Abstract
BACKGROUND
Food allergy (FA) and eosinophilic esophagitis (EE) are increasingly common clinical problems. Dendritic cells (DCs) are key regulators of the sensitization and effector phases of allergic immune responses, but their role in these diseases is largely unknown.
OBJECTIVE
To evaluate for alterations in the phenotype and function of DCs in children with IgE-mediated milk allergy or EE compared to their non-affected siblings.
METHODS
Plasmacytoid (pDCs) and monocytoid (mDCs) DCs were prepared from peripheral blood of children with milk allergy (FA), EE, and nonaffected siblings (CON). Purified pDCs and mDCs were cultured alone or with autologous CD4+ lymphocytes. Cytokine levels in plasma, or culture supernatants following stimulation, were measured using multiplex array immunoassay. Cell-surface molecule expression was determined by flow cytometry.
RESULTS
DCs from FA subjects produced greater levels of pro-inflammatory cytokines (IL-6, TNF-α), GM-CSF, and mDC-derived IL-10 compared to controls following allergen exposure. TH2 but not TH1 cytokines were spontaneously produced in DC-CD4+ T cell co-cultures from children with FA and were not significantly increased after stimulation with milk extract, suggesting an ongoing activation in vivo. This hypothesis was further supported by evidence for elevated IL-5 and IL-13 protein in the plasma of children with both FA and EE. The only significant DC phenotypic differences were: 1) reduced levels of CD80 in EE subjects and 2) FcεRI expression that correlated with serum IgE levels in both groups of subjects.
CONCLUSION
This study suggests that DCs from children with FA and EE produce more pro-inflammatory cytokines, and that their CD4+ T cells are spontaneously activated to produce TH2 cytokines in the presence of FcεRI-bearing DCs.
Keywords: food allergy, dendritic cell, Th2 cytokines, IgE receptor
INTRODUCTION
Food allergy (FA) affects about 6% of young children and 3–4% of adults in Westernized countries. Eosinophilic esophagitis (EE), characterized by infiltration of eosinophils within the esophagus, has also become a more common clinical problem and is often driven by abnormal immune responses to food proteins [1–3]. Multiple studies have suggested a role for TH2 CD4+ T cells in the pathogenesis of these diseases. Some studies attribute allergy to the absence of a TH1 response and others to an increased TH2 response after allergen exposure [4–8]. However, the participation of other cell types important in allergic immune responses, including dendritic cells (DCs), has been largely unexplored.
DCs are professional antigen-presenting cells (APCs) capable of activating naïve T cells and responding to innate immune stimuli. There are two major subtypes of immature DCs in the peripheral blood of humans – Blood Dendritic Cell Antigen (BDCA)2+, BDCA4+, CD123hi, CD11c− plasmacytoid DCs (pDCs) and BDCA1+ BDCA3+ CD123lo CD11c+ myeloid DCs (mDCs). pDCs and mDCs express the αγ2 variant of the high affinity IgE receptor FcεRI, which reportedly increases the efficiency of antigen presentation by up to 1000-fold through a mechanism known as antigen focusing [9, 10]. Both subtypes are important in the sensitization and effector phases of allergic asthma and can efficiently induce allergen-dependent TH2 responses [11]. APCs, including DCs, are also thought to play a central role in the induction and maintenance of oral tolerance [12–14].
Given these data, we hypothesize that DCs also play an integral role in the development of food allergy in general and EE in specific. In this study, we sought to identify phenotypic and functional biomarkers associated with DC activity in FA and EE by comparing these in children with either condition as well as to their non-affected siblings.
METHODS
Study subjects
Children (n=14; 7 male (M) and 7 female (F)) with IgE-mediated cow’s milk allergy (FA), 8 (6 M, 2F) with eosinophilic esophagitis (EE), and 11 (3M, 8F) non-affected siblings (CON) of the children with FA and EE were enrolled in this study. All experiments described were performed on all subjects, except in Supplementary Table 1 where limited cell numbers allowed us to do dose titration of allergen on only a subset of subjects (13 subjects with FA, 7 with EE, and 8 CONs). Patient and parental interviews and review of medical records provided details of the following information: (a) all foods being avoided by each subject at the time of enrollment into the study, (b) sensitization to foods (defined by food-specific IgE>0.35 kUA/L) that were currently being ingested on a regular basis, (c) current medications, and (d) the presence of other allergic diseases (eczema, allergic rhinitis, asthma). This information, along with other significant laboratory parameters, is summarized in Table 1. This study was approved by the Johns Hopkins Institutional Review Board.
Table 1.
Patient Characteristics
| Age | Milk IgE | Total IgE | Phad | Avoiding | Sens | Acute Rxn | Meds | Other | |
|---|---|---|---|---|---|---|---|---|---|
| FA1 | 9.3 | 1108.0 | 4318 | 9.12 | M, E, P, Se, T, O(3) | Y | Y | IC | A, AR |
| FA2 | 6.5 | 394.0 | 3634 | 5.24 | M, E, P, Se, T, O(3) | Y | Y | IC | A |
| FA3 | 6.9 | 5.4 | 335 | 2.63 | M, E, P,T | Y | Y | LRA | E, A, AR |
| FA4 | 8.4 | 94.5 | 4006 | 51.70 | M, E, P, Se, T, O(5) | Y | Y | LRA | E, A, AR |
| FA5 | 9.8 | 32.0 | 1171 | 77.00 | M, E, P, T, O(1) | Y | Y | IC | E, A, AR |
| FA6 | 10.8 | 904.0 | 4603 | 68.70 | M, E, P, Se, T, W, O(9) | Y | Y | NC | E, AR |
| FA7 | 8.3 | 77.4 | 998 | 8.84 | M, E, Se, W, O(9) | Y | Y | NC | E, A, AR |
| FA8 | 6.1 | 810.0 | 5960 | 82.30 | M, E, P,S, Se, T, W, O(*) | Y | Y | IC | E, A, AR |
| FA9 | 5.9 | 51.5 | 4469 | 614.00 | M, E, P, Se, T, O(1) | Y | Y | LRA | E, A |
| FA10 | 6.3 | 90.4 | 1423 | 41.00 | M, E, P, Se, T, W, O(1) | Y | Y | E, A, AR | |
| FA11 | 4.2 | 20.8 | 238 | 25.80 | M, T | Y | Y | E | |
| FA12 | 4.8 | 88.7 | 2545 | 49.20 | M, P,S, Se, T, W, O(2) | Y | Y | E, A, AR | |
| FA13 | 8.6 | 652.0 | 3998 | 91.10 | M, E, P, Se, T, O(*) | Y | Y | IC, NC | E, A, AR |
| FA14 | 5.8 | 133.2 | 1361 | 50.80 | M, P, Se, T, O(1) | Y | Y | LRA | E, A |
| Med | 6.7 | 92.5 | 3090 | 50.00 | |||||
| IQR | 2.6 | 529.5 | 3022 | 61.64 | |||||
| EE1 | 21.6 | 2.7 | 350 | 23.10 | M, E, P | Y | Y | SC | AR |
| EE2 | 2.2 | 5.3 | 548 | 23.30 | M, P,S, Se, T, W, O(§) | Y | Y | IC, NC, AH, LRA | E, A, AR |
| EE3 | 13.1 | 0.18 | 276 | 35.40 | M | Y | Y | SC | AR |
| EE4 | 5.9 | 3.99 | 1007 | 22.60 | M, P, T | Y | N‡ | AR | |
| EE5 | 18.0 | 0.92 | 67 | 2.21 | N† | Y | N‡ | SC | AR |
| EE6 | 10.5 | 6.78 | 553 | 49.60 | M, E, P,S, Se, T, O(2) | Y | Y | AH | E, A, AR |
| EE7 | 7.5 | 28.40 | 157 | 0.45 | M, E, P, T, W, O(3) | Y | Y | E, A, AR | |
| EE8 | 15.1 | 0.54 | 204 | 28.50 | M, E | Y | N‡ | SC | AR |
| Med | 11.8 | 3.34 | 313 | 23.20 | |||||
| IQR | 8.7 | 4.85 | 357 | 12.72 | |||||
| Con1 | 11.3 | 0.15 | 187 | 1.37 | N | NT | N | ||
| Con2 | 10.3 | 0.12 | 129 | 17.60 | N | NT | N | AR | |
| Con3 | 10.2 | 0.38 | 144 | 2.94 | N | NT | N | ||
| Con4 | 6.0 | 0.25 | 112 | 0.10 | N | NT | N | ||
| Con5 | 7.5 | 1.52 | 18.80 | <0.35 | N | NT | N | ||
| Con6 | 12.5 | 0.23 | 1056 | 19.10 | N | NT | N | ||
| Con7 | 13.4 | <0.10 | 16 | 0.15 | N | NT | N | ||
| Con8 | 2.2 | <0.10 | 161 | 0.15 | N | NT | N | ||
| Con9 | 17.2 | <0.10 | 9 | 0.48 | N | NT | N | AR | |
| Con10 | 14.0 | <0.10 | 27 | 0.16 | N | NT | N | AH | AR |
| Con11 | 16.9 | <0.10 | 4 | 0.27 | N | NT | N | AR | |
| Med | 11.3 | 0.12 | 112 | 0.27 | |||||
| IQR | 4.8 | 0.24 | 135 | 2.01 |
Subject was not following a food avoidance diet at time of enrollment into the study; now exclusively on elemental formula
These subjects experienced abdominal pain and/or vomiting after ingestion of concentrated or large quantities of milk
Diet limited to elemental formula exclusively
Diet limited to elemental formula and few foods (amarenth, tapicoa, rice, fruit, vegetables, chicken, turkey, pork)
Age: Age in years (y) at time of enrollment
Milk IgE: Milk-specific IgE (kUA/L) Total IgE: Total serum IgE (kU/L) Phad: Phadiatop for most common aeroallergens (kU/L)
Avoiding: Foods being avoided in the diet at the time of enrollment into the study M, milk; E, egg; P, peanut; S, soy; Se, sesame; T, tree nuts; W, wheat; O, other (number of other foods); N, none
Sens: Subject was (Y) or was not (N) regularly ingesting food(s) in their diet to which they were sensitized but clinically tolerant; NT, not tested
Acute Rxn: Subject did (Y, yes) or did not (N, no) experience acute hypersensitivity reaction after ingestion of milk
Meds: Current medications actively used at time of enrollment into the study: SC, swallowed corticosteroid; IC, inhaled corticosteroid; NC, intranasal corticosteroid; LRA, leukotriene receptor antagonist; AH, antihistamine
Other: Other atopic diseases diagnosed or reported: E, eczema; A, asthma; AR, allergic rhinitis
Cell preparation and cultures
Peripheral blood was collected in EDTA following venipuncture and subjected to double Percoll (Pharmacia Biotech) density centrifugation. Plasma was decanted and stored at −20° C. Cell isolation procedures have been described previously [15]. Briefly, the upper fraction of cells consisted of basophil-depleted mononuclear cells that were used to isolate pDCs using BDCA4+ magnetic bead selection (Miltenyi). Cells not retained on this column were then used to isolate mDCs with BDCA1+ selection (Miltenyi) after depletion of CD19+ B cells. Although the few numbers of DC subtypes isolated did not allow for routine testing of purity, periodic evaluations indicate enrichments achieving up to 95%. CD4+ T cells were prepared by positive selection (Miltenyi) from remaining cells after DCs removal.
DC subtypes (2.5X104 cells) were cultured in a final volume of 250μl of conditioned-Iscove Modified Dulbecco Media (C-IMDM; Invitrogen Life Technologies) that was supplemented with 5% fetal calf serum (FCS; Invitrogen Life Technologies), 1X nonessential amino acids (Invitrogen Life Technologies), and 10μg/mL gentamicin (Invitrogen Life Technologies), pH 7.2–7.4. Cultures were stimulated with 50μg/mL of an aqueous crude milk extract (Greer) or 5μg/mL of anti-human IgE antibody (prepared in-house) [16] for 24 hours in 96-well flat-bottom plates. This dose of milk extract was chosen as it was found to induce optimal T cell proliferation in DC-T cell co-cultures (data not shown). For DC-T cell co-cultures, 1×104 pDCs or mDCs were cultured with 1×105 autologous CD4+ T cells in a final volume of 250μl and stimulated with 50μg/mL or 10μg/mL of crude milk extract for 96 hours in 96-well round-bottom plates.
Cytokine measurements
Cytokines were measured using multiplex bead immunoassay (Bioplex, BioRad) according to the manufacturer’s directions. A human x-plex panel (consisting of TNF-α, IL-6, GM-CSF, and IL-10), the human Th1/Th2 panel, and the human 27-plex panel were used to evaluate supernatants from pure DC cultures, DC-T cell co-cultures, and plasma, respectively. Limits of detection for this assay are IL-1β 0.6 pg/mL, IL-1Ra 5.5 pg/mL, IL-2 1.6 pg/mL, IL-4 0.7 pg/mL, IL-5 0.6 pg/mL, IL-6 2.6 pg/mL, IL-7 1.1 pg/mL, IL-8 1.0 pg/mL, IL-9 2.5 pg/mL, IL-10 0.3 pg/mL, IL-12 (p70) 3.5 pg/mL, IL-13 0.7 pg/mL, IL-15 2.4 pg/mL, IL-17 3.3 pg/mL, eotaxin 2.5 pg/mL, FGF basic 1.9 pg/mL, G-CSF 1.7 pg/mL, GM-CSF 2.2 pg/mL, IFN-γ 6.4 pg/mL, IP-10 6.1 pg/mL, MCP-1 1.1 pg/mL, MIP-1α 1.6 pg/mL, MIP-1β 2.4 pg/mL, PDGF-BB 2.9 pg/mL, RANTES 1.8 pg/mL, TNF-α 6.0 pg/mL, and VEGF 3.1 pg/mL.
Flow cytometry
Mononuclear cell suspensions were fixed in buffered 4% paraformaldehyde and frozen below −70°C. Prior to and during staining, cells were blocked with FcR blocking reagent according to the manufacturer’s directions (Miltenyi). The following antibodies were used: BDCA2-FITC or -Biotin, BDCA1-FITC or -APC, (Miltenyi); CD80-Biotin, CD86-APC, CD19-Pacific Blue; (BD Pharmingen), and/or FcεRIα-PE (eBioscience). Streptavidin-PerCP (BD Pharmingen) was added following staining with the above biotin-conjugated antibodies. Stained cells were analyzed using an LSRII machine (BD Pharmingen). Percent positives were defined by first gating on pDCs or mDCs and then determining the percent of positively stained cells relative to nonstained cells or isotype controls (similar results were obtained when either control was used).
Serologic measurements
Measurements of total serum IgE, milk-specific IgE, and a multi-allergen screen were performed on plasma by the Johns Hopkins Dermatology Allergy and Clinical Immunology (DACI) Reference Laboratory (Baltimore, MD) using a fluorescent-based enzyme immunoassay (FEIA) performed on the ImmunoCAP 250 (Phadia, Kalamazoo, MI, USA). The Phadiatop is a single measurement that detects IgE antibody specific for any of 15 common aeroallergens.
Statistics
Not all data sets were normally distributed, including after logarithmic transformation; therefore, all comparisons were unpaired Wilcoxon rank-sum tests except for Table II where observations were paired by individual (KaleidaGraph, Synergy Software, Reading, PA). P values were exact except in the case of ties in which asymptotic statistics were used. Linear and Spearman or Pearson (based on whether the data was normally distributed) correlations were performed using Prism Software (GraphPad Software, San Diego, CA). The box defines the 25% and 75% quartiles, division within the box the median, and whiskers the range. Statistically significant p-values, defined as <0.05, are indicated.
Table 2.
Changes in cytokine secretion with milk stimulation. P values comparing difference in cytokine levels produced by pDCs or mDCs co-cultured with CD4+ T cells in the presence or absence of milk extract (50 μg/ml milk extract) using Wilcoxon Matched-Pairs Signed-Ranks Test.
| pDC | mDC | |||||
|---|---|---|---|---|---|---|
| FA | Con | EE | FA | Con | EE | |
| IL-5 | 0.6257 | 0.0320 | 0.9453 | 0.0166* | 0.0420 | 0.5469 |
| IL-13 | 0.0419 | 0.0010 | 0.3828 | 1.0000 | 0.0322 | 0.6400 |
| IL-4 | 0.0001 | 0.0059 | 0.0142 | 0.0002 | 0.1025 | 0.0156 |
| IL-10 | 0.0001 | 0.0010 | 0.0078 | 0.0001 | 0.0010 | 0.0078 |
| GM-CSF | 0.0001 | 0.0010 | 0.0078 | 0.0001 | 0.0010 | 0.0078 |
| IL-2 | 0.0001 | 0.0078 | 0.0010 | 0.0134 | 0.0010 | 0.0078 |
| IL-12 | 0.0001 | 0.0010 | 0.0078 | 0.0001 | 0.0010 | 0.0078 |
| TNF-α | 0.0001 | 0.0010 | 0.0078 | 0.0001 | 0.0010 | 0.0078 |
| IFN-γ | 0.0001 | 0.0010 | 0.0078 | 0.0017 | 0.0010 | 0.0078 |
levels statistically higher in media alone than after stimulation with milk extract
RESULTS
Clinical characteristics of subjects
Thirty-three subjects were enrolled (Table 1). Median total serum IgE levels were 3090 kU/L, 313 kU/L, and 112 kU/L for the FA, EE, and CON subjects, respectively. The corresponding milk-specific IgE levels were 92.5 kUA/L, 3.34 kUA/L, and 0.12 kUA/L. The diagnosis of food allergy was based on a convincing history of reaction following exposure to cow’s milk and a milk-specific IgE>0.35 kUA/L (ImmunoCAP, Phadia, USA). All EE subjects had active disease at the time of enrollment as defined by the presence of chronic gastrointestinal symptoms (including vomiting, dysphagia, abdominal pain, and / or failure to thrive) and >15 eosinophils per high-powered field on recent esophageal biopsies. While many of the subjects with EE experienced acute allergic reactions after ingestion of milk, three of the subjects had gastrointestinal symptoms only (Table 1). All EE subjects had detectable milk-specific IgE and histologic improvement with milk avoidance. All subjects with FA, and most subjects with EE, were actively avoiding multiple foods in addition to cow’s milk, and were also regularly ingesting foods to which they were sensitized but not clinically reactive (Table 1). Although several CON subjects had low positive levels of milk-specific IgE, none had a history suggesting allergy to any food nor had they avoided any foods.
DC cytokine responses to allergen stimulation
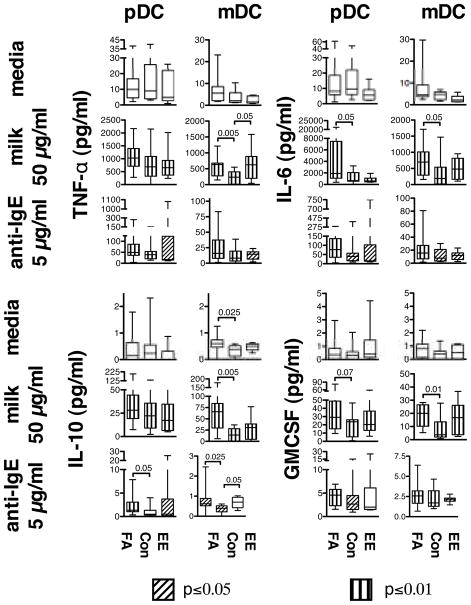
Previous studies have suggested that DCs are an important source of pro-inflammatory cytokines after treatment with artificial stimuli that crosslink the IgE receptor [16, 17]. As shown in Fig. 1, mDCs from children with FA and EE produced significantly greater quantities of TNF-α after stimulation with milk. Both pDCs and mDCs from children with FA also produced more IL-6 in response to milk, with much higher levels produced by pDCs compared to mDCs. No significant difference in IL-6 production was observed between EE subjects and CONs. Higher levels of GM-CSF were produced by mDCs from FA subjects, with a trend in this direction by pDCs after stimulation with allergen [18]. Interestingly, mDCs from children with FA, but not EE, also produced greater quantities of IL-10 in response to milk.
Fig. 1.
Cytokine secretion by purified DCs. pDCs or mDCs isolated from peripheral blood of FA, CON, and EE children were cultured in the presence of media alone, 50μg/mL of crude milk extract, or 5μg/mL of goat polyclonal anti-human IgE. TNF-α, IL-6, IL-10, and GM-CSF were measured 24 hours after stimulation. Levels of each cytokine under each condition tested were compared across groups and statistically significant differences are indicated. Levels of each cytokine following treatment with milk or anti-IgE to levels produced by cells cultured in media alone were also compared within each group of subjects, and significant differences are indicated by markings within the boxes according to the legend.
All three groups of subjects produced IL-6, TNF-α, GM-CSF, and IL-10 when stimulated with a goat anti-human IgE antibody [16] that results in cross-linking of the IgE receptor, suggesting that DCs from all subjects were capable of responding to this bivalent stimulus (Fig. 1). Levels of all four cytokines were significantly correlated with expression of FcεRI on pDCs but not mDCs in response to anti-IgE (Supplementary Fig. 1). Levels of IL-10, but not IL-6, TNF-α, or GM-CSF, were significantly higher in FA (pDCs and mDCs) and/or EE (mDCs) subjects compared to CONs following anti-IgE treatment. No significant differences in spontaneous production of IL-6, TNF-α, or GM-CSF by either DC subtype were observed in FA or EE subjects compared to CONs (Fig. 1). mDCs from children with FA produced slight but statistically higher spontaneous levels of IL-10 compared to controls, although the levels were still relatively low compared to those induced with anti-IgE stimulation. These data suggest that DCs from children with FA produce more pro-inflammatory cytokines (IL-6, TNF-α), GM-CSF, and IL-10 (from mDCs) than CONs after stimulation with allergen. These differences were less obvious for children with EE. For the most part, levels of all cytokines tested were significantly higher than media controls following stimulation with milk or anti-IgE in all three groups of subjects (Fig. 1).
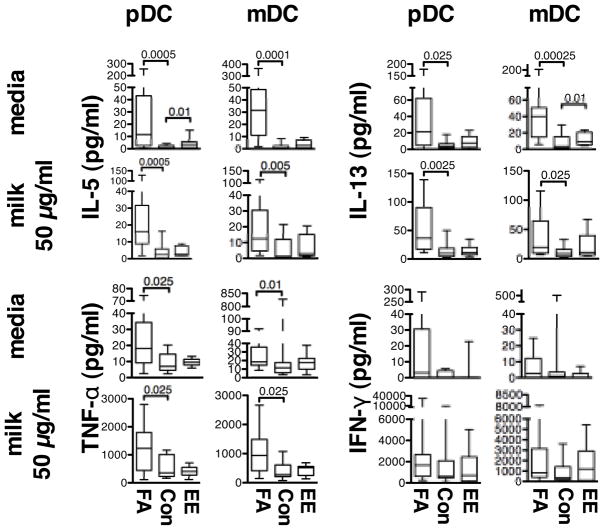
TH2 cytokine responses in DC-CD4+ T cell co-cultures
To investigate the role of DC subtypes in polarizing CD4+ T-cells toward a TH2 phenotype, purified pDCs or mDCs were cultured together with autologous CD4+ T cells, and cytokine production was evaluated 96 hours later (Fig. 2 and Supplementary Fig. 2). We observed that TH2 cytokines (IL-4, IL-5, IL-13, IL-10), IL-2, GM-CSF, and pro-inflammatory TNF-α were all spontaneously produced by both pDC- and mDC-T cell co-cultures from children with FA. To investigate this issue further, an additional 9 subjects with milk allergy were investigated and demonstrated little or no IL-13 production when CD4+ T cells were cultured alone without DCs (data not shown). Spontaneous production of these cytokines by subjects with EE was not statistically different from CONs, except for higher spontaneous release of IL-5 and IL-13. No significant difference in spontaneous production of IFN-γ, a classic TH1 cytokine, was seen across the three groups of subjects.
Fig. 2.
Cytokine secretion by DC-CD4+ T cell co-cultures. pDCs or mDCs from FA, CON, and EE subjects were cultured together with autologous CD4+ T cells in media alone or 50 μg/mL of crude milk extract. Indicated cytokines (pg/mL) were measured 96 hours after stimulation.
Following stimulation with crude milk extract, levels of IL-5, IL-13, IL-4, IL-10, IL-2 (in pDC-T cell co-cultures only), and TNF-α remained significantly higher in the FA group compared to CONs after antigen stimulation (Fig. 2 and Supplementary Fig. 2). In contrast, the EE subjects did not differ significantly from CONs for any cytokines tested after stimulating the cultures with milk extract, even though all EE subjects were clinically reactive to milk. After the addition of milk extract, levels of nearly all cytokines tested increased relative to levels in unstimulated cultures for all three groups of subjects, except for two notable exceptions (Table 2). IL-5 and IL-13 levels in the FA group failed to significantly rise following stimulation with milk extract relative to unstimulated cultures. Similar results were obtained when 10μg/mL of extract was used (Supplementary Table 1). These findings suggest that DCs from FA children can provide in vitro accessory cell activity to support the production of IL-5 and IL-13 by CD4 lymphocytes in the absence of exogenous allergen.
Plasma levels of TH2 cytokines
We next assayed for cytokines in plasma from our three groups of subjects with the intent of supporting the hypothesis that lymphocytes from FA and/or EE subjects exhibit ongoing TH2 cytokine production in vivo. As shown in Fig. 3, levels of IL-5 and IL-13 were significantly elevated in plasma from both FA and EE children compared to CONs. FA subjects also had higher plasma levels of IL-2. However, levels of many other cytokines and chemokines, including IL-1β, IL-1ra, IL-4, IL-6, IL-7, IL-8, IL-9, IL-10, IL12-p70, IL-15, IL-17, eotaxin, basic FGF, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α and VEGF, did not differ among the three groups of subjects. Collectively, these data support the conclusion that EE and FA subjects have ongoing production of Th2 cytokines (IL-5 and IL-13) in vivo and, as indicated by the in vitro data, possess circulating DCs that play a role in this activity.
Fig. 3.
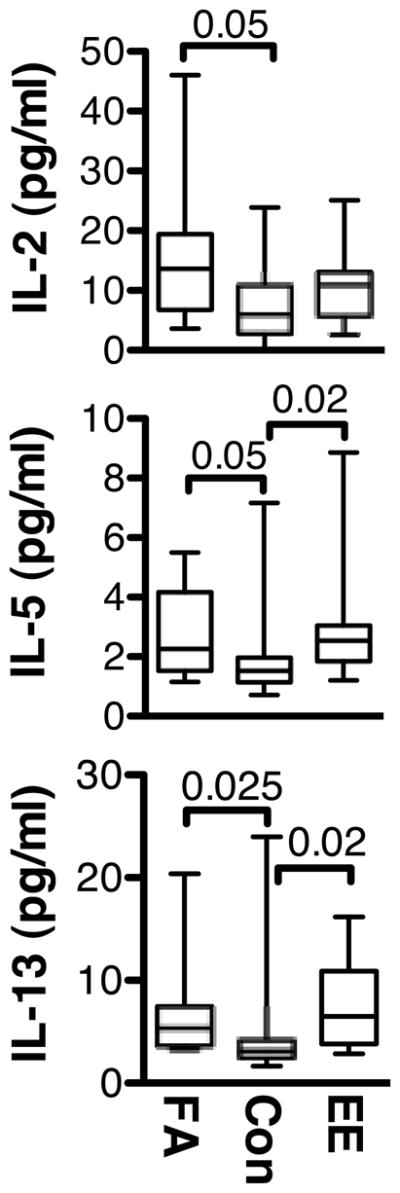
Plasma cytokine levels in children with FA, EE, and CONs. Plasma levels of IL-2, IL-5, and IL-13 (pg/mL) are indicated.
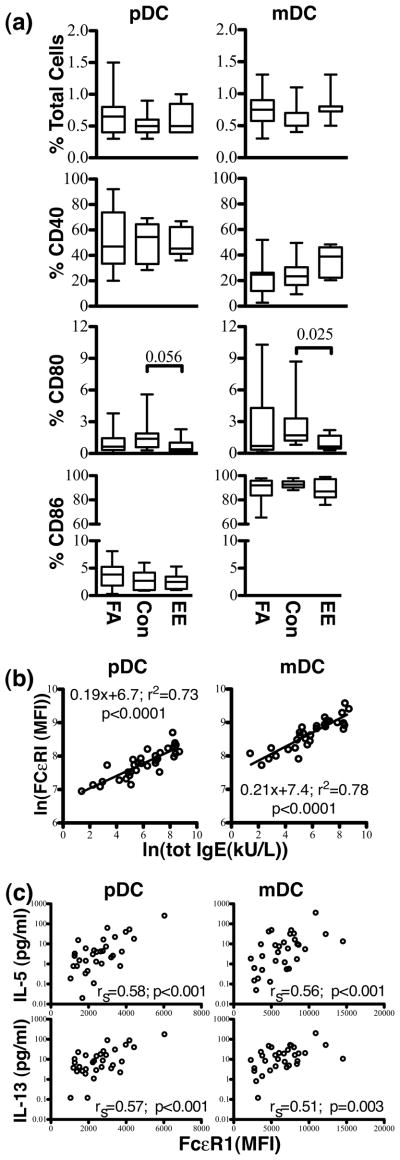
Comparison of co-stimulatory molecule expression on DCs from children with FA, EE, and CONs
Flow cytometry was performed on mononuclear cell suspensions to investigate whether the ongoing production of TH2 cytokines by children with FA and EE was associated with altered expression of co-stimulatory molecules on their DCs. (Fig. 4A). First, the frequencies of pDCs and mDCs did not differ significantly across the three groups of subjects. Fig. 4A shows that expression of CD40 and CD86 also did not differ among subjects on either DC subtype. However, children with EE had significantly less staining for CD80 on their mDCs and there was a similar trend on their pDCs. These data indicate that the greater spontaneous production of cytokines by allergic children was unlikely to be related to altered co-stimulatory molecule expression on their DCs.
Fig. 4.
Flow cytometric analysis of DCs from children with FA, EE, and CONs. (a) The relative frequency of pDCs and mDCs as a percentage of total mononuclear cells (% total cells), as well as the percent of pDCs and mDCs expressing CD40, CD80, and CD86 in FA, CON, and EE subjects, are represented. (b) Expression (mean fluorescence intensity; MFI) of FcεRIα on pDCs and mDCs. All subjects are represented (FA, EE, CON). Levels of FcεRIα expression on both pDCs and mDCs are highly correlated to total serum IgE levels (KU/L). (c) Spearman correlation was used to compare the level of IL-5 and IL-13 spontaneously produced in pDC- or mDC-CD4+ T cell co-cultures to the level of expression of FcεRI (expressed in MFI).
Relationship of DC FcεRI expression to serum IgE
Expression of FcεRIα on both subtypes of DCs in the three groups of subjects was highly correlated with serum levels of IgE (Fig. 4B) (pDCs, r2=0.73, p<0.0001; mDCs, r2=0.78, p<0.0001). Relative expression of FcεRIα on mDCs was approximately twice as great as that observed on pDCs. Expression of FcεRI on both pDCs and mDCs from children with food allergy (and mDCs only in the EE group) was higher than controls (Supplementary Fig. 3). Using Spearman correlation, spontaneous release of IL-5 and IL-13 from both pDCs and mDCs used as APCs was highly correlated to expression of FcεRI on the respective DC subtype (Fig 4C).
DISCUSSION
FA and EE both appear to be increasing in prevalence in developed countries. The pathogenesis of both FA and most cases of EE is thought to involve a failure of oral tolerance. DCs are known to be essential in both the induction and maintenance of oral tolerance in the intestine, where they play a primary role in presenting dietary antigens to T lymphocytes and thereby directing subsequent immune responses [12–14]. While many EE patients produce food-specific IgE, anaphylaxis to foods is less common. Like FA, EE is associated with a TH2 dominated response, and recent work has demonstrated a critical role for IL-13 in inducing an EE-specific transcriptome [19]. To our knowledge, the study herein is the first to examine how differences in DC phenotype and/or function may contribute to the pathogenesis of these diseases.
We have shown that DC-CD4+ T cell co-cultures from children with FA spontaneously produced relatively large quantities of TH2 cytokines in the absence of allergen exposure. EE patients similarly demonstrated ongoing production of IL-5 and IL-13. Of the phenotypic markers investigated, only FcεRI expression on the surface of both pDCs and mDCs correlated with the amount of spontaneously produced IL-5 and IL-13. This suggests an important role for the IgE receptor on the surface of DCs in inducing ongoing cytokine release from CD4+ T cells. Importantly, CD4+ T cells from children with FA, when cultured alone, spontaneously produced no or much lower amounts of TH2 cytokines, suggesting that DCs were required for this phenomenon. No further increase in IL-5 or IL-13 occurred in DC-T cell co-cultures from FA or EE children after stimulation with milk allergen. We conclude from this observation that T cells from these subjects are already activated in vivo in a response dependent on circulating DCs, and that DC/T cell co-cultures are unresponsive to additional allergen stimulation in vitro. Evidence that best supports this hypothesis was additionally seen with elevated IL-5 and IL-13 levels in the plasma of subjects with FA and EE –the same two cytokines produced in vitro in the DC/T cell co-cultures. Certainly, the role of DCs and their expression of FcεRI/IgE in helping to drive T cell cytokine responses have long been proposed. Moreover, this concept is further supported by our recent findings that reductions in IL-5 and IL-13 produced in response to allergen track with IgE neutralization and FcεRI reduction on DCs following in vivo treatment with omalizumab [20].
Unlike the FA group, levels of IL-5 and IL-13 produced in co-cultures stimulated with milk allergen from EE subjects did not differ significantly from controls, despite comparable levels of IL-5 and IL-13 in the plasma of FA and EE subjects. Several explanations seem possible. The median milk-specific IgE levels of subjects with EE in our study were almost 30-fold lower than that of the food allergic group. Alternatively, the majority of lymphocytes responsible for TH2 cytokine production in EE may be localized to the esophageal tissues and are no longer present in peripheral blood. Indeed, lymphocytes have been found to accumulate in the esophagi of patients diagnosed with EE [8, 21]. No difference in IFN-γ, a TH1 cytokine, was observed between FA or EE subjects and controls in either stimulated or unstimulated cultures. These data support earlier studies that have suggested the magnitude of the TH2 response, rather than the absence of a TH1 response, underlies clinical disease.
The spontaneous induction of TH2 cytokines in DC-CD4+ T cell co-cultures from FA and EE subjects was not associated with increased co-stimulatory molecule expression on DCs from these subjects prior to culture. In fact, EE subjects had significantly less staining for CD80. Alterations in CD80 have previously been implicated in allergic disease. After stimulation with Derp1, Charbonnier et al. [22] showed that CD86 and HLA-DR were upregulated equally on DCs in control and dust mite-allergic subjects, but only mDCs from normal subjects demonstrated increased expression of CD80. CD80 has also been shown to be required for induction of low-dose oral tolerance to peanut in mice [23]. The diminished expression of CD80 on DCs from EE patients may, therefore, contribute to their lack or oral tolerance.
Assuming that IgE is playing a role in the DC-dependent T cell cytokine responses observed among our FA and EE subjects, then it seems relevant to comment here on what has been known for decades regarding basophil function in these subjects. In particular, May et al. [24] reported in 1976 that up to 80% of children with food allergy have basophils that spontaneously release histamine. Subsequent studies by Sampson et al. [25] suggested a role for histamine releasing factor (HRF) (along with IgE) in this phenomenon. Histamine release reportedly returned to normal after children were placed on appropriate elimination diets [25]. Basophils from a majority of the FA children in our study also demonstrated spontaneous histamine release (unpublished data). All of them were regularly ingesting multiple foods to which they were sensitized but clinically tolerant. We propose that these food antigens may form IgE complexes in the systemic circulation that subsequently bind and activate FcεRI on basophils and DCs leading to their activation. Indeed, circulating basophils from allergic children have previously been shown to be activated, and our data suggests DCs from food allergic children could be as well, which subsequently facilitates T cell activation [26]. Although not tested here, this is certainly one mechanistic hypothesis that requires investigation in future studies.
We found that DCs from children with FA produced much higher quantities of IL-6 (pDCs and mDCs) and TNF-α (mDCs) after stimulation with milk allergen compared to their nonaffected siblings. Assuming an IgE-dependent activation, the lack of a significant difference in TNF-α production by pDCs from FA subjects could reflect differences in the kinetics of IL-6 and TNF-α secretion by pDCs. Schroeder et al. [16] have shown that pDC-derived TNF-α peaks much earlier (8 hours) and then declines, while IL-6 continues to increase at 24 hours (the time point chosen in this study) following IgE crosslinking. The higher expression of pro-inflammatory cytokines by DCs following food allergen exposure is likely to be an important mechanism by which DCs promote allergic inflammation in children with food allergy. The milk extract used in this study does contain endotoxin. While this may also contribute to the release of pro-inflammatory cytokines by mDCs after stimulation with allergen, based on our own experience and other published observations, human pDCs do not express TLR4, and therefore would not be expected to respond to endotoxin [27]. Treatment with artificial stimuli known to crosslink the IgE receptor on DCs does lead to release of IL-6 and TNF-α, and the greater expression of FcεRI on DCs from allergic subjects may contribute to the greater amount of pro-inflammatory cytokines released after treatment with allergen. Indeed, we did observe a positive correlation between expression of FcεRI and production of IL-6, TNFα, IL-10 and GM-CSF by pDCs in response to anti-IgE treatment. However, the three groups of subjects did not differ in the amount of IL-6 or TNF-α produced from DCs after treatment with anti-IgE. This suggests a critical role for other receptors on the DC in mediating the increased pro-inflammatory cytokines produced in response to milk in the children with FA and EE. Of note, although none of the control subjects in our study had food allergy, some might still be classified as allergic. A number of them had sensitivity to environmental allergens, and a few had symptoms of allergic rhinitis. The age and sex distribution of the groups studied also varied in some instances, and it is possible these disparities also contributed to the differences in DC cytokine production that was observed. Finally, although none of our control subjects reported a history of ever reacting to any foods, we cannot completely exclude the possibility that they may have outgrown a food allergy in the past.
Both DC subtypes from FA subjects also produced larger quantities of GM-CSF following stimulation with milk. GM-CSF is an important growth factor for eosinophils that can also activate APCs [28, 29]. This may represent another mechanism by which DCs promote TH2 responses in this disease. With the exception of greater TNF-α production by mDCs, no differences in secretion of pro-inflammatory cytokines, GM-CSF, or IL-10 by either pDCs or mDCs from subjects with EE were observed compared to nonaffected controls following milk stimulation. This is in spite of documented clinical reactivity to milk by all of the subjects with EE in this study. These findings may also suggest that the relevant pathogenic cells in subjects with EE are localized to the gastrointestinal tract, and therefore the function of peripheral DCs may be relatively spared.
Interestingly, mDCs from FA children produced greater quantities of IL-10. IL-10 may promote TH2 responses by decreasing secretion of IL-12 and thereby indirectly inhibit the differentiation of TH1 cells [30]. Alternatively, IL-10 has also been shown to have immunosuppressive effects on both TH1 and TH2 cells, and may indirectly interfere with IgE synthesis and eosinophil survival [31]. Recent work has suggested that autocrine secretion of IL-10 following FcεRI crosslinking on mDCs may be a mechanism to inhibit TNF-α secretion by these cells, and thereby diminish their pro-inflammatory activities following allergen exposure [17, 32].
In summary, we found that DCs from children with FA produced greater quantities of pro-inflammatory cytokines and GM-CSF after allergen stimulation that may promote allergic inflammation and TH2 responses in these diseases. Evidence for ongoing DC-dependent T cell production of TH2, but not TH1, cytokines was also evident in co-cultures from children with FA. Surprisingly, the IL-5 and IL-13 levels produced in these cultures were not increased after in vitro stimulation with milk. This observation was also associated with elevated IL-5/IL-13 protein in the plasma of FA (and EE) subjects compared to controls.
Of the phenotypic markers investigated, only FcεRI on the surface of both pDCs and mDCs correlated with the amount of “spontaneously” produced IL-5 and IL-13. Coupled with previous reports, these findings collectively suggest an important role for IgE (and its receptor, FcεRI) in promoting DC-dependent secretion of these cytokines by CD4+ T cells in children with multiple food allergies and/or EE.
Supplementary Material
Acknowledgments
This study was supported by the Exploratory Investigations in Food Hypersensitivity grant, AI079853 (JTS) from the NIAID, NIH, and, in part, by the Asthma and Allergic Diseases Cooperative Research Centers grant (AADCRC) U19AI070345-01 (project 3:JTS) from the NIAID, NIH, Bethesda, Maryland. P.A.F was supported by Training Grant T32AI007007, NIAID, NIH, Bethesda, Maryland.
Abbreviations
- FA
food allergy
- EE
eosinophilic esophagitis
- DC
dendritic cell
- APC
antigen-presenting cell
- BDCA
Blood Dendritic Cell Antigen
- pDC
plasmacytoid dendritic cell
- mDC
monocytoid dendritic cell
- CON
control
- HSHR
high spontaneous histamine release
- HRF
histamine releasing factor
- MFI
mean fluorescence intensity
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Spergel JM, Andrews T, Brown-Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed bya combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95:336–43. doi: 10.1016/S1081-1206(10)61151-9. [DOI] [PubMed] [Google Scholar]
- 2.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2002;109:363–8. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 3.Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2007;119:509–11. doi: 10.1016/j.jaci.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Bullock JZ, Villanueva JM, Blanchard C, Filipovich AH, Putnam PE, Collins MH, Risma KA, Akers RM, Kirby CL, Buckmeier BK, Assa’ad AH, Hogan SP, Rothenberg ME. Interplay of adaptive th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:22–31. doi: 10.1097/MPG.0b013e318043c097. [DOI] [PubMed] [Google Scholar]
- 5.Thottingal TB, Stefura BP, Simons FE, Bannon GA, Burks W, HayGlass KT. Human subjects without peanut allergy demonstrate T cell-dependent, TH2-biased, peanut-specific cytokine and chemokine responses independent of TH1 expression. The Journal of allergy and clinical immunology. 2006;118:905–14. doi: 10.1016/j.jaci.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Tiemessen MM, Van Ieperen-Van Dijk AG, Bruijnzeel-Koomen CA, Garssen J, Knol EF, Van Hoffen E. Cow’s milk-specific T-cell reactivity of children with and without persistent cow’s milk allergy: key role for IL-10. The Journal of allergy and clinical immunology. 2004;113:932–9. doi: 10.1016/j.jaci.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111:1065–72. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. The Journal of allergy and clinical immunology. 2001;108:954–61. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 9.Maurer D, Ebner C, Reininger B, Fiebiger E, Kraft D, Kinet JP, Stingl G. The high affinity IgE receptor (Fc epsilon RI) mediates IgE-dependent allergen presentation. J Immunol. 1995;154:6285–90. [PubMed] [Google Scholar]
- 10.Maurer D, Fiebiger S, Ebner C, Reininger B, Fischer GF, Wichlas S, Jouvin MH, Schmitt-Egenolf M, Kraft D, Kinet JP, Stingl G. Peripheral blood dendritic cells express Fc epsilon RI as a complex composed of Fc epsilon RI alpha- and Fc epsilon RI gamma-chains and can use this receptor for IgE-mediated allergen presentation. J Immunol. 1996;157:607–16. [PubMed] [Google Scholar]
- 11.Farkas L, Kvale EO, Johansen FE, Jahnsen FL, Lund-Johansen F. Plasmacytoid dendritic cells activate allergen-specific TH2 memory cells: modulation by CpG oligodeoxynucleotides. The Journal of allergy and clinical immunology. 2004;114:436–43. doi: 10.1016/j.jaci.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Dubois B, Joubert G, de Aguero MG, Gouanvic M, Goubier A, Kaiserlian D. Sequential Role of Plasmacytoid Dendritic Cells and Regulatory T Cells in Oral Tolerance. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 13.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–75. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Toes RE. Mechanisms of oral tolerance revisited. Arthritis Res Ther. 2008;10:108. doi: 10.1186/ar2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder JT, MacGlashan DW, Jr, Kagey-Sobotka A, White JM, Lichtenstein LM. IgE-dependent IL-4 secretion by human basophils. The relationship between cytokine production and histamine release in mixed leukocyte cultures. J Immunol. 1994;153:1808–17. [PubMed] [Google Scholar]
- 16.Schroeder JT, Bieneman AP, Xiao H, Chichester KL, Vasagar K, Saini S, Liu MC. TLR9- and FcepsilonRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol. 2005;175:5724–31. doi: 10.4049/jimmunol.175.9.5724. [DOI] [PubMed] [Google Scholar]
- 17.Le T, Tversky J, Chichester KL, Bieneman AP, Huang SK, Wood RA, Schroeder JT. Interferons modulate Fc epsilon RI-dependent production of autoregulatory IL-10 by circulating human monocytoid dendritic cells. The Journal of allergy and clinical immunology. 2009;123:217–23. doi: 10.1016/j.jaci.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, Bardelli M, Montagna D, Locatelli F, Wack A. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood. 2007;109:5371–9. doi: 10.1182/blood-2006-08-038422. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, Collins MH, Putnam PE, Wells SI, Rothenberg ME. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. The Journal of allergy and clinical immunology. 2007;120:1292–300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder JT, Bieneman AP, Chichester KL, Hamilton RG, Xiao H, Saini SS, Liu MC. Decreases in Human Dendritic Cell-dependent Th2-like Responses Following Acute in vivo IgE Neutralization. J Allergy Clin Immunol. 2009 doi: 10.1016/j.jaci.2009.10.021. (In Press) [DOI] [PubMed] [Google Scholar]
- 21.Lucendo AJ, Navarro M, Comas C, Pascual JM, Burgos E, Santamaria L, Larrauri J. Immunophenotypic characterization and quantification of the epithelial inflammatory infiltrate in eosinophilic esophagitis through stereology: an analysis of the cellular mechanisms of the disease and the immunologic capacity of the esophagus. Am J Surg Pathol. 2007;31:598–606. doi: 10.1097/01.pas.0000213392.49698.8c. [DOI] [PubMed] [Google Scholar]
- 22.Charbonnier AS, Hammad H, Gosset P, Stewart GA, Alkan S, Tonnel AB, Pestel J. Der p 1-pulsed myeloid and plasmacytoid dendritic cells from house dust mite-sensitized allergic patients dysregulate the T cell response. J Leukoc Biol. 2003;73:91–9. doi: 10.1189/jlb.0602289. [DOI] [PubMed] [Google Scholar]
- 23.van Wijk F, Nierkens S, de Jong W, Wehrens EJ, Boon L, van Kooten P, Knippels LM, Pieters R. The CD28/CTLA-4-B7 signaling pathway is involved in both allergic sensitization and tolerance induction to orally administered peanut proteins. J Immunol. 2007;178:6894–900. doi: 10.4049/jimmunol.178.11.6894. [DOI] [PubMed] [Google Scholar]
- 24.May CD, Remigio L. Observations on high spontaneous release of histamine from leucocytes in vitro. Clin Allergy. 1982;12:229–41. doi: 10.1111/j.1365-2222.1982.tb02523.x. [DOI] [PubMed] [Google Scholar]
- 25.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989;321:228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 26.James JM, Kagey-Sobotka A, Sampson HA. Patients with severe atopic dermatitis have activated circulating basophils. The Journal of allergy and clinical immunology. 1993;91:1155–62. doi: 10.1016/0091-6749(93)90318-a. [DOI] [PubMed] [Google Scholar]
- 27.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 28.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–12. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 29.van Rijt LS, Lambrecht BN. Role of dendritic cells and Th2 lymphocytes in asthma: lessons from eosinophilic airway inflammation in the mouse. Microsc Res Tech. 2001;53:256–72. doi: 10.1002/jemt.1092. [DOI] [PubMed] [Google Scholar]
- 30.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–35. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 31.O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–8. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faith A, Singh N, Chevretton E, Roberts D, Lee T, Corrigan C, Hawrylowicz C. Counter regulation of the high affinity IgE receptor, FcepsilonRI, on human airway dendritic cells by IL-4 and IL-10. Allergy. 2009 doi: 10.1111/j.1398-9995.2009.02060.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.