Abstract
In vitro metabolism of methadone was investigated in cytochrome P450 (CYP) Supersomes and phenotyped human liver microsomes (HLMs) to reconcile past findings on CYP involvement in stereo-selective metabolism of methadone. Racemic methadone was used for incubations; (R)-and (S)-methadone turnover and (R)- and (S)-EDDP formation were determined using chiral liquid chromatography-tandem mass spectrometry. CYP Supersome activity for methadone use and EDDP formation ranked CYP2B6>3A4>2C19>2D6>2C18, 3A7>2C8, 2C9, 3A5. After abundance scaling, CYP3A4, 2B6 and 2C19 accounted for 63–74, 12–32 and 1,4–14% of respective activity. CYP2B6, 2D6 and 2C18 demonstrated a preference for (S)-EDDP formation; CYP2C19, 3A7 and 2C8 for (R)-EDDP; 3A4 none. Correlation analysis with 15 HLMs supported the involvement of CYP2B6 and 3A. The significant correlation of S/R ratio with CYP2B6 activity confirmed its stereo-selectivity. CYP2C19 and 2D6 inhibitors and monoclonal antibody (mAb) did not inhibit EDDP formation in HLM. Chemical and mAb inhibition of CYP3A in high 3A activity HLM reduced EDDP formation by 60–85%; inhibition of CYP2B6 in 2B6 high activity HLM reduced (S)-EDDP formation by 80% and (R)-EDDP formation by 55%. Inhibition changed methadone metabolism in a stereo-selective manner. When CYP3A was inhibited, 2B6 mediated (S)-EDDP formation predominated; S/R stereo-selectivity increased. When 2B6 was inhibited (S)-EDDP formation fell and stereo-selectivity decreased. The results confirmed the primary roles of CYPs 3A4 and 2B6 in methadone metabolism; CYP2C8 and 2C9 did not appear involved; 2C19 and 2D6 have minimal roles. CYP2B6 is the primary determinant of stereo-selective metabolism; stereo-selective inhibition might play a role in varied plasma concentrations of the two enantiomers.
Methadone is a μ-opioid receptor agonist that is used for the treatment of chronic pain and opioid dependence. Clinically it is used in the racemic form; (R)-methadone has higher affinity at μ and δ opioid receptors [1] and greater analgesic activity [2]. Pharmacokinetic studies in humans have also found differences between the two isomers, with (R)-methadone having significantly longer elimination half-life, greater volume of distribution, and lower protein binding [3–5]. Methadone is subject to numerous pharmacokinetic drug interactions; these are thought to primarily occur at cytochrome P450 (CYP) sites of methadone metabolism [6–9]. The stereo-selectivity of the drug interaction may be of importance; interactions that increase or decrease (R)-methadone may lead to toxicity (i.e. respiratory depression) or withdrawal, respectively; interactions that increase (S)-methadone may increase the incidence of prolonged QT intervals [10].
Since the initial studies on CYP involvement in the in vitro metabolism of methadone [11,12], five other laboratories have investigated comparative involvement of different CYPs in methadone metabolism [13–19]. Most of these in vitro studies focused on the predominant metabolic pathway, N-demethylation followed by spontaneous cyclization to form 2-ethyl-1,5-dimethyl-3,3,-diphenylpyrrolidine (EDDP). The involvement of CYP3A4 was noted in all of these studies; and all that studied stereo-selectivity noted none for 3A4-mediated methadone N-demethylation [13,15,16,18,19]. Two laboratories also studied methadone depletion, with contrasting results; Wang and De Vane [15] found CYP3A4 depleted (R)-methadone at a much higher rate, Gerber et al. [16] found no difference in the depletion of (R)- and (S)-methadone by CYP3A4. As tools to study CYP2B6 became available, its important role in methadone N-demethylation was also noted [16,17], and studies on the stereo-selectivity of the reaction consistently showed higher rates of (S)-methadone metabolism by CYP2B6 [16,18,19]. Other CYPs have also been reported to be involved in methadone N-demethylation including: 2D6 [11,15,16], 2C8 [15], 2C9 [11–13,16] and 2C19 [11,13,16,18,19]. Among these CYPs, 2C19 (R > S) [16,18,19], 2C8 (R > S) and 2D6 (S > R) [15] displayed stereo-selectivity.
These studies leave some questions about the involvement of CYPs besides 3A4 and 2B6 in methadone N-demethylation and the stereo-selectivity of methadone depletion. Only one study on CYP2B6 stereo-selective methadone N-demethylation provided detailed evidence beyond metabolism in recombinant expressed CYP [19]. The current investigation has both confirmed and expanded on these findings. We have tested the stereo-selective metabolism of methadone in a large panel of CYP Supersomes with scaling of Supersome activities, correlation analysis and use of a number of specific chemical inhibitors and monoclonal antibodies. The relative contribution of 3A4 and 2B6, as well as other CYPs, to in vitro methadone metabolism and stereo-selective metabolism was determined.
Materials and Methods
Materials
Racemic methadone, racemic EDDP perchlorate and the corresponding d3-labeled analogues were purchased from Cerilliant (Round Rock, TX, USA). D-glucose 6-phosphate monosodium salt, glucose-6-phosphate dehydrogenase, β-NADP sodium salt, EDTA disodium salt, MgCl2, ketoconazole, isoniazid, troleandomycin, raloxifene, tamoxifen, diltiazem, N,N’N’-triethylenethiophosphoramide (Thio-TEPA), orphenadrine, ticlopidine, quinidine and erythromycin were obtained from Sigma/Aldrich Chemical Corp. (St. Louis, MO, USA). Fluoxetine was obtained from Research Biochemicals International (Natick, MA, USA). Verapamil was from Knoll Pharmaceutical Co. (Whippany, NJ, USA). Midazolam was from Hoffmann-LA Roche Inc. (Nutley, NJ, USA). Paroxetine was from GlaxoSmithKline Research & Development (Research Triangle Park, NC, USA). Insect cell cDNA-expressed human CYPs (Supersomes) and phenotyped HLMs were purchased from BD-Gentest (Woburn, MA, USA). Inhibitory mAbs to human CYP3A4/5, 2B6, 2C19 and 2D6 were provided by the National Cancer Institute of the National Institutes of Health (Bethesda, MA, USA). All aqueous reagents were prepared in purified water (specific resistance >18.2 mΩ/cm) obtained from a Milli-Q Plus water purification system.
In vitro incubation of methadone with recombinant human CYPs
The metabolism of methadone was evaluated in microsomes prepared from insect cells transfected with cDNAs encoding for human CYP1A2, 2A6, 2B6, 2C8, 2C9*1, 2C18, 2C19, 2D6*1, 2E1, 3A4, 3A5 and 3A7. All Supersomes co-expressed NADPH CYP reductase, and those that co-expressed cytochrome b5 were used where available, this was not the case for 1A2, 2C18 and 3A5. The incubation mixture (final volume 200 µl) contained incubation buffer (0.1 M phosphate buffer pH 7.4 with 1.0 mM EDTA and 5.0 mM MgCl2); an NADPH generating system (NADPH GS) composed of 10 mM glucose-6-phosphate, 1.2 mM NADP, and 1.2 units of glucose-6-phosphate dehydrogenase; 25 pmol P450 and 750 ng/ml methadone. The reaction was initiated by adding the NADPH GS, and incubated at 37°C in a shaking water bath for 30 min. This was repeated for 10 min. for 2B6 and 3A4. Control insect cell microsomes were used at the mean protein concentration averaged over all of the Supersomes. The reactions were stopped by the addition of 100 µl ice-cold methanol and the mixture was stored at −75°C until analysis. Scaling of activities was determined as: total activity = Σactivityi × abundancei, where i=individual CYP activities and abundance was determined from immunoquantitation of CYP or from the relative activity factor (RAF), where RAF = (mean activity for CYP-specific reaction in HLM) / (activity for CYP-specific reaction by cDNA-expressed CYP). Data for HLM immunoquantitations and CYP-specific activities were from a commercial (Gentest) database (except for CYP2B6 activities) as previously described [20].
Correlation studies
HLMs from 15 individual donors along with data for CYP-specific enzyme activities, provided by BD-Gentest, were used to study the relationship between the formation of (R)- and (S)-EDDP and the metabolism of selective CYP substrates. The ability of HLM from each donor to metabolize methadone to (R)- and (S)-EDDP, as well as the S/ R ratio, was correlated with CYP-specific enzyme activities. The experiment was performed using incubation conditions described above with 750 ng/ml methadone and 0.5 mg/ml HLM protein for 30 min.
Inhibition studies
The inhibition of methadone N-demethylation by chemical inhibitors and monoclonal antibodies was evaluated in CYP Supersomes and phenotyped HLMs. 1) Chemical inhibition. The selective chemical inhibitors included: CYP3A4/5 inhibitors ketoconazole (2 µM), midazolam (10–100 µM), fluoxetine (10 µM), erythromycin (50 µM), isoniazid (500 µM), troleandomycin (10–50 µM), raloxifene (30 µM), tamoxifen (10 µM), verapamil (10 µM) and diltiazem (10 µM); CYP2B6 inhibitors Thio-TEPA (0.1–100 µM) and orphenadrine (100 µM); CYP2C19 inhibitor ticlopidine (50 µM); 2D6 inhibitors quinidine (10 µM), fluoxetine (10 µM) and paroxetine (1 µM). The incubation was performed with or without a 15-min. pre-incubation for mechanism-based and non-mechanism-based inhibitors, respectively, with 25 pmol CYP Supersome or 0.5 mg/ml HLM protein, followed by the addition of methadone (750 ng/ml). CYP3A4 and 2B6 Supersomes and HLM with high 3A4 and 2B6 activity were incubated for 15 min.; 2D6 and 2C19 Supersomes and HLM with high CYP2D6 and 2C19 activities were incubated for 30 min. The incubation sample with addition of same amount of methanol served as control. 2) mAbs inhibition. The recommended volumes (10 µl) of mAbs specific for CYP3A4/5, 2B6, 2C19 and 2D6 were pre-incubated with 0.5 mg/ml HLM protein in 200 µl of incubation buffer for 5 min. at 37°C [21]. Tubes were then placed on ice and methadone at the final concentration of 750 ng/ml was added. The reaction was initiated by addition of the NADPH GS and incubated at 37°C for the times noted above. Ten microlitres of egg lyersozyme was used as a control.
Liquid chromatographic-electrospray ionization-tandem mass spectrometric analysis (LC-MS/MS)
The quantification of (R)- and (S)-methadone and (R)- and (S)-EDDP was performed using our previously described LC-ESI-MS/MS method [5]. In brief, 20 µl of 1 ng/µl (R)- and (S)-methadone-d3 and (R)- and (S)-EDDP-d3 were added to the incubation samples as internal standards followed by 25 µl of 1 N NaOH, and 3 ml of methyl t-butyl ether. After mixing and centrifugation, the organic layer was transferred to a clean 13×100 mm tube, acidified with 10 µl of 0.1 N HCl and mixed. The organic solvent was evaporated and the residues were reconstituted in 100 µL of methanol:10 mM ammonium acetate (1:9, v/v) and 10 µl was injected into the LC-MS/MS.
LC-MS/MS was performed on a Surveyor HPLC system and a TSQ Quantum triple quadrupole MS with ESI source (Thermo-Finnigan, San Jose, CA, USA). Chiral separations were conducted on a Chiral-AGP 50 × 2.0 mm, 5 µm column (Analytical, Pompton Plains, NJ, USA). The mobile phase was a gradient of 10 mM ammonium acetate (A) and methanol (B) with %A at 88, 88, 60, 60, 88 and 88 at 0, 2.5, 3.6, 9.0, 9.1 and 16 min., respectively. The multiple reaction monitoring of m/z 310 to 265, m/z 313 to 268, m/z 278 to 234 and m/z 281 to 234 were used to analyse methadone, methadone-d3, EDDP and EDDP-d3, respectively. Weighted 1/X2 quadratic curves were used for quantification over an enantiomer range from 2.5 to 500 ng/ml. Quality control samples prepared in HLM at 2.5, 5.0, 100 and 400 ng/ml (N=5 per concentration) were tested at initial validation and had % target accuracies within 16% at the lower limit and within 15% at higher concentrations; % CVs were within 13.8% [5]. Further validation data are available in our previous publication [5].
Results
Stereo-selective metabolism of methadone in CYP Supersomes
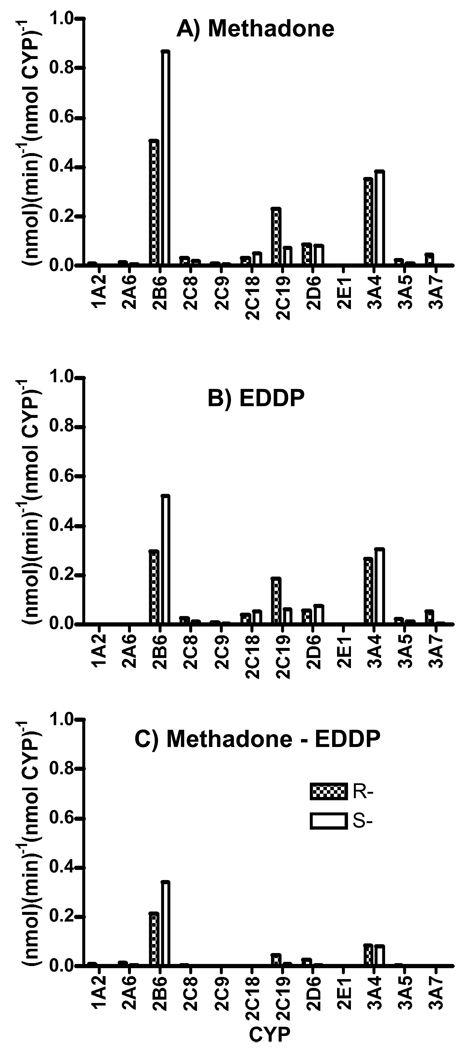
Incubation of racemic methadone with cDNA-expressed CYPs revealed that the catalytic activities of CYP Supersomes for the turnover of (R)-methadone was in the order of 2B6>3A4>2C19>2D6>3A7>2C18>2C8>3A5; for the utilization of (S)-methadone, it was in the order of 2B6>3A4>2D6>2C19>2C18>2C8> 3A5 (fig. 1A). The same order was observed for EDDP production (fig. 1B). The difference between methadone turnover and EDDP production was mainly found with 2B6 and 3A4 (fig. 1C), which indicated that some other metabolic pathways might be involved. Enantiomer selectivity was quantitated as (S-R)/R × 100%. For CYP3A4, 2B6, 2C19, 2D6, 2C18 and 2C8, the differences between two enantiomers for EDDP production were 15, 76, −67, 38, 41 and −44%, respectively. The respective differences of methadone turnover were 10, 71, −68, −1, 62 and −45%.
Figure 1.
Methadone turnover (A). EDDP production (B) and Methadone turnover minus EDDP production (C) in cDNA-expressed CYPs (Supersomes) incubated with 750 ng/ml methadone. Specific use of (R)- and (S)-methadone and production of (R)- and (S)-EDDP is indicated by speckled and hatched bars, respectively. Results are the mean of duplicate incubations. For CYPs with higher activities (2B6 and 3A4) 10 min incubations were used; 30-min. incubations were used for other CYPs.
Scaling
When activities were scaled based on relative abundance of CYPs in HLM determined by immunoquantitation or by RAF [20], the predominant enzyme contributing to methadone turnover and EDDP production was 3A4 (table 1). CYP2B6 showed the next highest relative activity with contribution to (S)-turnover/production (18–32%) exceeding the (R )-turnover/production (12–21%). For CYP2C19, the (R)-turnover/production (5–14%) exceeded (S)-turnover/production (2–4%). The method of determining abundance had some impact on absolute percentages, but not on the ranking of contribution (table 1).
Table 1.
Scaling of contribution of individual CYPs to the depletion of R- and S-methadone (meth) and formation of R- and S-EDDP.
| Abundance from Immunquantitation |
Abundance from Relative Activity Factor |
|||||||
|---|---|---|---|---|---|---|---|---|
| CYP |
(R)-Meth |
(S)-Meth |
(R)-EDDP |
(S)-EDDP |
(R)-Meth |
(S)-Meth |
(R)-EDDP |
(S)-EDDP |
| 1A2 | 0.7 | 0.3 | 0.0 | 0.0 | 1.1 | 0.4 | 0.0 | 0.0 |
| 2A6 | 1.0 | 0.4 | 0.0 | 0.0 | 2.0 | 0.6 | 0.0 | 0.0 |
| 2B6 | 14.3 | 23.0 | 11.5 | 18.5 | 21.3 | 31.9 | 17.8 | 26.7 |
| 2C8 | 0.4 | 0.2 | 0.4 | 0.2 | 8.1 | 0.4 | 0.9 | 0.4 |
| 2C9 | 1.2 | 0.5 | 1.2 | 0.4 | 3.4 | 1.4 | 3.7 | 1.1 |
| 2C18 | 0.4 | 0.6 | 0.7 | 0.9 | * | * | * | * |
| 2C19 | 13.0 | 3.9 | 14.4 | 4.4 | 5.0 | 1.4 | 5.8 | 1.6 |
| 2D6 | 1.1 | 0.1 | 1.0 | 1.3 | 0.5 | 0.4 | 0.5 | 0.6 |
| 3A4 | 67.9 | 70.2 | 70.7 | 74.3 | 66.0 | 63.4 | 71.3 | 69.6 |
Note – Scaling was performed using the Gentest liver bank data for immunquantitations and activities (except for CYP2B6 activity) as previously described [20].
Reference activity for 2C18 in HLM is not available.
Other metabolites
In an effort to understand the contribution of other metabolic pathways, we also monitored for 2-ethyl-5-methyl-3,3-diphenylpyraline (EMDP) in most incubates with a limit of detection of approximately 1.0 ng/ml; no EMDP production was noted under these incubation conditions. In selected samples, we also performed selective ion monitoring for hydroxyl metabolites of methadone and EDDP (M+16) and were not able to detect any peaks. Subsequent studies focused on (R)- and (S)-EDDP production.
Correlation analysis
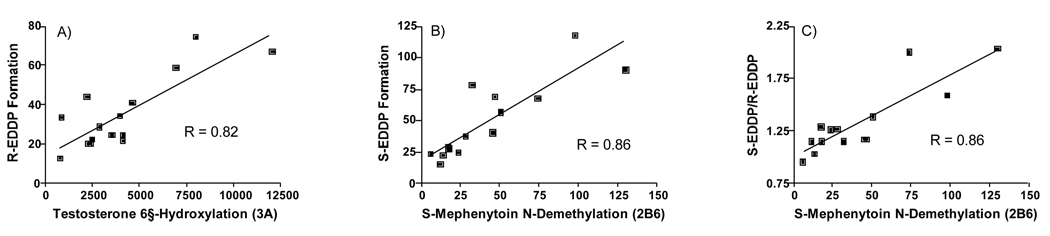
The rate of formation of (R)-EDDP and (S)-EDDP was also determined in 15 individual phenotyped HLMs and the data were correlated with CYP phenotyped activities provided by the vendor. Significant correlations (p<0.05) were observed for both (R)-EDDP (r = 0.82) and (S)-EDDP (r = 0.54) formation with testosterone 6β-hydroxylation catalyzed by CYP3A, and (R)-EDDP (r = 0.60) and (S)-EDDP (r = 0.86) (S)-mephenytoin N-demethylation catalyzed by 2B6 (fig. 2). The S/R ratio was significantly correlated (p<0.05) with CYP2B6. Significant correlations were also found between (R)-EDDP (r = 0.58) and (S)-EDDP (r = 0.68) formation with paclitaxel 6α-hydroxylation catalyzed by 2C8; this, however, appeared to arise from a significant correlation within the liver bank for 2B6 versus 2C8, r = 0.596. Other significant correlations between activities within the liver bank were as follows: 1A2 versus 2C19, r = 0.892; 2D6 versus 3A4, r = 0.593 (negative slope); and 2C9 versus 2E1, r = 0.515. There was no significant correlation between 3A4 and 2B6 activities (r = 0.09).
Figure 2.
Correlation of activities in 15 phenotyped HLM between testosterone 6βhydroxylation (3A) and R-EDDP formation (A), S-mephenytoin N-demethylation (2B6) and S-EDDP formation (B) and S-mephenytoin N-demethylation (2B6) and the ratio of S-EDDP/R-EDDP formation.
Chemical and immuno-inhibition of methadone N-demethylation mediated by 3A4
The chemical inhibitors ketoconazole (2 µM), midazolam (100 µM), erythromycin (50 µM), isoniazid (500 µM), troleandomycin (50 µM), raloxifene (30 µM), verapamil (10 µM) and diltiazem (10 µM) effectively inhibited (≥ 90% inhibition) (R)- and (S)-EDDP formation following incubation of methadone with CYP3A4 Supersome. The inhibition by midazolam was concentration-dependent and it increased from 30 to 90% as the concentration of midazolam increased from 10 to 100 µM. The inhibition by troleandomycin was over 88% at the lowest concentration tested (10 µM) and it slightly increased as the concentration increased to 50 µM. Tamoxifen (10 µM) and fluoxetine (10 µM) were weaker inhibitors (< 45%) under these conditions (table 2). The same inhibitors were used with a selected HLM with high 3A4 activity. Except for tamoxifen and fluoxetine, all the other inhibitors including mAb 3A4/5 reduced (R)- and (S)-EDDP production 52–93% and 43–86%, respectively. These data suggested 60 to 85% of the EDDP formation in this HLM was attributed to 3A.
Table 2.
Effect of chemical and monoclonal antibodies (mAb) inhibition on (R)- and (S)-EDDP production in HLM with high CYP3A4 activity and CYP3A4 Supersomes
| Inhibitor | µM | Pre- Incubation (min) |
HLM | Supersome | ||
|---|---|---|---|---|---|---|
| (R)-EDDP |
(S)-EDDP |
(R)-EDDP |
(S)-EDDP |
|||
| % Inhibition | ||||||
| Ketoconazole | 2 | 0 | 84.4 | 81.8 | 96.5 | 97.0 |
| Midazolam | 10 | 15 | nd | nd | 27.4 | 26.2 |
| 50 | 15 | nd | nd | 77.8 | 78.7 | |
| 100 | 15 | 86.4 | 82.3 | 90.3 | 91.6 | |
| Fluoxetine | 10 | 15 | 11.6 | 12.1 | 18.5 | 12.2 |
| Erythromycin | 50 | 15 | 68.4 | 56.4 | 93.9 | 94.0 |
| Troleandomycin | 10 | 15 | nd | nd | 91.4 | 91.2 |
| 20 | 15 | nd | nd | 93.5 | 93.6 | |
| 50 | 15 | 73.2 | 63.5 | 95.7 | 96.1 | |
| Isoniazid | 500 | 15 | 70.8 | 51.4 | 96.9 | 96.9 |
| Raloxifene | 30 | 15 | 93.2 | 86.2 | 96.8 | 97.2 |
| Tamoxifen | 10 | 15 | 9.6 | 10.6 | 42.1 | 38.3 |
| Verapamil | 10 | 15 | 63.2 | 53.9 | 93.0 | 93.3 |
| Ditilazem | 10 | 15 | 51.6 | 44.7 | 90.0 | 89.9 |
| mAb 3A4/5 | --- | 0 | 56.6 | 42.5 | nd | nd |
Note: Values are the mean of duplicate incubations in HLM 452018 (high CYP3A4/5 activity). The manufacturers stated activities for the tested CYPs were: S-mephenytoin N-demethylation (2B6), 32; S-mephenytoin 4-hydroxylation (2C19), 23; bufuralol 1’-hydroxylation (2D6), 28; testosterone 6β-hydroxylation (3A4/5), 12,000. Mean activities for (R)- and (S)-EDDP production were 11.8 and 15.6 nmol/min/mg prot. nd – not determined.
Chemical and immuno-inhibition of methadone N-demethylation mediated by 2B6
The inhibitory capability of methadone N-demethylation by CYP2B6-selective chemical inhibitors Thio-TEPA (50 µM) and orphenadine (100 µM) was evaluated initially in 2B6 Supersomes. Thio-TEPA was a strong 2B6 inhibitor that inhibited more than 85% of EDDP formation, while orphenadine was much weaker and inhibited less than 45%. In a selected HLM with high CYP2B6 activity, the inhibition by Thio-TEPA was concentration-dependent over the concentration range 0.1–100 µM with the inhibitory percentage up to 47 and 77% for (R)- and (S)-EDDP formation, respectively. mAb 2B6 inhibited up to 65% and 85% for (R)- and (S)-enantiomer, respectively (table 3).
Table 3.
Effect of chemical and monoclonal antibodies (mAb) inhibition on (R)- and (S)-EDDP production in HLM with high CYP2B6 activity and CYP2B6 Supersomes
| Inhibitor | µM | Pre- Incubation (min) |
HLM | Supersome | ||
|---|---|---|---|---|---|---|
| (R)-EDDP |
(S)-EDDP |
(R)-EDDP |
(S)-EDDP |
|||
| % Inhibition | ||||||
| Thio-TEPA c | 0.1 | 0 | 0.0 | 0.0 | nd | nd |
| 0.5 | 0 | 11.2 | 22.4 | nd | nd | |
| 1 | 0 | 24.1 | 37.6 | nd | nd | |
| 5 | 0 | 37.3 | 64.7 | nd | nd | |
| 10 | 0 | 39.3 | 69.6 | nd | nd | |
| 50 | 0 | 42.0 | 74.4 | 86.8 | 86.5 | |
| 100 | 0 | 47.5 | 77.4 | nd | nd | |
| Orphenadrine a | 100 | 15 | 39.1 | 38.0 | 44.9 | 43.0 |
| mAb 2B6 | --- | 0 | 64.7 | 84.5 | nd | nd |
Note: Values are the mean of duplicate incubations in HLM 452064 (high CYP2B6 activity). The manufacturers stated activities for the tested CYPs were: S-mephenytoin N-demethylation (2B6), 130; S-mephenytoin 4-hydroxylation (2C19), 14; bufuralol 1’-hydroxylation (2D6), 220; testosterone 6β-hydroxylation (3A4/5), 2,200. Mean activities for (R)- and (S)-EDDP production were 10.1 and 21.2 nmol/min/mg prot. nd – not determined.
Chemical and immuno-inhibition of methadone N-demethylation mediated by 2D6 and 2C19
The CYP2D6-selective chemical inhibitors quinidine (10 µM), fluoxetine (10 µM) and paroxetine (1 µM) were investigated in HLM and Supersome. Quinidine inhibited less than 30% of (R)- and (S)-EDDP formation in HLM, although control reactions showed that greater than 97% of Supersome 2D6 activity was inhibited under similar experimental conditions. No significant inhibition (<30%) was observed for fluoxetine and paroxetine in 2D6 Supersome or a HLM with high CYP2D6 activity at these concentrations. Less than 10% of (R)- and (S)-EDDP formation was inhibited by mAb 2D6 in this HLM (table 4).
Table 4.
Effect of chemical and monoclonal antibodies (mAb) inhibition on (R)- and (S)-EDDP production in HLM with high CYP2D6 and 2C19 activity and CYP2D6 and 2C19 Supersomes
| Inhibitor | µM | Pre- Incubation (min) |
HLM | Supersome | ||
|---|---|---|---|---|---|---|
| (R)-EDDP |
(S)-EDDP |
(R)-EDDP |
(S)-EDDP |
|||
| % Inhibition | ||||||
| CYP2D6 | ||||||
| Quinidine | 10 | 0 | 27.0 | 23.5 | 98.5 | 97.3 |
| Fluoxetine | 10 | 0 | 23.8 | 23.5 | 29.0 | 27.5 |
| Paroxetine | 1 | 15 | 16.0 | 25.5 | 9.9 | 7.6 |
| mAb 2D6 | --- | 0 | 6.7 | 6.3 | nd | nd |
| CYP2C19 | ||||||
| Ticlopidine | 10 | 0 | nd | nd | 87.8 | 81.8 |
| 20 | 0 | nd | nd | 91.1 | 84.8 | |
| 50 | 0 | 13.1 | 41.0 | 95.1 | 90.1 | |
| mAb 2C19 | --- | 0 | 13.9 | 0.0 | nd | nd |
Note: Values are the mean of duplicate incubations in HLM 452095 (high CYP2D6 activity) and HLM 452056 (high CYP2C19). For HLM 452095 the manufacturers stated activities for the tested CYPs were: S-mephenytoin N-demethylation (2B6), 12; S-mephenytoin 4-hydroxylation (2C19), 30; bufuralol 1’-hydroxylation (2D6), 160; testosterone 6β-hydroxylation (3A4/5), 760. Mean activities for R-EDDP and S-EDDP production were 1.58 and 1.94 nmol/min/mg prot. For HLM 452056 the manufacturers stated activities for the tested CYPs were: S-mephenytoin N-demethylation (2B6), 45; S-mephenytoin 4-hydroxylation (2C19), 410; bufuralol 1’-hydroxylation (2D6), 100; testosterone 6β-hydroxylation (3A4/5), 4000. Mean activities for (R)- and S-EDDP production were 4.78 and 5.98 nmol/min/mg prot. nd – not determined.
Ticlopidine (50 µM) inhibited more than 90% of R- and S-EDDP formation in Supersome 2C19. In a selected HLM with high CYP2C19 activity, less than 15% of (R)- and (S)-EDDP formation was inhibited by ticlopidine. mAb 2C19 inhibited approximately 14% of (R)-EDDP formation with no effect on (S)-EDDP formation (table 4).
Correlation of residual activity with percent inhibition of CYP3A4 and 2B6
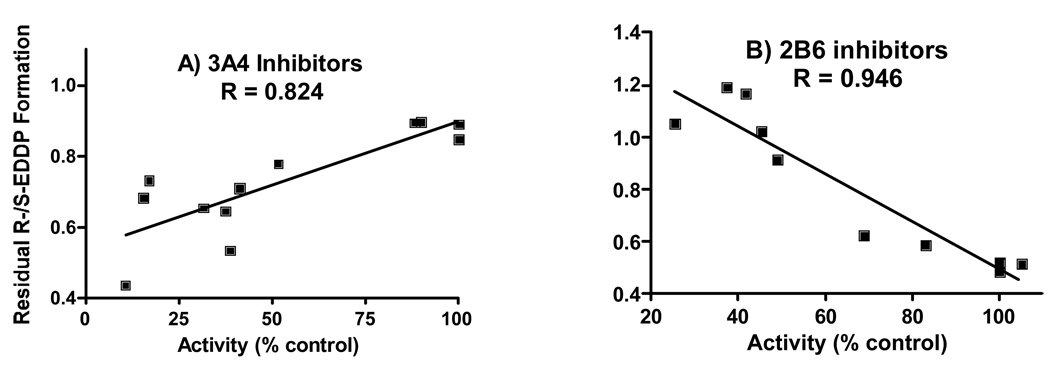
Variation in inhibition in the selected HLMs was compared to residual (R)- and (S)-EDDP formation (i.e., activity remaining after inhibition), expressed as the R-/S-EDDP ratio. In HLM enriched with CYP3A4 and CYP2B6, the respective control ratios were approximately 0.90 and 0.50. As inhibition of CYP3A4 increased in the 3A4-enriched HLM, the ratio decreased to those seen in control 2B6-enriched HLM; correlation between the ratio and extent of inhibition (R=0.824) was significant (fig. 3A). As inhibition of CYP2B6 increased in the 2B6-enriched HLM, the ratio increased to, and surpassed, those seen in control 3A4-enriched HLM; correlation between the ratio and extent of inhibition (R=0.946) was significant (fig. 3B).
Figure 3.
Correlation between the percent chemical and immuno-inhibition in HLM with high activities for CYP3A4 (A) and CYP 2B6 (B) with residual R/ S-EDDP production ratios (i.e., ratio of activities remaining after inhibition). Data are from tables 2 and 3, respectively; the mean inhibition of (R)- and (S)-EDDP production was used for correlation plots.
Discussion
We have employed an array of in vitro techniques to define the involvement of specific CYPs in the stereo-selective metabolism of methadone. The use of all of these methodologies in one study provides a stronger argument for CYP involvement, or non-involvement, and has allowed us to achieve our aim of reconciling previous results of in vitro studies. While the replicates used for each set of experiments were too few to permit statistical comparisons, the use of multiple approaches strongly demonstrates that on an average CYP3A, 2B6 and 2C19 contribute 60–75, 20–30 and 5–15% to methadone turnover and EDDP production with CYP2B6 the primary contributor to enantiomer-specific metabolism.
In the current study, the stereo-selective metabolism of methadone was examined in a panel of twelve CYP Supersomes. Previous studies have examined nine [11,17], seven [16], five [12,14] or three [13,15,18,19] cDNA expressed CYPs. With the exception of CYP2E1, all were able to turnover methadone, and all except 2E1, 1A2 and 2A6 produced EDDP under the conditions employed. We confirmed the R>S stereo-selective properties of 2C8 [15] and 2C19 [16,18,19], further refuting the observation of Foster et al. [13] that 2C19 was not stereo-selective. We also confirm the S>R stereo-selective properties of 2B6 [16,18,19] and 2D6 [15], and observe these for the first time for 2C18. Our study on lack of stereo-selectivity of methadone depletion by CYP3A4 agrees with Gerber et al. [16] as opposed to the stereo-selectivity observed by Wang et al. [15].
The use of a large number of CYPs in the screen permits a reliable comparison for scaling. This calculation expresses activity relative to abundance of CYPs in human liver, and has only previously been approached by Iribarne et al. [11], but they lacked information on CYP2B6 and 2C19. This calculation suggests that based on CYP mean abundance, CYP3A4 has an approximately 2–3-fold greater involvement in methadone turnover and EDDP formation than 2B6, and even greater so than 2C19 and 2D6. Contributions of other CYPs were almost negligible. The conditions used for our experiments in recombinant-expressed CYPs closely matched those used by Kharasch et al. [17] in regard to substrate concentration (≈ 2.5 µM) and source of enzymes (co-expression of CYP reductase and cytochrome b5 when available). In their experiment, the activity of CYP3A4 versus 2B6 was even higher, so scaling would suggest an even lower involvement of CYP2B6. It is worth noting that the ratio of abundance of CYP3A4 to 2B6 in our liver bank database of 6.9 does not differ too greatly from the 5.5 determined from the approximate slope of the liver bank used presented by Kharasch and colleagues in a later publication (fig. 2 in [19]). Differences in exact percent outcomes based on use of immunoquantitation versus RAF to estimate abundance have been discussed previously [20,22]; inclusion of holoenzyme during immunoquantitation and lack of complete specificity of CYP-specific activities for RAF determinations are respective limitations.
Only Iribarne et al. [11] have performed correlation analysis of a bank of HLM, and theirs was limited to CYP3A activity. Our correlation analysis strongly supports the role of CYP3A4 and the stereo-selective role of CYP2B6. A significant correlation was also found between CYP2C8 activity and the formation of S-EDDP as well as the ratio of S/R-EDDP formation. Based on other information that is negative for a significant contribution of CYP2C8 to methadone N-demethylation, this correlation appears to arise from the correlation within our liver bank of CYP2C8 activity with CYP2B6 activity. It might be premature to suggest some co-regulation of CYP2B6 and 2C8 based on this finding, as Totah et al. [19] reported a significant correlation between CYP2B6 and CYP3A4 immunoquantitations in their liver bank, which we could not reproduce between CYP2B6 and CYP3A4 activities.
For studies on the effect of immuno- and chemical-inhibition, we used phenotyped HLM that had relatively greater activity for the CYP under investigation. Only Foster et al. [13] have also used immuno-inhibition and those were limited to antibodies directed against CYP3A4 and 2E1. The use of mAb against CYP3A4 and 2B6 supports the prominent roles of these enzymes in methadone metabolism, while those against CYP2C19 and 2D6 support the minimal roles of these enzymes. This was substantiated by the impact of chemical inhibition. There were some limitations to these experiments as developments in selectivity of some of the inhibitors have evolved since we made initial protocols for our experiments. For example, while Ko et al. [23] demonstrated ticlopidine was a potent competitive and mechanism-based inhibitor of CYP2C19, it was subsequently found that ticlopidine was an even more potent competitive and mechanism-based inhibitor of CYP2B6 [24,25]. Thus, the inhibition of (S)-EDDP formation seen in table 3 was probably due more to inhibition of CYP2B6. The limited involvement of CYP2C19 is seen from the immuno-inhibition experiments. Also, thio-TEPA, while better known as a selective mechanism-based inhibitor of CYP2B6, has also been found to be a potent and selective reversible inhibitor [26], as used in this study. This study substantiates others that show orphenadrine is not an optimal selective inhibitor of CYP2B6 [27,28]. Not all inhibitors were chosen their potency (e.g. orphenadrine, fluoxetine, tamoxifen), as a spread of inhibition allowed the correlation between residual stereo-selectivity.
This correlation strongly supports the finding of Totah et al. that CYP2B6 is the major determinant of stereo-selective metabolism of methadone in HLM [19]. The significant negative correlation between R/S-EDDP ratio and residual enzyme activity in the presence of CYP2B6 inhibitors (fig. 3B) demonstrated that when the activity of 2B6 was inhibited, the priority of (S)-EDDP formation decreased and (R)/(S) ratio approached unity. The significant positive correlation between (R)/(S)-EDDP ratio and residual enzyme activity in the presence of CYP3A4 inhibitors (fig. 4A) demonstrated that when the activity of 3A4 was inhibited, the relative amount of 2B6 increased and its priority to (S)-EDDP formation became dominant. The relative abundance of 2B6 and 3A4 would appear to be the major factors determining the stereo-selectivity of microsomal metabolism of methadone.
This conclusion is consistent with recent in vivo studies that compare CYP genotypes to trough and peak concentrations of (R)- and (S)-methadone and (R)- and (S)-EDDP. These studies showed that genetic determinants of CYP2B6 influences (S)-methadone and (S)-EDDP plasma concentrations, while genotypic variations in CYP1A2, 2C9, 2C19 and 2D6 have little influence [29–31]. In addition, subjects phenotyped with lower CYP3A activity had higher steady-state trough (R,S)-methadone concentrations [30]. An exception for the involvement of CYP2D6 in methadone metabolism may exist for the ultrarapid metabolizers [32]. Here, the increased abundance of CYP2D6 may well make it a significant contributor.
Recently, Kharasch et al. have put forth the hypothesis that CYP3A4 is not involved in methadone clearance [33,34]. The involvement of both CYP3A4 and 2B6 in methadone metabolism, however, can, at least in part, explain these findings on methadone drug interactions with the antiretroviral, ritonavir. In primary cultures of human hepatocytes, ritonavir is a mixed inhibitor/inducer of CYP3A4, while it is an inducer of CYP2B6 [35]. When methadone was given with ritonavir (200 mg tid increased over time to 400 mg bid), methadone metabolism was induced in the presence of inhibition of CYP3A4; this was accompanied by a significant increase in the metabolic ratio AUC (EDDP/ methadone) that was much greater for the (S)-enantiomer [33]. When methadone was given with ritonavir (100 mg bid) combined with indinavir, methadone metabolism was essentially unchanged, while CYP3A4 was still inhibited and the metabolic ratio AUC (S)-EDDP/(S)-methadone was still significantly increased [34]. In both cases, ritonavir induced CYP2B6. In the first case where higher doses of ritonavir were used, the induction is sufficient to give overall induction even when CYP3A4 is inhibited; in the second case, with lower doses of ritonavir, the induction is only sufficient to offset the inhibition of CYP3A4.
Our current in vitro results are consistent with the importance of both CYP2B6 and CYP3A4 in the clearance of methadone. The potential of drug-drug interactions with methadone in vivo must take into consideration the effect on both. Because CYP2B6 has greater activity towards metabolism of (S)-methadone, an imbalance in the response of CYP2B6 and 3A4 during drug interactions may lead to differences between the (R)- and (S)- enantiomers. Even if the clearance of (R,S)-methadone was not altered, this could impact the therapeutic or toxic response to methadone treatment.
Acknowledgment
This study was supported by NIDA grant R01 DA10100.
References
- 1.Kristensen K, Christensen CB, Christrup LL. The mu1, mu 2, delta, kappa opioid receptor binding properties of methadone stereoisomers and morphine. Life Sci. 1995;56:45–50. doi: 10.1016/0024-3205(94)00426-s. [DOI] [PubMed] [Google Scholar]
- 2.Scott CC, Robbins EB, Chen KK. Pharmacological comparison of the optical isomers of methadone. J Pharmacol Exp Ther. 1948;92:282–286. [PubMed] [Google Scholar]
- 3.Kristensen K, Blemmer T, Angelo HR, Christrup LL, Drenck NE, Rasmussen SN, et al. Stereoselective pharmacokinetics of methadone in chronic pain patients. Ther Drug Monit. 1996;18:221–227. doi: 10.1097/00007691-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Foster DJR, Somogyi AA, Dyer KR, White JM, Bocher F. Steady-state pharmacokinetics of (R)- and (S)-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2000;50:427–440. doi: 10.1046/j.1365-2125.2000.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moody DE, Lin SN, Chang Y, Lamm L, Greenwald MK, Ahmed MS. An enantiomer selective liquid chromatography-tandem mass spectrometry method for methadone and EDDP validated for use in human plasma, urine and liver microsomes. J Anal Toxicol. 2008;32:208–219. doi: 10.1093/jat/32.3.208. [DOI] [PubMed] [Google Scholar]
- 6.Kreek MJ. Drug interactions with methadone. Ann NY Acad Sci. 1976;281:350–371. doi: 10.1111/j.1749-6632.1976.tb27945.x. [DOI] [PubMed] [Google Scholar]
- 7.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–1193. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari A, Coccia CPR, Bertolini A, Sternieri E. Methadone - metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551–559. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 9.McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: A review. Am J Addict. 2010;19:4–16. doi: 10.1111/j.1521-0391.2009.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eap CB, Crettol S, Rougier JS, Schlapfer J, Grilo LS, Deglon JJ, et al. Stereoselective block of hERG channel by (S)-methadone and QT interval prolongation in CYP2B6 slow metabolizers. Clin Pharmacol Ther. 2007;81:719–728. doi: 10.1038/sj.clpt.6100120. [DOI] [PubMed] [Google Scholar]
- 11.Iribarne C, Berthou F, Baird S, Dreano Y, Picart D, Bail JP, et al. Involvement of cytochrome P450 3A4 enzyme in the N-demethylation of methadone in human liver microsomes. Chem Res Toxicol. 1996;9:365–373. doi: 10.1021/tx950116m. [DOI] [PubMed] [Google Scholar]
- 12.Moody DE, Alburges ME, Parker RJ, Collins JM, Strong JM. The involvement of cytochrome P450 3A in the N-demethylation of l-α-acetylmethadol (LAAM), norLAAM and methadone. Drug Metab Dispos. 1997;25:1347–1353. [PubMed] [Google Scholar]
- 13.Foster DJR, Somogyi AA, Bochner F. Methadone N-demethylation in human liver microsomes: lack of stereoselectivity and involvement of CYP3A4. Br J Clin Pharmacol. 1999;47:403–412. doi: 10.1046/j.1365-2125.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prost F, Thormann W. Capillary electrophoresis to assess drug metabolism induced in vitro using single CYP450 enzymes (Supersomes ™): Application to the chiral metabolism of mephenytoin and methadone. Electrophoresis. 2003;24:2577–2587. doi: 10.1002/elps.200305493. [DOI] [PubMed] [Google Scholar]
- 15.Wang JS, DeVane CL. Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of (R)- and (S)-methadone in vitro. Drug Metab Dispos. 2003;31:742–747. doi: 10.1124/dmd.31.6.742. [DOI] [PubMed] [Google Scholar]
- 16.Gerber JG, Rhodes RJ, Gal J. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16:36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- 17.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition, and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–269. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED. Enantiomeric interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther. 2007;321:389–399. doi: 10.1124/jpet.106.117580. [DOI] [PubMed] [Google Scholar]
- 19.Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108:363–374. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- 20.Neff JA, Moody DE. Differential N-demethylation of l-α-acetylmethadol (LAAM) and norLAAM by cytochrome P450s 2B6, 2C18, and 3A4. Biochem Biophys Res Commun. 2001;284:751–756. doi: 10.1006/bbrc.2001.5054. [DOI] [PubMed] [Google Scholar]
- 21.Yang TJ, Krausz KW, Sai Y, Gonzalez FJ, Gelboin HV. Eight inhibitory monoclonal antibodies define the role of individual P-450s in human liver microsomal diazepam, 7-ethoxycoumarin, and imipramine metabolism. Drug Metab Dispos. 1999;27:102–109. [PubMed] [Google Scholar]
- 22.Venkatakrishnan K, von Moltke LL, Court MH, Harmatz JS, Crespi CL, Greenblatt DJ. Comparison between cytochrome P450 (CYP) content and relative activity approaches to scaling from cDNA-expressed CYPs to human liver microsomes: ratios of accessory proteins as sources of discrepancies between approaches. Drug Metab Dispos. 2000;28:1493–1504. [PubMed] [Google Scholar]
- 23.Ko JW, Desta Z, Soukhova NV, Tracy T, Flockhart DA. In vitro inhibition of the cytochrome P450 (CYP450) system by the antiplatelet drug ticlopidine: potent effect on CYP2C19 and CYP2D6. Br J Clin Pharmacol. 2000;49:343–351. doi: 10.1046/j.1365-2125.2000.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter T, Murdter TE, Heinkele G, Pleiss J, Tatzel S, Schwab M, et al. Potent mechanism-based inhibition of human CYP2B6 by clopidogrel and ticlopidine. J Pharmacol Exp Ther. 2004;308:189–197. doi: 10.1124/jpet.103.056127. [DOI] [PubMed] [Google Scholar]
- 25.Turpeinen M, Nieminen R, Juntunen T, Taavitsainen P, Raunio H, Pelkonen O. Selective inhibition of CYP2B6-catalyzed bupropion hydroxylation in human liver microsomes in vitro. Drug Metab Dispos. 2004;32:626–631. doi: 10.1124/dmd.32.6.626. [DOI] [PubMed] [Google Scholar]
- 26.Walsky RL, Obach RS. A comparison of 2-phenyl-2-(1-piperidinyl)propane (PPP), 1,1',1"-phosphinothioylidynetrisaziridine (ThioTEPA), clopidogrel, and ticlopidine as selective inactivators of human cytochrome P450 2B6. Drug Metab Dispos. 2007;35:2053–2059. doi: 10.1124/dmd.107.015883. [DOI] [PubMed] [Google Scholar]
- 27.Ekins S, VandenBranden M, Ring BJ, Wrighton SA. Examination of purported probes of human CYP2B6. Pharmacogenetics. 1997;7:165–179. doi: 10.1097/00008571-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Guo Z, Raeissi S, White RB, Stevens JC. Orphenadrine and methimazole inhibit multiple cytochrome P450 enzymes in human liver microsomes. Drug Metab Dispos. 1997;25:390–393. [PubMed] [Google Scholar]
- 29.Crettol S, Deglon JJ, Besson J, Croquette-Krokkar M, Gothuey I, Hammig R, et al. Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin Pharmacol Ther. 2005;78:593–604. doi: 10.1016/j.clpt.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Crettol S, Deglon JJ, Besson J, Croquette-Krokar M, Hammig R, Gothuey I, et al. ABCB1 and cytochrome P450 genotypes and phenotypes: Influence on methadone plasma levels and response to treatment. Clin Pharmacol Ther. 2006;80:668–681. doi: 10.1016/j.clpt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Shiran MR, Lennard MS, Iqbal MZ, Lagundoye O, Seivewright N, Tucker GT, et al. Contribution of the activities of CYP3A, CYP2D6, CYP1A2 and other potential covariates to the disposition of methadone in patients undergoing methadone maintenance treatment. Br J Clin Pharmacol. 2009;67:29–37. doi: 10.1111/j.1365-2125.2008.03312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eap CB, Broly F, Mino A, Hammig R, Deglon JJ, Uehlinger C, et al. Cytochrome P450 2D6 genotype and methadone steady-state concentrations. J Clin Psychopharmacol. 2001;21:229–234. doi: 10.1097/00004714-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84:497–505. doi: 10.1038/clpt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kharasch ED, Hoffer C, Whittington D, Walker A, Bedynek PS. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport. Anesthesiology. 2009;110:660–672. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: Implications for predicting clinical drug interactions. Drug Metab Dispos. 2007;35:1853–1859. doi: 10.1124/dmd.107.016089. [DOI] [PubMed] [Google Scholar]