Abstract
Problem
Experimental infection of cats with FIV-B-2542 produces high rates of fetal infection and reproductive failure. We hypothesized that dysregulation of placental cytokine expression occurs in FIV-infected queens, and aberrant expression potentiates inflammation and impacts pregnancy outcome. Our purpose was to quantify expression of representative pro-inflammatory cytokines (IL-6, IL-12p35, and IL-1β), IL-10 (anti-inflammatory), and the chemokine SDF-1α in early- and late-term placental tissues.
Methods
Real-time reverse-transcriptase-PCR was used to measure gene expression in placental tissues.
Results
Increased expression of IL-6 and IL-12p35 and decreased expression of IL-10 occurred in FIV-infected tissues at early pregnancy; at late gestation, IL-6 expression increased and IL-1β and SDF-1α decreased. At late pregnancy, IL-6 expression positively correlated with FIV load. IL-12:IL-10 ratios were higher in infected tissues at early, but not late pregnancy. Fetal nonviability accompanied decreased IL-12p35 and SDF-1α expression at both stages and decreased IL-12:IL-10 ratio at late pregnancy.
Conclusion
FIV infection caused a pro-inflammatory placental microenvironment at early, but not late pregnancy.
Keywords: Feline immunodeficiency virus, placenta, cytokines, inflammation
Introduction
Placental cytokines and chemokines are immunoregulatory molecules that are responsible for pregnancy maintenance. These immunomodulators are critical for embryo implantation, endometrial development,1 and the development of fetal placentation.2 Successful pregnancy is biased towards a Th2 immune response with a suppressed Th1 response at the maternal-fetal interface.3–6 Th1 and Th2 cytokines are mutually antagonistic.6, 7 The production of Th2 cytokines aids in the maintenance of pregnancy by downregulating Th1 cytokines, thereby preventing the formation of an inflammatory microenvironment in the placenta.8 The altered expression of the Th1 cytokine, IL-12 (a pro-inflammatory cytokine), and the Th2 cytokine, IL-10 (an anti-inflammatory cytokine), is a clear indicator of Th1:Th2 immune shifts and cytokine dysregulation.4, 6, 8, 9 Cytokine dysregulation in the placenta may result in poor reproductive outcome, including spontaneous abortion, preterm labor, preeclampsia, and intrauterine growth restriction.8, 10
Vertical transmission of HIV (defined as HIV transfer from mother-to-child during pregnancy, labor and delivery, or breastfeeding) accounts for more than 90% of pediatric infections worldwide. In the United States, mother-to-child transmission (MTCT) is the most common source of all AIDS cases in children and results in 100–200 infected infants annually.11 The rate of disease progression in infants is rapid, and about 50% of infected newborns will succumb to the disease before 24 months of age.12 In addition, HIV infection of pregnant women often results in reproductive failure, including low birth weight babies, preterm delivery, and an increased incidence of spontaneous abortions.13–15 Although it is evident that HIV has the potential to negatively impact reproductive outcome, the mechanisms of viral transplacental transfer and pregnancy perturbation associated with infection remain undefined.
HIV expression is augmented by the production of placental immunomodulators such as cytokines,16 and HIV infections can dysregulate cytokine networks in placental tissues.17, 18 Aberrant expression of cytokines may result in placental inflammation, and the presence of inflamed placental membranes is likely to facilitate viral transplacental transfer.17
FIV causes a natural infection of domestic cats that is clinically similar to HIV-1. Vertical transmission of FIV may occur in utero, producing infected offspring and frequent reproductive failure.19–22 We previously reported a high rate of reproductive failure in litters delivered at late gestation (week 8) by cesarean section in FIV-infected cats.21 In that report, vertical transmission of FIV occurred in nearly all pregnancies, and FIV was detected in tissues of both viable and nonviable kittens and fetuses. We found that Th1 and Th2 cytokine expression did not differ significantly in the placentas of infected versus uninfected cats.21 However, Th1 cytokine expression was significantly increased in placentas of FIV-infected, resorbed fetuses in comparison to non-resorbed placentas. Cytokine gene expression in early-term placental tissue was not evaluated in that study.
We hypothesized that dysregulation of placental cytokine expression occurs in FIV-infected queens, and aberrant expression of these cytokines may potentiate inflammation and impact pregnancy outcome. As a component of a larger project aimed at identifying specific placental cell populations, namely trophoblasts and regulatory T cells (Tregs), whose dynamics and function may be altered by FIV infection, our initial intent was to evaluate immune function at the level of whole placenta to determine 1) the expression of representative pro-inflammatory cytokines (IL-6, IL-12p35, and IL-1β), an anti-inflammatory cytokine (IL-10), and the pleiotropic chemokine SDF-1α (CXCL12), which binds the receptor CXCR4 expressed on activated T cells and trophoblasts, in early- and late-term placental tissues; 2) whether immunomodulator expression is related to FIV infection and pregnancy outcome. We report that FIV-infection during early and late gestation was associated with altered immunomodulator expression. The data indicate that FIV induces a pro-inflammatory placental microenvironment at early, but not late pregnancy.
Materials and Methods
Feline placental tissues
All procedures utilizing cats (Felis domesticus) were performed with approval of the Mississippi State University Institutional Animal Care and Use Committee. As previously reported,21, 22 cats were reproductively mature, specific-pathogen-free (SPF) animals of less than 12 months of age when obtained from a commercial cattery. Ten cats were inoculated intravenously with 1 ml of a plasma pool containing approximately 1.3 × 104 copies/ml of FIV-B-2542, provided by Dr. Edward A. Hoover.23 Uninfected cats served as normal controls. Infection was confirmed by detection of FIV provirus by PCR and for seroconversion by ELISA. All queens were allowed to naturally breed with SPF toms. Breeding was observed and pregnancy was confirmed by ultrasonography.
The time of FIV inoculation to delivery ranged from approximately 9.5 to 13.5 months (mean 11.14 months) for the early gestation study and 4.7 to 14.1 months (mean 9.5 months) for the late gestation study. Kittens were delivered by cesarean section during week 3–4 of gestation (early) and week 8 of gestation (late)21 from FIV-B-2542-infected and uninfected queens. Placentas were collected under sterile conditions using the following dissection procedure. The uteri were removed and individual fetal membranes were collected. Following rinses with sterile PBS, membranes were incised with a sterile scalpel, and fetuses and placentas were collected. Placental tissues were snap frozen in liquid nitrogen and cryopreserved at −80°C. The effect of FIV infection on pregnancy at early- and late-term gestation was previously reported. FIV provirus was detected in placental and fetal tissues by standard PCR.21, 22 For this study, we evaluated selected placental samples from both early- and late-term pregnancy, including placentas from both viable and nonviable pregnancies (Table 1).
Table 1.
Tissues Included in Study
RNA extraction
RNA was purified from frozen placental tissues of FIV-B-2542-infected and uninfected queens at early gestation (n = 27 and n = 42, respectively) and late gestation (n = 23 and n = 24, respectively), using TRIzol Reagent (Invitrogen, Corp. Carlsbad, CA) according to the manufacturer's instructions. The RNA extraction procedure was performed as previously reported.22
Primer design of immunomodulators
Cytokine and chemokine mRNA sequences for the cat and human were obtained from the National Center for Biotechnology Information (NCBI) and aligned using the European Molecular Biology Laboratory-European Bioinformatics Institute (EMBL-EBI) ClustalW alignment tool. The human mRNA was blasted against the human genome to locate the exon/intron boundaries. These boundaries were used to find the homologous boundaries in the feline sequence. We used Beacon Designer (PREMIER Biosoft) to design primer/probe sets targeting the representative pro-inflammatory cytokines IL-6, IL-12p35, and IL-1β, an anti-inflammatory cytokine IL-10, the chemokine SDF-1α, and the internal control gene (β-actin). All PCR amplicons spanned an intron-exon junction. The target probes were 5' labeled with the reporter dye FAM (6-carboxyfluorescein) and 3' labeled with the quencher dye TAMRA (6-carboxytetramethylrhodamine). The probe for the housekeeping gene was 5' labeled with the reporter dye HEX (hexachloro-6-carboxyfluorescein) and 3' labeled with the quencher dye TAMRA. Primers and probes were obtained commercially (MWG- BIOTECH, Inc., High Point, NC). Sequences of primers and probes used in these studies are shown in Table 2. Primers and probes used to detect FIV RNA in placental and fetal tissues were described previously.21
Table 2.
Primers and Probes used in Real Time RT-PCR to Determine Cytokine Gene Expression.
| Gene | Primer | Sequence (5'–3') | Length | Accession | Probe sequence (5'–3') |
|---|---|---|---|---|---|
| IL-10 | Forward | ACTTTCTTTCAAACCAAGGACGAG | 24 | AF060520 | TCTCGGACAAGGCTTGGCAACCCA |
| Reverse | GGCATCACCTCCTCCAAATAAAAC | 24 | |||
| IL-6 | Forward | GTGTGACAACTATAACAAATGTGAGG | 26 | L16914 | CAAGGAGGCACTGGCAGAAAACAACCT |
| Reverse | GTCTCCTGATTGAACCCAGATTG | 23 | |||
| IL-12p35 | Forward | ACACCAAGCCCAGGAATGTTC | 21 | U83185 | AACCACTCCCAAACCCTGCTGCGA |
| Reverse | TGGCCTTCTGAAGCGTGTTG | 20 | |||
| IL-1β | Forward | ATTGTGGCTATGGAGAAACTGAAG | 24 | M92060 | TTTGCCTGCTCACAACCCCTCCAG |
| Reverse | TCTTCTTCAAAGATGCAGCAAAAG | 24 | |||
| SDF-1α | Forward | GCTACAGATGTCCTTGCCGATTC | 23 | AB011965 | TCGAGAGCCACGTTGCCAGAGCCA |
| Reverse | TCTTCAGCCTCGCCACGATC | 20 | |||
| β-Actin | Forward | GACTACCTCATGAAGATCCTCACG | 24 | AB051104 | ACAGTTTCACCACCACCGCCGAGC |
| Reverse | CCTTGATGTCACGCACAATTTCC | 23 |
Intron/exon boundaries are in bold and underlined.
Real-time reverse transcriptase (RT)-PCR
The real-time RT –PCR used an iCycler (BioRad Laboratories, Valencia, CA): 50°C, 30 min; 95°C, 5 min; 45 × (95°C, 15 s; 60°C, 1 min). Each reaction contained 12.5 μl of the commercial reaction mix, 0.5 μl of Thermoscript™ Plus/Platinum® Taq Mix, 1 μl of forward and reverse β-actin primers (7.5 pmol/μl), 1μl of forward and reverse target primers (10 pmol/μl), 1 μl of the respective probe (100 fmol/μl), and 180–300 ng RNA. For every placental RNA sample, parallel reactions were performed in triplicate for each gene. Standard curves were generated from serially diluted, pooled RNA from uninfected cats and used to normalize for differences in PCR efficiency and RNA template as previously described.22
Statistical analysis
Statistical analysis of immunomodulator expression for early- and late-term control and FIV-B-2542-infected placental samples was done using single-factor ANOVA (Microsoft Excel-XP, Redmond, WA) followed by Wilcoxon Rank Sum Test (National Science Digital Library). Correlation of cytokine gene expression and FIV load was done using Spearman Rank Correlation.25
Results
Detection of FIV in placental and fetal tissues and reproductive outcome
Placentas from early24 and late21 gestation were probed for FIV RNA using standard and real time PCR as described previously. All early-gestation placentas tested and more than 93% of late-gestation placentas were FIV positive. Reproductive outcome was reported for early22 and late21 gestation studies. Briefly, fetal nonviability at early gestation was 4.7% and 22.2% in control and FIV-infected cats, respectively. At late gestation fetal nonviability was 3.2% and 60% in control and infected cats, respectively.
Expression of immunomodulators in placental tissues
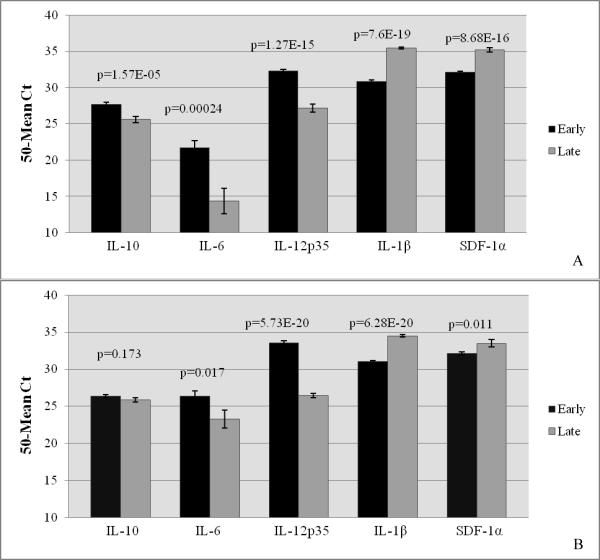
Immunomodulator mRNA was expressed in all early- and late-term placental tissues. To determine their normal feline placental expression between the two stages of pregnancy, control placentas from early- and late-term pregnancies were compared. There were significant differences in expression of all immunomodulators in control placentas. In normal placentas, higher levels of IL-10, IL-6 and IL-12p35 mRNA were expressed at early gestation, while expression of IL-1β and SDF-1α was higher at late pregnancy (Fig. 1A). Likewise, placentas from infected queens only were evaluated at early and late pregnancy, and the same pattern of cytokine expression was determined, with the exception of that of IL-10, which did not differ between early and late pregnancy (Fig. 1B).
Figure 1.
Relative Cytokine Expression in Feline Placental Tissue during Early and Late Gestation
Relative expression of feline immunomodulators was quantified from RNA extracted from whole placental tissue sections using real-time RT-PCR. The pro-inflammatory cytokines (IL-6, IL-12p35, and IL-1β), the anti-inflammatory cytokine (IL-10), and the chemokine (SDF-1α) were evaluated in control (A) and FIV-infected (B) placentas at early and late gestation. Bars represent mean Ct values substracted from a negative endpoint (50 − mean Ct), bracketed by standard errors of the mean. P values obtained from single factor ANOVA are noted. P ≤ 0.05 was considered significant.
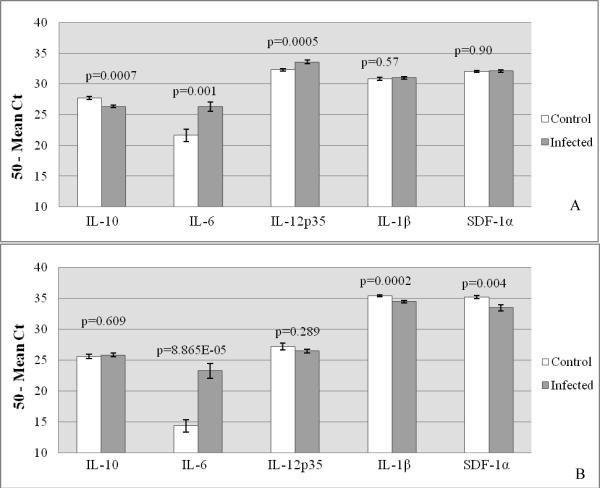
To determine the effect of FIV infection on placental cytokine expression, FIV-infected and control placentas from both early- and late-term gestation were compared. These data are summarized in Table 3. At early gestation, FIV-infection resulted in significantly-increased expression of the pro-inflammatory cytokine mRNAs for IL-6 and IL-12p35, decreased expression of mRNA for the anti-inflammatory cytokine IL-10 mRNA, and had no effect on the mRNA expression of IL-1β and SDF-1α (Fig. 2A). At late gestation, FIV-infection caused increased IL-6 mRNA expression, decreased expression of IL-1β and SDF-1α, and had no effect on mRNA expression of IL-10 and IL-12p35 (Fig. 2B).
Table 3.
Adjusted Mean Ct Values (50-Mean Ct) for Placental Tissues Collected from FIV-infected and Control Cats at Early and Late Pregnancy.
| Early Pregnancy | Late Pregnancy | |||||||
|---|---|---|---|---|---|---|---|---|
| Cytokine | Cont | FIV + | P value | Result* | Cont | FIV + | P value | Result |
| IL-10 | 27.70 | 26.36 | 0.0007 | < | 25.60 | 25.85 | 0.609 | ns |
| IL-6 | 21.68 | 26.35 | 0.001 | > | 14.36 | 23.27 | 8.86E-05 | > |
| IL-12p35 | 32.32 | 33.57 | 0.0005 | > | 27.19 | 26.48 | 0.289 | ns |
| IL-1β | 30.81 | 31.00 | 0.57 | ns | 35.43 | 34.50 | 0.0002 | < |
| SDF-1α | 32.08 | 32.11 | 0.90 | ns | 35.22 | 33.48 | 0.004 | < |
Adjusted mean Ct = mean of triplicate Ct values determined for placental samples by real time RT-PCR that were subtracted from a negative endpoint (50).
Cont = uninfected queens; FIV+ = queens experimentally infected with FIV-B-2542.
P values were determined using single-factor ANOVA; P ≤ 0.05 were significant.
Result = effect of FIV infection on gene expression (> = increased expression; < = decreased expression; ns = not significant).
Figure 2.
Relative Cytokine Expression in Control versus FIV-infected Feline Placental Tissue during Early and Late Gestation
Relative expression of feline immunomodulators was quantified from RNA extracted from whole placental tissue sections using real-time RT-PCR. The pro-inflammatory cytokines (IL-6, IL-12p35, and IL-1β), the anti-inflammatory cytokine (IL-10), and the chemokine (SDF-1α) were evaluated in control and FIV-infected placentas at early (A) and late (B) gestation. Bars represent mean Ct values substracted from a negative endpoint (50 − mean Ct), bracketed by standard errors of the mean. P values obtained from single factor ANOVA are noted. P ≤ 0.05 was considered significant.
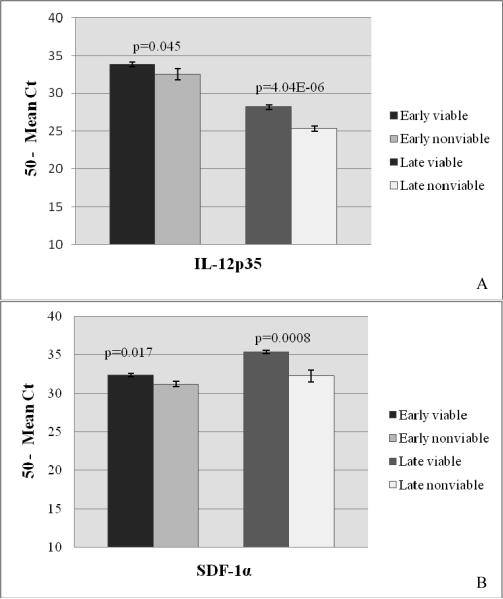
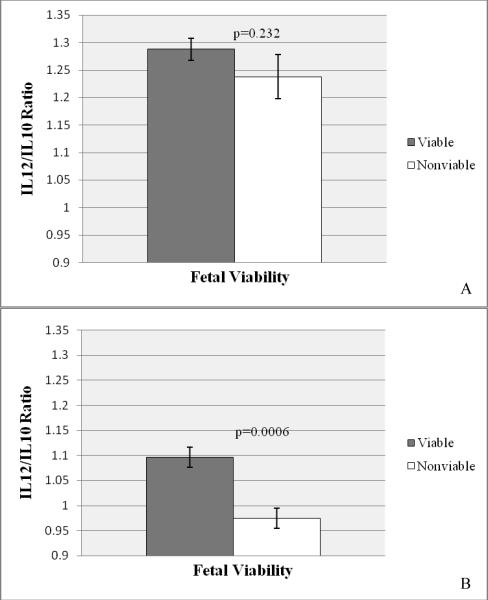
To determine whether cytokine expression was related to fetal viability, placentas from FIV-infected, viable pregnancies were compared to placentas from FIV-infected nonviable pregnancies for both early- and late-term gestation. Of all immunomodulators evaluated, only the expression of IL-12p35 and SDF-1α differed significantly between the two groups (Fig. 3A and B, respectively). Both the cytokine and chemokine were expressed to significantly higher levels in placentas from viable offspring at both stages of pregnancy.
Figure 3.
Relative Expression of Immunomodulators in Placentas from Viable and Nonviable Offspring at Early and Late Pregnancy
Relative expression of feline immunomodulators was quantified from RNA extracted from placental tissue sections using real-time RT-PCR. Tissues were collected from FIV-infected queens producing viable and nonviable offspring at early and late pregnancy. IL-12p35 (A) and SDF-1α (B) expression was measured from early placentas from viable pregnancies (n = 21) versus nonviable pregnancies (n = 6) and late placentas from viable pregnancies (n = 9) versus nonviable pregnancies (n = 14). Bars represent mean Ct values substracted from a negative endpoint (50 − mean Ct), bracketed by standard errors of the mean. P value for IL-12p35 at early pregnancy was determined by both single factor ANOVA (p=0.06) and Wilcoxon Rank Sum Analysis (P=0.045). P ≤ 0.05 was considered significant. (Comparisons for other cytokines showed no significant differences and are not shown).
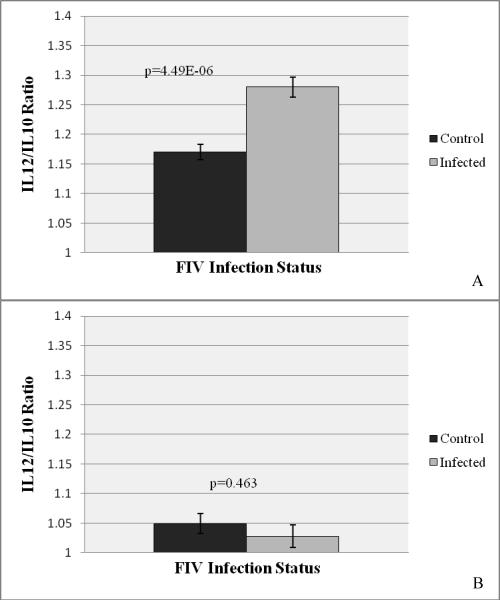
IL-12:IL-10 ratios were determined for control and infected tissues at both stages of pregnancy to measure the Th1:Th2 ratio, as expression of the two cytokines are mutually antagonistic. At early pregnancy, the IL-12:IL-10 ratio was 1.17 in control placentas and 1.28 in FIV-infected placentas, a significant increase (p=4.49E-06) that revealed a virus-induced pro-inflammatory microenvironment (Fig. 4A). At late pregnancy the IL-12:IL-10 ratio in control placentas approached neutrality (1.05), and did not differ significantly in infected placentas (Fig. 4B). A comparison of the IL-12:IL-10 ratio between placentas taken from viable and nonviable offspring did not differ significantly at early pregnancy (Fig. 5A), but the IL-12:IL-10 ratio was significantly higher in the viable group at late pregnancy (Fig. 5B).
Figure 4.
IL-12:IL-10 Ratios for Control and FIV-infected Placental Tissues at Early and Late Pregnancy
The ratio of IL-12 to IL-10 was calculated for each individual sample, and values were analyzed statistically. Bars represent mean IL12:IL10 ratios for control versus FIV-infected tissues at early (A) and late (B) pregnancy bracketed by the standard errors of the mean. P values obtained from single factor ANOVA are noted. P ≤ 0.05 was considered significant.
Figure 5.
IL-12:IL-10 Ratios for Placental Tissues from Viable and Nonviable Offspring at Early and Late Pregnancy
The ratio of IL-12 to IL-10 was calculated for each individual sample, and values were analyzed statistically. Bars represent mean IL12:IL10 ratios for viable versus nonviable pregnancies at early (A) and late (B) pregnancy bracketed by the standard errors of the mean. P values obtained from single factor ANOVA are noted. P ≤ 0.05 was considered significant.
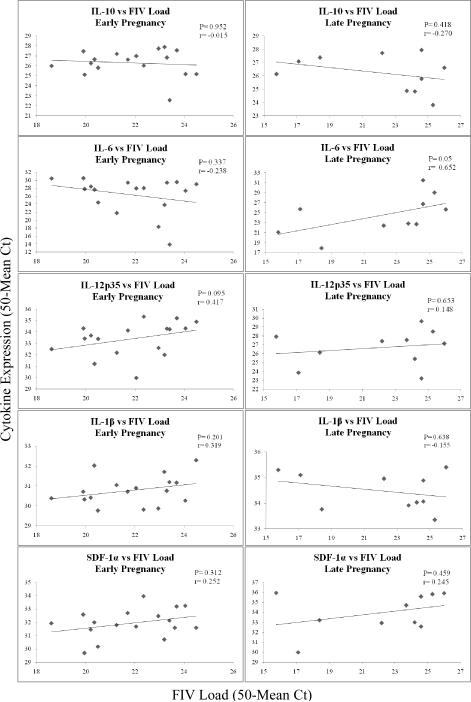
To determine whether cytokine gene expression correlated with the amount of FIV in placental tissues, individual placental samples were analyzed statistically using Spearman Rank Correlation (Fig. 6). A significant correlation between virus load and cytokine gene expression was noted for only one cytokine, IL-6. Increased expression of this cytokine was positively correlated with increasing virus load at late pregnancy only (P=0.05; r= 0.652). No other comparisons approached significance at the 95% confidence level.
Figure 6.
Correlation of FIV Load and Cytokine Gene Expression in Individual Placentas Obtained from FIV-infected Queens at Early and Late Pregnancy.
Adjusted mean Ct values (50-mean Ct) for FIVgag sequence (X) amplified from individual placental tissues were correlated with adjusted mean Ct values for cytokines (Y) amplified from the same tissues. Individual XY data points are plotted on scatter charts, and trend lines are shown. The data were analyzed using Spearman Rank Correlation. P ≤ 0.05 was considered significant.
Discussion
In the present study using the FIV-infected cat model to explore lentivirus-induced placental immunopathology, we used real-time RT-PCR to quantify the expression of representative pro-inflammatory cytokines IL-6, IL-12p35, and IL-1β, the Th2 cytokine IL-10, and the chemokine SDF-1α (the ligand for chemokine receptor CXCR4) in early- and late-term feline placental tissues. To determine the normal pattern of expression, we first compared data obtained from early and late placentas from control cats only. We detected cytokine mRNA in all placental tissues from both early- and late-term gestation. Two of four pro-inflammatory immunomodulators, IL-1β and SDF-1α, were expressed to higher levels at late gestation, while unexpectedly, the remaining two, IL-6 and IL-12p35, decreased from early to late pregnancy. As predicted, the Th2 cytokine IL-10 was decreased at late pregnancy. Placentas from FIV-infected queens yielded the same pattern of cytokine gene expression with the exception of IL-10, which did not differ from early to late pregnancy.
The role that these cytokines play in feline gestation is not yet known. The pattern of expression of IL-6 was similar to that reported by Moussa et al.26 who also found that IL-6 was expressed to higher levels in normal, first-trimester human chorionic villi than later in pregnancy. IL-6 is a pleiotropic cytokine produced by trophoblasts, macrophages, and Th2 cells. IL-6 regulates expression of human chorionic gonadotropin (HCG) from trophoblasts by activating IL-6 receptor-mediated signal transduction,27 and it contributes to trophoblast invasion.28 Therefore, its expression during early pregnancy is essential. Cats do not express chorionic gonadotropin; whether this cytokine plays a role in feline reproductive hormone expression is unknown.
HIV infection alters cytokine expression in placental tissue. Lee et al18 reported increased levels of IL-1β, IL-6, and TNF-α in trophoblasts isolated from HIV-infected human term placentas collected immediately after delivery. On the other hand, other investigators reported decreased expression of IL-6 and other inflammatory cytokines and β-chemokines in HIV-infected term placentas.26 In a recent report29, increased expression of the pro-inflammatory cytokines TNF-α, IL-16, and RANTES was detected in chorionic villi obtained at delivery from HIV-infected women as compared to those obtained from uninfected women. We report that FIV infection altered placental cytokine expression, as compared to controls, but viral effects differed with gestational stage. At early pregnancy, placentas from infected animals had significantly reduced IL-10 mRNA and increased IL-6 and IL-12p35 mRNA, indicating that the virus produced a pro-inflammatory environment at this stage. While the cellular source of IL-12 was not defined, it is known to be a product of activated macrophages and a potent inducer of the Th1 response and NK cell activation.30 At late pregnancy, IL-6 increased dramatically in infected cats, while IL-1β and SDF-1α actually decreased, and IL-10 and IL-12p35 were unchanged. Only IL-6 was consistently increased as a result of FIV infection, regardless of pregnancy stage. Other investigators reported increased levels of IL-631, 32 and IL-1233 in the peripheral circulation of FIV-infected, symptomatic cats.
IL-6 expression showed a strong, positive correlation with FIV load, but only at late pregnancy. The reason why increasing virus load did not positively correlate with IL-6 expression at early pregnancy as well is unclear at this time. This disparity may be explained by a differential impact of the virus on the function of early- versus late-gestation placental cells expressing IL-6 or perhaps those cells prohibiting expression of this inflammatory cytokine, such as Tregs. These findings suggest that IL-6 may be a key player in FIV-induced placental immunopathology. For all other cytokines, gene expression neither positively nor negatively correlated with the amount of FIV in placental tissue. Thus, altered cytokine expression occurring in infected tissues may be independent of virus load. However, variability in gene expression between different cats and even between different placentas from the same cat confounded the interpretation of the correlation analysis. Inter- and intra-cat (different time points) variability in cytokine gene expression in peripheral blood monocytes, determined by real time PCR, was documented previously.34
The Th1:Th2 ratio has been used as an indicator of a pro- or anti-inflammatory microenvironment at the maternal-fetal interface. The pro-inflammatory (Th1) cytokine IL-12 and the anti-inflammatory (Th2) cytokine IL-10 are antagonistic, and thus, the IL-12:IL-10 ratio provided a measure of the inflammatory status of our tissues. The Th1 cytokine was favored during early pregnancy (IL-12:IL-10 = 1.17) in normal placentas, and the ratio was significantly enhanced in FIV-infected queens. At late pregnancy the Th1:Th2 ratio was essentially neutral (IL-12:IL-10 = 1.05) in normal placentas and did not differ significantly in infected placentas. Collectively, the data support a virus-induced, pro-inflammatory placental microenvironment at early, but not late pregnancy.
Clearly, numerous additional cytokines could have been evaluated in the present study. For example, evaluation of expression of IFN-γ in early placentas may have been useful, as we previously reported enhanced expression of this cytokine in late-term placentas from failed pregnancies in the FIV-infected cat model.21 Likewise, higher expression of this cytokine was associated with reproductive failure in mice35 and humans.36 However, it was not our intent to provide an exhaustive analysis of cytokine expression in these tissues, but rather, to evaluate a limited number of cytokines that would provide an assessment of a pro- or anti-inflammatory placental environment. Expression of a more comprehensive array of cytokines is presently being evaluated in microdissected trophoblasts and Tregs.
Our previous reports indicate that reproductive failure in the FIV-infected cat occurs during early pregnancy, as a high rate of fetal demise was detected at week 3–4 of gestation in infected cats,22 and the majority of nonviable fetuses collected at late pregnancy (week 8) were resorptions.21 When cytokine gene expression was compared in placentas from FIV-infected queens producing viable versus nonviable offspring, two pro-inflammatory immunomodulators, IL-12p35 and SDF-1α, were expressed to significantly higher levels in the viable group at both stages of pregnancy. No differences were observed with other cytokines. This result was surprising, as we expected to see higher levels of pro-inflammatory mediators associated with nonviable pregnancy. We are not yet able to explain this occurrence. Likewise, we previously reported higher levels of expression of CXCR4 in placentas from viable pregnancies at late term;37 thus, both chemokine and chemokine receptor expression were associated with pregnancy viability.
The chemokines RANTES, MIP-1α, and MIP-1β, which bind CCR5, and SDF-1α, which binds CXCR4, are well-described antagonists for HIV infection of target cells that express these chemokine receptors.38 Therefore, aside from their role in inflammation, we were particularly interested in expression of CXCR4 and SDF-1α in placentas because CXCR4 is the co-receptor utilized by FIV for target cell binding. Thus, the binding of CXCR4 by its natural ligand, SDF-1α, could potentially block FIV infection of target cells. We previously speculated that cells expressing CXCR4 are important to pregnancy maintenance in the cat model.37 The present data allow for the possibility that SDF-1α may limit FIV infection of such cells by binding to CXCR4, contributing to successful pregnancy. The non-significant correlation of SDF-1α expression and FIV load renders it difficult to determine the relationship between the two parameters.
In a recent report,39 SDF-1 gene expression was quantified in decidua-derived mesenchymal stem cells from normal and pre-eclamptic women. SDF-1 was expressed to higher levels in the normal population. In placental tissues, SDF-1α is best known to function in trophoblast invasion via “cross talk” between the chemokine expressed by decidual stromal cells and CXCR4 on the trophoblast40 and to promote trophoblast survival by stimulating pathways which inhibit apoptosis.41 Its pro-inflammatory function in the periphery includes T cell migration (reviewed by N. Karin).42 However, an anti-inflammatory function for this chemokine was recently reported in autoimmune disease, the SDF-1α/CXCR4 interaction polarizing Th1 cells into antigen-specific regulatory T cells.43 While this anti-inflammatory mechanism has not been explored in placental tissues, the cat may provide a suitable model to examine whether decidual T regs are impacted by this chemokine.
The IL-12:IL-10 ratio was significantly depressed in the nonviable group at late gestation, but it did not reach a statistically significant decrease at early pregnancy. These findings were unexpected, as we predicted that fetal nonviability would be accompanied by increased placental inflammation. The diminished expression of these cytokines may suggest the reduction in number or function of inflammatory cells expressing these cytokines as a result of viral infection, since all placentas in the viable versus nonviable comparison were collected from FIV-infected queens. Alternatively, the lower levels of expression of the cytokines may be a consequence, rather than a cause of pregnancy failure. Other investigators reported similar inflammatory cytokine expression patterns associated with human spontaneous abortion and in abortion-prone mice,44, 45 which led to challenges to the Th1:Th2 paradigm of pregnancy.46, 47
The effects of FIV infection on placental cytokine expression at late pregnancy reported herein differ from those of our prior report utilizing many of the same late-term tissues.21 We previously found that pro-inflammatory cytokines interferon (IFN)-γ and IL-1β were significantly increased in placentas from resorbed versus nonresorbed offspring, predicting a pro-inflammatory status that may have compromised pregnancy. However, in that study, we grouped total viable and total nonviable tissues, including those from both control and infected queens, while in the present study we evaluated only those from FIV-infected animals. This change may have produced the different result.
An important advantage of the FIV-infected cat model of HIV MTCT is the ability to collect and evaluate placentas and fetuses at defined time points from both infected and control cats, which is not possible with humans. While human placental tissues may be obtained following miscarriages or elective abortions, we have no control over timing of those events, and collecting tissues from infected and uninfected women at the same gestational stage would be very difficult. Moreover, in developed countries, HIV-infected women are generally treated with anti-retroviral therapy, confounding the interpretation of any virological or immunological data. Collectively, the data that we have obtained using the FIV-infected cat model parallel those reported for HIV, revealing a lentivirus-induced pro-inflammatory placental environment at early pregnancy. The vulnerability of fetal development to inflammation at early gestation is well documented,3 and thus, our findings implicate inflammation as a contributor to the high rate of pregnancy failure that we detected at early pregnancy in this animal model. These findings reinforce the utility of the FIV-infected cat as a model for HIV vertical transmission and reproductive failure.
A limitation of this study is that we evaluated target gene mRNA only, which may be transiently expressed. Given that gene expression may be regulated at the translational level, as well as the transcriptional level, we may have obtained an incomplete picture of expression of one or more genes of interest. Quantification of cytokine proteins by immunoassay is a preferred means of measuring gene expression. However, our ability to perform these immunological assays is limited by the lack of commercially-available antibodies to feline cytokines and immunological markers.
Key to understanding the relevance of these data is to identify the placental or decidual cells that are a source of these cytokines. Trophoblasts are fetal-derived cells that produce numerous cytokines that regulate placental function.48–52 Maternal Tregs, located in the deciduum, produce cytokines that suppress inflammation.53 Both of these cell populations can be infected by HIV, and feline Tregs were shown to support the replication of FIV.54, 55 In the event that these cell populations were infected with FIV in our cats, then viral infection leading to depletion or altered function of these cells may explain abnormal cytokine patterns. Our laboratory is currently exploring the role of trophoblasts and decidual Tregs in lentivirus-induced immunopathology and pregnancy failure.
Acknowledgements
We thank Dr. Shane C. Burgess and Leslie A. Shack for laboratory facility use and quantitative PCR advisement. We are grateful to Dr. Edward A. Hoover, Colorado State University, for providing the FIV-B-2542 inoculum. We thank the veterinary staff at the College of Veterinary Medicine, Mississippi State University for assistance with animal care and surgeries. This project was supported by the National Institutes of Health (2R15AI048419-02A1) and (3R15AI048419-02A1S1).
References
- 1.Saito S. Cytokine cross-talk between mother and the embryo/placenta. J Reprod Immunol. 2001;52:15–33. doi: 10.1016/s0165-0378(01)00112-7. [DOI] [PubMed] [Google Scholar]
- 2.Clark DA, Chaouat G. What do we know about spontaneous abortion mechanisms? Am J Reprod Immunol. 1989;19:28–37. doi: 10.1111/j.1600-0897.1989.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 3.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, III, Petraglia F. Inflammation and Pregnancy. Reproductive Sciences. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 4.Costeas PA, Koumouli A, Giantsiou-Kyriakou A, Papaloizou A, Koumas L. Th2/Th3 cytokine genotypes are associated with pregnancy loss. Hum Immunol. 2004;65:135–141. doi: 10.1016/j.humimm.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–482. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 6.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 7.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 8.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 9.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 10.Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol. 2008;20:462–469. doi: 10.1111/j.1365-2826.2008.01668.x. [DOI] [PubMed] [Google Scholar]
- 11.CDC . CDC HIV/AIDS Fact Sheet. Department of Health and Human Services; USA: 2007. Mother-to-Child (Perinatal) HIV Transmission and Prevention. [Google Scholar]
- 12.UNAIDS/WHO . Towards Universal Access. Joint United Nations Programme on HIV/AIDS; 2009. [Google Scholar]
- 13.D'Ubaldo C, Pezzotti P, Rezza G, Branca M, Ippolito G. Association between HIV-1 infection and miscarriage: a retrospective study. DIANAIDS Collaborative Study Group. Diagnosi Iniziale Anomalie Neoplastiche AIDS. Aids. 1998;12:1087–1093. doi: 10.1097/00002030-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Kumar RM, Uduman SA, Khurranna AK. Impact of maternal HIV-1 infection on perinatal outcome. Int J Gynaecol Obstet. 1995;49:137–143. doi: 10.1016/0020-7292(95)02356-h. [DOI] [PubMed] [Google Scholar]
- 15.Langston C, Lewis DE, Hammill HA, Popek EJ, Kozinetz CA, Kline MW, Hanson IC, Shearer WT. Excess intrauterine fetal demise associated with maternal human immunodeficiency virus infection. J Infect Dis. 1995;172:1451–1460. doi: 10.1093/infdis/172.6.1451. [DOI] [PubMed] [Google Scholar]
- 16.Kedzierska K, Crowe SM. Cytokines and HIV-1: interactions and clinical implications. Antivir Chem Chemother. 2001;12:133–150. doi: 10.1177/095632020101200301. [DOI] [PubMed] [Google Scholar]
- 17.Shearer WT, Reuben J, Lee BN, Popek EJ, Lewis DE, Hammill HH, Hanson IC, Kline MW, Langston C. Role of placental cytokines and inflammation in vertical transmission of HIV infection. Acta Paediatr Suppl. 1997;421:33–38. doi: 10.1111/j.1651-2227.1997.tb18317.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee BN, Ordonez N, Popek EJ, Lu JG, Helfgott A, Eriksen N, Hammill H, Kozinetz C, Doyle M, Kline M, Langston C, Shearer WT, Reuben JM. Inflammatory cytokine expression is correlated with the level of human immunodeficiency virus (HIV) transcripts in HIV-infected placental trophoblastic cells. J Virol. 1997;71:3628–3635. doi: 10.1128/jvi.71.5.3628-3635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Neil LL, Burkhard MJ, Hoover EA. Frequent perinatal transmission of feline immunodeficiency virus by chronically infected cats. J Virol. 1996;70:2894–2901. doi: 10.1128/jvi.70.5.2894-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allison RW, Hoover EA. Covert vertical transmission of feline immunodeficiency virus. AIDS Res Hum Retroviruses. 2003;19:421–434. doi: 10.1089/088922203765551764. [DOI] [PubMed] [Google Scholar]
- 21.Weaver CC, Burgess SC, Nelson PD, Wilkinson M, Ryan PL, Nail CA, Kelly-Quagliana KA, May ML, Reeves RK, Boyle CR, Coats KS. Placental immunopathology and pregnancy failure in the FIV-infected cat. Placenta. 2005;26:138–147. doi: 10.1016/j.placenta.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Boudreaux CE, Lockett NN, Chemerys DN, Clay BT, Scott VL, Willeford B, Brown T, Coats KS. Maternal hematological and virological characteristics during early feline immunodeficiency virus (FIV) infection of cats as predictors of fetal infection and reproductive outcome at early gestation. Vet Immunol Immunopathol. 2009;131:290–297. doi: 10.1016/j.vetimm.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers AB, Hoover EA. Maternal-fetal feline immunodeficiency virus transmission: timing and tissue tropisms. J Infect Dis. 1998;178:960–967. doi: 10.1086/515692. [DOI] [PubMed] [Google Scholar]
- 24.Lockett NN, Scott VL, Boudreaux CE, Clay BT, Pruett SB, Ryan PL, Coats KS. Expression of regulatory T cell (Treg) activation markers in endometrial tissues from early and late pregnancy in the feline immunodeficiency virus (FIV)-infected cat. Placenta. 2010 doi: 10.1016/j.placenta.2010.06.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wessa P. Free Statistics Software. version 1.1.23-r6 Office for Research Development and Education; 2010. [Google Scholar]
- 26.Moussa M, Roques P, Fievet N, Menu E, Maldonado-Estrada JG, Brunerie J, Frydman R, Fritel X, Herve F, Chaouat G. Placental cytokine and chemokine production in HIV-1-infected women: trophoblast cells show a different pattern compared to cells from HIV-negative women. Clin Exp Immunol. 2001;125:455–464. doi: 10.1046/j.1365-2249.2001.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masuhiro K, Matsuzaki N, Nishino E, Taniguchi T, Kameda T, Li Y, Saji F, Tanizawa O. Trophoblast-derived interleukin-1 (IL-1) stimulates the release of human chorionic gonadotropin by activating IL-6 and IL-6-receptor system in first trimester human trophoblasts. J Clin Endocrinol Metab. 1991;72:594–601. doi: 10.1210/jcem-72-3-594. [DOI] [PubMed] [Google Scholar]
- 28.Dubinsky V, Poehlmann TG, Suman P, Gentile T, Markert UR, Gutierrez G. Role of regulatory and angiogenic cytokines in invasion of trophoblastic cells. Am J Reprod Immunol. 2010;63:193–199. doi: 10.1111/j.1600-0897.2009.00778.x. [DOI] [PubMed] [Google Scholar]
- 29.Kfutwah A, Mary JY, Lemen B, Leke R, Rousset D, Barré-Sinoussi F, Nerrienet E, Menu E, Ayouba A. Plasmodium falciparum Infection Significantly Impairs Placental Cytokine Profile in HIV Infected Cameroonian Women. PLoS ONE. 2009;4:e8114. doi: 10.1371/journal.pone.0008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutterwala FS, Mosser DM. The taming of IL-12: suppressing the production of proinflammatory cytokines. J Leukoc Biol. 1999;65:543–551. [PubMed] [Google Scholar]
- 31.Ohashi T, Goitsuka R, Watari T, Tsujimoto H, Hasegawa A. Elevation of feline interleukin 6-like activity in feline immunodeficiency virus infection. Clin Immunol Immunopathol. 1992;65:207–211. doi: 10.1016/0090-1229(92)90148-h. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence CE, Callanan JJ, Willett BJ, Jarrett O. Cytokine production by cats infected with feline immunodeficiency virus: a longitudinal study. Immunology. 1995;85:568–574. [PMC free article] [PubMed] [Google Scholar]
- 33.Dean GA, Pedersen NC. Cytokine response in multiple lymphoid tissues during the primary phase of feline immunodeficiency virus infection. J Virol. 1998;72:9436–9440. doi: 10.1128/jvi.72.12.9436-9440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kipar A, Leutenegger CM, Hetzel U, Akens MK, Mislin CN, Reinacher M, Lutz H. Cytokine mRNA levels in isolated feline monocytes. Veterinary Immunology and Immunopathology. 2001;78:305–315. doi: 10.1016/s0165-2427(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 35.Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, Wegmann TG. Control of fetal survival in CBA × DBA/2 mice by lymphokine therapy. J Reprod Fertil. 1990;89:447–458. doi: 10.1530/jrf.0.0890447. [DOI] [PubMed] [Google Scholar]
- 36.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott VL, Burgess SC, Shack LA, Lockett NN, Coats KS. Expression of CD134 and CXCR4 mRNA in term placentas from FIV-infected and control cats. Vet Immunol Immunopathol. 2008;123:90–96. doi: 10.1016/j.vetimm.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verani AaL, P. Chemokines as natural HIV antagonists. Current Molecular Medicine. 2002;2:691–702. doi: 10.2174/1566524023361862. [DOI] [PubMed] [Google Scholar]
- 39.Hwang JH, Lee MJ, Seok OS, Paek YC, Cho GJ, Seol HJ, Lee JK, Oh MJ. Cytokine expression in placenta-derived mesenchymal stem cells in patients with pre-eclampsia and normal pregnancies. Cytokine. 2010;49:95–101. doi: 10.1016/j.cyto.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Zhou WH, Du MR, Dong L, Yu J, Li DJ. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal cells in human first-trimester pregnancy. Hum Reprod. 2008;23:2669–2679. doi: 10.1093/humrep/den308. [DOI] [PubMed] [Google Scholar]
- 41.Jaleel MA, Tsai AC, Sarkar S, Freedman PV, Rubin LP. Stromal cell-derived factor-1 (SDF-1) signalling regulates human placental trophoblast cell survival. Mol Hum Reprod. 2004;10:901–909. doi: 10.1093/molehr/gah118. [DOI] [PubMed] [Google Scholar]
- 42.Karin N. The multiple faces of CXCL12 (SDF-1{alpha}) in the regulation of immunity during health and disease. J Leukoc Biol. 2010 doi: 10.1189/jlb.0909602. [DOI] [PubMed] [Google Scholar]
- 43.Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. 2008;205:2643–2655. doi: 10.1084/jem.20080730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenclussen AC, Fest S, Busse P, Joachim R, Klapp BF, Arck PC. Questioning the Th1/Th2 paradigm in reproduction: peripheral levels of IL-12 are down-regulated in miscarriage patients. Am J Reprod Immunol. 2002;48:245–251. doi: 10.1034/j.1600-0897.2002.01136.x. [DOI] [PubMed] [Google Scholar]
- 45.Ostojic S, Dubanchet S, Chaouat G, Abdelkarim M, Truyens C, Capron F. Demonstration of the presence of IL-16, IL-17 and IL-18 at the murine fetomaternal interface during murine pregnancy. Am J Reprod Immunol. 2003;49:101–112. doi: 10.1034/j.1600-0897.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 46.Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol. 2004;134:93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- 47.Chaouat G, Zourbas S, Ostojic S, Lappree-Delage G, Dubanchet S, Ledee N, Martal J. A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical Th1/Th2 dichotomy. J Reprod Immunol. 2002;53:241–256. doi: 10.1016/s0165-0378(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 48.Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–548. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilczynski JR. Th1/Th2 cytokines balance--yin and yang of reproductive immunology. Eur J Obstet Gynecol Reprod Biol. 2005;122:136–143. doi: 10.1016/j.ejogrb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Bennett WA, Lagoo-Deenadayalan S, Brackin MN, Hale E, Cowan BD. Cytokine expression by models of human trophoblast as assessed by a semiquantitative reverse transcription-polymerase chain reaction technique. Am J Reprod Immunol. 1996;36:285–294. doi: 10.1111/j.1600-0897.1996.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 51.Bennett WA, Lagoo-Deenadayalan S, Stopple JA, Barber WH, Hale E, Brackin MN, Cowan BD. Cytokine expression by first-trimester human chorionic villi. Am J Reprod Immunol. 1998;40:309–318. doi: 10.1111/j.1600-0897.1998.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 52.Guilbert L, Robertson SA, Wegmann TG. The trophoblast as an integral component of a macrophage-cytokine network. Immunol Cell Biol. 1993;71(Pt 1):49–57. doi: 10.1038/icb.1993.5. [DOI] [PubMed] [Google Scholar]
- 53.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joshi A, Vahlenkamp TW, Garg H, Tompkins WA, Tompkins MB. Preferential replication of FIV in activated CD4(+)CD25(+)T cells independent of cellular proliferation. Virology. 2004;321:307–322. doi: 10.1016/j.virol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Joshi A, Garg H, Tompkins MB, Tompkins WA. Preferential feline immunodeficiency virus (FIV) infection of CD4+ CD25+ T-regulatory cells correlates both with surface expression of CXCR4 and activation of FIV long terminal repeat binding cellular transcriptional factors. J Virol. 2005;79:4965–4976. doi: 10.1128/JVI.79.8.4965-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]