than inhibition of retinal neovascularization.
3.3 Calpain inhibitors suppress formation of abnormal vascular tufts, reduce vascular leak, and improve vascular perfusion by circulating tracer
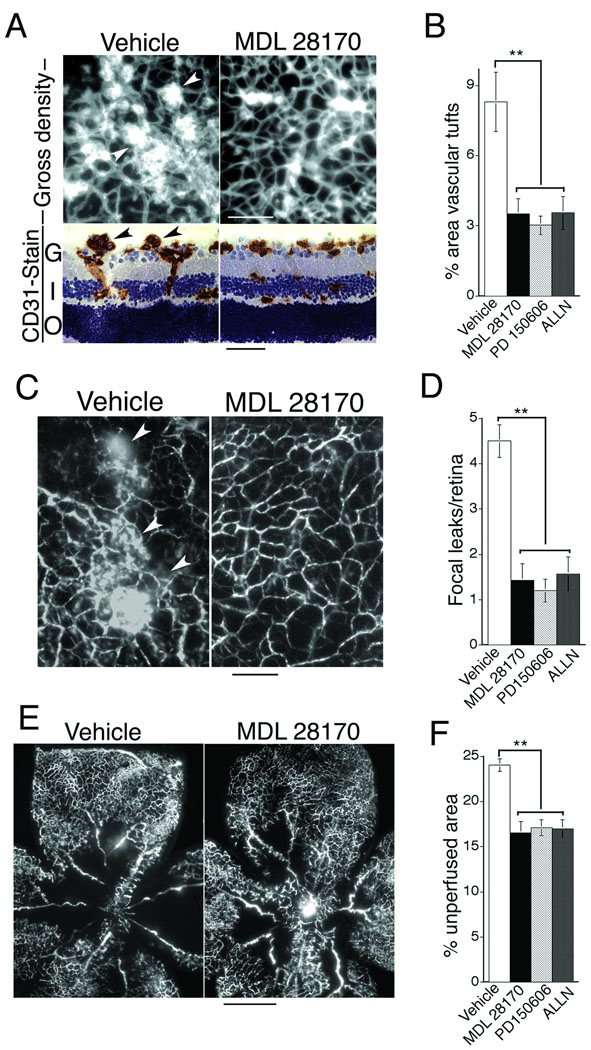
To examine neovascular architecture in greater detail, we quantified abnormal vascular tufts in retinal flat mounts and analyzed vascular tufts in cross section. Optimum doses of calpain inhibitors MDL 28170, PD 150606, and ALLN each markedly reduced the formation of prominent abnormal vascular tufts within 5 days of treatment (P17) (Fig. 3A, B). Also, we examined the effect of calpain inhibitors on dysfunctional vascular leakage. Consistent with improved vascular architecture and reduction in vascular tufts that often leak, calpain inhibitors MDL 28170, PD150606, and ALLN each markedly reduced focal leakage in retina within 5 days (P17) (Fig. 3 C, D). Finally, we tested simple perfusion of the retinal vasculature with tracer injected intravenously. Consistent with improved vascular re-growth and reduced hypoxia, calpain inhibitors improved overall perfusion of retina with tracer at P17 (Fig. 3E, F). Not surprisingly, improvement in tracer perfusion provided by calpain inhibitors was somewhat modest in comparison with reduction of hypoxia (Fig. 2). This is expected because perfusion with tracer does not measure improvement in blood flow. By contrast, reduction in abnormal vascular tufts (Fig. 3A, B) and reduction in vascular leak (Fig. 3C, D) each of which are expected to improve blood flow, were closely similar to reduction in hypoxia (Fig. 2).
Figure 3. Moderate doses of calpain inhibitors reduce abnormal vascular tufts and reduce vascular leakiness associated with retinopathy and also improve vascular perfusion of retina.
Animals were treated with vehicle or optimal doses of calpain inhibitors (see Fig. 2) for 5 days beginning with the onset of retinopathy (P12) and harvested at P17. (A) Imaging of vascular tufts. Upper panels: whole mount retina vasculature stained with Bandeiraea simplicifolia TRITC-lectin. Lower panels: retina cross-sections stained for endothelial cells with CD31 antibody (brown color). Arrows denote vascular tufts. G = ganglion layer, I = inner nuclear layer, O = outer nuclear layer. Scale bars: upper panels, 200 µm; lower panels, 50 µm. (B) Quantification of vascular tufts in whole mount retinas at P17: calpain inhibitors MDL 28170, PD 150606, and ALLN similarly reduced % retina area containing vascular tufts relative to controls (**p<0.01, n≥9 animals for each group). (C) Analyses of vascular leakiness. Left panel: typical vascular leaks (arrows) in whole mount retina of vehicle control of animal perfused with FITC-dextran tracer. Right panel: typical absence of leak in whole mount retina of animal treated with calpain inhibitor MDL 28170. Scale bar, 100 microns. (D) Quantification of vascular leak in whole mount retinas. Relative to controls, calpain inhibitors MDL 28170, PD 150606, and ALLN similarly reduced # focal leakage points/retina (**p<0.01, n≥9 animals for each group). (E) Analysis of retinal vascular perfusion: whole mounted retinal vasculature following live perfusion with FITC-labeled dextran tracer. Scale bar, 500 µm. (F) Quantification of % unperfused area following treatment with vehicle control or calpain inhibitors MDL 28170, PD150606, and ALLN (**p<0.01, n≥9 animals for each group).
3.4 Calpain inhibitors enhance capillary morphogenesis and organization of the actin cytoskeleton; modest (30–35%) inhibition of calpain activity provides optimal improvement
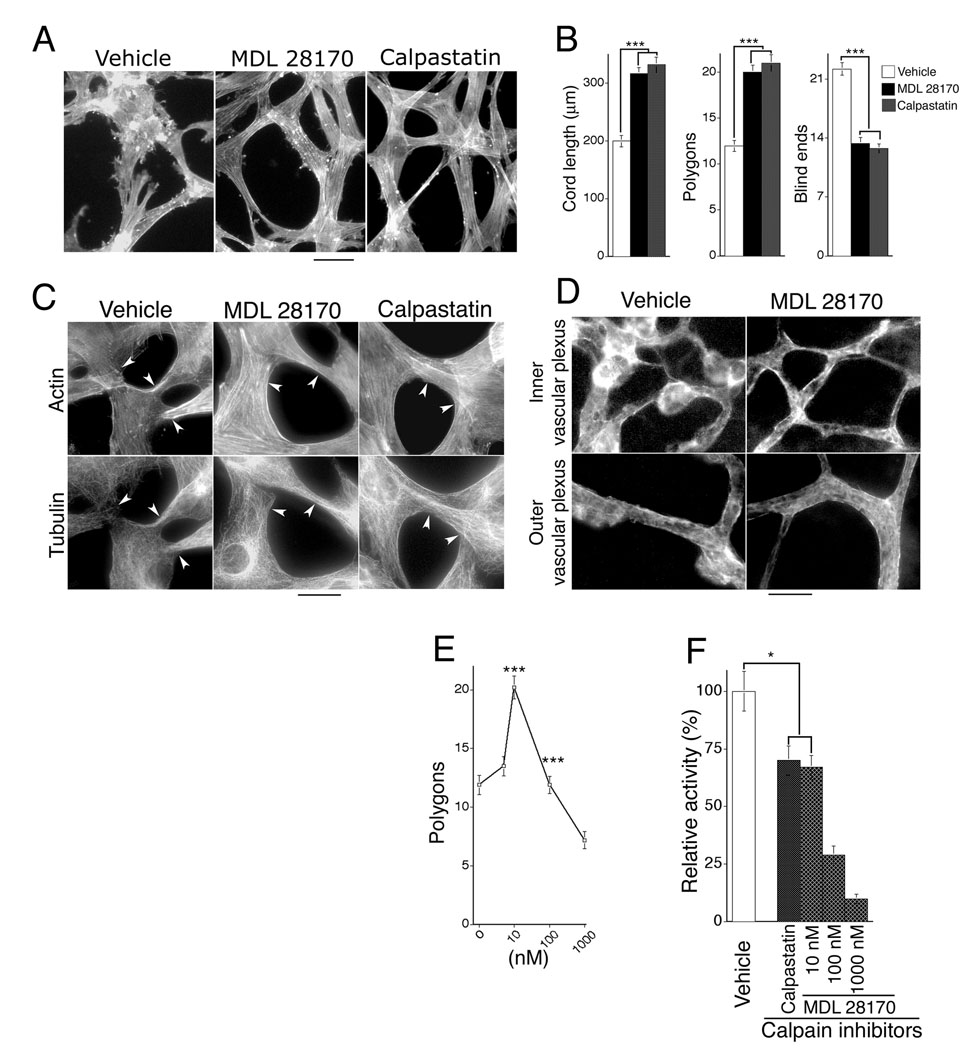
To investigate mechanism(s) by which calpain inhibitors improve neovessel architecture, we examined retinal microvascular endothelial cells (MVECs) undergoing capillary morphogenesis in vitro with models previously shown to correlate with neovascular architecture in vivo [26]. Confluent human retinal MVECs were “overlaid” (Fig. 4A) or “sandwiched” (supplementary Fig. 2) with interstitial collagen type I, which provoked organization of the endothelial cell monolayer into solid pre-capillary cords that are the precursors to capillary-like tubes with lumens (reviewed [27]). Calpain inhibitor MDL 28170 and calpastatin peptide, a highly specific 27-residue cell-permeable calpain inhibitory peptide representing the functional inhibitory domain of the natural endogenous calpain inhibitor [24, 28], each improved organization of retinal MVECs into capillary cords, - as measured by increased cord length, reduced blind ends, and overall network organization (polygons) (Fig. 4A, B; supplementary Fig. 2). These inhibitors also improved alignment of actin cables during cord formation (Figs. 4A, 4C; supplementary Fig. 2) and prevented collapse of microtubules at cell-cell junctions (Fig. 4C), consistent with increased cord length and reduction in blind ends. Similarly, we found that calpain inhibitors also improved organization of the actin cytoskeleton in newly formed blood vessels in vivo (Fig. 4D). Thus, these experiments link calpain inhibition to improved cytoskeletal organization, improved capillary morphogenesis, and improved neovascular architecture.
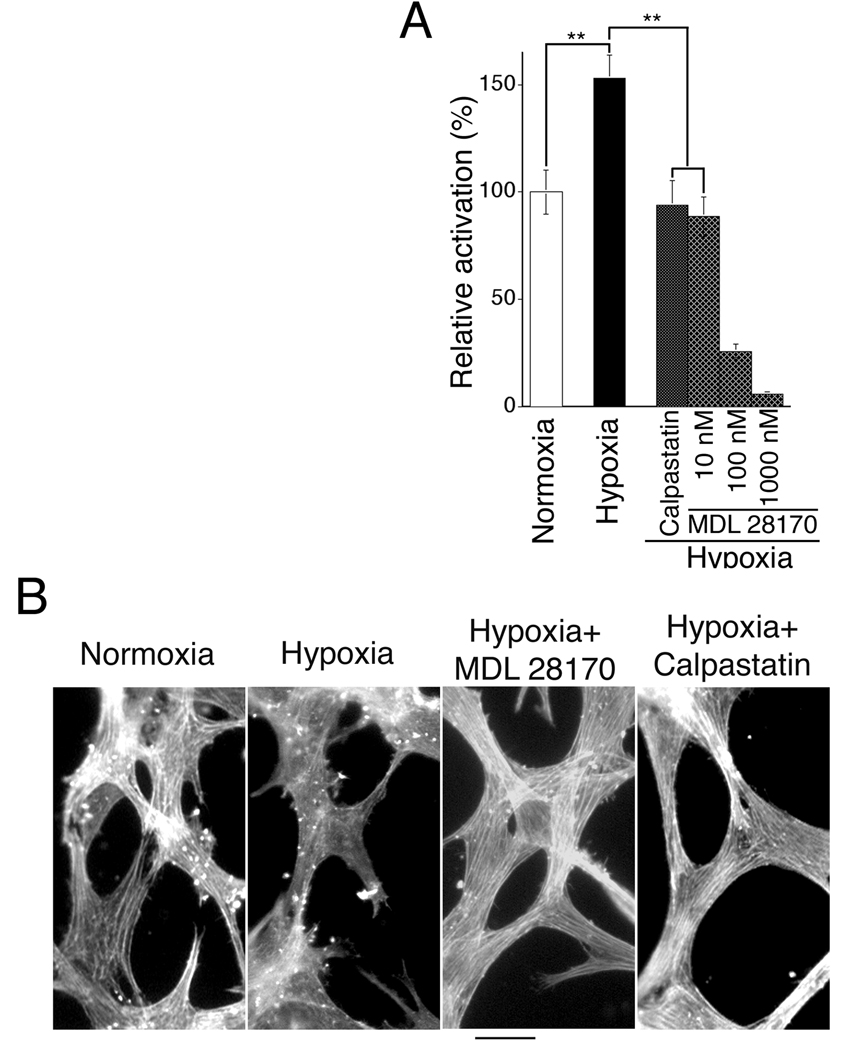
Figure 4. Calpain inhibitors improve organization and alignment of endothelial cell actin cables during capillary morphogenesis in vitro and neovascularization in vivo; optimal improvement is achieved with 30–35% inhibition of calpain activity.
(A) F-actin (stained with FITC-phalloidin) in retinal MVECs undergoing capillary morphogenesis in vitro in response to overlay with collagen I. Scale bar, 25 µm. Calpain inhibitor doses: MDL 28170 (10 nM), calpastatin peptide (200 nM). (B) Calpain inhibitors improve capillary morphogenesis in vitro as measured by increased cord length, increased formation of inter-connected polygon networks, and reduction in blind ends. ***p<0.001, n=21 for each group; no significant difference is observed between calpain inhibitors, calpastatin peptide and MDL 28170. (C) Consistent with reduction in blind ends during capillary morphogenesis, calpain inhibitors simultaneously improve stability of actin and tubulin in vitro, particularly at cell/cell junctions (see arrows) that are critical for stable inter-connection of vascular networks. Scale bar, 20 µm. (D) In vivo, during retinal neovascularization, calpain inhibitor MDL 28170 (0.25 mg/kg, once daily) improves organization and alignment of vascular actin similar to findings in vitro. Animals were treated beginning with the onset of retinopathy for 5 days (P12–17), and whole mount retinas were stained for F-actin with FITC-phalloidin. Scale bar: 25 µm. (E) Maximal improvement of capillary morphogenesis in vitro, as measured by closed polygons/microscopic field, is achieved with MDL 28170 at a concentration of 10 nM. Lower and higher concentrations are less effective or inhibitory (***p<0.001 for 10 nM in comparison with control and 100 nM dose; n≥15 for each group). Similarly, the optimum dose of calpastatin peptide was determined to be 200 nM. (F) Assay of calpain activity in live retinal MVECs; doses of calpain inhibitors MDL 28170 (10 nM) and calpastatin (200 nM) that provide maximal improvement of capillary morphogenesis in vitro correspond to 30–35% inhibition of calpain activity. *p<0.05.
Findings that calpain inhibitors improve capillary morphogenesis in vitro provided the opportunity to estimate the amount of calpain inhibition required for optimal improvement. Dose experiments established that MDL 28170 maximally improved capillary morphogenesis at 10 nM which is less than that required for half-maximum inhibition of calpains [29, 30] (Fig. 4E). Comparable improvement of capillary morphogenesis was achieved with calpastatin peptide at 200 nM. With an established calpain fluorescent substrate assay applied to live retinal MVECs in culture, we determined that these concentrations of inhibitors each corresponded to ~30–35% inhibition of total calpain activity in retinal MVECs (Fig. 4F).
3.5 Hypoxia activates calpain in retinal endothelial cells and disrupts the actin cytoskeleton
Because hypoxia is a key parameter in ischemic retinopathy, we also investigated a possible relationship between hypoxia, calpain activity, and cytoskeletal instability in retinal MVECs. Retinal MVECs were subjected to hypoxia and calpain activity was assayed in live cells with an established fluorometric substrate in combination with calpain inhibitors. We found significant (~50%) induction of calpain substrate-cleaving activity with hypoxia, and calpain inhibitors blocked this activity (Fig. 5A). Most significantly, we found that hypoxia severely disrupted actin cables in retinal MVECs undergoing capillary morphogenesis and that calpain inhibitors not only restored the actin cytoskeleton during hypoxia but also promoted coordinate alignment of actin cables between neighboring MVECs within vascular network (Fig. 5B). Therefore, these studies link hypoxia-induction of calpain activity to destabilization of the actin cytoskeleton in retinal MVECs. Also, these observations in vitro are consistent with our findings that calpain inhibitors simultaneously reduce retinal hypoxia (Fig. 2) and improve the vascular actin cytoskeleton of neovasculature in vivo (Fig. 4D). Collectively, the in vitro and in vivo data indicate that calpain inhibitors promote cytoskeletal stabilization directly in retinal MVECs under both hypoxic (Fig. 5B) and normoxic (Fig. 4A, 4C) conditions. In addition, by reducing retinal hypoxia (Fig. 2) and thereby reducing hypoxia-induced calpain activity (Fig. 5A) that disrupts actin cables (Fig. 5B), calpain inhibitor treatment likely provides further stabilization of the endothelial cell cytoskeleton and neovascular networks.
Figure 5. Hypoxia induces calpain activity and disassembles the actin cytoskeleton in retinal MVECs; moderate calpain inhibition restores actin cables in hypoxia.
(A) Retinal MVECs cultured in normoxia (21 % O2) were subjected to hypoxia (5% O2) for 2 h in the presence or absence of calpain inhibitors (calpastatin 200 nM or MDL 28170, at doses indicated) and assayed for calpain activity as described in methods. **p < 0.01, n≥17. (B) The actin cytoskeleton of retinal MVECs undergoing capillary morphogenesis induced by collagen I was visualized by staining with FITC-phalloidin. Cells were in normoxia (21% O2) or hypoxia (5% O2), and calpain inhibitors MDL 28170 (10 nM) or calpastatin (200 nM) were added, as indicated. These calpain inhibitor doses are moderate and inhibit calpain activity only partially (see Panel A). In comparison with cells in normoxia, actin cables were nearly absent in cells under hypoxic conditions. However, moderate doses of calpain inhibitors strongly restored actin cables in hypoxia and also promoted cable alignment. Scale bar: 20 µm.
4. Discussion
In this study, with a mouse model of retinopathy of pre-maturity that exhibits the key vascular defects associated with ischemic retinopathies, we found that administration of calpain inhibitors in moderation profoundly improved neovascular architecture and function, as measured by reduction in abnormal vascular tufts and reduction in vascular leakage. Administration of calpain inhibitors also significantly improved vascular re-growth, as measured by improved vascular coverage of retina. Most importantly, the improvement in neovascularization provided by calpain inhibitors resulted in marked reduction of underlying retinal hypoxia. Alleviation of underlying retinal hypoxia is critically important because hypoxia drives recurring pathological neovascularization, ultimately resulting in tractional retinal detachment and blindness [1]. Remarkably, in controls, much of the retinal area that experienced vascular re-growth remained hypoxic, indicating that neovascularization per se is insufficient to alleviate hypoxia. The vascular architecture in these hypoxic regions was highly abnormal, consistent with poor vascular function. By contrast, revascularized regions of the retinas of animals treated with optimally moderate doses of calpain inhibitors were primarily normoxic. Thus, administration of calpain inhibitors promoted re-growth of architecturally improved blood vessels that alleviated retinal hypoxia, whereas vascular re-growth without such treatment was comparatively inefficient.
In retinopathies, including the mouse model of retinopathy employed here, hypoxia induces expression of HIF-regulated cytokines such as VEGF that drive neovascularization [1, 20–23, 31]. It seems counterintuitive that hypoxia would induce a neovasculature that is architecturally abnormal and leaky and, as shown here, unable to restore normal oxygenation. Thus, the question arises as to why retinal hypoxia induces an abnormal neovasculature. Two of our findings suggest the intriguing hypothesis that abnormalities in retinal neovascularization in response to hypoxia may be due, at least in part, to hyper-activation of calpain. First, as summarized above, moderate calpain inhibition improves hypoxia-induced neovascular architecture and function in vivo; and secondly, hypoxia induces calpain activity in retinal MVECs in vitro. Consistent with our observations that hypoxia induces calpain activity in retinal MVECs, calpain activation has been demonstrated previously in hypoxic retina in vivo [13, 14], and hypoxia has been shown also to activate calpain in endothelial cells from lung [32] and umbilical vein [33].
Calpains cleave a large number of proteins that regulate cytoskeletal architecture, including cytoskeletal elements and proteins that link cytoskeleton to membrane [5, 34, 35]. In particular, our data indicate a functional relationship between calpain activity and disruption of the retinal MVEC cytoskeleton. We found that calpain inhibitors strongly improved organization of actin cables and microtubules in retinal MVECs during capillary morphogenesis in vitro. Furthermore, hypoxia severely disrupted the actin cytoskeleton of retinal MVECs in vitro, and calpain inhibitors restored actin cables. Similarly, we found that calpain inhibitors strongly improved organization of the endothelial actin cytoskeleton in newly formed blood vessels in vivo. Thus, it is likely that improvement in neovascular architecture provided by calpain inhibitors is attributable to improved cytoskeletal organization and/or stability. Most importantly, our data illustrate that optimal moderation of calpain activity with calpain inhibitors restores cytoskeletal organization, improves capillary morphogenesis, and promotes formation of a functionally improved neovasculature that reduces underlying hypoxia. Consequently, calpain inhibitors offer a treatment strategy for alleviating the persistent hypoxia that causes recurring cycles of abnormal neovascularization.
It is important to emphasize that these studies identified moderate inhibition rather than severe inhibition of calpain as important for achieving vascular improvements in hypoxic retina. In vitro capillary morphogenesis experiments indicated that optimal improvements were observed at 30–35% calpain inhibition. In experiments in vitro and in vivo we observed inhibition of capillary morphogenesis and inhibition of neovascularization with calpain inhibitor doses higher than that required for optimal improvement. Given the importance of calpain activity for basic cellular functions, suppression of angiogenesis with higher doses of calpain inhibitors is not surprising. For example, inhibition of calpain activity can interfere with normal cell adhesion and migration [8, 36]; and, in mice, deletion of the small subunit that is common to both calpain I and calpain II results in embryonic death at mid-gestation [37]. Others have reported that calpain inhibition suppresses angiogenesis in vivo [38], and blocks capillary morphogenesis in vitro [39]; and our findings with the higher doses of calpain inhibitors are consistent with these previous observations. However, and most importantly, experiments described here establish that daily administration of calpain inhibitors at appropriately moderate doses improves the function of neovessels rather than inhibits neovascularization.
Finally, in sharp contrast to “anti-angiogenesis” treatment strategies such as those targeting VEGF or VEGF receptors (reviewed [40]), the calpain inhibitor strategy described here does not inhibit angiogenesis but rather corrects pathological neovascularization to reduce hypoxia. Inhibition of neovascularization cannot alleviate underlying hypoxia; moreover, inhibition of VEGF or VEGF signaling may harm neural retina that is VEGF-dependent [41]. Alleviation of hypoxia is of paramount importance in ischemic retinopathy because hypoxia destroys photoreceptors and ganglion cells and also drives formation of a pathological, leaky vasculature that causes retinal detachment and blindness [1–4]. Accordingly, appropriately moderate calpain inhibition, to improve functional neovascularization and thereby remedy underlying hypoxia, offers a distinct and rational advantage over “anti-angiogenesis” strategies for treating ischemic retinopathies. In addition, this strategy suggests a new therapeutic paradigm for treating retinal ischemia, based on improvement rather than antagonism of new blood vessel growth.
Supplementary Material
Acknowledgements
We thank the V. Kann Rasmussen Foundation for financial support. This work also was supported by NIH grants CA129339 and NS064498 (DRS), NIH grants EY017017 and EY017017-S (LEHS), The Roche Foundation for Anemia Research (LEHS), and a Research to Prevent Blindness Senior Investigator Award (LEHS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamis AP, Aiello LP, D'Amato RA. Angiogenesis and ophthalmic disease. Angiogenesis. 1999;3:9–14. doi: 10.1023/a:1009071601454. [DOI] [PubMed] [Google Scholar]
- 2.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 3.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 5.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53 Suppl 1:S12–S18. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- 7.Potter DA, Tirnauer JS, Janssen R, Croall DE, Hughes CN, Fiacco KA, Mier JW, Maki M, Herman IM. Calpain regulates actin remodeling during cell spreading. J Cell Biol. 1998;141:647–662. doi: 10.1083/jcb.141.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulkarni S, Saido TC, Suzuki K, Fox JE. Calpain mediates integrin-induced signaling at a point upstream of Rho family members. J Biol Chem. 1999;274:21265–21275. doi: 10.1074/jbc.274.30.21265. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt A, Kaverina I, Otey C, Huttenlocher A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J Cell Sci. 2002;115:3415–3425. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- 10.Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 11.Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JS, Elce JS, Huttenlocher A. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem. 2001;276:48382–48388. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- 12.Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima E, David LL, Bystrom C, Shearer TR, Azuma M. Calpain-specific proteolysis in primate retina: Contribution of calpains in cell death. Invest Ophthalmol Vis Sci. 2006;47:5469–5475. doi: 10.1167/iovs.06-0567. [DOI] [PubMed] [Google Scholar]
- 14.Azuma M, Shearer TR. The role of calcium-activated protease calpain in experimental retinal pathology. Surv Ophthalmol. 2008;53:150–163. doi: 10.1016/j.survophthal.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 16.D'Amato R, Wesolowski E, Smith LE. Microscopic visualization of the retina by angiography with high-molecular-weight fluorescein-labeled dextrans in the mouse. Microvasc Res. 1993;46:135–142. doi: 10.1006/mvre.1993.1042. [DOI] [PubMed] [Google Scholar]
- 17.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoang MV, Senger DR. In vivo and in vitro models of mammalian angiogenesis. Methods Mol Biol. 2005;294:269–285. doi: 10.1385/1-59259-860-9:269. [DOI] [PubMed] [Google Scholar]
- 19.Rosser BG, Gores GJ. Cellular in vivo assay of calpain activity using a fluorescent substrate. Application to study of anoxic liver injury. Methods Mol Biol. 2000;144:245–259. doi: 10.1385/1-59259-050-0:245. [DOI] [PubMed] [Google Scholar]
- 20.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shima DT, Adamis AP, Ferrara N, Yeo KT, Yeo TK, Allende R, Folkman J, D'Amore PA. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med. 1995;1:182–193. [PMC free article] [PubMed] [Google Scholar]
- 22.Pe'er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995;72:638–645. [PubMed] [Google Scholar]
- 23.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci U S A. 1996;93:4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donkor IO. A survey of calpain inhibitors. Curr Med Chem. 2000;7:1171–1188. doi: 10.2174/0929867003374129. [DOI] [PubMed] [Google Scholar]
- 25.Wang KK, Nath R, Posner A, Raser KJ, Buroker-Kilgore M, Hajimohammadreza I, Probert AW, Jr, Marcoux FW, Ye Q, Takano E, Hatanaka M, Maki M, Caner H, Collins JL, Fergus A, Lee KS, Lunney EA, Hays SJ, Yuen P. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc Natl Acad Sci U S A. 1996;93:6687–6692. doi: 10.1073/pnas.93.13.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoang MV, Whelan MC, Senger DR. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci U S A. 2004;101:1874–1879. doi: 10.1073/pnas.0308525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whelan MC, Senger DR. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem. 2003;278:327–334. doi: 10.1074/jbc.M207554200. [DOI] [PubMed] [Google Scholar]
- 28.Maki M, Bagci H, Hamaguchi K, Ueda M, Murachi T, Hatanaka M. Inhibition of calpain by a synthetic oligopeptide corresponding to an exon of the human calpastatin gene. J Biol Chem. 1989;264:18866–18869. [PubMed] [Google Scholar]
- 29.Lubisch W, Hofmann HP, Treiber HJ, Moller A. Synthesis and biological evaluation of novel piperidine carboxamide derived calpain inhibitors. Bioorg Med Chem Lett. 2000;10:2187–2191. doi: 10.1016/s0960-894x(00)00430-3. [DOI] [PubMed] [Google Scholar]
- 30.Lee KS, Seo SH, Lee YH, Kim HD, Son MH, Chung BY, Lee JY, Jin C, Lee YS. Synthesis and biological evaluation of chromone carboxamides as calpain inhibitors. Bioorg Med Chem Lett. 2005;15:2857–2860. doi: 10.1016/j.bmcl.2005.03.095. [DOI] [PubMed] [Google Scholar]
- 31.Adamis AP, Shima DT, Tolentino MJ, Gragoudas ES, Ferrara N, Folkman J, D'Amore PA, Miller JW. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996;114:66–71. doi: 10.1001/archopht.1996.01100130062010. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Patel JM, Block ER. Hypoxia-specific upregulation of calpain activity and gene expression in pulmonary artery endothelial cells. Am J Physiol. 1998;275:L461–L468. doi: 10.1152/ajplung.1998.275.3.L461. [DOI] [PubMed] [Google Scholar]
- 33.Aono Y, Ariyoshi H, Tsuji Y, Ueda A, Tokunaga M, Sakon M, Monden M. Localized activation of m-calpain in human umbilical vein endothelial cells upon hypoxia. Thromb Res. 2001;102:353–361. doi: 10.1016/s0049-3848(01)00238-9. [DOI] [PubMed] [Google Scholar]
- 34.Tompa P, Buzder-Lantos P, Tantos A, Farkas A, Szilagyi A, Banoczi Z, Hudecz F, Friedrich P. On the sequential determinants of calpain cleavage. J Biol Chem. 2004;279:20775–20785. doi: 10.1074/jbc.M313873200. [DOI] [PubMed] [Google Scholar]
- 35.Pfaff M, Du X, Ginsberg MH. Calpain cleavage of integrin beta cytoplasmic domains. FEBS Lett. 1999;460:17–22. doi: 10.1016/s0014-5793(99)01250-8. [DOI] [PubMed] [Google Scholar]
- 36.Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- 37.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su Y, Cui Z, Li Z, Block ER. Calpain-2 regulation of VEGF-mediated angiogenesis. Faseb J. 2006;20:1443–1451. doi: 10.1096/fj.05-5354com. [DOI] [PubMed] [Google Scholar]
- 39.Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006;98:617–625. doi: 10.1161/01.RES.0000209968.66606.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shojaei F, Ferrara N. Antiangiogenesis to treat cancer and intraocular neovascular disorders. Lab Invest. 2007;87:227–230. doi: 10.1038/labinvest.3700526. [DOI] [PubMed] [Google Scholar]
- 41.Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D'Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS ONE. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.