Abstract
Purpose
This study was performed to determine the spinal cord tolerance to single-fraction, partial-volume irradiation in swine.
Methods/Materials
A 5 cm long cervical segment was irradiated in 38-47 week old Yucatan minipigs using a dedicated, image-guided radiosurgery linear accelerator. Radiation was delivered to a cylindrical volume approximately 5 cm in length and 2 cm in diameter that was positioned lateral to the cervical spinal cord resulting in a dose distribution with the 90%, 50% and 10% isodose lines traversing the ipsilateral, central and contralateral spinal cord, respectively. Dose was prescribed to the 90% isodose line. Twenty-six pigs were stratified into 8 dose groups from 12-47 Gy. The mean maximum spinal cord doses were 16.9±0.1, 18.9±0.1, 21.0±0.1, 23.0±0.2, and 25.3±0.3 Gy in the 16, 18, 20, 22 and 24 Gy dose groups, respectively. The mean percentage spinal cord volumes receiving >= 10 Gy for the same groups were 43%±3, 48%±4, 51%±2, 57%±2 and 59%±4. The study endpoint was motor neurologic deficit determined by a change in gait during a one year follow-up period.
Results
A steep dose response curve was observed with an ED50 (95% CI) for the maximum dose point of 20.0 Gy (18.3-21.7). Excellent agreement was observed between the occurrence of neurologic change and the presence of histological change. All animals with motor deficits showed some degree of demyelination and focal white matter necrosis on the irradiated side with relative sparing of the gray matter while histology was unremarkable in animals with normal neurologic status.
Conclusions
Results indicate that for a dose distribution with a steep lateral gradient, pigs have a lower ED50 for paralysis than has been observed in rats and more closely resembles that for rats, mice and guinea pigs receiving uniform spinal cord irradiation.
Keywords: spinal cord tolerance, stereotactic spinal radiosurgery, swine
Introduction
In recent years, many investigators have pursued the use of image-guided stereotactic radiosurgery (SRS) to escalate single-fraction doses (> 8 Gy) to spinal metastases resulting in an exponential increase in clinical experience. Prior to 2003, the entire spinal SRS experience reported in the literature for all methodologies included approximately 50 patients1-4. As of 2010, the published image-guided spinal SRS experience has grown to more than 1100 lesions treated by the two largest reporting groups alone5,6. Dose escalation has occurred over time as experience with spinal radiosurgery has increased; prescription doses of 10-12 Gy were used 5-6 years ago but currently doses of 16-24 Gy are common. Spinal SRS results in impressive durable pain responses (approximately 90%) and high rates of local control (approximately 80% or greater) when sufficient doses are delivered7-9. Only four clinical cases of myelopathy have been reported following varied doses from single-fraction spinal radiosurgery making it difficult to draw firm conclusions regarding spinal cord tolerance and underscoring the need for further investigation10,11.
Spinal cord tolerance generally dictates the dose that is prescribed to spinal and paraspinal tumors, as excessive radiation to the spinal cord results in devastating transient or permanent myelopathy. Unfortunately, despite the increased clinical application of spinal radiosurgery, little is known regarding the tolerance of the spinal cord to high-dose single-fraction irradiation. The maximum tolerated spinal cord dose-volume relationship and/or single point dose are unknown and are currently a subject of debate. Ryu, et. al. 6 have reported that the partial-volume tolerance of the spinal cord is at least 10 Gy to 10% of the spinal cord volume when the spinal cord volume is defined to extend 6 mm superior and inferior to the radiosurgery target. Sahgal, et. al.11 recommend a dose threshold of 10 Gy to the thecal sac while Gibbs, et. al.5 recommend caution when considering radiosurgery plans that expose more than 1.0 cm3 of spinal cord to greater than 8 Gy dose equivalent.
In contrast to the limited knowledge on single-fraction spinal cord tolerance in humans, there is a wealth of data from pre-clinical studies. Volume-effect studies have been performed by varying both the length and width of irradiated spinal cord but lateral dose-volume effects are most relevant to the present study. Lateral dose-volume effects in spinal cord irradiation have been investigated by van Luijk, et. al. who irradiated the lateral half of rat cervical spinal cords to a length of 2.0 cm with 150 MeV protons12. A penumbra width of 1 mm made it possible to create an extremely high dose gradient across the 3.5 mm wide spinal cord so that the contralateral edge of the spinal cord received < 10% of the dose while the ipsilateral edge received 100%. The resulting dose response curve showed an ED50 (95% confidence interval) of 30.0 Gy (26.3-31.3 Gy) while the corresponding ED50 for full-width irradiation was 20.4 Gy (19.6-21.1 Gy)12. A more extensive follow-up study further demonstrated large regional differences in radiosensitivity within the rat cervical spinal cord, indicating that the lateral white matter is more radiosensitive than the central part13. The potential existence of a lateral dose-volume effect has important clinical implications, suggesting that the spinal cord tolerance increases as much as 50% when the spinal cord is irradiated with an inhomogeneous dose distribution similar to that achieved in spinal radiosurgery.
Methods and Materials
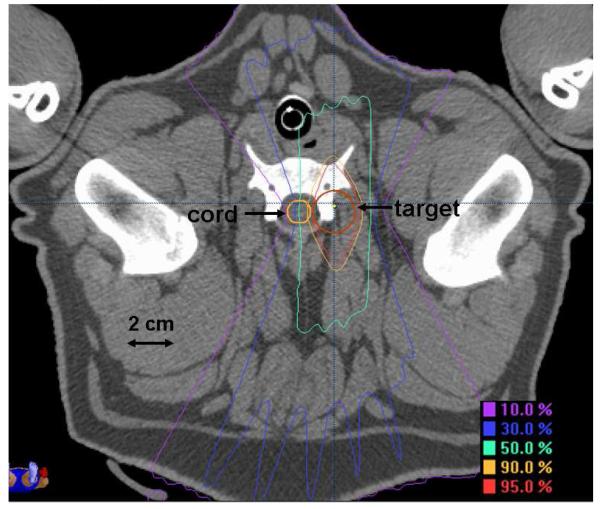
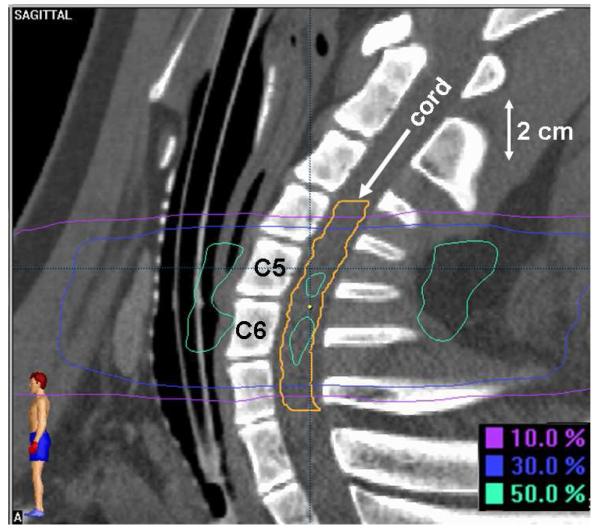
This study conformed to all national and local regulations regarding the use of animals for research and was approved by the Institutional Animal Care and Use Committee. The cervical spinal cords of twenty-six female, 40-47 week old, Yucatan minipigs were irradiated, one additional animal served as an unirradiated control. All animals received a treatment planning CT scan with 1.0-1.5 mm thick slices and a 350-400 cm field of view. Treatment planning calculations were performed using Brainscan 5.31 software (BrainLAB, AG). Radiation was delivered in a single dose to a cylindrical target volume approximately 5 cm in length and 2 cm in diameter that was positioned lateral to the cervical spinal cord. In the rostral/caudal direction, the target volume was centered at the level of the rostral end of the sixth cervical vertebral body and extended from mid-C4 through mid-C7. Dose distributions in the axial and sagittal planes are shown in Figures 1 and 2. The sagittal plane shown (Fig. 2) is through the midline of the spine rather than the center of the target volume to illustrate the level and extent of the spinal cord irradiated. Dynamically-shaped arcs were used to create a dose distribution with the 90%, 50% and 10% isodose lines traversing the ipsilateral, central and contralateral spinal cord, respectively, equating to a lateral dose gradient of approximately 8% per millimeter. The spinal cord was contoured 4.5-7.5 mm beyond the irradiated volume in the rostral and caudal directions and the dose calculation grid resolution through the spinal cord ranged from 1.4-1.6 mm. Dose distributions were normalized to the global plan maximum and dose was prescribed to the 90% isodose line. Animals were stratified into 8 prescription dose groups as follows: 47 Gy (1), 36 Gy (1), 24 Gy (4), 22 Gy (4), 20 Gy (5), 18 Gy (5), 16 Gy (5), and 12 Gy (1). Treatment planning dose-volume histogram statistics for the spinal cord are summarized in Table 1 including: a) maximum dose, b) percentage volume to receive ≥10 Gy, c) percentage volume to receive ≥14 Gy, d) dose to 10% volume, and e) dose to 1 cc volume.
Figure 1.
Dose distribution in the axial plane.
Figure 2.
Dose distribution in the sagittal plane.
Table 1.
Dose-volume histogram statistics for the spinal cord.
| Dose Group (Gy) |
Mean Maximum Cord Dose (Gy) |
Mean Percentage Volume >= 10 Gy |
Mean Percentage Volume >= 14 Gy |
Mean Dose to 10% Volume (Gy) |
Mean Dose to 1.0 cc |
|---|---|---|---|---|---|
| 16 (n=5) | 16.9±0.1 | 43±3 | 20±2 | 15.7±0.2 | 12.9±0.5 |
| 18 (n=5) | 18.9±0.1 | 48±4 | 29±5 | 17.5±0.2 | 14.3±1.1 |
| 20 (n=5) | 21.0±0.1 | 51±2 | 35±1 | 19.5±0.2 | 15.5±0.8 |
| 22 (n=4) | 23.0±0.2 | 57±2 | 40±2 | 21.2±0.2 | 16.8±1.2 |
| 24 (n=4) | 25.3±0.3 | 59±4 | 42±2 | 23.5±0.4 | 19.3±0.4 |
For all procedures, animals were anesthetized with isoflurane and positioned supine in a body-length, vacuum-molded immobilization cushion. Individual immobilization cushions were molded for each animal and kept unchanged between simulation and radiosurgery. Image-guided localization was performed using stereoscopic kilovoltage x-rays (Novalis Body X-ray 6D, BrainLAB AG). A pair of digital radiographs was exposed and automatically fused with digitally reconstructed radiographs (DRR’s) generated from the pre-treatment CT scan to determine if any positioning adjustments were necessary. After visual evaluation of the fusion results, the treatment table was shifted until the actual position and required treatment position differed by less than 1 mm in the three primary axes. Rotations along the axis of the treatment table were corrected to less than one degree and rotations along the other two axes were corrected to less than two degrees. The image-guidance process was repeated following table adjustments to ensure that shifts were made correctly. Biplanar megavoltage portal images were acquired and evaluated to verify positioning before radiosurgery and kilovoltage image-guidance was repeated midway through the treatment to verify positioning during radiosurgery. Irradiation was performed using a 6 MV image-guided radiosurgery linear accelerator (Novalis, BrainLAB AG). Dose was delivered at a rate of 800 monitor units per minute that equated to an instantaneous dose rate of 4.0-7.3 Gy/min at the spinal cord, varying with the depth of overlying tissue. A beam configuration with three dynamic arcs was used to expedite overall delivery time (first beam on to last beam off). Overall delivery time varied with prescription dose but a 24 Gy prescription was typically delivered in 10-15 minutes.
After radiosurgery, animals were followed for a minimum of one year or until a neurologic response was observed. The general health of animals was observed daily with attention toward unusual restlessness, vocalizing, loss of mobility, licking, biting, or guarding of a painful area, failure to groom, unkempt appearance, open sores, skin lesions, loss of appetite, and weight loss. Gait was observed approximately weekly either with the animal walking freely in a large space or on an exercise treadmill. Response was defined as any study-related change in gait whether the change was permanent or transient. Animals recognized to have a change in gait were evaluated by a veterinarian for symptoms indicative of pain. After euthanasia, the cervical spinal cords of all 27 study animals were removed and fixed in formalin before being sectioned and processed for embedding in paraffin. Five uniformly distributed axial sections through the irradiated volume of each animal were cut and stained with a Luxol fast blue/periodic acid Schiff combination and were examined for histology. The dose-related incidence of motor deficit was used as an endpoint to obtain quantal data that was then analyzed by probit analysis12,14 to establish a dose-response curve and to calculate the probability of a deficit at increasing dose levels with the associated 95% confidence bounds.
Results
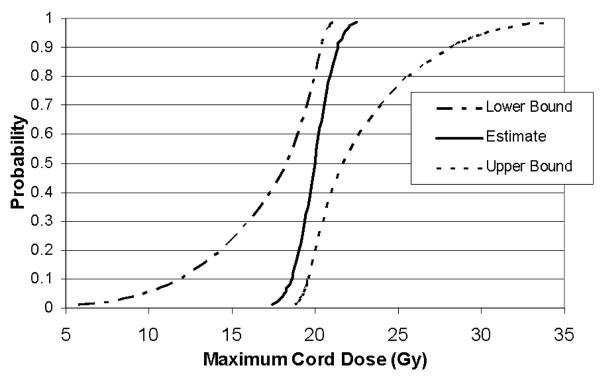
Parameters such as prescription dose, maximum spinal cord dose, age at radiosurgery, length of follow-up and latency until response are shown for individual animals in Table 2. Fourteen of twenty-six pigs developed front limb motor changes on the irradiated side (left), eleven pigs never developed any detectable deficits and one pig (#26) was euthanized due to a non-neurologic complication. No deficits were noted on the unirradiated side (right) in any animal. Five of the fourteen animals that developed front motor deficits were noted to develop hind limb changes within four weeks of the front limb change. Motor deficits presented initially as a mild limp and progressed to changes ranging from mild paresis (carpal knuckling) to flexion contracture. No animal exhibited behavior indicative of pain throughout this study. The latent period for initial onset of motor deficit ranged from 10-23 weeks with the exception of one animal in the 20 Gy group (#14) with a transient motor deficit thirty-five weeks after radiosurgery that resolved after two weeks and remained normal until the study conclusion. A steep dose response curve emerged, with no response observed when the maximum point dose in the spinal cord was <19 Gy and 100% response when the maximum dose was >21 Gy. The dose-response curve showing ‘maximum spinal cord dose’ versus ‘probability of motor neurologic deficit’ along with the upper and lower 95% confidence bounds is shown in Figure 3. Calculated estimates of the maximum spinal cord doses resulting in 1, 5, 10, 50 and 90% probability of paralysis along with the associated 95% confidence bounds are shown in Table 3; the dose associated with a 50% incidence of paralysis (ED50) is 20.0 Gy. A Pearson goodness-of-fit chi-square indicated (p=0.991) that the probit model is an excellent fit.
Table 2.
Dose and time parameters for individual irradiated animals.
| ID# | Rx Dose (Gy) |
Maximum Cord Dose (Gy) |
Age at SRS (weeks) |
Follow-up (weeks) |
Latency (weeks) |
|---|---|---|---|---|---|
| 1 | 12 | 12.9 | 44 | 56 | NA* |
| 2 | 16 | 16.7 | 44 | 57 | NA* |
| 3 | 16 | 16.9 | 42 | 53 | NA* |
| 4 | 16 | 17.1 | 45 | 53 | NA* |
| 5 | 16 | 16.9 | 47 | 54 | NA* |
| 6 | 16 | 16.9 | 45 | 53 | NA* |
| 7 | 18 | 18.8 | 43 | 58 | NA* |
| 8 | 18 | 19 | 40 | 53 | NA* |
| 9 | 18 | 19 | 45 | 53 | NA* |
| 10 | 18 | 18.8 | 47 | 53 | NA* |
| 11 | 18 | 19 | 43 | 27 | 23 |
| 12 | 20 | 20.9 | 43 | 75 | NA* |
| 13 | 20 | 21.1 | 42 | 16 | 15 |
| 14 | 20 | 20.9 | 43 | 53 | 35 |
| 15 | 20 | 21.1 | 40 | 20 | 18 |
| 16 | 20 | 21.1 | 40 | 27 | 20 |
| 17 | 22 | 23.2 | 43 | 11 | 10 |
| 18 | 22 | 23.0 | 41 | 12 | 10 |
| 19 | 22 | 22.7 | 43 | 15 | 13 |
| 20 | 22 | 23.0 | 38 | 18 | 18 |
| 21 | 24 | 25.6 | 42 | 33 | 13 |
| 22 | 24 | 25.3 | 44 | 31 | 14 |
| 23 | 24 | 25.3 | 43 | 15 | 14 |
| 24 | 24 | 24.8 | 41 | 14 | 12 |
| 25 | 36 | 38.4 | 44 | 25 | 19 |
| 26 | 47 | 49.8 | 40 | 8 | NA* |
No motor neurologic deficits were observed in stated follow-up period.
Figure 3.
Dose response curve for motor neurologic deficit with 95% confidence bounds.
Table 3.
Probit analysis estimates for the probability of myelopathy at a given maximum point dose.
| 95% Confidence Limits (Gy) | |||
|---|---|---|---|
| Probability | Dose (Gy) | Lower bound | Upper Bound |
| 0.01 | 17.4 | 5.8 | 18.8 |
| 0.05 | 18.2 | 9.8 | 19.3 |
| 0.10 | 18.6 | 11.9 | 19.6 |
| 0.50 | 20.0 | 18.3 | 21.7 |
| 0.90 | 21.4 | 20.3 | 28.1 |
Excellent agreement was observed between the occurrence of neurologic change and the presence of histological change. All animals with motor deficits showed some degree of demyelination and focal white matter necrosis on the irradiated side with relative sparing of the gray matter while histology was unremarkable in animals with normal neurologic status. In a few cases near the threshold dose for myelopathy, histology only showed some very small foci of demyelination. In addition to diffuse demyelination of the white matter, foci of white matter necrosis were observed increasingly with dose. As reported in most species, including man, focal white matter necrosis is the most common histology underlying radiation myelopathy of the spinal cord15. The only animal in the 18 Gy group (pig #11 in Table 3) with mild neurological signs (a limp) had some minor demyelination and gliosis in the outer lateral part of the white matter near the dorsal root entry zone. At a dose of 20 Gy, all animals except one (pig #12 in Table 3) showed extensive lateral demyelination but necrosis was limited to small foci. Histology for pig #12 was unremarkable. A dose of 36 Gy (pig #25 in Table 3) resulted in more severe necrosis, including extensive vascular damage that extended into the gray matter, though primarily limited to the irradiated half of the spinal cord.
Discussion
No previous partial-cord irradiation study has included a pre-clinical model in which the spinal cord was similar in size to humans and the irradiation conditions were similar to those encountered in clinical spinal radiosurgery. It is unknown exactly how the physical size of the spinal cord influences radiation tolerance in the setting of partial-cord irradiation, but if viable oligodendrocytes and oligodendrocyte precursor cells can only migrate 2-3 mm from unirradiated tissue, as suggested16-19, the size of the human spinal cord could limit the extent of remyelination and the encouraging results from rat studies may be less relevant.
The complex reactions of biological systems to radiation made this study impossible to perform in non-biological models. The Yucatan minipig was the animal of choice for this study. The pig has been used for radiobiological studies involving many anatomical sites of interest including the skin20, kidney21, lung22 and spinal cord23 because these structures have many anatomical and physiological similarities to their counterparts in humans 24. Mature pigs used to study the effects of irradiated length on the response of the spinal cord showed frank paralysis, vascular changes, white matter demyelination and necrosis comparable to those seen in humans14,25,26. The spinal cord tolerance to single-fraction, uniform irradiation was investigated in two previous pig studies at Oxford University. The first study compared the response of “mature” (37-42.5 weeks) versus “immature” (15.5-23 weeks) animals23 and the second study14 investigated the “length effect.” Pigs defined as “mature” (40-47 weeks) were used in the current study because one Oxford study23 found that only transient neurologic changes occurred in “immature” pigs after doses that paralyzed mature animals suggesting that young pigs have a greater ability to repair or compensate for radiation damage.
While many studies have been performed to investigate parameters that affect spinal cord tolerance, only the most relevant studies are compared here. Outlines of study designs from Bijl, et. al.13, the Oxford study14, and the current study are shown in Table 4. Based on results of the Oxford study in pigs14 and on studies in rats by Bijl, et. al.13, and van Luijk, et. al.12, it was hypothesized that the ED50 for spinal cord tolerance to lateral partial-cord irradiation in pigs would be at least 30 Gy. It is unclear why the ED50 for the present study was only 20.0 Gy. Migration of functional cells or their precursors from uninjured tissue at the periphery of a radiation injury has been proposed as one mechanism that accounts for an increase in spinal cord tolerance when short lengths are irradiated18,27. The ability of normal remyelinating cells to migrate up to 2 mm from a narrow rim of healthy tissue surrounding an area of demyelination has been shown by Franklin et. al. in a rat model19 while Bijl, et. al. have suggested a critical migration distance of 2-3 mm18. It may be that the steep dose gradient created by using the ‘shoot through’ method with a proton beam12 coupled with the relatively small diameter of the rat spinal cord (3.5 mm) allowed for migration of remyelinating cells from the non-irradiated side of the spinal cord to the irradiated side whereas the pig spinal cord is too large (8-11 mm diameter) for effective migration. This explanation conflicts somewhat with the “bath and shower” experiments by Bijl, et. al., who showed even doses as low as 4 Gy to tissue surrounding a high-dose radiation spinal cord injury negatively influence the ability of the high-dose lesion to recover17. Preliminary results (n=22) from our companion study investigating spinal cord tolerance to uniform irradiation, suggest that the advent of motor deficit appears to be independent of irradiated volume in the lateral direction.
Table 4.
Spinal cord tolerance study parameter comparison.
| Parameter | Current Study | van den Aardweg, et. al.14 | Bijl, et. al.13 |
|---|---|---|---|
| Species | Yucatan Minipig | Large White Pig | Wistar Rat |
| Cord Coverage | Lateral Gradient | Uniform | Lateral Gradient |
| Dose Rate | 4.0-7.3 Gy/minute | 0.21-0.30 Gy/minute | 10-15 Gy/min |
| Energy | 6MV LINAC | 60-Co | 150 MeV protons |
| Planning Images | CT | CT/fluoroscopy mix | N/A |
| Positioning Method | Stereoscopic xray | Conventional | Planar xray |
| Field Size(s) | 2.5 × 5 cm (w × l) | 5×10, 5×5, 5×2.5 cm (w × l) | 2×2 cm |
| Field Arrangement | Dynamic arc | Parallel opposed | Single Posterior |
| Target | mid-C4 to mid-C7 | C2-C5 | C1-T2 |
| Follow-up | 53-58 weeks* | 70-110 weeks | 52 weeks |
| Latency | 10-23 weeks** | 7.5-16.5 weeks | 18-23 weeks |
| Sex | Female | Female | male |
| Age | 40-47 weeks | 37-43 weeks | 12-14 weeks |
| ED50 | 20.0 Gy | 27 Gy*** | 29-33 Gy |
One exception at 75 weeks.
One exception at 35 weeks.
Response not adjusted for low dose rate.
The current study is similar to the Oxford study14 in that both studies incorporated mature female pigs but most other aspects differ. The most significant differences are the dose distribution and dose rate used. While the Oxford study used parallel opposed fields to create a uniform dose distribution across the spinal cord, the current study delivered a steep dose gradient (≈8% per mm) across the spinal cord in the lateral direction. The ED50 of the present study (20.0 Gy) is much less than reported for the Oxford study14 (27-28 Gy) despite the steep dose gradient used in the present study. This finding is most likely due to the low dose rate (0.21-0.30 Gy/min) used in the Oxford study14. Spinal cord tolerance has been shown to increase dramatically from 21.3 Gy to 27.2 Gy as dose rate is reduced from 1.79 Gy/min to 0.245 Gy/min in a rat model 28,29. Spinal cord tolerance appears to be constant in the range of typical clinical dose rates from 1.79 Gy/min up to 10-15 Gy/min18,27,28. The ED50 (20.0 Gy) for the current study is in good agreement with published spinal cord tolerance data for pigs (if adjusted for the dose-rate effect)14, guinea pigs30, rats18,27,28 and mice31 that receive uniform spinal cord irradiation under similar conditions (field length ≥ 1.6 cm and dose rate > 1.7 Gy/min).
The follow-up period of the current study (53-75 weeks) is considered long enough to observe the initial phase of radiation myelopathy (4-6 months) as commonly reported for guinea pigs30, rats18,27,28, mice31 and pigs14. The latent period observed for the onset of motor deficits in the current study (10-35 weeks) was in good agreement with the Oxford study14 (7.5-16.5 weeks). The latency period for pigs is shorter than the mean response time (10.3 months) reported for three cases of human myelopathy following single-fraction spine radiosurgery but response times do overlap11. It is possible that more cases of radiation myelopathy would have been observed with a longer follow-up period; the Oxford study reported two late responding pigs at 65 and 75 weeks following irradiation to doses very close to ED50. The one non-responding animal in the 20 Gy dose group (pig #12, Table 2) of the current study was followed out to 75 weeks but no neurologic changes were noted.
The histological changes observed in the lateral white matter are very similar to lesions observed in other studies in pigs23, monkeys26 and rats32. Lesions consist of diffuse demyelination, with focal areas of coalescing necrosis in the higher dose groups. In the current study, the animal showing transient neurological signs and subsequently followed for at least a year after irradiation, showed some degree of glial scar formation, the extent and location of which are in agreement with the transient and relatively minor character of the motor impairment.
The spine of the mature Yucatan minipig is similar in size to humans allowing for inclusion of the same image-guided positioning and irradiation conditions encountered in clinical spinal radiosurgery. The accuracy of the Novalis Body image-guidance system has been reported by Verellen, et. al., as a mean three-dimensional displacement vector of 0.6 mm with an overall standard deviation of 0.9 mm when 2 mm thick CT slices are used33. Solberg, et. al.34 used the Novalis Body system to target an anthropomorphic head phantom and observed a mean (Std. Dev.) three-dimensional vector displacement of 1.1 mm (0.42 mm) in end-to-end positioning verification tests. Vinci, et. al., compared measured versus calculated positions of the 80% isodose line following Novalis Body image-guided irradiation of a head phantom and concluded that the addition of 1.25, 1.0, and 1.0 mm margins (A-P,R-L,S-I) is appropriate for a gross tumor volume/planning tumor volume expansion to cover delivery error35. The shape of the dose distribution used in the current study makes the dose-volume histogram relatively insensitive to 1-2 mm shifts in the anterior/posterior and rostral/caudal directions. A lateral 1 mm shift of the treatment isocenter towards the spinal cord would result in an increase in the maximum spinal cord dose of approximately 1-2% while a 1 mm shift in the opposite direction would result in a decrease of the maximum spinal cord dose of 3-4%. Respiration-induced spinal motion was not considered in this study because it has been reported to be negligible in swine under similar setup conditions36.
In conclusion, results indicate that for a dose distribution with a steep lateral gradient (≈8% per mm), pigs have an ED50 that closely resembles that for pigs (if adjusted for the dose-rate effect)14, guinea pigs30, rats18,27,28 and mice31 that receive uniform spinal cord irradiation. While this study does not ultimately determine the tolerance of the human spinal cord to radiosurgery, this study could not be performed in a human model. Studies have shown that pigs have many anatomic and physiologic similarities to humans24 and results of radiobiological studies have shown good agreement with the limited data from irradiated humans23. The porcine model provides a homogeneous population to perform a systematic investigation of radiation dose-volume effects on the spinal cord under conditions encountered in clinical spinal stereotactic radiosurgery and continuing studies are likely to elucidate the mechanism of injury and opportunities for intervention.
Acknowledgments
This project was funded in its entirety by the National Institute of Neurological Disorders and Stroke, R01 NS049517.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at:
49th Annual Meeting of the American Society for Radiation Oncology, Los Angeles, 2007
50th Annual Meeting of the American Society for Radiation Oncology, Boston, 2008
51st Annual Meeting of the American Society for Radiation Oncology, Chicago, 2009
Conflicts of Interest Notification
Paul Medin and Timothy Solberg teach in radiosurgery courses sponsored by BrainLAB AG. All remaining authors have no conflicts to report.
Contributor Information
Paul M Medin, Department of Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, USA.
Ryan D Foster, Department of Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, USA.
Albert J van der Kogel, Department of Radiation Oncology, Radboud University Medical Center Nijmegen, The Netherlands.
James W Sayre, Departments of Biostatistics of Radiology, University of California Los Angeles, CA, USA.
William H McBride, Department of Radiation Oncology, University of California Los Angeles, CA, USA.
Timothy D Solberg, Department of Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, USA.
References
- 1.Takacs I, I, Hamilton AJ, Lulu B, Fosmire H, Johnson P, Stea B, et al. Frame based stereotactic spinal radiosurgery: experience from the first 19 patients treated. Stereotact Funct Neurosurg. 1999;73(1-4):69. doi: 10.1159/000029755. [DOI] [PubMed] [Google Scholar]
- 2.Murphy MJ, Chang S, Gibbs I, Le QMD, Kim D. Image-Guided Radiosurgery in the Treatment of Spinal Metastases. Neurosurgical Focus. 2001;11(6) doi: 10.3171/foc.2001.11.6.7. [DOI] [PubMed] [Google Scholar]
- 3.Ryu SI, Chang SD, Kim DH, Murphy MJ, Le QT, Martin DP, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49(4):838–846. doi: 10.1097/00006123-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Yin FF, Ryu S, Ajlouni M, Zhu J, Yan H, Guan H, et al. A technique of intensity-modulated radiosurgery (IMRS) for spinal tumors. Med Phys. 2002;29(12):2815–2822. doi: 10.1118/1.1521722. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs IC, Patil C, Gerszten PC, Adler JR, Jr., Burton SA. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64(2 Suppl):A67–A72. doi: 10.1227/01.NEU.0000341628.98141.B6. [DOI] [PubMed] [Google Scholar]
- 6.Ryu S, Jin JY, Jin R, Rock J, Ajlouni M, Movsas B, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109(3):628–636. doi: 10.1002/cncr.22442. [DOI] [PubMed] [Google Scholar]
- 7.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32(2):193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 8.Ryu S, Fang YF, Rock J, Zhu J, Chu A, Kagan E, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97(8):2013–2018. doi: 10.1002/cncr.11296. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Bilsky MH, Lovelock DM, Venkatraman ES, Toner S, Johnson J, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71(2):484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S42–S49. doi: 10.1016/j.ijrobp.2009.04.095. [DOI] [PubMed] [Google Scholar]
- 11.Sahgal A, Ma L, Gibbs I, Gerszten PC, Ryu S, Soltys S, et al. Spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77(2):548–553. doi: 10.1016/j.ijrobp.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 12.van Luijk P, Bijl HP, Coppes RP, van der Kogel AJ, Konings AW, Pikkemaat JA, et al. Techniques for precision irradiation of the lateral half of the rat cervical spinal cord using 150 MeV protons [corrected] Phys Med Biol. 2001;46(11):2857–2871. doi: 10.1088/0031-9155/46/11/307. [DOI] [PubMed] [Google Scholar]
- 13.Bijl HP, van Luijk P, Coppes RP, Schippers JM, Konings AW, Der Kogel AJ. Regional differences in radiosensitivity across the rat cervical spinal cord. Int J Radiat Oncol Biol Phys. 2005;61(2):543–551. doi: 10.1016/j.ijrobp.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 14.van den Aardweg GJ, Hopewell JW, Whitehouse EM. The radiation response of the cervical spinal cord of the pig: effects of changing the irradiated volume. Int J Radiat Oncol Biol Phys. 1995;31(1):51–55. doi: 10.1016/0360-3016(94)E0306-5. [DOI] [PubMed] [Google Scholar]
- 15.Wong CS, van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv. 2004;4(5):273–284. doi: 10.1124/mi.4.5.7. [DOI] [PubMed] [Google Scholar]
- 16.Withers R. Migration and myelination. Int J Radiat Oncol Biol Phys. 2003;57(1):9–10. doi: 10.1016/s0360-3016(03)00530-3. [DOI] [PubMed] [Google Scholar]
- 17.Bijl HP, van Luijk P, Coppes RP, Schippers JM, Konings AW, van der Kogel AJ. Unexpected changes of rat cervical spinal cord tolerance caused by inhomogeneous dose distributions. Int J Radiat Oncol Biol Phys. 2003;57(1):274–281. doi: 10.1016/s0360-3016(03)00529-7. [DOI] [PubMed] [Google Scholar]
- 18.Bijl HP, van Luijk P, Coppes RP, Schippers JM, Konings AW, van der Kogel AJ. Dose-volume effects in the rat cervical spinal cord after proton irradiation. Int J Radiat Oncol Biol Phys. 2002;52(1):205–211. doi: 10.1016/s0360-3016(01)02687-6. [DOI] [PubMed] [Google Scholar]
- 19.Franklin RJ, Gilson JM, Blakemore WF. Local recruitment of remyelinating cells in the repair of demyelination in the central nervous system. J Neurosci Res. 1997;50(2):337–344. doi: 10.1002/(SICI)1097-4547(19971015)50:2<337::AID-JNR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Hopewell JW, Barnes DW, Robbins ME, Corp M, Sansom JM, Young CM, et al. The relative biological effectiveness of fractionated doses of fast neutrons (42 MeVd----Be) for normal tissues in the pig. II. Late effects on cutaneous and subcutaneous tissues. Br J Radiol. 1990;63(754):760–770. doi: 10.1259/0007-1285-63-754-760. [DOI] [PubMed] [Google Scholar]
- 21.Robbins ME, Barnes DW, Campling D, Hopewell JW, Knowles JF, Sansom JM, et al. The relative biological effectiveness of fractionated doses of fast neutrons (42 MeVd----Be) for normal tissues in the pig. IV. Effects on renal function. Br J Radiol. 1991;64(765):823–830. doi: 10.1259/0007-1285-64-765-823. [DOI] [PubMed] [Google Scholar]
- 22.Hopewell JW, Rezvani M, Moustafa HF. The pig as a model for the study of radiation effects on the lung. Int J Radiat Biol. 2000;76(4):447–452. doi: 10.1080/095530000138439. [DOI] [PubMed] [Google Scholar]
- 23.van den Aardweg GJ, Hopewell JW, Whitehouse EM, Calvo W. A new model of radiation-induced myelopathy: a comparison of the response of mature and immature pigs. Int J Radiat Oncol Biol Phys. 1994;29(4):763–770. doi: 10.1016/0360-3016(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 24.Swindle MM, Smith A. In: Informational Resources on Swine in Biomedical Research. Smith C, editor. USDA Agricultural Research Service; 2000. (AWIC Resource Series No. 11). [Google Scholar]
- 25.Fajardo LF, Berthrong M. Vascular lesions following radiation. Pathol Annu. 1988;23(Pt 1):297–330. [PubMed] [Google Scholar]
- 26.Schultheiss TE, Stephens LC, Jiang GL, Ang KK, Peters LJ. Radiation myelopathy in primates treated with conventional fractionation. Int J Radiat Oncol Biol Phys. 1990;19(4):935–940. doi: 10.1016/0360-3016(90)90015-c. [DOI] [PubMed] [Google Scholar]
- 27.Hopewell JW, Morris AD, Dixon-Brown A. The influence of field size on the late tolerance of the rat spinal cord to single doses of X rays. Br J Radiol. 1987;60(719):1099–1108. doi: 10.1259/0007-1285-60-719-1099. [DOI] [PubMed] [Google Scholar]
- 28.Scalliet P, Landuyt W, Van der SE. Repair kinetics as a determining factor for late tolerance of central nervous system to low dose rate irradiation. Radiother Oncol. 1989;14(4):345–353. doi: 10.1016/0167-8140(89)90147-3. [DOI] [PubMed] [Google Scholar]
- 29.Pop LA, van der PM, Ruifrok AC, Schalkwijk LJ, Hanssen AE, van der Kogel AJ. Tolerance of rat spinal cord to continuous interstitial irradiation. Int J Radiat Oncol Biol Phys. 1998;40(3):681–689. doi: 10.1016/s0360-3016(97)00852-3. [DOI] [PubMed] [Google Scholar]
- 30.Knowles JF. The radiosensitivity of the guinea-pig spinal cord to X-rays: the effect of retreatment at one year and the effect of age at the time of irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1983;44(5):433–442. doi: 10.1080/09553008314551411. [DOI] [PubMed] [Google Scholar]
- 31.Lo YC, McBride WH, Withers HR. The effect of single doses of radiation on mouse spinal cord. Int J Radiat Oncol Biol Phys. 1992;22(1):57–63. doi: 10.1016/0360-3016(92)90982-n. [DOI] [PubMed] [Google Scholar]
- 32.van der Kogel AJ. Radiation tolerance of the rat spinal cord: time-dose relationships. Radiology. 1977;122(2):505–509. doi: 10.1148/122.2.505. [DOI] [PubMed] [Google Scholar]
- 33.Verellen D, Soete G, Linthout N, Van Acker S, De Roover P, Vinh-Hung V, et al. Quality assurance of a system for improved target localization and patient set-up that combines real-time infrared tracking and stereoscopic X-ray imaging. Radiother Oncol. 2003;67(1):129–141. doi: 10.1016/s0167-8140(02)00385-7. [DOI] [PubMed] [Google Scholar]
- 34.Solberg TD, Medin PM, Mullins J, Li S. Quality assurance of immobilization and target localization systems for frameless stereotactic cranial and extracranial hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;71(1 Suppl):S131–S135. doi: 10.1016/j.ijrobp.2007.05.097. [DOI] [PubMed] [Google Scholar]
- 35.Vinci JP, Hogstrom KR, Neck DW. Accuracy of cranial coplanar beam therapy using an oblique, stereoscopic x-ray image guidance system. Med Phys. 2008;35(8):3809–3819. doi: 10.1118/1.2955751. [DOI] [PubMed] [Google Scholar]
- 36.Medin PM, Solberg TD, De Salles AA, Cagnon CH, Selch MT, Johnson JP, et al. Investigations of a minimally invasive method for treatment of spinal malignancies with LINAC stereotactic radiation therapy: accuracy and animal studies. Int J Radiat Oncol Biol Phys. 2002;52(4):1111–1122. doi: 10.1016/s0360-3016(01)02762-6. [DOI] [PubMed] [Google Scholar]