Abstract
Background
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are sequelae of severe trauma. It is unknown if certain races are at greater risk of developing ALI/ARDS, and once established, if there are racial differences in the severity of lung injury or mortality.
Methods
Retrospective cohort study of 4,397 trauma patients (1,831 Caucasians, 871 African-Americans, 886 Hispanics, and 809 Asian/Pacific Islanders) requiring ICU admission between 1996-2007 at an urban level I trauma center.
Results
African-American patients were most likely to present in shock with penetrating trauma and receive a massive transfusion. The incidence of ALI/ARDS was similar by race (p=0.99). Among patients who developed ALI/ARDS, there was no evidence to support a difference in initial PaO2/FiO2 (p=0.33), lung injury score (p=0.67) or mortality (p=0.78) by race.
Conclusions
Despite differences in baseline characteristics, the incidence of ALI/ARDS, severity of lung injury, and mortality were similar by race.
Keywords: acute respiratory distress syndrome, acute lung injury, trauma, race, epidemiology, adult
Introduction
Racial and ethnic differences are well documented for some chronic diseases. For example, African-Americans have a higher prevalence of essential hypertension than Caucasians (1), as well as a higher incidence of heart failure (2). However, only recently have investigators begun to examine whether racial differences influence clinical outcomes in the critical care setting, and specifically among patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS).
Recently, two studies reported higher mortality rates in African-American (3, 4) and Hispanic patients (3) with ALI/ARDS. One study used a national administrative database consisting only of patients who had died, so it was not possible to determine if there were racial differences in the incidence of ARDS or to identify which factors led to the mortality difference (4). The second study used the National Heart Lung and Blood Institute’s ARDS Network data and controlled for confounding factors. The increased mortality in African-American patients in that study was explained by a greater severity of illness (3). Interestingly, Hispanic ethnicity was an independent risk factor for mortality; unlike African-American patients, the increased mortality in Hispanics was not explained by either severity of illness or co-morbidities (3).
Both of these studies included all patients with ALI/ARDS, resulting in a heterogeneous sample, as ALI/ARDS has many causes. Although, trauma is a well-recognized cause of ALI/ARDS, trauma patients accounted for less than 9% of the ARDS Network sample (3). Some studies have suggested that from both a pathophysiologic and a clinical standpoint, patients who develop ALI/ARDS as a result of trauma may differ from those whose ALI/ARDS is due to other causes (5, 6). Furthermore, these studies of racial differences focused exclusively on mortality differences in patients with established ALI/ARDS (3, 4). In trauma patients, it is unknown if certain races are at greater risk of developing ALI/ARDS, and once ALI/ARDS is established, if there are racial differences in the severity of lung injury or mortality. To answer these questions, we analyzed a racially and ethnically diverse cohort of trauma patients over an 11-year period. We hypothesized that injury patterns and treatment, namely mechanism of injury, injury severity, and the amount of blood transfused, may differ by race, and that these differences might account for a higher incidence of ALI/ARDS and worse outcomes among certain races.
Materials and Methods
Patient sample, study design, and clinical data
We assembled a retrospective cohort that included all trauma patients ≥14 years old who were either admitted to the intensive care unit (ICU) or went to the operating room after evaluation in the Emergency Department (ED) at San Francisco General Hospital, an urban level I trauma center, between April 1, 1996 and December 31, 2007. The cohort consisted of 4,397 patients: 1,831 Caucasians, 871 African-Americans, 886 Hispanics, and 809 Asian/Pacific Islanders (Figure I). Data on race were missing for 421 trauma patients who were removed from the cohort so that only patients whose race was identified were included. The Institutional Review Board of the University of California, San Francisco approved this study.
Figure I.
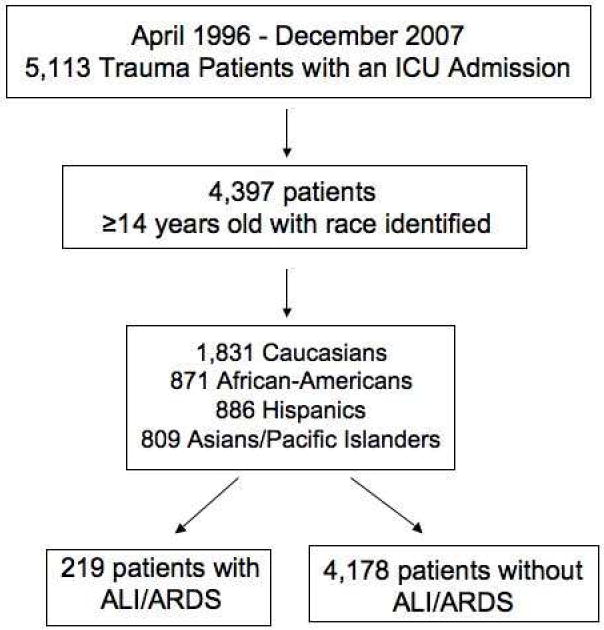
Flow diagram depicting the inclusion and exclusion criteria used to assemble the cohort of trauma patients requiring ICU admission between 1996-2007.
Demographic data consisting of age, gender, and race, and clinical data consisting of mechanism of injury, Injury Severity Score, Abbreviated Injury Score, and physiologic variables were obtained from the trauma registry. Mechanism of injury was classified as either penetrating or blunt trauma. In addition, the Abbreviated Injury Scores were used to determine whether chest trauma was present. The number of units of packed red blood cells (PRBCs) transfused within the first 24 hours of admission was also documented.
For the analyses, the Injury Severity Score and the number of units of PRBCs transfused within the first 24 hours of admission were divided into clinically relevant categories. Injury severity score was categorized as <15, 15-25, and >25. The number of units of PRBCs transfused was categorized as 0, 1-2 units, 3-9 units, and ≥10 units, which we designated as massive transfusion. Shock was defined as a systolic blood pressure ≤90 mmHg when the patient presented to the ED.
A single investigator prospectively identified all patients with ALI and ARDS (RHK). Over the study period, ICU patients were screened for ALI/ARDS either for ARDS Network clinical trials or for ICU quality assurance purposes. The American-European Consensus Conference criteria were used to define the cases (7). Only patients who met the criteria for ALI or ARDS within the first 72 hours of admission were included to ensure that trauma was the cause of ALI/ARDS. We chose this time interval because the classic studies of trauma-related ALI/ARDS observed that ALI/ARDS typically developed within the first 1-3 days after injury (8, 9). The first ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen (PaO2/FiO2) upon meeting ALI/ARDS criteria was available for all 219 patients with ALI/ARDS. The Lung Injury Score (LIS) was available for 149 patients.
Statistical analyses
The cumulative incidence of ALI/ARDS was determined for the entire cohort and the chi-squared test was used to test the hypothesis that there is a difference in the incidence of ALI/ARDS by race. One-way analysis of variance was used to compare the PaO2/FiO2 and lung injury score by race to test the hypothesis that there is a difference in the severity of lung injury by race. The chi-squared test was used to test the hypothesis that there is a difference in mortality by race among patients who developed ALI/ARDS. STATA/SE 10.1 (College Station, TX) was used for all statistical analyses, which were reviewed by a biostatistician.
Multivariable logistic regression was done to determine whether race was a predictor for the development of ALI/ARDS while controlling for confounding variables. First, bivariate analyses of the following eleven predictors were done: age, gender, race, Injury Severity Score, number of units of PRBCs transfused within the first 24 hours of admission, penetrating mechanism of injury, presence of chest trauma, presence of shock upon presentation to the ED, and the first heart rate, respiratory rate, and Glasgow coma score in the ED. All variables considered clinically important on an a priori basis and those variables with p<0.2 in bivariate analyses were included in the multivariable logistic regression model. The likelihood ratio test was used to compare nested models for groups of nominal categorical variables. The final model was evaluated using the Hosmer-Lemeshow goodness of fit test and the linktest.
Results
We found significant racial differences in the mechanism of injury (penetrating versus blunt), presence of shock upon presentation to the ED, number of units of PRBCs transfused, and demographics (Table I). Approximately half of the African-American patients and a quarter of the Hispanic patients presented with penetrating trauma. In addition, African-American patients were most likely to present with shock and to receive a massive transfusion compared to patients of other races. Furthermore, African-Americans and Hispanics were younger and more likely to be male than Caucasians or Asian/Pacific Islanders. Among those who developed ALI/ARDS the mean number of units of PRBCs transfused within the first 24 hours of admission was 16.5 for African-Americans, 14.5 for Hispanics, 9.9 for Asian/Pacific Islanders, and 7.2 for Caucasians (p=0.007).
Table I.
Baseline Characteristics of the Entire Trauma Cohort by Race
| Characteristic | Caucasian (n=1831) | African American (n=871) | Hispanic (n=886) | Asian/Pacific Islander (n=809) |
|---|---|---|---|---|
| Age, in years | 47 ± 201 | 36 ± 16 | 37 ± 17 | 52 ± 23 |
| Male | 74% | 86% | 85% | 61% |
| Massive Transfusion2 | 6% | 14% | 9% | 9% |
| Number of units of PRBCs transfused3 | 2.2 ± 4.9 | 4.5 ± 10.0 | 3.2 ± 8.4 | 2.9 ± 6.4 |
| Penetrating Mechanism of Injury | 8% | 48% | 25% | 10% |
| Presence of Chest Trauma | 35% | 35% | 34% | 31% |
| Injury Severity Score | 22 ± 13 | 20 ± 13 | 20 ± 13 | 23 ± 13 |
| Shock | 11% | 18% | 13% | 11% |
| Heart Rate | 96 ± 26 | 97 ± 29 | 96 ± 24 | 93 ± 24 |
| Respiratory Rate | 18 ± 8 | 18 ± 8 | 18 ± 8 | 18 ± 8 |
| Glasgow Coma Score | 11.8 ± 5 | 12.5 ± 4 | 11.9 ± 4 | 11.7 ± 5 |
data are shown as means ± SD or percentages
≥10 units of packed red blood cells transfused within the first 24 hrs of admission
total number of units of packed red blood cells transfused within the first 24 hrs of admission
The overall incidence of ALI/ARDS was 5% and was similar by race (Figure II). Caucasian patients were used as the reference group in the multivariable logistic regression and their incidence of ALI/ARDS was 4.8%. To estimate the upper limit of incidence of ALI/ARDS by race, we used the upper limit of the 95% confidence interval (CI) of the odds ratio for each race. Among African-Americans, this was 1.2. Therefore, at most, the risk of developing ALI/ARDS in African-Americans is 1.2 times (20%) greater than in Caucasians. However, since the incidence of ALI/ARDS is only 4.8% in Caucasians, a 20% greater incidence is equal to 5.8%, a clinically insignificant absolute difference. This same principle holds true for Hispanics: 1.5 times (50%) greater incidence, 7.2% incidence of ALI/ARDS and Asian/Pacific Islanders: 1.6 times (60%) greater incidence, 7.7% incidence of ALI/ARDS. Furthermore, the 95% confidence interval for each race included the value 1, which indicates no difference when compared to the reference group.
Figure II.
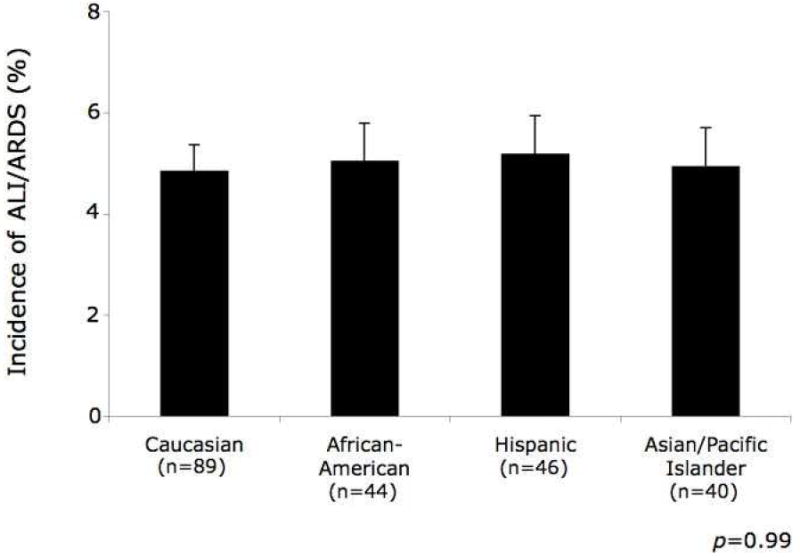
Incidence of acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) by race. The bars represent the proportion of patients within each race who developed ALI/ARDS and the error bars represent the standard error surrounding the point estimate of the incidence.
Among patients who developed ALI/ARDS, there was no evidence to support a difference in the severity of lung injury (Table II) or mortality by race (Figure III). For African-Americans, the odds of death were 0.8 times that of Caucasians (95% CI 0.3-2.0). For Hispanics, the odds of death were 1.3 times that of Caucasians (95% CI 0.6-2.9). For Asians/Pacific Islanders, the odds of death were 0.8 times that of Caucasians (95% CI 0.3-2.0).
Table II.
Severity of Lung Injury by Race
| Clinical Criteria | Caucasian | African American | Hispanic | Asian/Pacific Islander | p value |
|---|---|---|---|---|---|
| Initial PaO2/FiO2, mean (95% CI) | 161 (148-175) | 155 (132-178) | 148 (126-171) | 140 (116-164) | 0.33 |
| Lung Injury Score, mean (95% CI) | 2.5 (2.3-2.6) | 2.6 (2.3-2.8) | 2.6 (2.3-2.9) | 2.6 (2.3-2.8) | 0.67 |
Figure III.
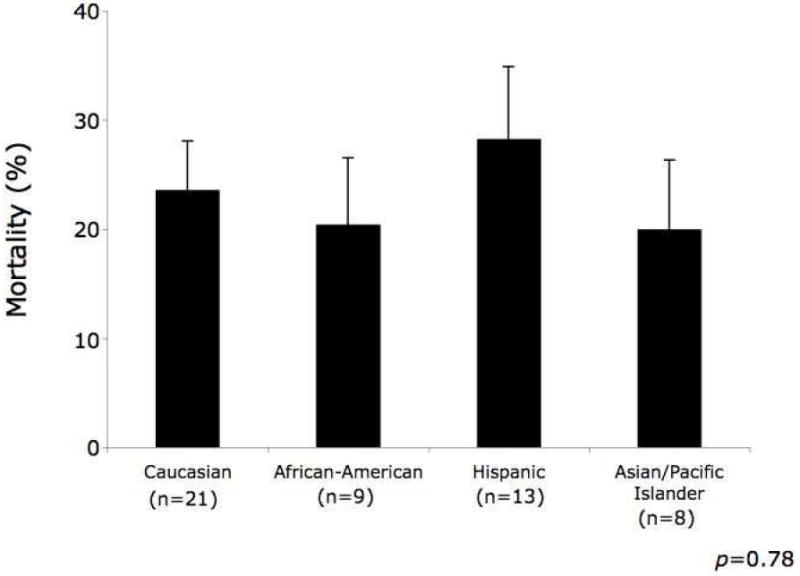
Mortality among patients who developed acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) by race. The bars represent the proportion of patients within each race who died and the error bars represent the standard error surrounding the point estimate of mortality.
The independent predictors of developing ALI/ARDS included Injury Severity Score ≥15, transfusion of ≥3 units of PRBCs within the first 24 hours of admission, chest trauma, presence of shock upon presentation to the ED, and the first heart rate and respiratory rate in the ED (Table III).
Table III.
Multivariable Analysis: Predictors of Developing Acute Lung Injury and Acute Respiratory Distress Syndrome
| Predictor | N (%) | Adjusted OR | p value | 95% CI |
|---|---|---|---|---|
| Race | ||||
| Caucasian | 1,831 (42) | Reference | ||
| African-American | 871 (20) | 0.8 | 0.3 | (0.5-1.2) |
| Hispanic | 886 (20) | 1.0 | 0.9 | (0.6-1.5) |
| Asian/Pacific Islander | 809 (18) | 1.0 | 0.9 | (0.7-1.6) |
| Age4 | 4,397 (100) | 0.9 | 0.09 | (0.9-1.0) |
| Male | 3,337 (76) | 1.2 | 0.3 | (0.8-1.8) |
| Injury Severity Score | ||||
| <15 | 1,334 (30) | Reference | ||
| 15-25 | 1,562 (36) | 2.4 | 0.007 | (1.3-4.4) |
| >25 | 1,501 (34) | 3.8 | <0.001 | (2.1-7.1) |
| Number of units of PRBCs | ||||
| 0 | 2,651 (60) | Reference | ||
| 1-2 | 540 (12) | 1.4 | 0.3 | (0.8-2.4) |
| 3-9 | 811 (18) | 3.2 | <0.001 | (2.1-4.8) |
| ≥10 | 388 (9) | 6.5 | <0.001 | (4.2-10.1) |
| Presence of Chest Trauma | 1,498 (34) | 2.9 | <0.001 | (2.1-4.1) |
| Penetrating Mechanism of Injury | 866 (20) | 0.8 | 0.2 | (0.5-1.2) |
| Shock5 | 561 (13) | 1.5 | 0.03 | (1.1-2.1) |
| Heart Rate6 | 4,376 (99) | 1.2 | 0.004 | (1.1-1.3) |
| Respiratory Rate7 | 4,388 (99) | 1.2 | 0.03 | (1.0-1.4) |
per 10 year increase in age
defined as systolic blood pressure ≤90 mmHg
per 20 beats/minute increase in heart rate
per 10 breaths/minute increase in respiratory rate
Discussion
Despite significant differences in baseline characteristics by race, namely age, mechanism of injury (penetrating versus blunt), and in particular shock upon presentation to the ED and the number of units of PRBCs transfused, the incidence of ALI/ARDS was similar by race. In this study as well as in prior trauma studies, shock (10) and transfusion of PRBCs (10, 11) are independent predictors for developing ALI/ARDS. Furthermore, the risk of developing ALI/ARDS increases as the number of units of PRBCs transfused increases (11). Therefore, we expected that African-American patients would be more likely to develop ALI/ARDS.
One possible explanation for the similar incidence of ALI/ARDS by race is that the risk factors for developing ALI/ARDS were balanced between different races. For example, although African-American patients received the most units of PRBCs, they were also younger and therefore less likely to have co-morbidities. Co-morbidities may place patients at higher risk of developing ALI/ARDS (3). Similarly, although Caucasian and Asian/Pacific Islander patients were older and therefore more likely to have co-morbidities they were less likely to present to the ED in shock and received fewer units of PRBCs. In addition to increasing co-morbidities with age, age itself is an independent risk factor for ALI/ARDS (12). Our analyses are limited by not having the Acute Physiology and Chronic Health Evaluation II (APACHE II) scores or co-morbidity data for each patient, which would have allowed us to control for these potential confounding factors.
Although our findings suggest that race does not contribute to the development of ALI/ARDS, it is well known that the etiology of ALI/ARDS is multigenic and multifactorial (13). Recently, candidate genes for ALI/ARDS have been identified, replicated in independent populations, and clustered according to specific pathways involved in the development of ALI/ARDS (13). The next step is to do genome wide association studies in which large numbers of single nucleotide polymorphisms (SNPs) are examined to detect associations between ALI/ARDS and known and previously unsuspected genes (14).
Our investigation differs from prior studies of racial differences in ALI/ARDS in two important ways. First, other investigators focused on differences in mortality by starting with a cohort in which every patient had ALI/ARDS (3, 4). Therefore, these studies could not determine if there was a difference in the incidence of ALI/ARDS by race. In our study, by examining a cohort of 4,397 trauma patients, we determined that the incidence of ALI/ARDS was similar by race. Second, prior studies included heterogeneous samples of patients with ALI/ARDS due to a variety of causes (3, 4). Trauma patients with ALI/ARDS may differ from patients with ALI/ARDS secondary to other causes (5, 6). For example, patients with ALI/ARDS due to trauma have lower mortality rates than patients with ALI/ARDS due to sepsis (6, 15). In addition, the development of ALI/ARDS does not further increase the risk of death in trauma patients (16, 17). Moreover, studies have shown that there is less epithelial and endothelial cell injury, the hallmark of ALI/ARDS, in trauma patients with ALI/ARDS than in patients with ALI/ARDS from other causes (6, 15, 18). Our findings provide further evidence suggesting that trauma patients with ALI/ARDS may be fundamentally different than non-trauma patients with ALI/ARDS. Considering this mounting evidence, perhaps clinical studies of patients with ALI/ARDS should consider only enrolling subgroups of patients with one cause of ALI/ARDS rather than enrolling such a heterogeneous patient sample. Conceivably, certain therapies for ALI/ARDS may be more effective for some subgroups of patients with ALI/ARDS than others.
Even within our sample of trauma patients with early ALI/ARDS secondary to major trauma, there may be heterogeneity. Early ALI/ARDS secondary to major trauma may differ in presentation, disease course and outcome depending on the nature, severity, and distribution of injuries. The most important distinction is direct lung injury from chest trauma versus indirect injury from abdominal trauma or extensive orthopedic fractures. Traumatic injury may be isolated to one body region, but more commonly, patients with severe trauma who are at greatest risk of developing ALI/ARDS present with injuries to multiple body regions. In some patients, the clinical picture may be further complicated by severe neurologic injury and neurogenic pulmonary edema.
In addition to major trauma, blood transfusions are another cause of ALI/ARDS in trauma patients. Blood transfusion is an independent risk factor for ALI/ARDS and can also lead to transfusion-related acute lung injury (TRALI). TRALI is defined as non-cardiogenic pulmonary edema associated with the transfusion of blood products (19). According to the two-hit hypothesis of TRALI, trauma is recognized as a potential “first hit” as neutrophils are primed and adhere to the pulmonary endothelium. Transfusion of blood products delivers the “second hit” by activating the primed neutrophils, leading to TRALI (19). TRALI is under diagnosed (20) because diagnostic confirmation requires examining the donor and recipient for passively transfused antibodies (19) which is not typically done. At our institution, only a few cases of TRALI have been reported to the blood bank over the 11-year period of this study. Therefore, we could not determine whether any patients in our cohort developed TRALI. Furthermore, some trauma patients with hemorrhagic shock receive numerous units of blood products and it would be difficult to differentiate trauma-related ALI/ARDS from TRALI in these patients.
The incidence of ALI/ARDS in our cohort was relatively low compared to some studies of trauma patients with an ICU admission (11, 16, 17) but was consistent with one study (10). The incidence of ALI/ARDS cited in clinical studies varies according to the cohort under investigation. For example, one study only included intubated patients whose Injury Severity Score was ≥16; the incidence of ARDS in this cohort was 34% (11). Our cohort included all trauma patients who spent a portion of their hospitalization in the ICU soon after presentation to the ED. Therefore, we included patients with a greater range of injury severity scores; who may or may not have required mechanical ventilation. Furthermore, in our study, a single investigator identified patients with ALI/ARDS prospectively over the entire 11-year period. This investigator excluded patients who may have originally met the American-European Consensus Conference criteria for ALI/ARDS if a follow-up chest radiograph within the next 24 hours no longer showed bilateral infiltrates or if the PaO2/FiO2 increased to >300. Even if the incidence of ALI/ARDS were underestimated in our cohort, it would not affect our ability to determine whether the incidence of ALI/ARDS differs by race because cases of ALI/ARDS would be similarly underestimated within each race.
There are some limitations to our study. First, although this cohort included patients over an 11-year period, it was done at only one major trauma center. This may limit the external validity of our findings. However, a strength of our single center study is that San Francisco General Hospital, the only designated level I trauma center in the city of San Francisco, has the ideal population for this type of study given the racial and ethnic diversity of its patients. Each of the major races was well represented in this study. Second, among the 219 patients who developed ALI/ARDS only 23% died. This small number of patients limits our ability to determine the predictors of mortality in this group because we would be at risk of committing a type II error.
Conclusions
In conclusion, despite racial differences in the mechanism of injury, shock upon presentation to the ED, and the number of units of PRBCs transfused within the first 24 hours of admission, the incidence of ALI/ARDS was similar by race in this trauma cohort. Furthermore, among patients who developed ALI/ARDS, there was no evidence to support a difference in the severity of lung injury or mortality by race.
Acknowledgments
Financial Support: Lisa M Brown, MD supported by NIH T32 GM008258-21
Michael A. Matthay, MD supported by HL51856
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ergul A. Hypertension in black patients: an emerging role of the endothelin system in salt-sensitive hypertension. Hypertension. 2000;36(1):62–7. doi: 10.1161/01.hyp.36.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson SE, Shlipak MG, Martin GS, et al. Racial and ethnic disparities in mortality from acute lung injury. Crit Care Med. 2009;37(1):1–6. doi: 10.1097/CCM.0b013e31819292ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979- 1996) Crit Care Med. 2002;30(8):1679–85. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Dicker RA, Morabito DJ, Pittet JF, Campbell AR, Mackersie RC. Acute respiratory distress syndrome criteria in trauma patients: why the definitions do not work. J Trauma. 2004;57(3):522–6. doi: 10.1097/01.ta.0000135749.64867.06. discussion 526-8. [DOI] [PubMed] [Google Scholar]
- 6.Calfee CS, Eisner MD, Ware LB, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35(10):2243–50. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 8.Blaisdell FW, Schlobohm RM. The respiratory distress syndrome: a review. Surgery. 1973;74(2):251–62. [PubMed] [Google Scholar]
- 9.Gomez AC. Pulmonary insufficiency in nonthoracic trauma. J Trauma. 1968;8(5):656–86. doi: 10.1097/00005373-196809000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Miller PR, Croce MA, Kilgo PD, Scott J, Fabian TC. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. Am Surg. 2002;68(10):845–50. discussion 850-1. [PubMed] [Google Scholar]
- 11.Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma. 2005;59(3):717–23. [PubMed] [Google Scholar]
- 12.Johnston CJ, Rubenfeld GD, Hudson LD. Effect of age on the development of ARDS in trauma patients. Chest. 2003;124(2):653–9. doi: 10.1378/chest.124.2.653. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Barnes KC. Recent advances in genetic predisposition to clinical acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296(5):L713–25. doi: 10.1152/ajplung.90269.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong MN. Gene association studies in acute lung injury: replication and future direction. Am J Physiol Lung Cell Mol Physiol. 2009;296(5):L711–2. doi: 10.1152/ajplung.00080.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisner MD, Thompson T, Hudson LD, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231–6. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 16.Treggiari MM, Hudson LD, Martin DP, Weiss NS, Caldwell E, Rubenfeld G. Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med. 2004;32(2):327–31. doi: 10.1097/01.CCM.0000108870.09693.42. [DOI] [PubMed] [Google Scholar]
- 17.Salim A, Martin M, Constantinou C, et al. Acute respiratory distress syndrome in the trauma intensive care unit: Morbid but not mortal. Arch Surg. 2006;141(7):655–8. doi: 10.1001/archsurg.141.7.655. [DOI] [PubMed] [Google Scholar]
- 18.Moss M, Gillespie MK, Ackerson L, Moore FA, Moore EE, Parsons PE. Endothelial cell activity varies in patients at risk for the adult respiratory distress syndrome. Crit Care Med. 1996;24(11):1782–6. doi: 10.1097/00003246-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Looney MR, Gropper MA, Matthay MA. Transfusion-related acute lung injury: a review. Chest. 2004;126(1):249–58. doi: 10.1378/chest.126.1.249. [DOI] [PubMed] [Google Scholar]
- 20.Kopko PM, Marshall CS, MacKenzie MR, Holland PV, Popovsky MA. Transfusion-related acute lung injury: report of a clinical look-back investigation. JAMA. 2002;287(15):1968–71. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]


