Abstract
Purpose
To develop a split-luciferase based reporter system that allows for non-invasive monitoring of activation of the Her2/neu pathway in vivo in a quantitative and sensitive manner.
Methods and Materials
Fusion proteins of the ErbB2/Her2/neu receptor to the N-terminal fragment of luciferase as well as of its downstream binding partner Shc to the C-terminal fragment of luciferase have been engineered based on the rationale that upon activation and binding of the Her2 receptor molecule to Shc, luciferase function will be reconstituted. Thus the resulting bioluminescence signals can serve as a surrogate measure of receptor activation.
Results
We show that our reporter systems functions well in vitro in breast cancer cells and in vivo in xenograft tumors. In particular, the activities of Her2/neu in xenograft tumors could be monitored serially for an extended period of time after radiotherapy.
Conclusions
We believe that the novel ErbB2/Her2/neu reporter presented here is a powerful tool to study the biology of the Her2-neu pathway in vitro as well as in vivo. It should also facilitate the development and rapid evaluation of new Her2/neu targeted therapeutics.
Keywords: radiotherapy, ErbB2/Her2/neu, reporter, luciferase imaging
Introduction
Tracking signaling pathway activation in living cells and particularly in experimental animals over time is a very powerful way to evaluate new treatment regimens and decipher biological mechanisms. Technical advances in imaging technologies, especially bioluminescence imaging, have allowed for the imaging of labeled cells in living animals (1). This has allowed for tumor growth, forming of metastases and tumor regression to be followed with great sensitivity(2). Furthermore, through sophisticated genetic engineering, luciferase imaging has been adapted so that it can provide functional information about various signaling pathways in tumor development as well as in their treatment. Examples of these include imaging of various gene reporters(3, 4) (5). More advanced forms of imaging allowed for protein-protein interactions (6–8), or activities of proteasome-mediated protein degradation to be imaged (9). These new bioluminescence imaging techniques provide unprecedented insights into tumor biology.
ErbB2 is a member of the EGFR receptor family and has been shown to be over-expressed in a variety of tumors with a bad prognosis (10, 11). In the clinic erbB2 inhibitors are shown to be effective in inhibiting tumor growth and metastasis in ErbB2 expressing breast cancer patients (12). In addition, other ErbB2 over-expressing cancers, e.g. pancreatic cancer, are likely to respond to ErbB2 inhibition as well (13, 14).
All members of the EGF receptor family (ErbB1 (EGFR), ErbB2 (Her2/neu), ErbB3 and ErbB4) form hetero- and/or homodimers upon activation, typically by ligand binding to any of these receptors. ErbB2 itself does not have a known specific ligand, however it is the preferred heterodimerization partner of ErbB3 as well as EGFR (15–18). Upon ligand binding to either of these receptors, heterodimers with ErB2 are formed resulting in cross-phosphorylation of both receptors and subsequent phosphorylation of Shc which is triggering the downstream signaling cascade. Although the downstream signaling pathway of all hetero- and homodimer combinations formed by members of the ErbB is shared, their signaling potency is very different. Interestingly, heterodimerization of the ligandless ErbB2 receptor with the kinase deficient ErbB3 receptor has been shown to be the most potent signaling complex (19, 20).
In the present study, we have generated a split-luciferase reporter system which can specifically report receptor kinase activities of ErbB2/Her2/Neu. We have engineered fusion proteins of the ErbB2 receptor and one of its downstream partners, Shc, to the N- and C-terminal fragments of luciferase, respectively. We show that upon ErbB2 activation, luciferase activities could indeed be reconstituted and quantified non-invasively in cell culture as well as in tumor xenografts.
Material and Methods
Construction of the plasmids
Full length ErbB2 was amplified using PCR from plasmid pErbB2 which was kindly provided by Dr. Bolin Liu, University of Colorado School of Medicine. Xho1 and Mlu1 restriction sites were introduced at either end of the gene by incorporating them in the forward and reverse primers, respectively.
The PCR product as well as the target vector pN1-EGFR-NLuc (8) were cut with XhoI and MluI, thus excising the EGFR insert of pN1-EGFR-NLuc. After gel purification, vector and insert were ligated overnight using T4 DNA Ligase resulting in pN1-Her2-NLuc, and sequenced. Her2-Nluc was excised with SpeI and NotI and transferred into the lentiviral vector pLex (Open Biosystems, Huntsville, Alabama), which was cut with NheI (which is compatible with SpeI) and NotI, to derive pLex-Her2-Nluc. The Shc-Cluc reporter plasmid pLex-Shc-CLuc has been published previously (8).
Generation of stable cell lines
Cells with stable integration of the Her2 reporter was generated through lentiviral mediated gene transfer. To generate the respective viruses, 293T cells were transfected with the lentiviral vectors pLex-Her2-NLuc or pLex Shc-CLuc, along with the packaging plasmid pSpax and the envelope plasmid pMD2.G using calcium phosphatefollowing standard protocols (21). The target breast cancer cells MDA231 and BT474 were infected with both of the viruses (encoding either Her2-Nluc or Shc-Cluc) and selected using puromycin. Clonal cell populations carrying both reporter genes were obtained by limiting dilution of 100–300 cells in three 96-well plates. After 3–6 weeks single clones were analyzed for positive luciferase signals. Positive clones were expanded for further testing.
Cell culture and western blotting
293T, MDA-MB231, and BT474 cell lines were maintained in DMEM supplemented with 10% FBS. Western blotting was performed using standard procedures as described. The following primary antibodies were used: ErbB2 (Calbiochem, San Diego, California) 1:1000, pN2A (phosphor-ErbB2, Neomarkers, Fremont, California) 1:1000, Shc, Phospho-Shc, mitogen-activated protein kinase (MAPK) and P-MAPK (Cell Signaling Technology, Beverly, Massachusetts) 1:1000, Beta Actin (Lab Vision Corp, Fremont, CA) 1:1000.
Imaging of Her2 reporter transduced cells in tissue culture
For ligand induction experiments, cells were serum starved overnight and incubated with either EGF (Cell Signal Technology) at 50ng/ml or Heregulin β1 (Thermo Scientific) at 20ng/ml for 1 hour. 10min prior to imaging, cells were incubated with luciferin (Caliper Life Sciences) at a concentration of 6mg/ml. Bioluminescent imaging was carried out using an IVIS200 imager (Caliper Life Sciences). Photographic images were superimposed with pseudocolored bioluminescent images acquired subsequently with an exposure time of 1–2 minutes at medium (4×4) or large (16×16) binning.
Animal procedures and in vivo imaging
Athymic nude mice were obtained from the National Cancer Institute through Charles River Laboratories and maintained in the Vivarium of the Anschutz Medical Campus in accordance with University of Colorado Institutional Animal Care and Use Committee guidelines. Xenograft tumors were established by subcutaneous injection of 5×105 cells suspended in a 50ul Matrigel/PBS solution (1:3) in each hind leg. For imaging, mice were intraperitoneally (i.p.) injected with 200ul of luciferin at a concentration of 15mg/ml in H2O 10 min prior to image acquisition and anaesthetized using isofluorane inhalation.
Statistical analysis
Statistical analysis was performed using Microsoft excel. Student’s t-test was performed to calculate p-values. Values <0.05 were considered significant. Error bars represent standard error of the mean.
Results
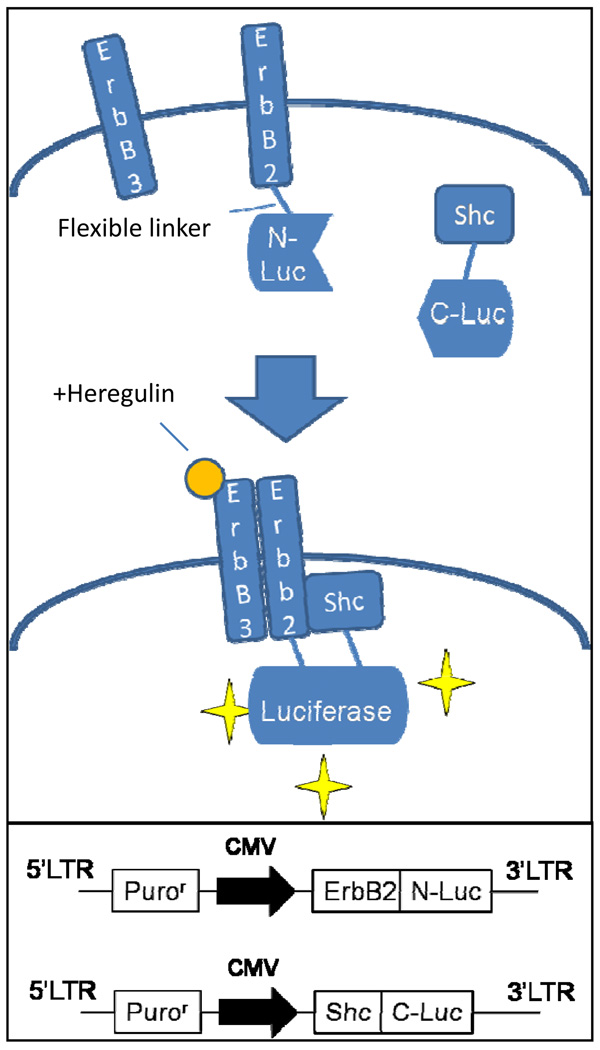
In order to generate a reporter for Her2/ErbB2 pathway activation which can be used in vitro as well as in vivo, a bi-fragment luciferase reconstitution system was used. This system is based on the assumption that luciferase activity is reconstituted when the N- and C- terminal fragments of luciferase which are fused to ErbB2 and its downstream binding partner Shc, respectively, come in close proximity to each other as a result of ErbB2 activation and its subsequent binding to Shc. In the presence of luciferin, the substrate of luciferase, bioluminescence is emitted which can be captured using a bioluminescence imaging device (see Figure 1 for schematic illustration).
Figure 1.
Principle of reporter functioning: see text for details. Lower box: structure of lentiviral vectors encoding fusion reporter proteins depicted above.
Functional analysis of the ErbB2 reporter in vitro
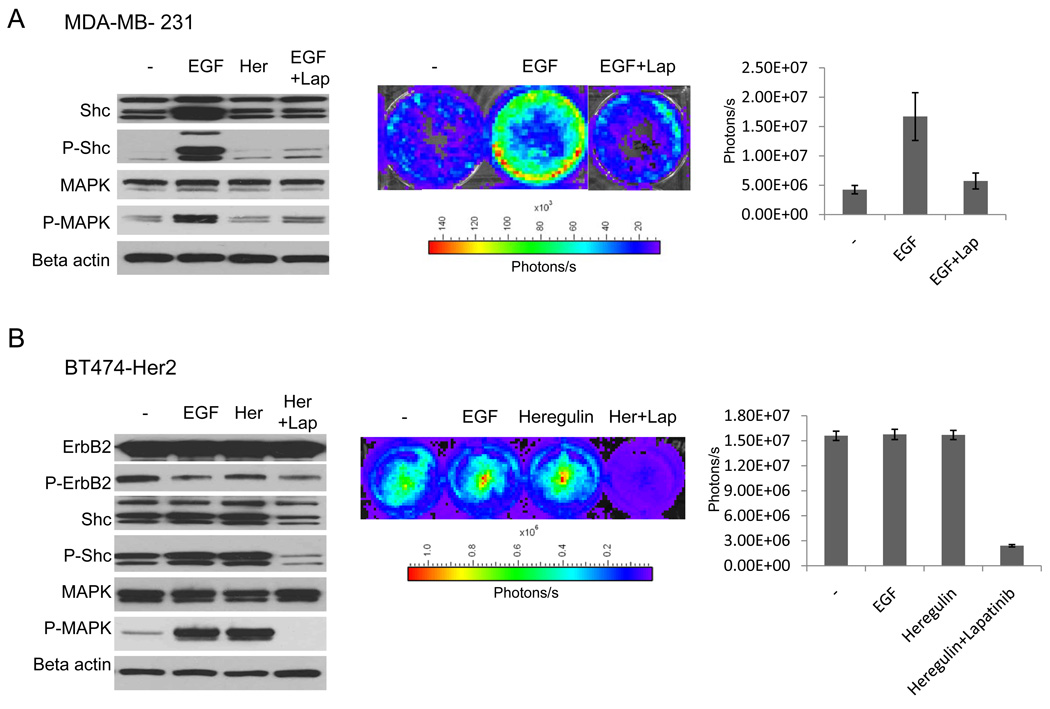
Two different breast cancer cell lines with our Her2 split luciferase reporter system, BT474 (22) (high endogenous ErbB2 expression) and MDA-MB231 (undetectable endogenous ErbB2 expression) were used. MDA-MB231 cells do not express Her2/ErbB2, but express robust levels of EGFR which becomes the predominant heterodimerization partner of transgenic ErbB2. Therefore, theoretically our ErbB2 reporter construct has no competition from endogenous Her2/ErbB2 proteins. However, because exogenously expressed ErbB2 can form heterodimers with endogenous EGFR, we believe that this cell line represents a good experimental system to study ErbB2 activation in response to different stimuli. As can be seen in Figure 2A (left panel), addition of EGF, but not heregulin, results in phosphorylation of both Shc as well as MAPK in MDA-MB231 cells, confirming the downstream activation of the Her2 signaling cascade. The lack of response to heregulin is consistent with the lack of expression of ErbB3, which is required for formation of heterodimers with ErbB2, in response to heregulin binding.
Figure 2.
Data demonstrating the functionality of Her2 reporter in breast cancer cell lines in culture.
A) MDA-MB231 cells transduced with split luciferase Her2 reporter. Left panel: western Blot analysis of phosphorylation status of Shc and MAPK after induction with EGF (2nd lane), Heregulin (3rd lane) and EGF or Heregulin in the presence of lapatinib. Her = Heregulin. Middle panel: representative pseudocolored bioluminescent images of the MDA-MB231-Her2-Luc cell line after incubation with ligand with or without the specific ErbB2 inhibitor lapatinib (1uM) for 1h. Cells have been serum starved overnight, then incubated with the ligand for 1h, and imaged after preincubation with luciferin for 10min. Cells were imaged using a Xenogen IVIS imager. Right panel: quantitative analysis of signal intensities of triplicate experiments.
B) BT474 transduced with split-luciferase Her2 reporter. Cellular treatment and analytical conditions were similar to those of MDA-MB231 cells. Error bars represent standard error of the mean of three individual experiments.
Consistent with the western blot results, exposure of MDA-MB231 ErbB2-Shc reporter cells to EGF (at 50ng/ml) induced a 3–4-fold activation (Figure 2A, middle and right panels). On the other hand, treatment with the ErbB3 ligand heregulin, which requires expression of both, ErbB3 and ErbB2, did not induce any induction, again consistent with the western blot results. Another piece of evidence confirming the functionality of our Her2 reporter came from the use of lapatinib, a small molecule drug that specifically targets EGFR/Her2 by interfering with the ATP binding of the tyrosine kinase domain. Our data indicate that treatment with lapatinib (1µM) significantly inhibits both Her2 reporter luciferase activities (p=0.03) as well as Shc phosphorylation (Figure 2A).
In the BT474 cell line, ErbB3 is the predominant heterodimerization partner of ErB2 (17). However, as shown in the western blot analysis in Figure 2B (left panel), addition of heregulin did not cause any increases in ErbB2 and Shc phosphorylation. This is perhaps a reflection of the already very high baseline phosphorylation level in untreated cells (Figure 2B, left panel). Consistent with the western blot results, exposure of BT474 cells to either heregulin or EGF has no effect on Her2 reporter activities. Interestingly, downstream activation of MAPK was detectable after addition of both, heregulin and EGF, which might be due to indirect activation by a pathway other than Her2 receptor activation. However, incubation of the cells with lapatinib effectively inhibited phosphorylation of Shc, MAPK as well as luciferase reporter activities (p<0.01) (Figure 2B), demonstrating functionality of our Her2 reporter in this cell line.
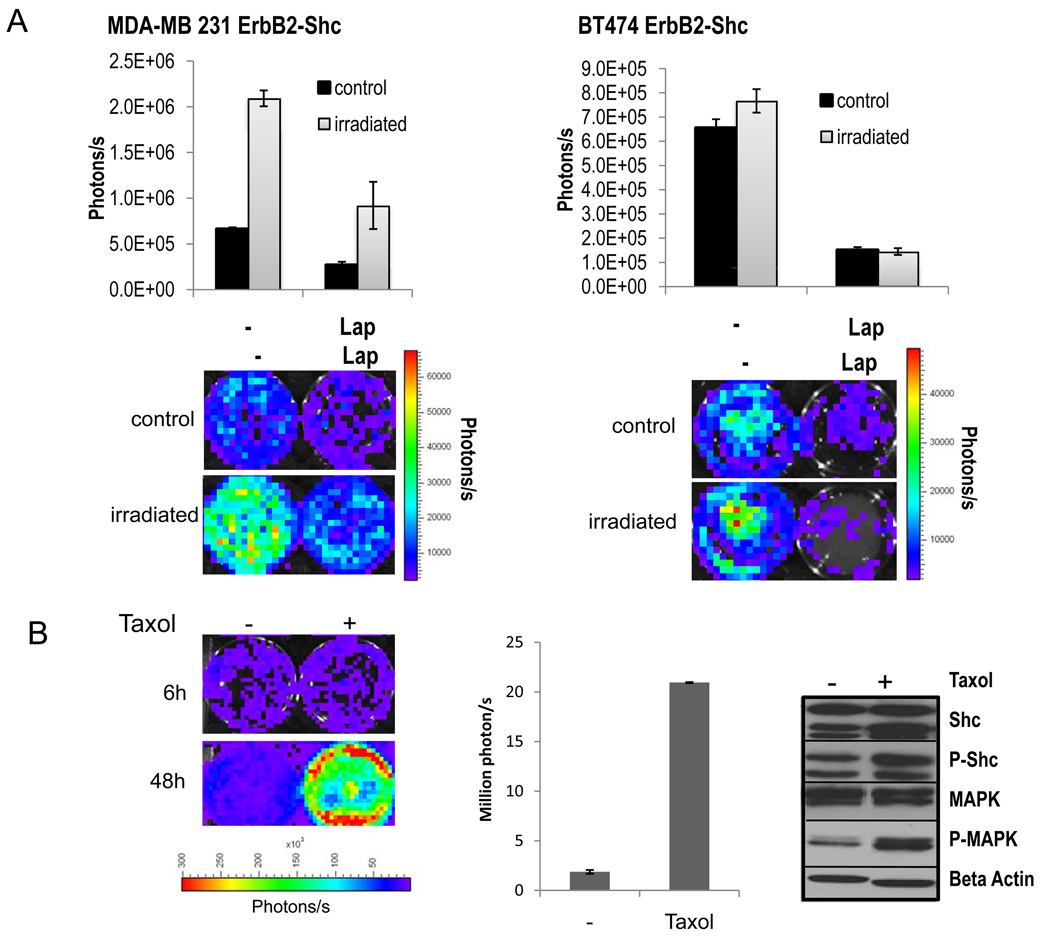
Induction of Her2 reporter activities in response to cytotoxic treatment
In order to further evaluate the functionality of our Her2 reporter cell lines, both cell lines were subjected to irradiation and tested if they could report receptor activation in response to ionizing radiation. Cells were irradiated with 5Gy and imaged in 24h intervals. Ninety-six hours after irradiation a significant induction of ErbB2 signaling was detected in both cell lines (p<0.01). In those wells where lapatinib had been added, induction was significantly attenuated (Figure. 3A).
Figure 3.
Monitoring erbB2/Her2 reporter activity in cancer cells exposed to cytotoxic treatments.
A). Her2-luc reporter activity in response to irradiation in MDA231-ErbB2-shc cells (left) and BT474-ErbB2-shc cells (right). Top panels: quantitative analysis of luciferase signals 72h after cells had been subjected to irradiation with 5Gy. Lower panels: representative bioluminescent images of irradiated (5Gy) and non-irradiated cells at 72h after treatment.
B). Induction of the ErbB2 pathway in response to treatment with taxol at a concentration of 0.5uM. Luciferase signals (left panel) and quantitative analysis (middle panel) of MDA-MB231 ErbB2-Shc cells 6h and 48h after incubation with taxol. Middle panel: Quantitative analysis. Right panel: Western blot analysis of downstream targets of the ErbB2 pathway. Phospho-specific and non-specific antibodies directed against Shc and MAPK were used to assess the phosphorylation status of Shc and MAPK after taxol incubation for 48h. Error bars represent standard error of the mean of three individual experiments.
To test if our reporter system is able to detect changes in ErbB2 pathway activation during cytotoxic drug treatment we treated MDA-MB231 ErbB2-Shc cells with Taxol, a commonly used drug for breast cancer treatment As can be seen in Figure 3B (left and middle panels), cells treated with Taxol (500nM) exhibited a profound activation of the ErbB2 signaling pathway in response to Taxol treatment 48 hours after addition of the drug (p<0.01). To confirm these findings western blot analysis of downstream targets of the Her2 pathway after taxol treatment was performed. Both Shc as well as MAPK became phosphorylated 48h after addition of taxol (Figure 3B, right panels), which is consistent with the results obtained by the luciferase reporter assay showing that our reporter system can track ErbB2 signaling in vitro in response to cytotoxic treatment in a sensitive manner over time.
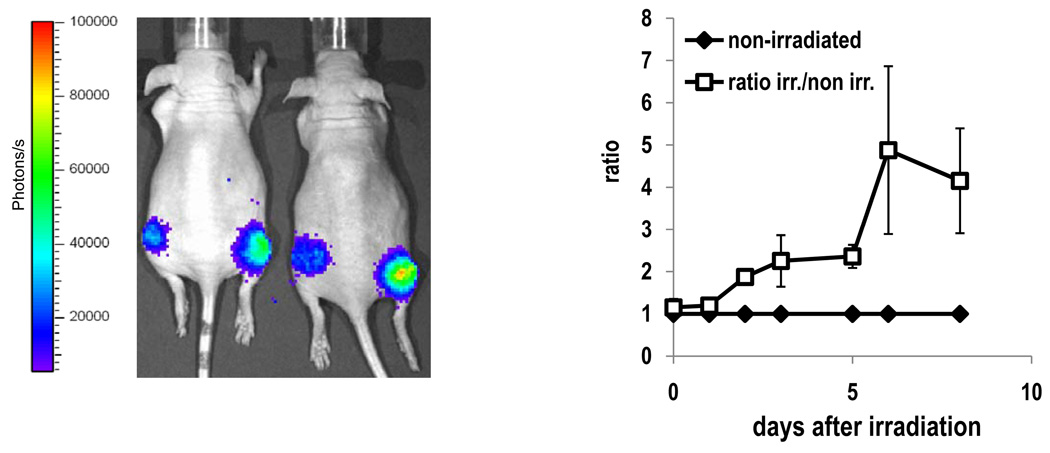
Validation of ErbB2 reporter in vivo during radiotherapy
In order to test if ErbB2 pathway activation could be monitored in vivo over an extended period of time e.g. following irradiation treatment, we established xenograft tumors by injecting MDAMB231 Her-Shc cells (1*105 cells) in both hind legs of athymic nude mice subcutaneously. When tumors reached a size of about 8mm in diameter, the right hind leg was irradiated with a single dose of 5Gy. Mice were imaged regularly over a period of 9 days following treatment. The non-irradiated leg of each mouse was used as internal control. Luciferase Signal intensities were normalized to the tumor volume and the ratios of signal intensities of the irradiated and the nonirradiated legs were calculated and are plotted in Figure 4. Induction of the irradiated leg was detectable at 5 hours after irradiation and further increased, peaking at 6 days after irradiation (about 5-fold peak induction compared to the non-irradiated leg).
Figure 4.
Non-invasive imaging of ErbB2 activation after irradiation. Mice (n=5) have been injected with 3×105 cells in each hind leg. When tumors reached a size of 7–8mm in diameter, the right hind leg was irradiated with a dose of 5Gy. Mice were imaged over a period of 9 days and tumor sizes measured using a caliper. Signal intensities were normalized to tumor size and the ratios of the irradiated vs. the non-irradiated side calculated (right panel). Error bars represent standard error of the mean. Left panel: representative pseudocolored bioluminescent image of tumor bearing mice at day three after irradiation.
Discussion
Targeting tyrosine kinases either by directly targeting the receptor or components of the intracellular signal transduction cascade has become a very important strategy in the treatment of a multitude of malignant tumors and currently a great number of drugs are in use or being evaluated in the clinic. In the treatment of breast cancer Her2 inhibitors have significantly improved the prognosis of Her2 positive patients and other inhibitors which target various RTK signaling cascade further downstream are beginning to find their way into the clinic. With an increasing number of these drugs being evaluated, there is a clear demand for better methods to assess the efficacy of these inhibitors both in vitro and in vivo. The Her2/erbB2 reporter described in this study is a significant step towards the development of a non-invasive, quantitative imaging approach to evaluate the efficacy of Her2-targeted therapeutics in tissue culture and in mouse tumor models.
One of the great advantages of using a luciferase-based reporter is that receptor activation can be monitored in vivo and ‘in real time’ i.e. at multiple timepoints in the same animal before, during and after treatment, thus minimizing inter-sample variability and greatly reducing the number of animals required for an experiment. Therefore, our reporter system should be very useful in evaluating new therapeutics targeted at the Her2/erbB2 pathway.
In addition to evaluating available therapeutics, our reporter system can also serve as a valuable tool for drug development. For example, it could be used to screen large chemical libraries for ErbB2 inhibition in a highly sensitive and rapid manner in vitro. Identified candidate drugs could then be further tested in vivo using the same reporter, thus providing valuable information on kinetics, bioavailability, and efficacy of the drug under physiological conditions.
The successful development of our Her2/erbB2, together with our earlier success in developing an EGFR reporter (8), firmly establish the split-luciferase system as a powerful approach to image and quantify RTK activities in vivo. We predict that such an approach will find wider applications in a range biological research fields beyond cancer research.
In summary, an effective and easy to use reporter system to monitor ErbB2 signaling in vitro as well as in vivo was established. Using this system, it was shown that ErbB2 activation could be detected non-invasively in a highly sensitive manner.
Acknowledgements
We thank Dr. Bolin Liu of University of Colorado Denver for providing us with the erbB2-encoding plasmid, anti-erbB2 antibody, and the BT474 breast cancer cell line. This project has been funded in part by an Erwin Schroedinger Stipend of the Austrian Science Fund (FWF). It was also supported in part by grant CA131408 and CA CA136748 from the US National Cancer Institute (to C-Y Li), and grant NNX09AH19G (to C-Y Li) from NASA Space Radiation Biology Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None of the authors has any financial interest in the work described in this study.
References
- 1.Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nat Rev Immunol. 2006;6:484–490. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- 2.Edinger M, Sweeney TJ, Tucker AA, et al. Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia. 1999;1:303–310. doi: 10.1038/sj.neo.7900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamei K, Ishikawa TO, Herschman HR. Transgenic mouse for conditional, tissue-specific Cox-2 overexpression. Genesis. 2006;44:177–182. doi: 10.1002/dvg.20199. [DOI] [PubMed] [Google Scholar]
- 4.Lyons SK, Lim E, Clermont AO, et al. Noninvasive bioluminescence imaging of normal and spontaneously transformed prostate tissue in mice. Cancer Res. 2006;66:4701–4707. doi: 10.1158/0008-5472.CAN-05-3598. [DOI] [PubMed] [Google Scholar]
- 5.Klopstock N, Levy C, Olam D, et al. Testing transgenic regulatory elements through live mouse imaging. FEBS Lett. 2007;581:3986–3990. doi: 10.1016/j.febslet.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Ozawa T, Kaihara A, Sato M, et al. Split luciferase as an optical probe for detecting protein-protein interactions in mammalian cells based on protein splicing. Anal Chem. 2001;73:2516–2521. doi: 10.1021/ac0013296. [DOI] [PubMed] [Google Scholar]
- 7.Villalobos V, Naik S, Piwnica-Worms D. Current state of imaging protein-protein interactions in vivo with genetically encoded reporters. Annu Rev Biomed Eng. 2007;9:321–349. doi: 10.1146/annurev.bioeng.9.060906.152044. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Li F, Huang Q, et al. Noninvasive imaging and quantification of epidermal growth factor receptor kinase activation in vivo. Cancer Res. 2008;68:4990–4997. doi: 10.1158/0008-5472.CAN-07-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li F, Sonveaux P, Rabbani ZN, et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 11.Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15:2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 12.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 13.Haller DG. Future directions in the treatment of pancreatic cancer. Semin Oncol. 2002;29:31–39. doi: 10.1053/sonc.2002.37381. [DOI] [PubMed] [Google Scholar]
- 14.Wolff RA. Novel therapies for pancreatic cancer. Cancer J. 2001;7:349–358. [PubMed] [Google Scholar]
- 15.Stern DF, Kamps MP. EGF-stimulated tyrosine phosphorylation of p185neu: a potential model for receptor interactions. EMBO J. 1988;7:995–1001. doi: 10.1002/j.1460-2075.1988.tb02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–6114. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 17.Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzahar E, Pinkas-Kramarski R, Moyer JD, et al. Bivalence of EGF-like ligands drives the ErbB signaling network. EMBO J. 1997;16:4938–4950. doi: 10.1093/emboj/16.16.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alimandi M, Romano A, Curia MC, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–1821. [PubMed] [Google Scholar]
- 20.Holbro T, Beerli RR, Maurer F, et al. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zufferey R, Nagy D, Mandel RJ, et al. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 22.Pasleau F, Grooteclaes M, Gol-Winkler R. Expression of the c-erbB2 gene in the BT474 human mammary tumor cell line: measurement of c-erbB2 mRNA half-life. Oncogene. 1993;8:849–854. [PubMed] [Google Scholar]