Abstract
Problem
The NFκB pathway is a major source of pro-inflammatory cytokines, which may contribute to cancer chemoresistance. We showed that constitutive NFκB activity is characteristic of the ovarian cancer stem cells (OCSCs). The aim of this study is to determine if the inhibition of NFκB by Eriocalyxin B (EriB) in the OCSCs may induce cell death in otherwise chemoresistant cells.
Methods
OCSCs and mature ovarian cancer cells (mOCCs) were treated with increasing concentrations of EriB. Cell viability was measured using the Celltiter 96 assay and caspase activity was quantified using Caspase-Glo™ assay. Cytokine levels were quantified using xMAP technology.
Results
EriB decreased the percent of viable cells in all cultures tested with GI50 of 0.5 −1µM after 48h of treatment. The intracellular changes associated with EriB-induced cell death are: 1) inhibition of NF-κB activity; 2) decreased cytokine production; 3) activation of caspases; and 4) down-regulation of XIAP. In addition, EriB is able to sensitize OCSCs to TNFα and FasL-mediated cell death.
Conclusions
Inhibition of the NFκB pathway induces cell death in the OCSCs. Since the OCSCs may represent the source of recurrence and chemoresistance, the use of NFκB inhibitors like EriB may prevent recurrence in ovarian cancer patients.
Keywords: inflammation, nuclear factor kappa B, TNF-a, cancer stem cells, ovarian cancer, ovarian cancer stem cells
INTRODUCTION
Epithelial ovarian cancer (EOC) is the most lethal of all gynecologic malignancies. In 2009, it was estimated that 21,550 new cases were to be diagnosed and 14,600 deaths will result from this disease1. Newly diagnosed ovarian cancer patients usually respond to surgery and chemotherapy but more than 80% of these responders eventually recur with chemo-resistant disease 2, 3. Thus, in EOC, the source of high mortality is disease recurrence. Unfortunately, the source of recurrence is unknown and therapies that can prevent recurrent disease are currently lacking.
Clinical and epidemiologic studies have suggested a strong association between chronic inflammation and cancer 4. Chronic inflammation has been shown to play a critical role in initiating, sustaining, and advancing the growth of several cancers, including EOC 5, 6. A key molecular link between inflammation and cancer is the NF-κB pathway. NF-κB controls many of the properties of cancer cells by regulating the transcriptional activation of genes associated with cell proliferation, angiogenesis, metastasis and suppression of apoptosis. Therefore, specific inhibition of NF-κB has been suggested as a potential therapeutic target.
Growing number of scientific evidence suggests that the tumor represents a heterogeneous population of cells where a specific subgroup, the cancer stem cells (CSCs), has the potential to recreate the original tumor 7. Our group recently reported the identification and characterization of the ovarian cancer stem cells (OCSCs) using the cell surface marker CD44 8, 9. These cells are chemoresistant and have the potential to recreate the original patient tumor in animal models. Thus, this cell population may have the capacity to survive treatment, rebuild the tumor, and initiate recurrence.
A major characteristic of the CD44+ OCSCs is the occurrence of a constitutive NF-κB pathway, which can be enhanced by ligation of Toll-like Receptor 4 (TLR4) and Tumor Necrosis Factor α (TNFα) receptor 5, 10, 11. In this study, we tested the hypothesis that the inhibition of the NF-κB pathway may have a significant effect on the OCSCs. We used the compound Eriocalyxin B (EriB), which is an analogue of oridonin, a natural ent-kaurene diterpene compound purified from Isodon ericalyx var. This natural product has been widely used in Chinese medicine as an anti-inflammatory and antibacterial agent 12, 13. Recent studies have shown that EriB has anti-tumoral effects in models of acute myeloid leukemia and has significant inhibitory effect on cell growth in several cancer cell lines 12.
In our study we demonstrate that EriB can inhibit both the constitutive and TNFα-induced NF-κB activation in the OCSCs. More importantly, we demonstrate that the inhibition of the NF-κB pathway promotes apoptosis in these cells. These findings suggest that inhibition of the NF-κB pathway may be an approach to prevent OCSC survival and therefore prevent ovarian cancer recurrence.
MATERIALS and METHODS
Cell lines and culture conditions
Ovarian cancer cells were isolated from malignant ovarian ascites or from ovarian tumors as previously described 14, 15. All patients signed consent forms, and the use of patient samples was approved under Yale University’s Human Investigations Committee (HIC #10425). OCSCs and CD44-/mature ovarian cancer cells (mOCCs) were isolated from these primary cultures and cultured as previously described 16
Growth curves and cell viability assay
Cells (4500 cells/well for OCSCs and 8000 cells/well for mOCCs) were plated in a 96-well plate. After 24h, the medium was replaced with OptiMem (Gibco, Invitrogen, Carlsbad, CA) for 4 hours followed by treatment. Growth curves were determined by placing treatment plates in the Incucyte system (Essen Instruments, Ann Arbor, MI), where the growth curves are constructed from confluence measurements acquired during round-the-clock kinetic imaging. Cell viability was determined using CellTiter 96 Assay (Promega, Madison, WI).
Protein preparation and cellular fractionation
Protein was extracted and measured as previously described (ref). Cytoplasmic and nuclear fractions were separated using the NE-PER Nuclear and Cytoplasmic Extraction (Pierce Biotechnology, Inc., Rockford, IL) according to manufacturer’s instructions.
Caspase-3/7, -8, and -9 activity assay
The activities of caspases-3/7, -8, and -9 were measured using Caspase-Glo™ 3/7, 8, and 9 (Promega, Madison, Wisconsin), respectively, as previously described 17.
Mitochondrial depolarization assay
After treatment, cells were trypsinized and stained with JC-1 dye using the Mitocapture™ mitochondrial apoptosis detection kit (BioVision Research Products Mountain View, CA) according to the manufacturer’s instructions. Data was gathered using the FACS Calibur System and analyzed using CellQuest software (BD Biosciences, Rockville, MD).
SDS-PAGE and Western blots
Gel electrophoresis and western blots were performed as previously described 17.
Quantification of NF-kB activity
NF-κB activity was measured using a luciferase reporter construct, pBII-LUC containing two κB sites before a Fos essential promoter (a gift from Dr. S. Ghosh, Yale University). Cells were transfected and luciferase activity measured as previously described 10.
Quantification of cytokines
Cytokine levels were measured from cell-free supernatants using Beadlyte® Human Multi-Cytokine Beadmaster™ Kit according to the manufacturer’s instructions. Data was acquired using the Bio-Rad 100 IS System (Bio-Rad, Hercules, CA).
Immuohistochemistry
Cells were cultured in 4 wells chamber slides and fixed with 4% paraformaldehyde for 20 minutes at room temperature. Afterwards, cells were stained with specific mouse monoclonal antibody for p65 as previously described 18. Slides were then incubated with Alexa Fluor546 anti-mouse IgG and counterstained with Hoechst 33342 dye (Molecular probes, Eugene, OR)
RESULTS
EriB induces cell death in EOC cells
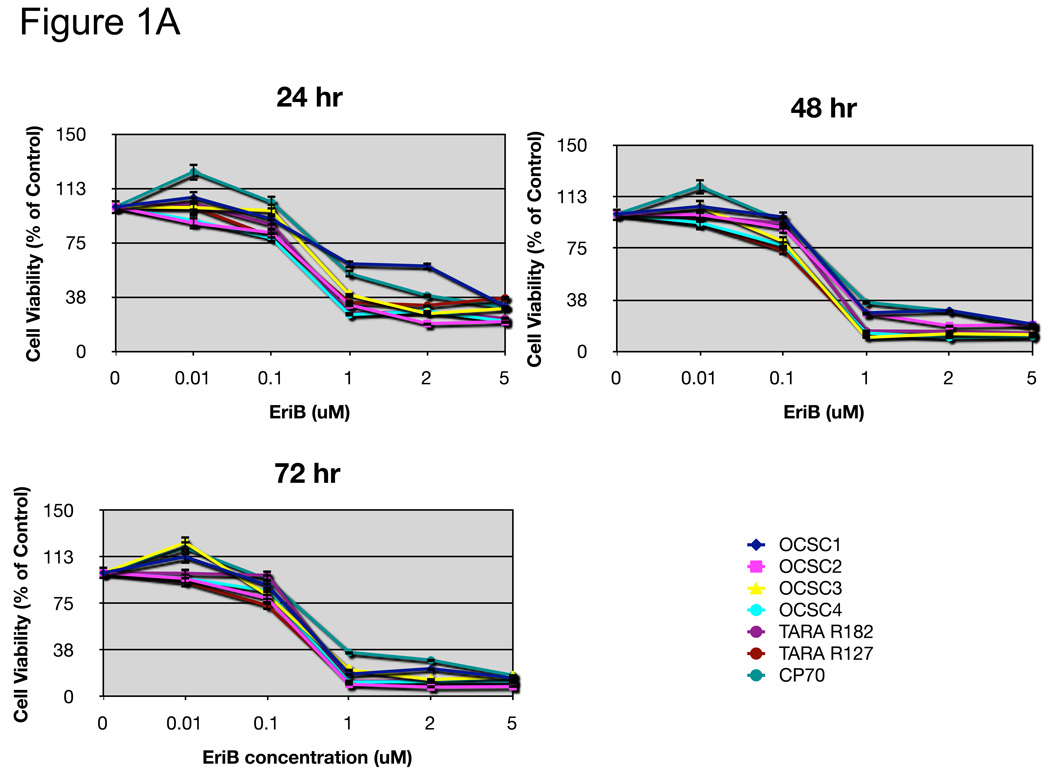
NF-κB has been shown to promote cell survival through the induction of target genes that can inhibit apoptosis 19. Therefore, our first objective was to evaluate the effect of EriB on EOC cell survival. For this, we used a panel of both mOCCs and OCSCs isolated from either malignant ascites or ovarian cancer tumors 15 16. The effect on cell viability was evaluated using the CellTiter 96 assay. EriB significantly reduced the number of viable cells in a dose and time dependent manner (Fig. 1A).
Figure 1.
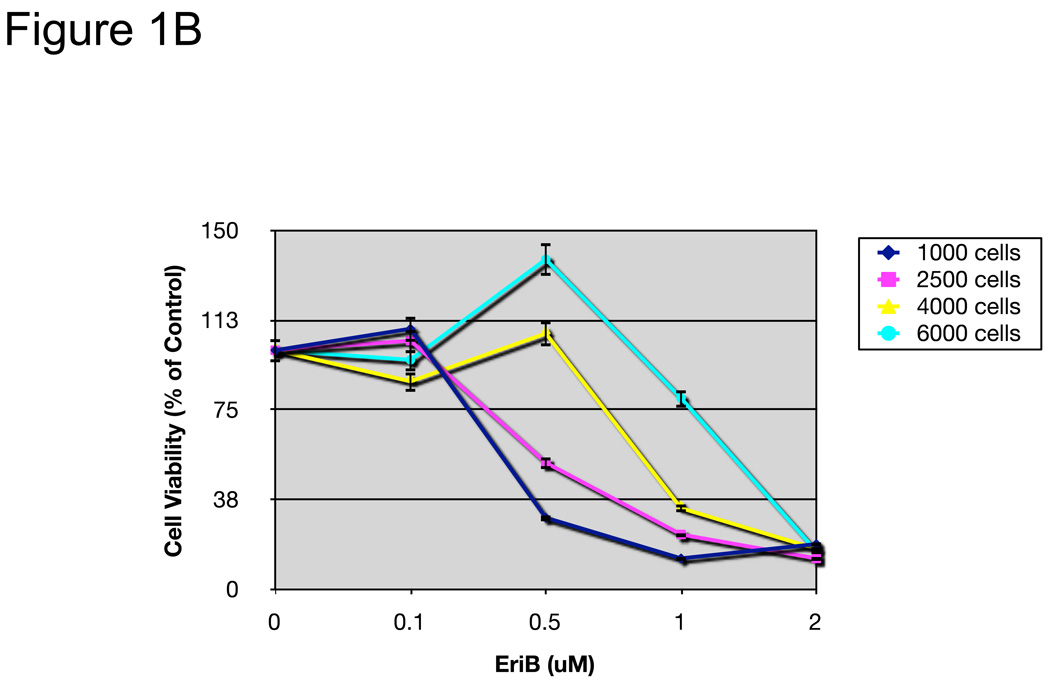
EriB induces cell death in EOC cells. A panel of OCSCs and mOCCs were treated with increasing concentration of EriB. (a) percentage of viable cells was quantified after 24, 48, and 72h using Celltiter 96 assay, (b) OCSC1 was treated at different confluence and effect on viability determined after 48h.
Interestingly, in the OCSCs, the effect of EriB was density dependent, with increasing resistance as cell density increased (Fig. 1B).
EriB-induced cell death is associated with caspase activation and loss of mitochondrial membrane potential
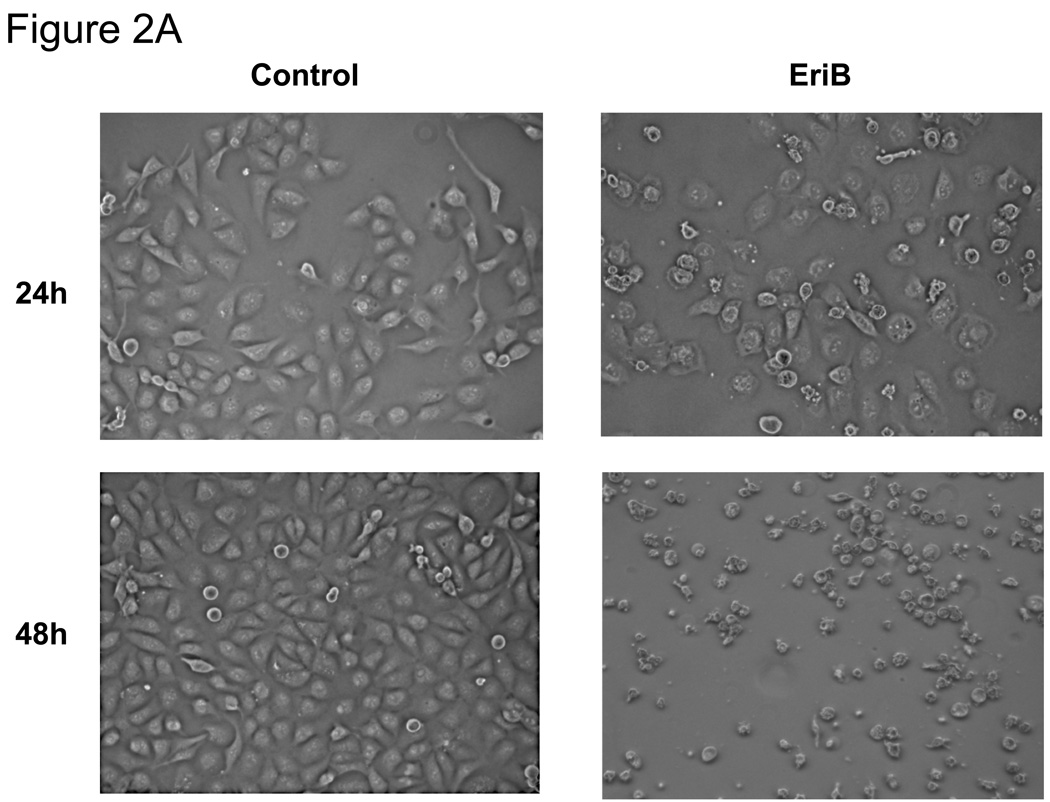
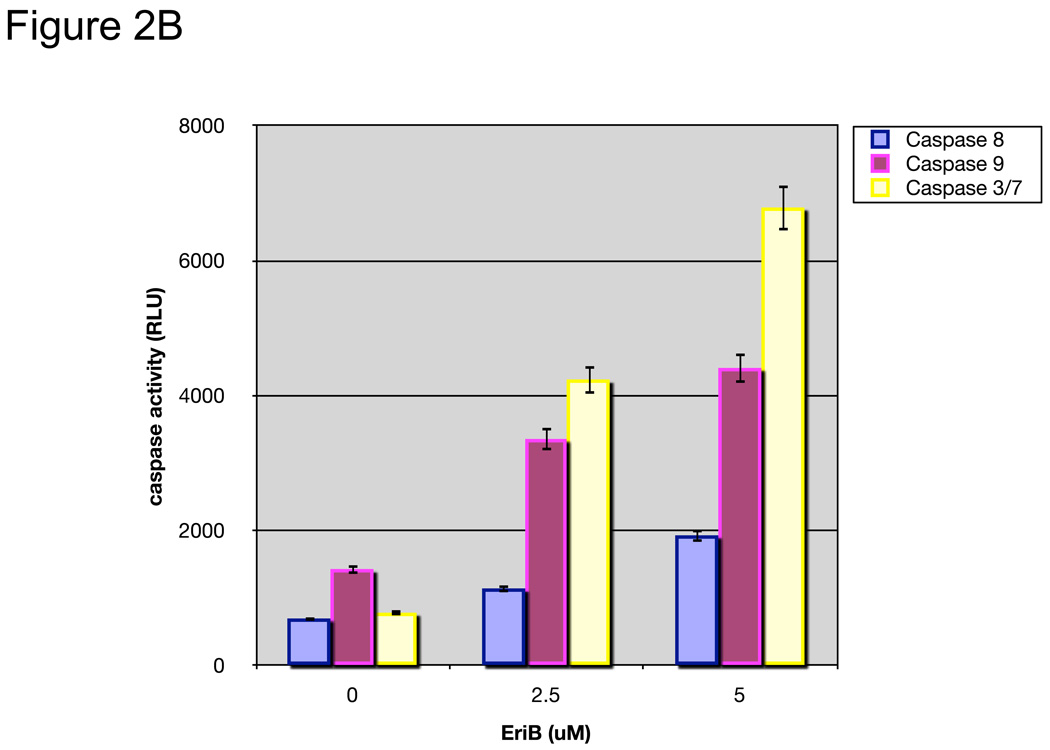
To determine whether the reduction in number of viable cells was caused by apoptosis, we evaluated cell morphology and caspase activity. As shown in Figure 2A, cells treated with EriB showed typical morphology associated with apoptosis such as contracted cytoplasm, condensed chromatin, and the formation of apoptotic bodies. In addition, we found a significant increase in caspase 8, caspase 9, and caspase 3/7 activities in the treated cells in a dose-dependent manner (Fig. 2B).
Figure 2.
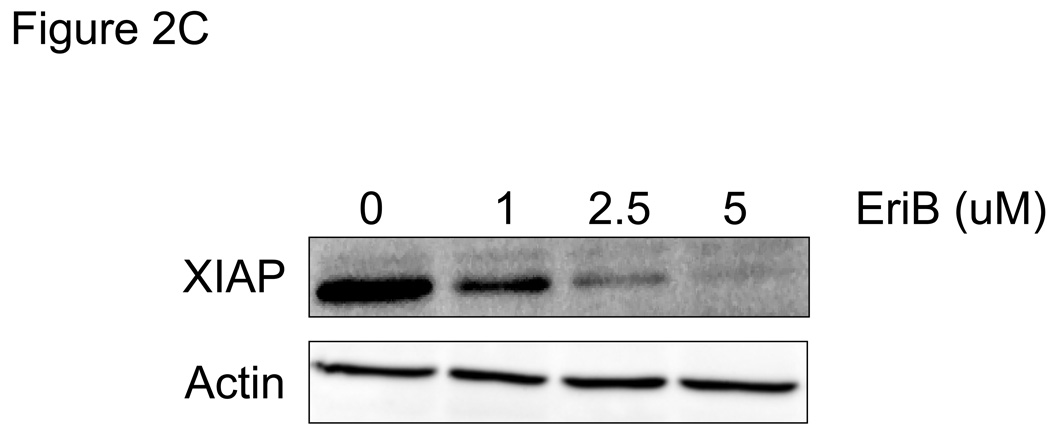
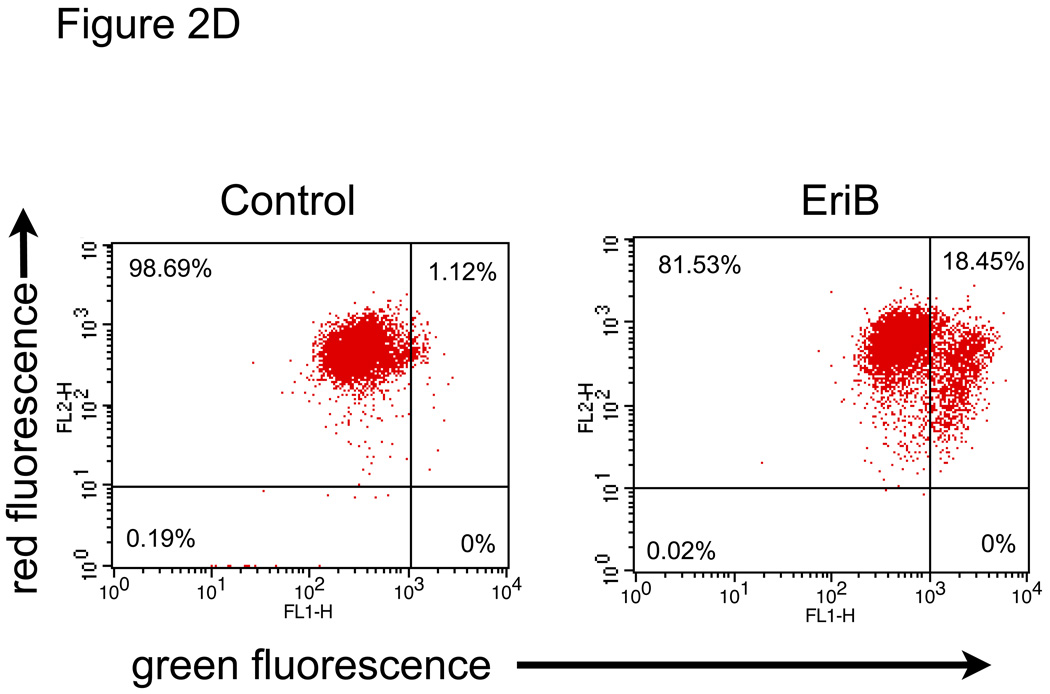
EriB induces apoptosis in EOC cells. A panel of OCSCs and mOCCs were treated with increasing concentration of EriB for 24h. (a) effect on morphology was evaluated using light microscopy; (b) activity of caspases 8, 9, and 3/7 was quantified using Caspase Glo assay; (c) levels of XIAP was determined by Western blot analysis; (d) effect on mitochondrial membrane stability was determined using the JC1 dye. Results shown are from OCSC1. Similar results were obtained with other cell cultures tested.
As mentioned above, one of the most documented functions of NF-κB is its ability to promote cell survival through the induction of target genes, which inhibit the apoptotic machinery 19. X-linked Inhibitory of Apoptosis Protein (XIAP) is an antiapoptotic protein which inhibits caspase 3 and caspase 9, and is highly expressed in EOC cells 14, 20. In addition, NF-κB has been shown to enhance the expression of XIAP 21. Our next objective was to determine if EriB-induced cell death is associated with any changes in the levels of XIAP. We showed that EriB treatment significantly reduced the levels of XIAP in the EOC cells (Fig. 2C) and that the decrease in XIAP correlates with caspase activation.
Another well-characterized down-stream target of NF-κB is the anti-apoptotic protein Bcl2, which functions to stabilize the mitochondria and therefore prevent the activation of caspases 22, 23. We next determined if EriB treatment has any effect on mitochondrial membrane integrity. We used the JC1 dye, which fluoresces bright red upon accumulation in intact mitochondria, but switches to green fluorescence once it is leaked into the cytoplasm upon the loss of mitochondrial membrane integrity 24. Treatment with EriB for 12h induced a significant shift in the JC1 dye fluorescence from red to green (Fig. 2D). This suggests that EriB induced NF-κB inhibition alters the balance of proteins involved in the protection of the mitochondria leading to destabilization of the mitochondrial membrane and therefore triggering caspase dependent cell death.
EriB inhibits constitutive NF-κB activity and constitutive cytokine production in the OCSCs
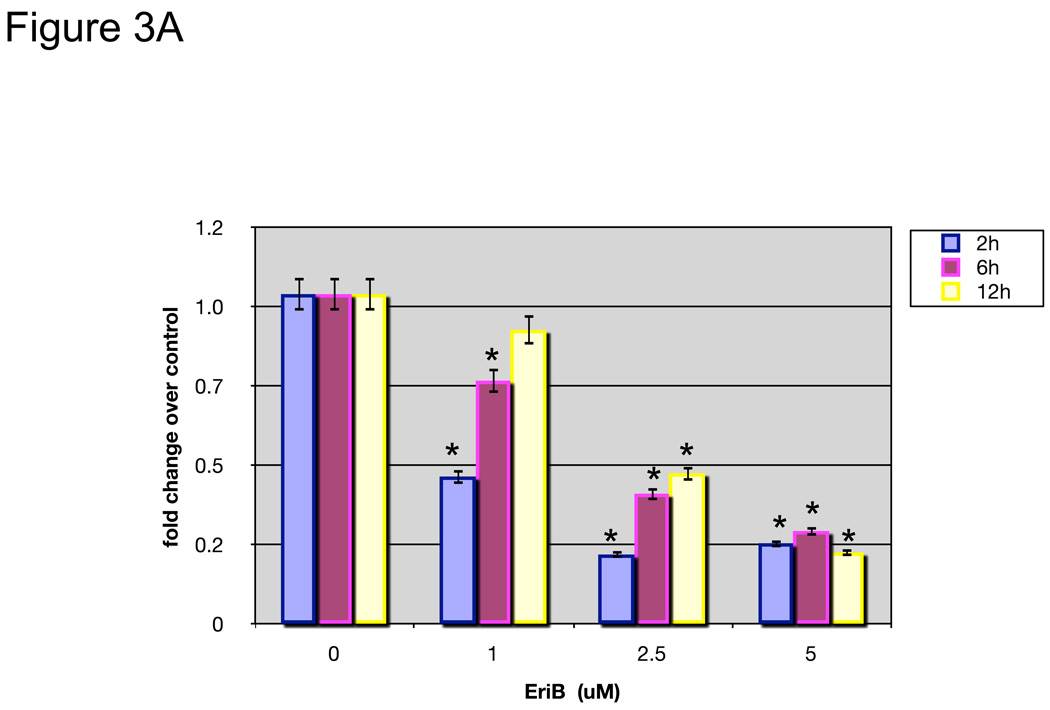
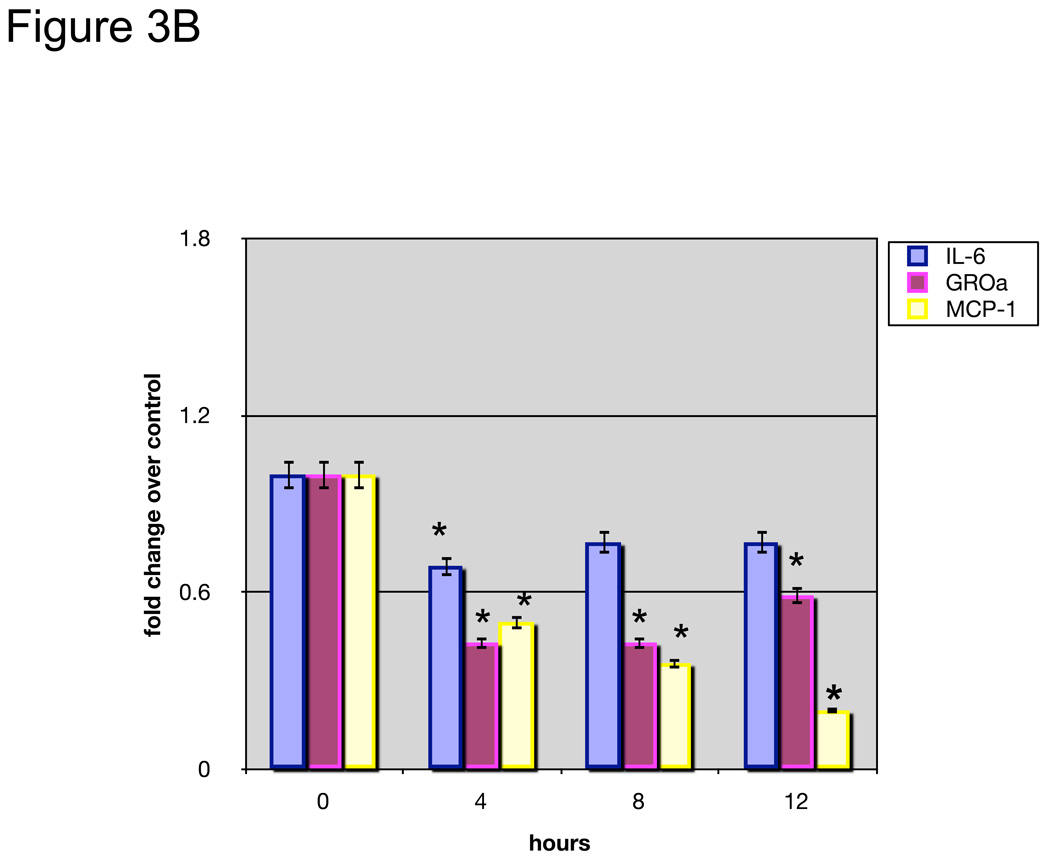
To further determine whether EriB’s effect on OCSCs is associated with its capacity to inhibit NF-κB activity, we transfected OCSCs with a luciferase reporter linked to two NF-κB promoter elements 10. Figure 3A shows that EriB is able to inhibit baseline NF-κB activity in the OCSCs in a dose and time dependent manner. Low doses have a significant but transient effect in the first 2 hours but the higher doses are able to maintain the inhibition for 12 hours (Fig. 3A). These results confirm the specific effect of EriB on NF-κB. Moreover, this effect on NF-κB activity is reflected on the levels of secreted cytokines; when we evaluated the constitutive production of IL-6, MCP-1 and GRO-α in the OCSCs, we observed a significant decrease in these cytokines as early as 4 hours after EriB treatment (Fig. 3B).
Figure 3.
EriB inhibits constitutive NF-κB activity and constitutive cytokine production in the OCSCs. OCSCs were treated with increasing concentrations of EriB at the shown time points. (a) NF-κB activity was measured using a luciferase reporter system as described in the Methods section; (b) levels of IL-6, GRO-α, and MCP-1 were quantified using xMAP technology. Results shown are from OCSC1. Similar results were obtained with other OCSCs tested. (*) p<0.0001.
EriB inhibits TNFα-induced NF-κB activation and cytokine production in the OCSCs
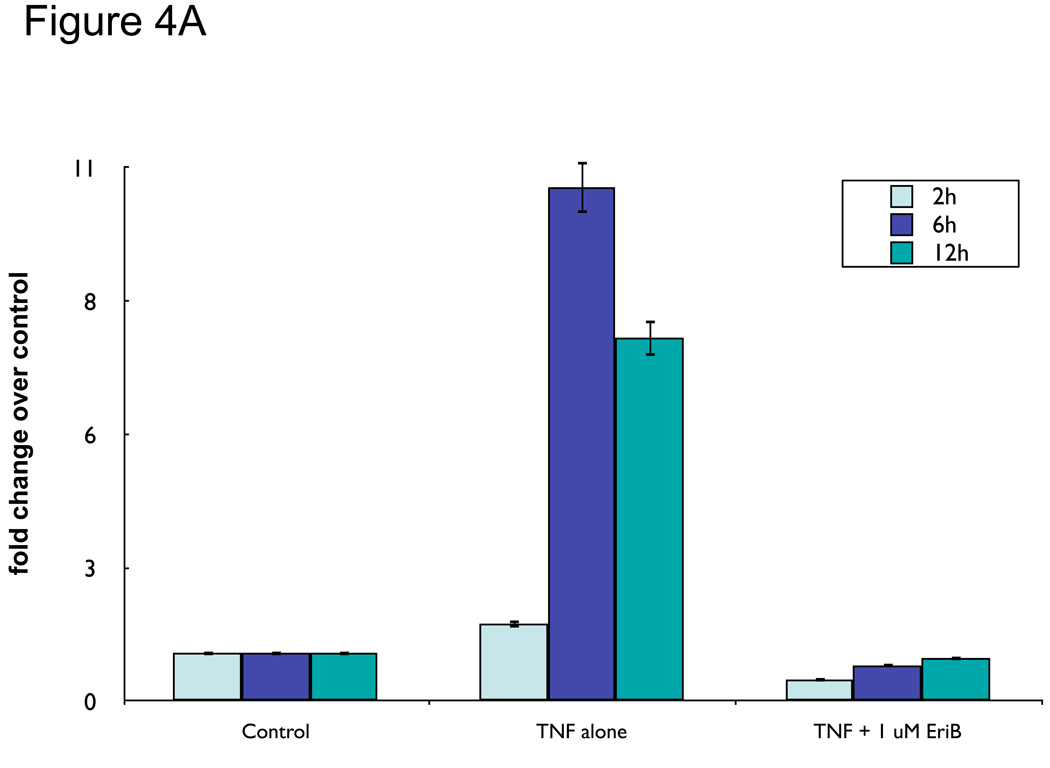
So far we have shown that EriB can inhibit OCSCs baseline NF-κB activity. Our next objective was to determine if EriB could also inhibit induced NF-κB activity. TNFα is an endogenous stimuli known to induce NF-κB activity in numerous cell types 25. We previously showed that instead of inducing cell death, TNFα is able to induce NF-κB activation and cytokine production in the OCSCs 10. Thus, we pre-treated OCSCs with increasing concentrations of EriB prior to TNFα treatment. As shown in Figure 4A, TNFα alone (10 ng/ml) induced a significant increase in luciferase activity in a time dependent-manner, demonstrating NF-κB activation. This effect was blocked by pretreatment with EriB even at the lower doses (1 µM). These results suggest that EriB can inhibit both constitutive and induced NF-κB activity.
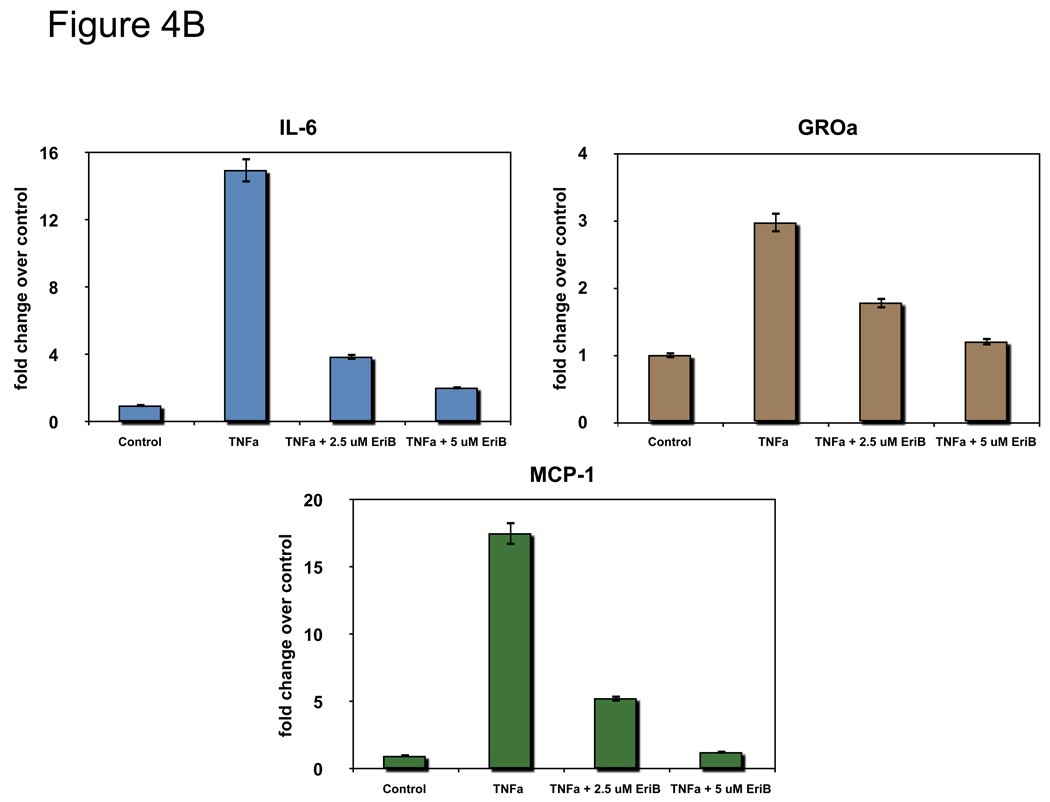
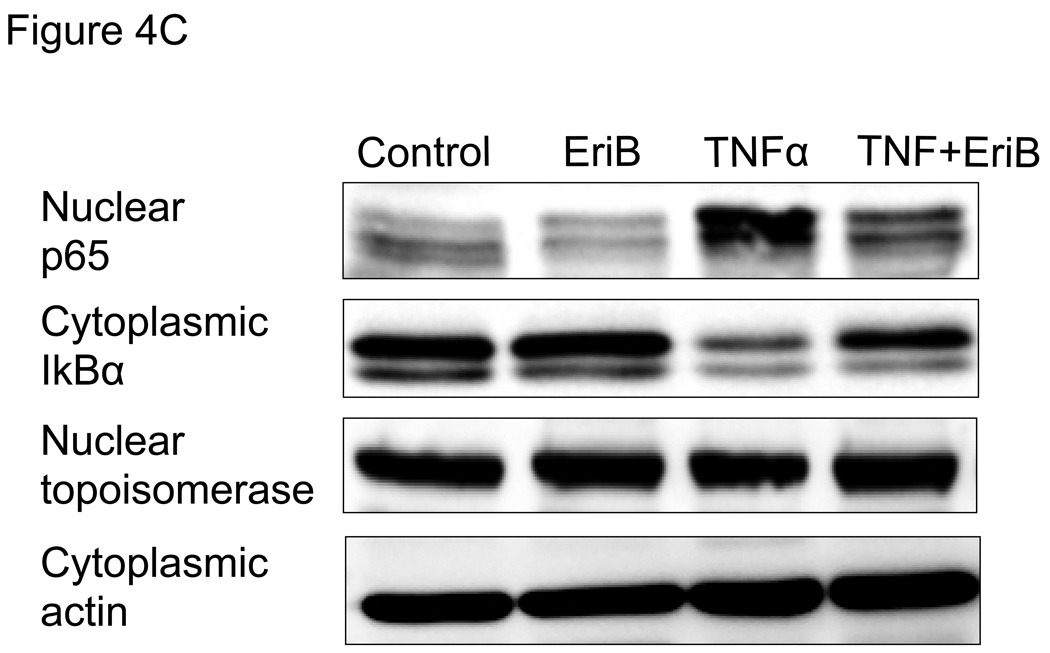
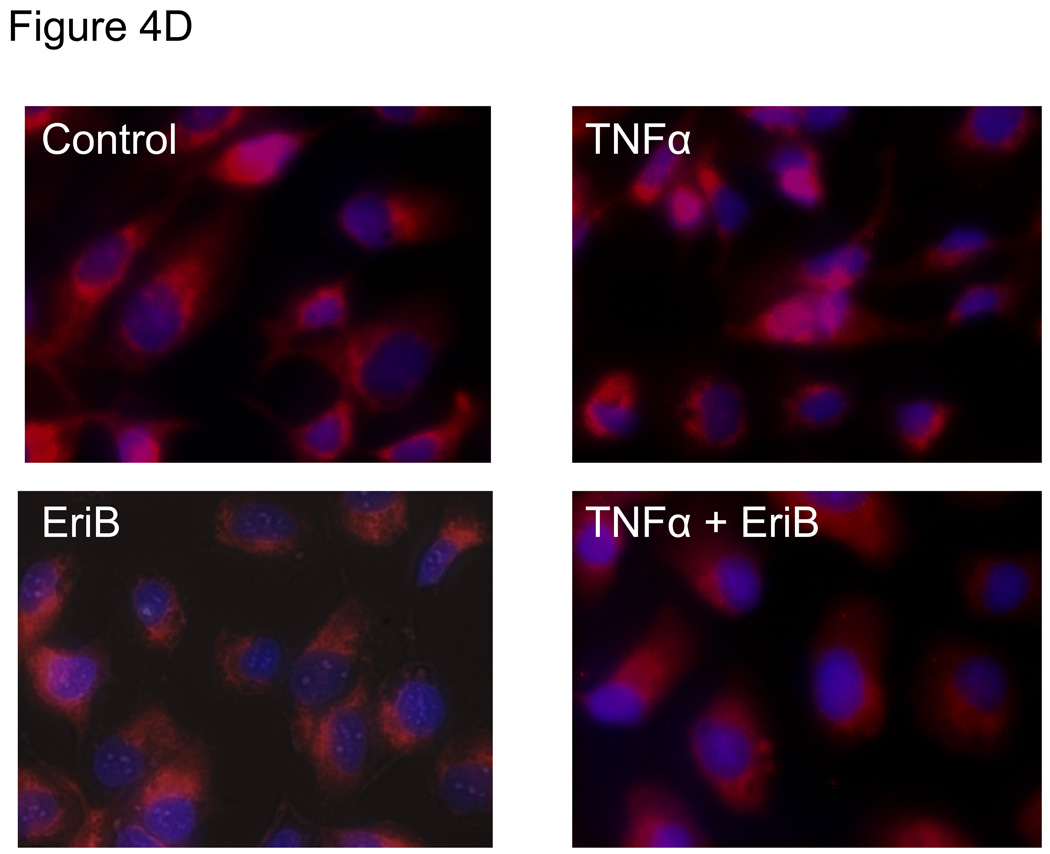
Figure 4.
EriB inhibits TNFα-induced NF-κB activation and cytokine production in the OCSCs. OCSCs were pre-treated with different doses EriB for 4h prior to treatment with 10 ng/ml of TNFα for 2, 6, and 12 additional hours. (a) NF-κB activity was measured using the luciferase reporter system described above; (b) levels of IL-6, GROα, and MCP-1 were quantified after 12h of EriB treatment using xMAP technology; (c) levels of nuclear p65 and total IκB were determined using western blot analysis; (d) co-localization of p65 and DAPI staining was determined using immunofluorescence. Results shown are from OCSC1. Similar results were obtained with other OCSCs tested.
To determine if the inhibition by EriB of TNFα-induced NF-κB activity translates to inhibition of cytokine production the levels of IL-6, GRO-α, and MCP-1 were measured in OCSC supernatants. Similar to what was observed with the NF-κB luciferase assay, pre-treatment with EriB was able to down-regulate TNFα-induced increase in all these cytokines (Fig. 4B).
EriB inhibits p65 nuclear translocation
To confirm the findings obtained with the luciferase reporter system and to determine where in the NF-κB pathway EriB may exert its action, we evaluated the nuclear translocation of p65, which is which is a subunit of the NF-κB complex and a classical indicator of NF-κB activity. We evaluated both constitutive as well TNFα-induced p65 nuclear levels. We observed a decrease in the basal levels of nuclear p65 in the OCSCs after EriB treatment (Fig. 4C). In addition, EriB was also able to inhibit TNFα-induced increase in nuclear p65 (Fig. 4C). These results were further confirmed using immunocytochemistry where we observed a decrease in both constitutive and TNFα-induced nuclear p65 immuno-reactivity (Fig. 4D).
Our next objective was to determine whether the decrease in nuclear p65 is associated with changes in IκBα levels. It has been shown that following stimulation with a broad range of stimuli, such as TNFα, IκBα is phosphorylated by the IKK complex leading to the ubiquitination and proteosomal degradation of IκBα 26, 27. As shown in Figure 4C, TNFα-induced p65 nuclear translocation corresponded with a significant decrease on cytoplasmic IκBα. Interestingly, co-treatment with EriB prevented the TNFα-induced decrease in IκBα. This suggests that EriB may block p65 nuclear translocation by preventing the degradation of IκBα.
EriB is able to sensitize OCSCs to TNFα- and FasL-induced cell death
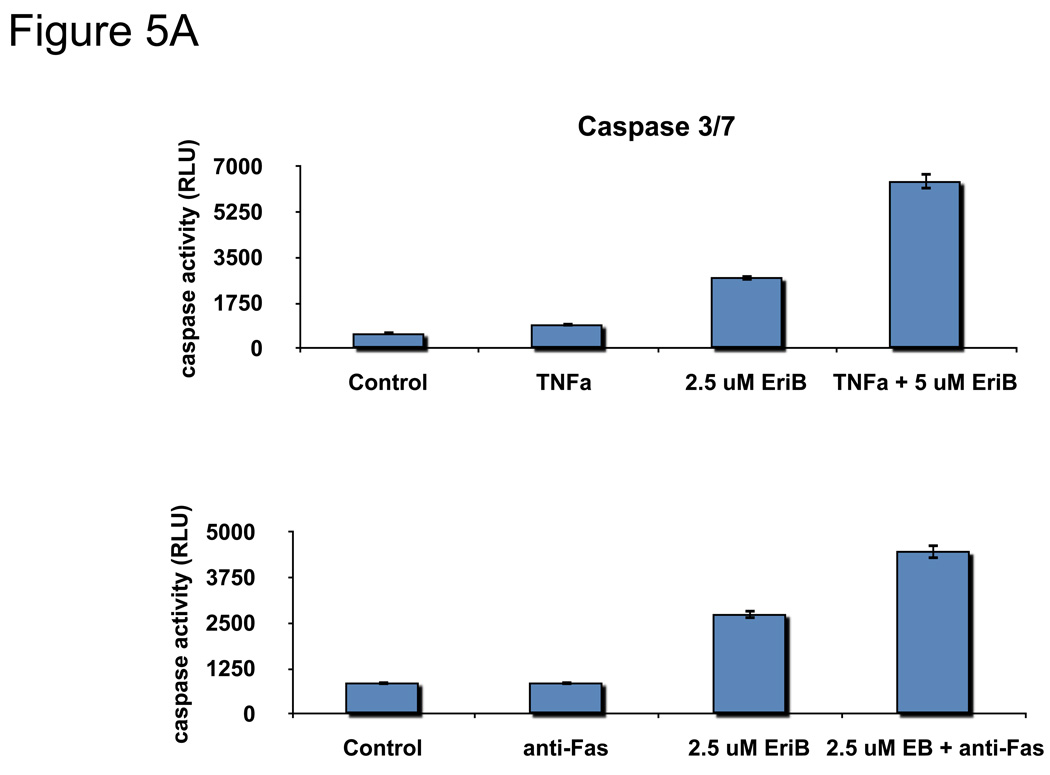
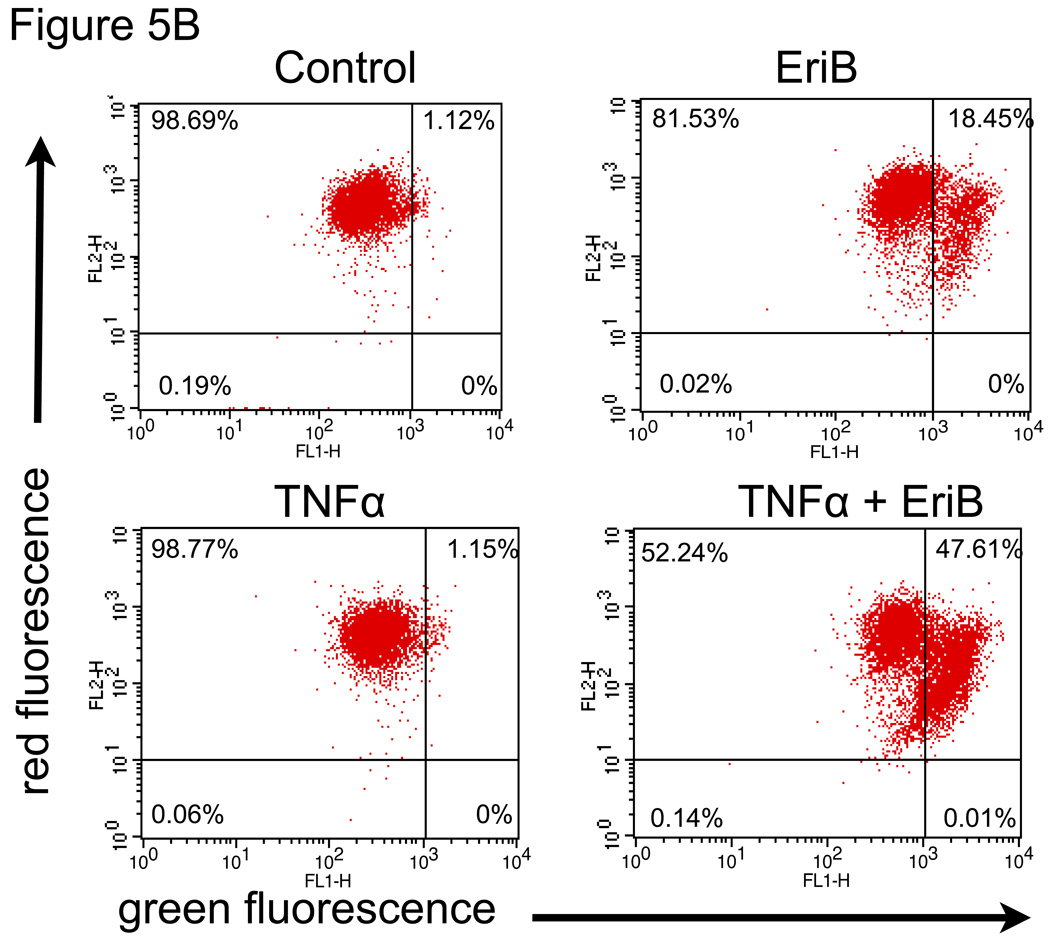
OCSCs are resistant to TNFα-induced cell death 14, 28 and instead respond to TNFα treatment by upregulating NF-κB activity and enhancing cytokine production. Our next objective was to determine if the inhibition of NF-κB by EriB can sensitize OCSCs to TNFα-induced cell death. OCSCs were pre-treated with EriB prior to TNFα treatment and the activation of the apoptotic pathway was determined by measuring caspase 3/7 activity and determining the effect on the mitochondrial membrane integrity. Our results show that TNFα alone was not able to induce the activation of caspase 3/7 in the OCSCs (Fig. 5A), nor was it able to destabilize the mitochondria (Fig. 5B). However, pre-treatment with EriB prior to TNFα significantly increased caspase 3/7 activity and also significantly induced a green shift in the JC1 dye (Fig. 5B,C) indicative of loss of mitochondrial membrane potential.
Figure 5.
EriB sensitizes OCSCs to TNFα- and Fas-induced apoptosis. OCSCs were pretreated with increasing concentrations of EriB for 4h prior to treatment with 10 ng/mL TNFα for additional 12h, (a) caspase 3/7 activity was quantified using Caspase Glo assay; (b) effect on mitochondrial membrane stability was determined using the JC1 dye; (c) OCSCs were pretreated with increasing concentrations of EriB for 4h prior to treatment with 500ng/mL anti-Fas for additional 24h and caspase 3/7 activity was quantified using Caspase Glo assay. Results shown are from OCSC1. Similar results were obtained with other OCSCs tested.
FasL is another pro-apoptotic receptor member of the TNFα family and its function is regulated by the NF-κB pathway. We previously showed that EOCs in general are resistant to FasL-induced cell death 14. To determine if EriB is also able to sensitize EOC cells to FasL-mediated apoptosis, OCSCs were pre-treated with EriB followed by an anti-Fas monoclonal antibody which mimics FasL activity. Similar to what was observed with TNFα, anti-Fas alone was not able to induce caspase 3/7 activity in any of the cell cultures tested (Fig. 5C). However, pre-treatment with EriB was able to sensitize EOC cells to FasL-induced apoptosis. These results demonstrate that inhibition of NF-κB by EriB is able to reactivate both the TNFα and FasL apoptotic cascade in the OCSCs.
DISCUSSION
In the present study we describe the effects of NF-κB inhibition by EriB on the OCSCs. We demonstrated that EriB, by blocking NF-κB activity, is able to suppress OCSC-mediated inflammation through the inhibition of cytokine production. More importantly, we show that it promotes OCSC death, therefore depleting the tumor of the possible source of tumor repair and recurrence.
CSCs or tumor-initiating cells represent a population of the tumor, which are chemoresistant and can undergo both processes of self-renewal and differentiation 11, 29, 30. These cells can therefore survive chemotherapy, and undergo self-renewal and differentiation to rebuild the tumor, thus leading to disease recurrence 31. Targeting these cells is thus pivotal for the prevention of cancer recurrence and metastasis and the improvement of patient survival.
We previously showed chemoresistance and differentiation capacity to be some key features of the OCSCs 16, 24. Thus it is possible that these cells are the source of EOC recurrence. Further studies showed that another remarkable characteristic of the OCSCs is constitutive NF-κB activity, which can be enhanced by TNFα ligation 9, 11. Moreover, these cells have a functional TLR4 pathway which can also contribute to enhanced NF-κB activity and cytokine production 32. Therefore, in addition to being the possible source of recurrence, OCSCs may also be the source of a chronic inflammatory microenvironment.
Acute inflammation represents a critical signal to adult stem cells to initiate the process of tissue repair, which involves self-renewal (maintaining the pool of stem cells) and tissue renewal (differentiation on tissue specific cells) 7, 33. In this setting, the source of the inflammatory signals has been associated with immune infiltrates, primarily macrophages, which respond to tissue damage by differentiating into the M2 type and secreting pro-inflammatory cytokines and chemokines 34–36. Chronic inflammation on the other hand, may have detrimental effects by directly affecting abnormal progenitor cells and promoting tumorigenicity. Over two decades ago, Dvorak and co-authors described the tumor as an unhealed wound that produces a continuous source of inflammatory mediators (cytokines and chemokines) 37. This inflammatory environment promotes cell survival, enhances cell proliferation and may even affect anti-tumoral immunologic response. Therefore the demonstration that OCSCs can create a chronic inflammatory microenvironment through the NF-κB pathway, provided us with a specific target for treatment.
We found that EriB is able to inhibit NF-κB activity, followed by suppression of cytokine production. This observation is relevant since cytokines are important for the process of tissue renewal, and in the case of the tumor, tumor recurrence. Targeting this pathway may therefore allow prevention of recurrence and may also affect the protumoral immunologic environment.
Interestingly, targeting the NF-κB pathway in the OCSCs induced apoptosis. These findings are in accordance with the role of NF-κB as a regulator of anti-apoptotic genes such as XIAP. Treatment with EriB induces a significant decrease in XIAP, which can then remove the inhibitory effects on the caspases. XIAP has been shown to be a central regulator of cell survival in EOC and major attempts have been made, without much success, to develop XIAP inhibitors 38–41.
We also found that inhibition of NF-κB can reverse the resistance of OCSCs to TNFα and FasL. TNFα has been shown to promote, in some types of tumors, cell growth instead of apoptosis 42. We previously demonstrated that while TNFα can induce cell death in the mOCCs, it can enhance growth of the OCSCs 10. This pro-apoptotic effect of EriB is not only associated with TNFα but also with FasL, another membranal receptor. Like TNFα, FasL is also ineffective in inducing cell death in the OCSCs. However, pre-treatment with EriB is able to sensitize OCSCs to FasL.
Finally we evaluated how EriB inhibits NF-κB activity. Our data demonstrated that EriB decreased the levels of nuclear p65 in the OCSCs, both at the baseline level and after TNFα treatment. Since NF-κB is a transcription factor, the inhibition of its nuclear translocation could explain the decrease in cytokines observed in the OCSCs after EriB treatment.
The nuclear translocation of p65 depends on its association with IκB 43. Upon its phosphorylation of IκB, it gets degraded and this allows the dissociation and nuclear translocation of p65. As stated previously, OCSCs have constitutive NF-κB activity and the IκB levels in these cells cycles over a 24h period. We found the inhibition of this cycle after the OCSCs were treated with EriB. This suggests that EriB is able to prevent IκB degradation.
In summary, we report a novel compound, which induces death in the OCSCs by inhibiting the NFκB pathway – a pathway which is highly activated in the OCSCs cells. Identifying and understanding the pathways in the OCSCs that can confer survival, resistance to apoptosis, and capacity for repair is relevant to improve survival of ovarian cancer patients.
Acknowledgements
This study was supported in part by grants from NCI/NIH RO1CA127913, RO1CA118678, The Janet Burros Memorial Foundation, The Sands Family Foundation and the Discovery To Cure Research Program.
Bibliography
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics,2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med. 2009;361:170–177. doi: 10.1056/NEJMcp0901926. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002;107:99–118. doi: 10.1007/978-1-4757-3587-1_4. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R, Alvero AB, Silasi DA, Mor G. Inflammation, cancer and chemoresistance: taking advantage of the toll-like receptor signaling pathway. Am J Reprod Immunol. 2007;57:93–107. doi: 10.1111/j.1600-0897.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen R, Alvero AB, Silasi DA, Steffensen KD, Mor G. Cancers take their Toll--the function and regulation of Toll-like receptors in cancer cells. Oncogene. 2008;27:225–233. doi: 10.1038/sj.onc.1210907. [DOI] [PubMed] [Google Scholar]
- 7.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 8.Alvero AB, O'Malley D, Brown D, Kelly G, Garg M, Chen W, Rutherford T, Mor G. Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer. 2006;106:599–608. doi: 10.1002/cncr.21633. [DOI] [PubMed] [Google Scholar]
- 9.Alvero AB, Fu HH, Holmberg J, Visintin I, Mor L, Marquina CC, Oidtman J, Silasi DA, Mor G. Stem-like Ovarian Cancer Cells can Serve as Tumor Vascular Progenitors. Stem Cells. 2009 doi: 10.1002/stem.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R, Alvero AB, Silasi DA, Kelly MG, Fest S, Visintin I, Leiser A, Schwartz PE, Rutherford T, Mor G. Regulation of IKKbeta by miR-199a affects NF-kappaB activity in ovarian cancer cells. Oncogene. 2008;27:4712–4723. doi: 10.1038/onc.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin G, Chen R, Alvero AB, Fu HH, Holmberg J, Glackin C, Rutherford T, Mor G. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene. 2010 doi: 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Zhao WL, Yan JS, Liu P, Sun HP, Zhou GB, Weng ZY, Wu WL, Weng XQ, Sun XJ, Chen Z, Sun HD, Chen SJ. Eriocalyxin B induces apoptosis of t(8;21) leukemia cells through NF-kappaB and MAPK signaling pathways and triggers degradation of AML1-ETO oncoprotein in a caspase-3-dependent manner. Cell Death Differ. 2007;14:306–317. doi: 10.1038/sj.cdd.4401996. [DOI] [PubMed] [Google Scholar]
- 13.Ikezoe T, Chen SS, Tong XJ, Heber D, Taguchi H, Koeffler HP. Oridonin induces growth inhibition and apoptosis of a variety of human cancer cells. Int J Oncol. 2003;23:1187–1193. [PubMed] [Google Scholar]
- 14.Kamsteeg M, Rutherford T, Sapi E, Hanczaruk B, Shahabi S, Flick M, Brown D, Mor G. Phenoxodiol-an isoflavon analogue-induces apoptosis in chemo-resistant ovarain cancer cells. Oncogene. 2003;22:2611–2620. doi: 10.1038/sj.onc.1206422. [DOI] [PubMed] [Google Scholar]
- 15.Flick MB, O’Malley D, Rutherford T, Rodov S, Kamsteeg M, Hao XY, Schwartz PE, Kacinski BM, Mor G. Apoptosis-Based Evaluation Of Chemosensitivity In Ovarian Cancer Patients. J Soc Gynecol Inv. 2004;11:252–259. doi: 10.1016/j.jsgi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I, Mor G. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvero AB, Montagna MK, Chen R, Kim KH, Kyungjin K, Visintin I, Fu HH, Brown D, Mor G. NV-128, a novel isoflavone derivative, induces caspase-independent cell death through the Akt/mammalian target of rapamycin pathway. Cancer. 2009;115:3204–3216. doi: 10.1002/cncr.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth C, Manzur A, Oyarzun E, Romero R, Mor G. Viral Infection of the Placenta Leads to Fetal Inflammation and Sensitization to Bacterial Products Predisposing to Preterm Labor. J Immunol. 2010;185 doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo JL, Kamata H, Karin M. The anti-death machinery in IKK/NF-kappaB signaling. J Clin Immunol. 2005;25:541–550. doi: 10.1007/s10875-005-8217-6. [DOI] [PubMed] [Google Scholar]
- 20.Straszewski-Chavez SL, Abrahams VM, Funai EF, Mor G. X-linked inhibitor of apoptosis (XIAP) confers human trophoblast cell resistance to Fas-mediated apoptosis. Mol Hum Reprod. 2004;10:33–41. doi: 10.1093/molehr/gah001. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 23.Jin HS, Lee DH, Kim DH, Chung JH, Lee SJ, Lee TH. cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activation. Cancer Res. 2009;69:1782–1791. doi: 10.1158/0008-5472.CAN-08-2256. [DOI] [PubMed] [Google Scholar]
- 24.Green JM, Alvero AB, Kohen F, Mor G. 7-(O)-Carboxymethyl daidzein conjugated to N-t-Boc-hexylenediamine: a novel compound capable of inducing cell death in epithelial ovarian cancer stem cells. Cancer Biol Ther. 2009;8:1747–1753. doi: 10.4161/cbt.8.18.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 26.Tang ED, Wang CY, Xiong Y, Guan KL. A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha. J Biol Chem. 2003;278:37297–37305. doi: 10.1074/jbc.M303389200. [DOI] [PubMed] [Google Scholar]
- 27.Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 28.Sapi E, Chen W, O’Malley D, Hao X, Dwipoyono B, Garg M, Kamsteeg M, Rutherford T, Mor G. Resistance of Ovarian Cancer Cells to Docetaxel is XIAP Dependent and Reversible by Phenoxodiol. Anti-Cancer Drugs. 2004;14:567–578. doi: 10.3727/0965040042707943. [DOI] [PubMed] [Google Scholar]
- 29.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 30.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 32.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 33.Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 34.Lin EY, Pollard JW. Macrophages: modulators of breast cancer progression. Novartis Found Symp. 2004;256:158–168. discussion 168-172, 259-169. [PubMed] [Google Scholar]
- 35.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 36.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 38.Cheng JQ, Jiang X, Fraser M, Li M, Dan HC, Sun M, Tsang BK. Role of X-linked inhibitor of apoptosis protein in chemoresistance in ovarian cancer: possible involvement of the phosphoinositide-3 kinase/Akt pathway. Drug Resist Updat. 2002;5:131–146. doi: 10.1016/s1368-7646(02)00003-1. [DOI] [PubMed] [Google Scholar]
- 39.Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–261. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y, Cherton-Horvat G, Dragowska V, Baird S, Korneluk RG, Durkin JP, Mayer LD, LaCasse EC. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res. 2003;9:2826–2836. [PubMed] [Google Scholar]
- 41.Alvero AB, Chen W, Sartorelli AC, Schwartz P, Rutherford T, Mor G. Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) induces apoptosis in ovarian cancer cells. J Soc Gynecol Investig. 2006;13:145–152. doi: 10.1016/j.jsgi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Ditsworth D, Zong WX. NF-kappaB: key mediator of inflammation-associated cancer. Cancer Biol Ther. 2004;3:1214–1216. doi: 10.4161/cbt.3.12.1391. [DOI] [PubMed] [Google Scholar]
- 43.Birbach A, Bailey ST, Ghosh S, Schmid JA. Cytosolic, nuclear and nucleolar localization signals determine subcellular distribution and activity of the NFkappaB inducing kinase NIK. J Cell Sci. 2004;117:3615–3624. doi: 10.1242/jcs.01224. [DOI] [PubMed] [Google Scholar]