Abstract
BACKGROUND
Prokineticin-1 (PROK1) and connective tissue growth factor (CTGF) are expressed in human endometrium and first-trimester decidua and have individually been proposed to have roles in implantation and placentation. We have recently demonstrated that CTGF may be a target gene for PROK1 in gene array analysis of a prokineticin receptor-1 stably transfected Ishikawa endometrial epithelial cell line (PROKR1-Ishikawa). The first aim of the study was to determine the effect of PROK1 on CTGF expression in PROKR1-Ishikawa cells and first-trimester decidua samples. Secondly, the effect of CTGF on trophoblast-derived HTR-8/SVneo cell adhesion and network formation was investigated.
METHODS AND RESULTS
Real-time qPCR showed that CTGF expression is elevated in first-trimester decidua compared with non-pregnant endometrium. In decidua, CTGF co-localized with PROKR1 to the glandular epithelium and a subset of stromal cells. PROK1 increased CTGF mRNA and protein expression in PROKR1-Ishikawa cells and first-trimester human decidua (8–12 weeks gestation). Knock down of endogenous PROK1 using micro RNA constructs targeted at PROK1, resulted in decreased expression of CTGF mRNA and protein in decidua. Inhibitors of specific cell signalling molecules demonstrated that PROK1 regulates CTGF expression via the Gq, phospholipase C (PLC), cSrc, epidermal growth factor receptor (EGFR), mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) kinase pathway activation. Treatment of trophoblast-derived HTR-8/Svneo cells with 1 µg/ml CTGF significantly increased adhesion to collagen IV, and differentiation of the cells into tube-like structures in matrigel.
CONCLUSIONS
CTGF expression in early pregnancy decidua is regulated by PROK1, via activation of the Gq, PLC, cSrc, EGFR, MAPK/ERK kinase pathway. CTGF in turn may contribute to the regulation of trophoblast conversion of maternal spiral arteries.
Keywords: prokineticin 1, connective tissue growth factor, decidua, trophoblast, cell adhesion and network formation
Introduction
During the first trimester of normal pregnancy, extravillous trophoblast (EVT) cells attach to and then invade the maternal decidua where they undergo endovascular transformation. These processes occur firstly in order to anchor the fetus to the maternal endometrium and secondly to remodel and replace the normal musculo-elastic structure of the maternal spiral arteries with fibrinoid material containing trophoblast. This results in conversion of the arteries into large diameter, low resistance vessels that provide steady perfusion of the villous trophoblast with maternal blood (Pijnenborg et al., 2006). The process of trophoblast invasion and endovascular transformation are tightly controlled spatially and temporally by factors released by decidua and trophoblast including fibroblast growth factor (FGF) (Anteby et al., 2004), vascular endothelial growth factor-A (Schiessl et al., 2009) and transforming growth factor-β1 (TGF-β1) (Lash et al., 2005). Failure of trophoblast invasion and spiral artery transformation has been implicated in the pathogenesis of conditions such as pre-eclampsia and intrauterine growth restriction.
Prokineticin-1 (PROK1) is a pleiotropic protein that has recently been described as having an important role in early pregnancy (Hoffmann et al., 2006, 2009; Evans et al., 2008, 2009). During the first trimester of pregnancy, expressions of PROK1 and its receptor prokineticin receptor-1 (PROKR1) are increased in decidua compared with non-pregnant endometrium (Battersby et al., 2004; Evans et al., 2008). Gene array analysis of an endometrial epithelial cell line stably expressing PROKR1 (PROKR1-Ishikawa) recently demonstrated that PROK1 differentially regulates a number of genes which are known to be important in implantation and early pregnancy, including cyclooxygenase-2, leukaemia inhibitory factor, interleukin (IL)-6, IL-8 and IL-11 (Evans et al., 2008, 2009; Cook et al., 2010). This therefore led to the hypothesis that PROK1 may play an important role in the establishment of early pregnancy (Evans et al., 2008).
The same gene array also identified connective tissue growth factor (CTGF) as a target gene for PROK1 (Evans et al., 2008). CTGF is a heparin-binding 38 kDa cysteine rich peptide that belongs to the CCN (Cyr61, CTGF, Nov) family of secretory proteins. With a broad spectrum of biological activities including cellular proliferation, differentiation, adhesion, chemotaxis, migration, apoptosis and extracellular matrix production, CTGF has major roles in many physiological as well as pathological processes, including angiogenesis, tissue repair, chondrogenesis, osteogenesis, cancer and fibrosis (Bradham et al., 1991; Brigstock, 2003). It is produced by, and capable of acting upon numerous cell types, including human (Uzumcu et al., 2000), mouse (Surveyor et al., 1998) and porcine (Moussad et al., 2002) uterine tissue. Studies have also suggested that CTGF may have a role in regulating implantation and placentation (Roh et al., 2005; Rimon et al., 2008) with expression being increased in placentae from women with pre-eclampsia compared with an uncomplicated pregnancy (Oh et al., 2009). However, whether CTGF has a direct effect on trophoblast biology has not been studied.
The current study therefore investigated PROK1 regulation of CTGF and examined PROKR1 and CTGF localization in human pregnant and non-pregnant endometrial tissues. Furthermore, the signalling pathway of PROK1-mediated CTGF expression was determined and the potential effects of CTGF on important physiological events during early pregnancy were investigated, specifically cell adhesion and network formation, using the trophoblast-derived HTR-8/SVneo cell line.
Materials and Methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) F-12 Glutamax and RPMI-1640 culture medium were purchased from Invitrogen Life Technologies (Paisley, UK). Penicillin–streptomycin and fetal calf serum (FCS) were purchased from PAA Laboratories Limited (Middlesex, UK). Inhibitors of Gq (YM-254890, final concentration 1 µM), phospholipase C (PLC, U73122, final concentration 10 µM), cSrc [4-amino- 5-(4-chlorophenyl)-7-(t-butyl)pyrazolo(3,4-d) pyrimidine (PP2), final concentration 10 µM], matrix metalloprotease (MMP, GM6001, final concentration 10 µM), epidermal growth factor receptor (EGFR, AG1478, final concentration 200 nM), mitogen-activated protein kinase (MEK, PD98059, final concentration 50 µM) and nuclear factor of activated T cells (NFAT, INCA 6, final concentration 20 µM) were all purchased from Calbiochem (Nottingham, UK) except YM-254890, a kind gift from M Taniguchi, Astellas Pharma Inc. (Tsukuba, Japan). Prokineticin receptor 1 antibody was purchased from Lifespan Biosciences (Atlanta, GA, USA) and the CTGF antibody was purchased from AbD Serotec (Kidlington, UK) Recombinant human PROK1 and CTGF were purchased from Peprotech (London, UK) and AbD Serotec, respectively. Alkaline phosphatase secondary antibodies and bovine serum albumin (BSA) were purchased from Sigma (Dorset, UK).
Patients and tissue collection
Endometrial biopsies (n = 35) were collected with an endometrial suction curette (Pipelle, Laboratoire CCD, Paris, France) from women of reproductive age with regular menstrual cycles (21–30 days) who were undergoing surgery for benign gynaecological conditions. All women had not taken any exogenous hormones in the 3 months preceding biopsy collection and none had any significant gynaecological pathology, i.e. no confirmed endometriosis or any fibroids greater than 3 cm in diameter. Biopsies were dated by a pathologist according to the histological criteria of Noyes et al. (1975). Furthermore, circulating progesterone and estradiol levels, measured in serum samples collected from each patient at the time of endometrial biopsy, were consistent for both the stated last menstrual period and the histological assessment (data not shown). First-trimester decidua (7–12 weeks gestation, n = 25) was collected from women undergoing elective first-trimester surgical termination of pregnancy. Ethical approval was obtained from Lothian Research Ethics Committee, and written informed consent obtained before tissue collection.
At the time of collection, tissue biopsies were: (i) fixed in neutral-buffered formalin (4%) for 24 h, stored in 70% ethanol and wax embedded; (ii) immersed in RNAlater (Ambion, Austin, TX, USA) and stored at −80°C for subsequent RNA extraction and/or (iii) chopped finely with scissors and transferred into serum-free DMEM-F-12 GlutaMAX, containing 100 IU penicillin and 100 µg/ml streptomycin for culture.
Cell/tissue culture and treatment
Human Ishikawa endometrial epithelial cells (European Collection of Cell Culture, Health Protection Agency, Porton Down, Wiltshire, UK), stably transfected with PROKR1 (PROKR1-Ishikawa cells) were maintained in DMEM F-12 GlutaMAX culture medium with 10% FCS, 100 IU penicillin and 100 µg streptomycin and 200 µg/ml G418 antibiotic, at 37°C and 5% CO2 as previously described (Evans et al., 2008). The human EVT cell-line HTR-8/SVneo was a kind gift from Prof. Charles H. Graham, Queen's University, Kingston, Ontario, Canada and was maintained in RPMI containing 5% FCS, 100 IU penicillin and 100 µg streptomycin at 37°C and 5% CO2. First-trimester decidua isolated at surgical termination of pregnancy was chopped finely with scissors and maintained in serum-free DMEM F-12 GlutaMAX containing 100 IU penicillin, and 100 µg/ml streptomycin overnight at 37°C and 5% CO2. Tissue was divided into equal portions the following day for experimental procedures.
PROKR1-Ishikawa cells and decidua explants were incubated in serum-free DMEM F-12 GlutaMAX containing 100 IU penicillin, and 100 µg/ml streptomycin overnight at 37°C and 5% CO2, before treatment with 40 nM PROK1 or vehicle, in the presence or absence of cell signalling inhibitors. The treatment dose of PROK1 was based upon previous dose–response findings of inositol phosphate mobilization in response to PROK1 treatment in PROKR1-Ishikawa cells (Evans et al., 2008). Cells were pre-treated with inhibitors for 1 h prior to PROK1 stimulation. Cells, tissue and culture medium were harvested and RNA was extracted for Taqman quantitative reverse transcription-PCR (qRT–PCR) and media was stored for CTGF ELISA analysis.
Decidua tissue samples were infected with lentivirus expressing PROK1 miRNA constructs for 72 h as described by Evans et al. (2009). Oligonucleotides encoding human PROK1 miRNA constructs were obtained from Invitrogen and inserted into the pcDNA6.2-GW/EmGFP-miR vector and used for transient transfections. These were recombined to create plenti6/V5-EmGFP-miR negative control and pLenti6/V5-EmGFP-hum-PROK1-72 and -287 (Evans et al., 2009). Following infection, tissue and medium were harvested, and RNA was extracted for taqman qRT–PCR and medium was stored for CTGF ELISA analysis.
Taqman qRT–PCR
CTGF expressions in PROKR1-Ishikawa cells and first-trimester decidua were measured by qRT–PCR analysis. RNA was extracted with Total RNA Isolation reagent (Sigma) as per the manufacturer's guidelines using phase lock tubes (Eppendorf, Cambridge, UK). RNA samples were reverse transcribed using MgCl2 (5.5 mM), deoxy (d)-NTPs (0.5 mM each), random hexamers (2.5 µM), ribonuclease inhibitor (0.4 U/µl) and multiscribe reverse transcriptase (1.25 U/µl; all from PE Applied Biosystems, Warrington, UK) and samples were incubated for 90 min at 25°C, 45 min at 48°C and 5 min at 95°C. A tube with no reverse transcriptase was included to control for DNA contamination. RT–PCR analysis was carried out using an ABI Prism 7900 (Applied Biosystems, Foster City, CA, USA) with CTGF primers and FAM (6-carboxyfluorescein)-labelled probe (forward: 5′-TGCACCGCCAAAGATGGT-3′, reverse: 5′-GGCACGTGCACTGGTACTTG-3′, probe: 5′-TCCCTGCATCTTCGGTGGTACGGT-3′). Gene expression was normalized to RNA loading using primers and VIC (Applied Biosystems)-labelled probe for ribosomal 18S as an internal standard (forward: 5′-CGGCTACCACATCCAAGGAA-3′, reverse: 5′-GCTGGAATTACCGCGGCT-3′, probe: 5′-TGCTGGCACCAGACTTGCCCTC-3′). Results are expressed as relative to a positive RNA standard (cDNA obtained from a single endometrial tissue) included in all reactions.
CTGF ELISA
CTGF protein secretion was analysed in the culture medium from PROKR1-Ishikawa cells and decidua explants using an ELISA development kit based on a sandwich ELISA format from Peprotech EC (London, UK), as per the manufacturer's instructions. Absorbance was detected by spectrophotometry, at 405 nm, with wavelength correction set at 650 nm. The detection sensitivity for CTGF was 63 pg/ml. Data are presented as mean ± SEM from at least three individual experiments.
Immunofluorescent microscopy
Co-localization of PROKR1 and CTGF in non-pregnant endometrium and first-trimester decidua was performed by dual fluorescent immunohistochemistry. Paraffin-embedded sections of 5 μm were dewaxed and rehydrated in graded ethanol. Antigen retrieval was performed by boiling in 0.01-M citrate buffer for 5 min, before endogenous peroxidase activity was quenched with 3% H2O2/MeOH solution. Non-specific binding sites were blocked using 5% normal goat serum, and then sections were incubated with rabbit anti-CTGF (1:10 000 in blocking serum) overnight at 4°C. Control sections were incubated with an equivalent concentration of normal immunoglobulin G from the same host species or blocking serum without antibody to confirm antibody specificity. CTGF immunoreactivity was detected with peroxidase-labelled goat anti-rabbit antibodies (1:500 in blocking serum), followed by incubation with fluorochrome TSA-plus fluorescein system (1:50 in substrate), producing a green fluorescence. Sections were then boiled again in 0.01-M citrate buffer for 5 min to prevent non-specific binding of the second peroxidise antibody, before re-blocking with 5% normal goat serum and incubation with rabbit anti-human PROKR1 (1:500) overnight at 4°C. PROKR1 immunoreactivity was detected with peroxidase-labelled goat anti-rabbit antibodies (1:500) followed by fluorochrome TSA-plus cyanide 3 System (1:50 in substrate), producing a red fluorescence. Sections were washed well with phosphate-buffered saline (PBS) in between antibody incubations, and counterstained with the nuclear specific fluorescent label 4′,6′-diamidino-2-phenylindole (Sigma 1:1000 in PBS) and mounted in PermaFluor fluorescence mounting medium. Fluorescent images were captured using a confocal laser scanning microscope (Meta Confocal; Carl Zeiss, Jena, Germany).
HTR-8/SVneo adhesion assay
HTR-8/Svneo cells in suspension were treated with CTGF (1 µg/ml), PROK1 (40 nM) or vehicle for 1 h, containing 1% serum. The concentrations of CTGF and PROK1 treatment were based on dose–response findings of cell adhesion to the extracellular matrix proteins (data not shown). Cells were plated in triplicate at 5 × 104 cells/well on 96-well collagen I, collagen IV, laminin or BSA (control)-coated CytoMatrix cell adhesion strips (Chemicon International, Inc.) according to the manufacturer's instructions and incubated at 37°C for 45 min. Cells were rinsed three times with PBS containing calcium and magnesium and stained for 5 min with 0.2% crystal violet dissolved in 10% ethanol. After five washes with PBS, crystal violet was eluted with 0.1 M NaH2PO4 containing 50% ethanol for 15 min and the optical densities were measured at 570 nm. Data are presented as mean ± SEM from at least three individual experiments.
HTR-8/SVneo network assay
Remodelling of maternal spiral arteries involves trophoblast invasion and EVT differentiation, whereby EVT form a lining of the uterine vessels. Previous reports have shown that primary cytotrophoblast and HTR-8/Svneo cells show endothelial cell-like behaviour in their ability to form networks of tube-like structures when grown on matrigel (Dokras et al., 2001). We used an in vitro culture system to investigate the effects of CTGF and PROK1 on HTR-8/Svneo cell network formation. Transwell cell culture inserts (diameter 12 mm, pore size 0.4 µM; Corning, Fisher Scientific UK Ltd., Loughborough, UK) were coated with 100 µl growth factor reduced Matrigel (BD Biosciences, Oxford, UK) and allowed to set at 37°C for at least 30 min. 2.5 × 104 HTR-8/SVneo cells in cell culture medium + 1% FCS were added to each insert and placed into a 12-well plate containing culture medium and either vehicle, 1 µg/ml CTGF, 40 nM PROK1 or 50 ng/ml FGF-2 as a positive control in the lower chamber. The concentrations of CTGF and PROK1 treatment were based on dose–response findings of network formation (data not shown). Cells were incubated at 37°C with 5% CO2 for 18 h, then fixed in methanol, stained with haematoxylin and five areas from each well were imaged using an Axiovert microscope (Zeiss) with ×10 magnification. Images were blinded and the number of cell–cell protracted contacts was counted as representations of the number of capillary-like networks present in each field. Each experiment was performed in duplicate. Data are presented as the average number of networks per field ± SEM from at least three separate experiments.
Statistics
All data are expressed as mean ± SEM. Results were subjected to statistical analysis by one-way analysis of variance or Student's t-tests as appropriate using Prism 5.0c (GraphPad Prism, San Diego, CA, USA) and significance was accepted when P < 0.05.
Results
PROK1 increases CTGF via Gq, PLC, cSrc, EGFR, MEK signalling
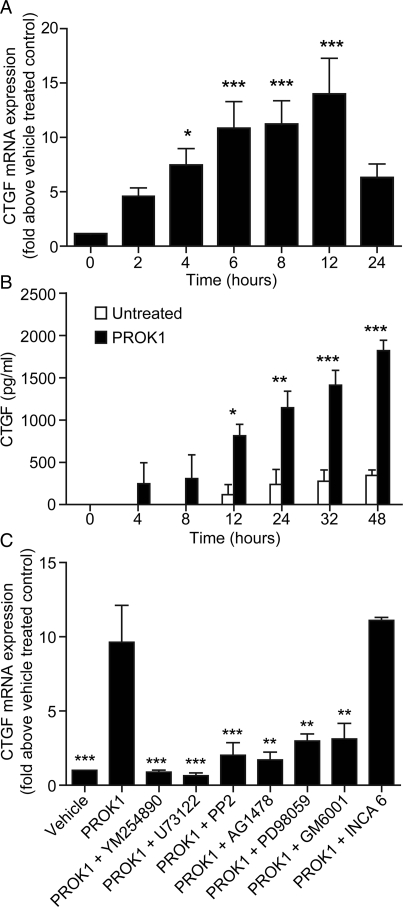
To determine the effect of PROK1 on CTGF expression and release, PROKR1-Ishikawa cells were treated with 40 nM PROK1 for up to 48 h. After 4 h treatment with PROK1, there was a significant fold-increase in CTGF mRNA expression compared with vehicle-treated cells (7.4 ± 1.6-fold; Fig. 1A; P < 0.05), which peaked by 12 h (13.9 ± 3.3-fold; P < 0.001). CTGF protein secretion was significantly elevated after 12–48 h of treatment (48 h: 1819.1 ± 122.7 versus 345.3 ± 64.4 pg/ml; Fig. 1B; P < 0.001). CTGF is a TGF-β1 inducible gene in fibroblasts, activated via a signalling cascade involving PLC, cSrc and MEK (Kucich et al., 2001). To examine whether PROK1 activates CTGF expression through a similar pathway, PROKR1-Ishikawa cells were treated with 40 nM PROK1 alone or with a panel of chemical inhibitors of cell signalling (Fig. 1C). Co-treatment with PROK1 and inhibitors of Gq (YM-254890), PLC (U73122), cSrc (PP2), MMP (GM6001), EGFR (AG1478) or MEK (PD98059), for 6 h significantly inhibited PROK1-mediated CTGF mRNA expression (P < 0.05). An inhibitor of NFAT-calcineurin association (INCA6) had no significant effect on PROK1-mediated CTGF mRNA expression.
Figure 1.
PROK1 increases CTGF mRNA and protein expression, via Gq, PLC, cSrc, EGFR and MEK signalling in PROKR1-Ishikawa cells. (A) CTGF mRNA and (B) protein expression in PROKR1-Ishikawa cells after treatment with 40 nM PROK1. CTGF mRNA expression peaked after 12 h and protein expression peaked after 48 h of PROK1 treatment. (C) Expression of CTGF mRNA was measured after treatment with vehicle or 40 nM PROK1 in the absence or presence of the specific cell signalling inhibitors YM-254890 (Gq inhibitor; YM), U73122 (PLC inhibitor; U7), PP2 (cSrc inhibitor), GM1489 (matrix metalloproteinase inhibitor; GM), AG1478 (EGFR inhibitor; AG), PD98059 (MEK inhibitor; PD) or inhibitor of INCA6 (NFAT cells, inhibitor, INCA 6). PROK1-induced CTGF mRNA expression was significantly inhibited in the presence of these inhibitors with the exception of INCA 6. Each bar represents the mean ± SEM of at least three independent experiments. ***P < 0.001, **P < 0.01, *P < 0.05 compared with vehicle treated cells (A and B) or PROK1 treatment alone (C), as determined by one-way ANOVA.
CTGF is expressed in endometrium and first-trimester decidua and regulated in decidua by PROK1
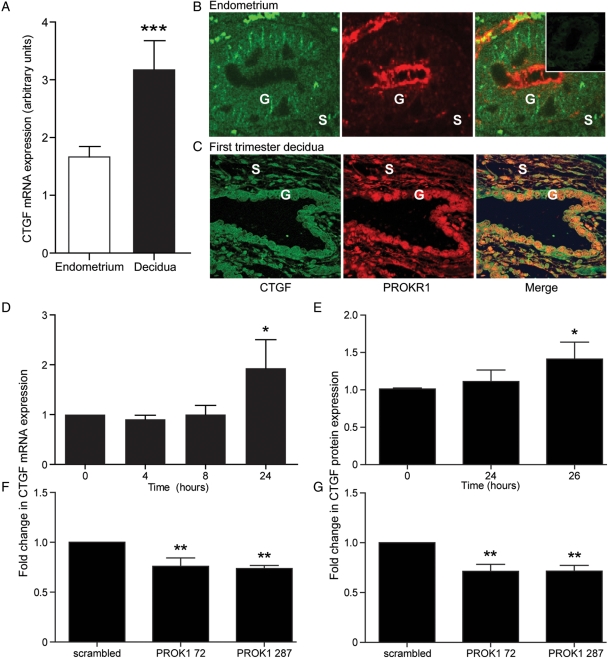
Since PROK1 and PROKR1 expressions are significantly elevated in first-trimester decidua compared with non-pregnant endometrium, we sought to investigate whether CTGF follows a similar pattern. Relative expression of CTGF mRNA expression was significantly higher in first-trimester decidua compared with non-pregnant endometrium (1.9 ± 0.49-fold; P < 0.01, Fig. 2A). There was no difference in CTGF mRNA expression across different stages of the menstrual cycle (data not shown). Immunofluorescent histochemistry and confocal microscopy were used to investigate the site of expression of CTGF in relation to PROKR1. CTGF and PROKR1 both localized to glandular epithelial cells in non-pregnant endometrium (Fig. 2B) and co-localized to a subset of stromal cells in first-trimester decidua (Fig. 2C).
Figure 2.
CTGF expression and localization in human non-pregnant endometrium and first-trimester decidua and regulation by PROK1 in first-trimester decidua. (A) CTGF mRNA expression is elevated in first-trimester decidua (n = 42) compared with non-pregnant endometrium (n = 30). (B) In non-pregnant endometrium (n = 4; representative sections shown), double fluorescence immunohistochemistry demonstrated that CTGF (green panel) and PROKR1 (red panel) both localized to the glandular epithelium (G) and a subset of stromal cells (S). Negative control (−ve) is indicated in the figure insert. (C) CTGF and PROKR1 co-localized in the glandular epithelium (G) and a subset of stromal cells (S) in first-trimester decidua. Co-localization shown in merge panel (×20 magnification). Treatment of first-trimester decidua with 40 nM PROK1-increased CTGF mRNA (n = 10) (D) and protein expression (n = 7) (E), after 24 and 26 h of treatment respectively. Conversely, first-trimester decidua infected with lentivirus encoding miRNA constructs targeting PROK1 (72 or 287)-decreased CTGF mRNA (F) and protein expression (G) compared with control tissue infected with a scrambled miRNA sequence. ***P < 0.001, **P < 0.01, *P < 0.05 compared with non-pregnant endometrium (A), vehicle-treated tissue (D and E) or compared with scrambled siRNA PROK1 construct (F and G), as determined by Student's t-test (A) or one-way ANOVA (D–G).
We subsequently investigated the effect of PROK1 on CTGF expression and release from first-trimester decidua. Treatment of first-trimester decidua explants with 40 nM PROK1-increased CTGF mRNA expression and protein secretion compared with vehicle-treated controls after 24 (1.93 ± 1.62-fold, Fig. 2D, P < 0.05) and 26 h, (1.56 ± 0.19-fold; Fig. 2E; P < 0.05; Fig. 2E), respectively. We confirmed that PROK1 regulates expression of CTGF in first-trimester decidua using PROK1-targeted miRNA lentivirus. First-trimester decidua was infected with one of two miRNA constructs targeting PROK1 (pLenti6/V5-EmGFP-hum-PROK1-72 or-287) or a negative scrambled control lentivirus (as described in Evans et al., 2009). Infection of first-trimester decidua with either the pLenti6/V5-EmGFP-hum-PROK1-72 or -287 significantly reduced levels of both CTGF mRNA (0.76 ± 0.08-fold and 0.74 ± 0.03-fold; P < 0.01; Fig. 2F) and secreted CTGF (0.81 ± 0.07-fold and 0.72 ± 0.06-fold; Fig. 2G) when compared with the negative scrambled control construct.
CTGF promotes HTR-8/SVneo cell adhesion to extracellular matrix
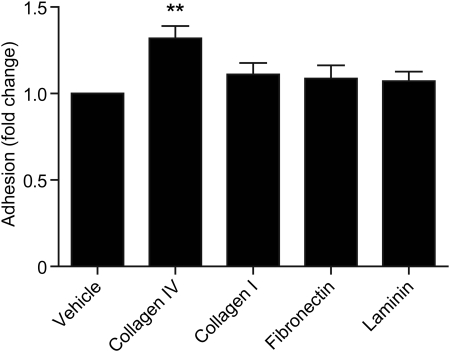
To investigate whether CTGF had an effect on the adhesion of trophoblast to different extracellular matrix components, HTR-8/Svneo cells were incubated on collagen I, collagen IV, fibronectin or laminin coated or uncoated wells. Treatment with 1 µg/ml CTGF significantly increased HTR-8/SVneo adhesion to collagen IV (1.4 ± 0.07-fold compared with vehicle-treated control; Fig. 3; P < 0.01), but not to collagen I, fibronectin or laminin (all P > 0.05). Similar studies using 40 nM PROK1 had no significant effect on HTR-8/SVneo cell adhesion to any of the matrices described (data not shown).
Figure 3.
CTGF enhances HTR-8/Svneo cell adhesion. Treatment of HTR-8/SVneo cells with 1 µg/ml CTGF-increased cell adhesion to collagen IV, but not collagen I, fibronectin or laminin. **P < 0.01 compared with uncoated wells. Each experiment was carried out in triplicate, and each bar represents the mean ± SEM of at least three different experiments.
CTGF promotes HTR-8/SVneo cell network formation
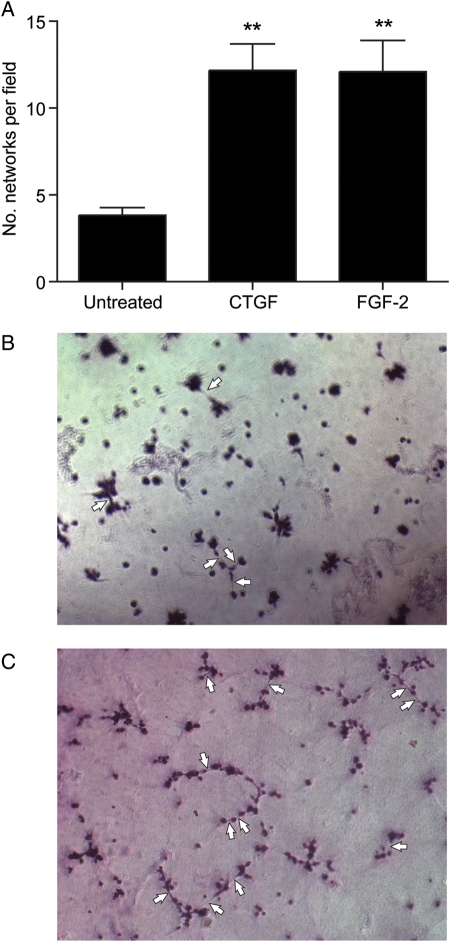
HTR-8/SVneo cells in matrigel were treated with 1 µg/ml CTGF or 50 ng/ml FGF-2, a positive angiogenic control (Hoffmann et al., 2009). Compared with vehicle-treated controls, the number of networks was significantly increased in cells treated with CTGF (9.3 ± 1.1 versus 3.1 ± 0.5 networks per field; Fig. 4A–C; P < 0.01) to a similar level as treatment with FGF-2 (FGF-2; 9.3 ± 1.1 versus 9.7 ± 2.5 networks per field; Fig. 4A, P < 0.01) for 18 h. Similar studies using 40 nM PROK1 had no effect on HTR-8/SVneo cell network formation (data not shown).
Figure 4.
CTGF induces network formation in trophoblast cell line HTR-8/SVneo. (A) Treatment of HTR-8/SVneo cells with CTGF increased the number of capillary-like network formations, as scored by the number of protracted cell–cell contacts formed following 18 h of treatment, to a similar level as caused by treatment with the known pro-angiogenic factor FGF-2. Each experiment was carried out in duplicate and each bar represents the mean ± SEM of at least three different experiments. **P < 0.01 compared with vehicle-treated controls representative images of capillary-like network formations by HTR-8/SVneo cells treated with vehicle (B) or CTGF (C). Examples of capillary-like network formations are highlighted by arrows in (B) and (C).
Discussion
This study demonstrates a novel role for PROK1 in early pregnancy in mediating expression of CTGF. CTGF in turn may regulate important physiological events in early pregnancy including trophoblast cell adhesion and network formation involved in endovascular transformation of EVT. Using the PROKR1-Ishikawa cell line, we demonstrated that PROK1 regulates CTGF expression via Gq, PLC, cSrc, EGFR and MEK signalling. Next, we demonstrated that CTGF expression is elevated in first-trimester decidua compared with non-pregnant endometrium and CTGF and PROKR1 proteins are both localized to glandular epithelium and a subset of stromal cells in human non-pregnant endometrium and co-localized in first-trimester decidua. Treatment of decidua with PROK1-increased CTGF mRNA and protein expression and conversely, inhibition of PROK1 expression reduced CTGF expression. Finally, using the HTR-8/SVneo trophoblast cell line, we demonstrated that CTGF promotes key processes involved in spiral artery remodelling, including trophoblast adhesion to extracellular matrix, and network formation.
Previous work from our group has demonstrated that PROK1 has an important role in early pregnancy by up-regulating key genes involved in implantation and early placentation (Evans et al., 2008, 2009; Cook et al., 2010). Our studies have also suggested that PROK1 may regulate the expression of CTGF. CTGF has been postulated to have a role in implantation and placentation (Roh et al., 2005; Rimon et al., 2008), however to our knowledge in humans, CTGF regulation and function during the menstrual cycle and early pregnancy had not been investigated. We found that PROK1 regulates CTGF expression and secretion in cultured endometrial epithelial cells.
As the signalling pathway of TGF-β regulation of CTGF, involving PLC, cSrc and MEK, is well documented in fibroblasts (Kucich et al., 2001; Leask et al., 2003), we sought to investigate whether the same signalling pathway regulated PROK1-mediated CTGF expression in endometrial epithelial cells. We demonstrate that PROKR1, a Gq-coupled receptor that mediates extracellular signal-regulated kinase 1/2 phosphorylation (Negri et al., 2005) also induces CTGF expression via a cSrc-EGFR-MEK signalling pathway in PROKR1-Ishikawa cells. In addition, inhibition of MMP, thereby preventing cleavage of heparin-bound EGF and binding of EGF to its receptor, also prevented PROK1-mediated CTGF expression, further confirming that PROK1 signals via this pathway to regulate CTGF expression. However, inhibition of the calcium-calcineurin–NFAT signalling pathway had no effect on PROK1-mediated CTGF regulation. Although it is possible that the signalling pathways found to be active in our PROKR1-Ishikawa cell line may have been influenced by their malignant transformation or genetic modification to express PROKR1, previous studies in our lab have shown that responses from these cells are replicable in ex vivo explant studies (Evans et al., 2008, 2009; Maldonado-Pérez et al., 2009).
We also showed that CTGF expression is elevated in first-trimester decidua compared with non-pregnant endometrium. This finding is consistent with our previous observations that PROK1 expression is elevated in decidua compared with non-pregnant endometrium (Evans et al., 2008) and further supports our findings that PROK1 is responsible for elevated CTGF expression in first-trimester decidua. In contrast to PROK1, we saw no temporal regulation of CTGF across the menstrual cycle, suggesting that CTGF is not under steroidal or PROK1 regulation in human non-pregnant endometrium. CTGF is regulated by TGF-β1 in other systems (Grotendorst et al., 1996; Kothapalli et al., 1998; Hishikawa et al., 1999) and in pigs, CTGF expression is highly correlated with that of TGF-β1 in uterine tissues, indicating that CTGF may mediate some of the functions of TGF-β1 in the reproductive tract during the oestrous cycle and pregnancy, such as stromal remodelling and angiogenesis (Moussad et al., 2002). CTGF is also regulated by TNF-α (Cooker et al., 2007) and EGF (Wenger et al., 1999), cytokines which are also elevated in early pregnancy, therefore it is possible that PROK1 is not the only regulator of uterine CTGF expression in early pregnancy.
We have shown CTGF protein expression in glandular epithelium of non-pregnant endometrium, and glandular epithelium and stroma of decidua from early pregnancy using fluorescent immunohistochemistry. These findings are in agreement with those of Uzumcu et al. (2000) and of Surveyor et al. (1998) in mice. We have further demonstrated that CTGF co-localizes with PROKR1 in the glandular epithelium of first-trimester decidua, supporting a role for PROK1 regulation of CTGF in these cells in vivo during early pregnancy.
In order to confirm the physiological relevance of our data, we investigated the effect of PROK1 on CTGF expression in first-trimester decidua explants. Consistent with our observations in the PROKR1-Ishikawa cell line, we showed that PROK1 regulates CTGF mRNA expression and protein secretion in first-trimester decidua explants. We further confirmed that PROK1 regulates CTGF expression in first-trimester decidua using miRNA constructs targeting PROK1. In accordance with our finding that exogenous PROK1 increases CTGF expression and protein secretion in decidua explants, abrogation of endogenous PROK1 resulted in a decrease in CTGF expression and protein secretion, confirming that PROK1 regulates basal expression and secretion of CTGF in first-trimester decidua.
Our data show that CTGF, but not PROK1, promotes trophoblast-derived HTR-8/SVneo cell adhesion and formation of capillary-like networks. Indeed CTGF increased the number of network formations by HTR-8/SVneo cells to a similar level as the angiogenic factor FGF-2. Although the higher number of networks may not necessarily equate to functionality, the network formation assay demonstrates the capacity of HTR-8/SVneo cells to exhibit an endovascular cell-like phenotype. Functionally, therefore, we propose two novel activities of PROK1-mediated CTGF up-regulation, first, establishing the ability of CTGF to promote trophoblast cell adhesion to the extracellular matrix component collagen IV, and second, promoting EVT cell tube-like network organization. For conversion of the spiral arteries into wide bore low resistance vessels during the first trimester of pregnancy, EVT cells invade through the maternal decidua to the spiral arteries, where they differentiate into tubule structures, lining the wall of the arteries. Consistent with these requisite cellular processes, CTGF has been shown to promote endothelial cell adhesion, migration and proliferation in culture and to induce angiogenesis in vivo (Kireeva et al., 1997; Shimo et al., 1998, 1999; Babic et al., 1999).
Together these findings indicate that PROK1-mediated CTGF secretion in endometrial epithelial cells may act upon trophoblast cells in a paracrine manner to promote requisite cellular steps of trophoblast conversion of maternal spiral arteries. Therefore disrupted expression of PROK1 expression or signalling, and therefore of its downstream target CTGF, may have implications for inadequate conversion of the maternal spiral arteries, leading to placental abnormalities or pre-eclampsia. This is consistent with the hypothesis proposed by Oh et al. (2009) that CTGF may have a role in the pathophysiology of placental injury or its sequelae including pre-eclampsia. However, given the broad cellular range of CTGF functions, there are likely to be many other biological processes, such as decidualization, tissue remodelling and placentation that PROK1-mediated CTGF expression may influence, and these remain to be investigated.
In conclusion, our findings extend previous work on the role of PROK1 in early pregnancy, and suggest that PROK1 may be important in regulating trophoblast conversion of maternal spiral arteries via CTGF.
Funding
This study was supported by MRC core funding to F.C.D. and H.N.J. Funding to pay the Open Access publication charges for this article was provided by the Medical Research Council.
Acknowledgements
We thank Sharon McPherson and Katie Cairns for patient recruitment and assistance with tissue collection, Pamela Brown for preparation of viral constructs and Ted Pinner for graphical assistance.
References
- Anteby EY, Greenfield C, Natanson-Yaron S, Goldman-Wohl D, Hamani Y, Khudyak V, Ariel I, Yagel S. Vascular endothelial growth factor, epidermal growth factor and fibroblast growth factor-4 and -10 stimulate trophoblast plasminogen activator system and metalloproteinase-9. Mol Hum Reprod. 2004;10:229–235. doi: 10.1093/molehr/gah031. [DOI] [PubMed] [Google Scholar]
- Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby S, Critchley HO, Morgan K, Millar RP, Jabbour HN. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2463–2469. doi: 10.1210/jc.2003-032012. [DOI] [PubMed] [Google Scholar]
- Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Cook IH, Evans J, Maldonado-Perez D, Critchley HO, Sales KJ, Jabbour HN. Prokineticin (PROK1) modulates interleukin (IL)-11 expression via prokineticin receptor (PROKR1) and the calcineurin/NFAT signalling pathway. Mol Hum Reprod. 2010;16:158–169. doi: 10.1093/molehr/gap084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooker LA, Peterson D, Rambow J, Riser ML, Riser RE, Najmabadi F, Brigstock D, Riser BL. TNF-alpha, but not IFN-gamma, regulates CCN2 (CTGF), collagen type I, and proliferation in mesangial cells: possible roles in the progression of renal fibrosis. Am J Physiol. 2007;293:F157–F165. doi: 10.1152/ajprenal.00508.2006. [DOI] [PubMed] [Google Scholar]
- Dokras A, Gardner LM, Seftor EA, Hendrix MJ. Regulation of human cytotrophoblast morphogenesis by hepatocyte growth factor/scatter factor. Biol Reprod. 2001;65:1278–1288. doi: 10.1095/biolreprod65.4.1278. [DOI] [PubMed] [Google Scholar]
- Evans J, Catalano RD, Morgan K, Critchley HO, Millar RP, Jabbour HN. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology. 2008;149:2877–2887. doi: 10.1210/en.2007-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, Jabbour HN. Prokineticin 1 mediates fetal–maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009;23:2165–2175. doi: 10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]
- Hishikawa K, Nakaki T, Fujii T. Transforming growth factor-beta(1) induces apoptosis via connective tissue growth factor in human aortic smooth muscle cells. Eur J Pharmacol. 1999;385:287–290. doi: 10.1016/s0014-2999(99)00763-3. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology. 2006;147:1675–1684. doi: 10.1210/en.2005-0912. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Saoudi Y, Benharouga M, Graham CH, Schaal JP, Mazouni C, Feige JJ, Alfaidy N. Role of EG-VEGF in human placentation: physiological and pathological implications. J Cell Mol Med. 2009;13:2224–2235. doi: 10.1111/j.1582-4934.2008.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF. Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res. 1997;233:63–77. doi: 10.1006/excr.1997.3548. [DOI] [PubMed] [Google Scholar]
- Kothapalli D, Hayashi N, Grotendorst GR. Inhibition of TGF-beta-stimulated CTGF gene expression and anchorage-independent growth by cAMP identifies a CTGF-dependent restriction point in the cell cycle. FASEB J. 1998;12:1151–1161. doi: 10.1096/fasebj.12.12.1151. [DOI] [PubMed] [Google Scholar]
- Kucich U, Rosenbloom JC, Herrick DJ, Abrams WR, Hamilton AD, Sebti SM, Rosenbloom J. Signaling events required for transforming growth factor-beta stimulation of connective tissue growth factor expression by cultured human lung fibroblasts. Arch Biochem Biophys. 2001;395:103–112. doi: 10.1006/abbi.2001.2571. [DOI] [PubMed] [Google Scholar]
- Lash GE, Otun HA, Innes BA, Bulmer JN, Searle RF, Robson SC. Inhibition of trophoblast cell invasion by TGFB1, 2, and 3 is associated with a decrease in active proteases. Biol Repro. 2005;73:374–381. doi: 10.1095/biolreprod.105.040337. [DOI] [PubMed] [Google Scholar]
- Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J Biol Chem. 2003;278:13008–13015. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- Maldonado-Pérez D, Brown P, Morgan K, Millar RP, Thompson EA, Jabbour HN. Prokineticin 1 modulates IL-8 expression via the calcineurin/NFAT signaling pathway. Biochim Biophys Acta. 2009;1793:1315–1324. doi: 10.1016/j.bbamcr.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussad EE, Rageh MA, Wilson AK, Geisert RD, Brigstock DR. Temporal and spatial expression of connective tissue growth factor (CCN2; CTGF) and transforming growth factor beta type 1 (TGF-beta1) at the utero-placental interface during early pregnancy in the pig. Mol Pathol. 2002;55:186–192. doi: 10.1136/mp.55.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, Colucci MA, Mignogna G, Barra D, Grohovaz F, Codazzi F, Kaiser A, Kreil G, et al. Biological activities of Bv8 analogues. Brit J Pharmacol. 2005;146:625–632. doi: 10.1038/sj.bjp.0706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Oh SY, Song SE, Seo ES, Kim KH, Choi SJ, Suh YL, Sadovsky Y, Roh CR. The expression of connective tissue growth factor in pregnancies complicated by severe preeclampsia or fetal growth restriction. Placenta. 2009;30:981–987. doi: 10.1016/j.placenta.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Rimon E, Chen B, Shanks AL, Nelson DM, Sadovsky Y. Hypoxia in human trophoblasts stimulates the expression and secretion of connective tissue growth factor. Endocrinology. 2008;149:2952–2958. doi: 10.1210/en.2007-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh CR, Budhraja V, Kim HS, Nelson DM, Sadovsky Y. Microarray-based identification of differentially expressed genes in hypoxic term human trophoblasts and in placental villi of pregnancies with growth restricted fetuses. Placenta. 2005;26:319–328. doi: 10.1016/j.placenta.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Schiessl B, Innes BA, Bulmer JN, Otun HA, Chadwick TJ, Robson SC, Lash GE. Localization of angiogenic growth factors and their receptors in the human placental bed throughout normal human pregnancy. Placenta. 2009;30:79–87. doi: 10.1016/j.placenta.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Kimura Y, Nishida T, Ishizeki K, Matsumura T, Takigawa M. Inhibition of endogenous expression of connective tissue growth factor by its antisense oligonucleotide and antisense RNA suppresses proliferation and migration of vascular endothelial cells. J Biochem. 1998;124:130–140. doi: 10.1093/oxfordjournals.jbchem.a022071. [DOI] [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, et al. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- Surveyor GA, Wilson AK, Brigstock DR. Localization of connective tissue growth factor during the period of embryo implantation in the mouse. Biol Repro. 1998;59:1207–1213. doi: 10.1095/biolreprod59.5.1207. [DOI] [PubMed] [Google Scholar]
- Uzumcu M, Homsi MF, Ball DK, Coskun S, Jaroudi K, Hollanders JM, Brigstock DR. Localization of connective tissue growth factor in human uterine tissues. Mol Hum Reprod. 2000;6:1093–1098. doi: 10.1093/molehr/6.12.1093. [DOI] [PubMed] [Google Scholar]
- Wenger C, Ellenrieder V, Alber B, Lacher U, Menke A, Hameister H, Wilda M, Iwamura T, Beger HG, Adler G, et al. Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene. 1999;18:1073–1080. doi: 10.1038/sj.onc.1202395. [DOI] [PubMed] [Google Scholar]