Abstract
Aim
To examine variation in positive and negative subjective effects to alcohol, tobacco, and marijuana and covariation between these three drugs and each effect.
Design
Retrospective self-reports of subjective effects were collected to estimate the genetic and environmental influences and the extent of their specificity across three drugs.
Participants
Data were drawn from 1299 adolescent and young adult same- and opposite sex twin- and sibling-pairs participating in the Colorado Center for Antisocial Drug Dependence (CADD).
Measurement
Subjective effects were assessed using a 13-item questionnaire that included positive and negative responses to alcohol, tobacco, and marijuana.
Findings
Heritable influences contributed moderately (additive genetic effects 17% to 56%) to positive and negative subjective effects to all three drugs and did not differ for males and females. Genetic and environmental contributions to positive and negative subjective effects are largely non-overlapping for tobacco and marijuana. Multivariate genetic modeling indicated subjective effects to alcohol, tobacco, and marijuana share a common, heritable etiology and that drug-specific genetic influences were an important contributor to individual differences in drug response.
Conclusions
Results from our genetic analyses suggest that subjective effects to these commonly used and misused drugs are heritable and that the genetic and environmental influences on effects to one drug also influence subjective effects to other drugs.
Keywords: Subjective effects, genetics, alcohol, tobacco, marijuana
Psychoactive drug use begins early in adolescence and, for many people, continues into adulthood. A common observation in both epidemiological and clinical samples during these ages has been the subsequent use of multiple drugs following initiation of one drug [1-3]. The most commonly used psychoactive drugs includes alcohol, tobacco, and marijuana, with estimated prevalence rates of 91.6%, 73.6%, and 42.4%, respectively, for lifetime use [4]. Among users of these psychoactive drugs, few users limit their involvement to a single substance, and many use a different combination of drugs, often leading to more problematic involvement with one or both drugs [5-8]. As the same neural systems are impacted by different psychoactive substances [9], how an individual responds to one drug may be indicative of how they will respond to other drugs. An important issue in the etiology of use and problem use is whether responses to drugs of different pharmacological classes have a distinct or shared etiology.
Responses or subjective effects [SE] to psychoactive drug use are commonly thought to reflect the physiological and pharmacological actions of a substance. Subjective effects can generally be grouped into pleasant or positive and adverse or negative categories. These often weakly correlated response types have been examined in studies of tobacco, marijuana, and alcohol in adolescent and adult samples [10-15]. Prior studies of SE have typically been substance-specific and examined in the context of the varying patterns of substance-specific use. In general, positive effects to a particular drug are predictive of a greater and more problematic use of the same drug while more mixed findings have emerged for negative effects [15-22]. A few studies have examined cross drug associations, finding that positive SE for one substance predict problematic use of another substance in the same or different pharmacological class [13, 14, 20, 23].
There is relatively little known regarding the genetic and environmental influences on SE to substances. To our knowledge, four studies of SE conducted using a genetically informative design have been reported. Two of these studies have been conducted in samples of adolescents using the children-of-twins [14] and discordant twin study [24] designs. While both designs are powerful methods to test environmental influences, they control for genetic effects and thus provide little information about the magnitude or nature of genetic influences. Of the remaining two studies, one examined SE to marijuana use in an adult sample of twins while the other compared the level of response to alcohol among siblings that differed in their degree of relatedness [25, 26]. Although both studies suggested a moderate heritable influence on SE, additional studies are needed to understand the nature and magnitude of genetic and environmental influences on SE to substance use. Moreover, given the within- and cross-drug relationships between positive and negative SE and patterns of use, the specificity of genetic and environmental influences on subjective responses remains unclear.
In the current report we examine, in both male and female same- and opposite-sex twin and full-sibling pairs, the interrelationship of genetic and environmental risk factors on SE to two licit (alcohol, tobacco) drugs and one illicit (marijuana) drug. We seek to address two sets of questions. First, what are the magnitudes of the genetic and environmental influences on SE, do they differ between the sexes, and are they the same or different for positive and negative responses? Second, to what extent are the genetic and environmental influences on SE across drugs of the same and different pharmacological class common or drug-specific?
Methods
Subjects
Subjects were participants in the Colorado Center on Antisocial Drug Dependence (CADD), a large collaborative study that includes family, twin, adoption, and clinical samples. Community samples were drawn from the Colorado Twin Registry (CTR) [27], the Colorado Adoption Project (CAP) [28], and the Colorado Adolescent Substance Abuse Family Study (ASA) [29]. Following the work by Zeiger et al [30] we combined this community sample with two clinical samples drawn from adolescents in treatment for substance abuse and delinquency, recruited as part of the ASA, and additional adolescents who had been convicted and placed on probation (e.g. adjudicated) in the Denver metropolitan area [31].
The total available sample size for which we had data on multiple pairs of siblings within a family was 1299. Sixty-one percent were drawn from our community based studies. In order to examine potential sex differences, we included same- and opposite-sex siblings. A total of 310 same-sex monozygotic twins (MZ; M: 152, F: 158), 188 same-sex dizygotic twins (DZ; M: 95, F: 93), and 430 same-sex full-siblings (FS; M: 336, F: 94) were examined. Our sample of opposite-sex siblings included 88 DZ twins and 283 full-siblings. The majority of participants self-reported either White (77.0%) or Hispanic (17.0%) ethnicity and were an average of 20.6 (± 3.8 years) at the time of assessment. Females accounted for 55.2% of our sample.
Assessment
Retrospectively reported subjective effects to tobacco were collected only if participants used every day for 30 days. For alcohol and marijuana, subjective responses were collected from those who reported using these drugs six or more times. Subjective effects were collected using a questionnaire developed by Lyons and colleagues [25]. For each drug, participants were asked “in the period shortly after you used [substance name], did it make you feel [subjective effect]”.
Based on confirmatory factor analyses and Mokken Scale Analysis (MSA) [32] in a non-genetic sample of respondents from our community and clinical studies [30], the current study examines 13 of the 23 original items on the Lyons questionnaire. These 13 items retained the original dimensionality reported previously [25], with average H coefficients of 0.45, 0.48, and 0.43 for the positive effects scales and 0.42, 0.52, and 0.54, for the negative effects of alcohol, tobacco, respectively. Estimates of KR20 were 0.69, 0.58, 0.65 for the positive effects and 0.75, 0.63, and 0.67 for the negative effects of alcohol, tobacco, and marijuana, respectively. The Mokken derived positive effects scale included the items: creative, euphoric, relaxed, increased sex drive, energetic, and sociable. Negative effects scales included the items: drowsy, unable to concentrate, dizzy, out of control, lazy and nauseous. Responses were scored as 0/1, with 1 indicating they had such an experience. Mean endorsement rates for these 13 subjective experiences were invariant to both age and clinical status [30]. Total scores on the positive and negative experiences scales could range between 0 and 6 and 0 and 5, respectively.
Statistical Analysis
Polychoric correlations for each SE scale were estimated taking into account the non-independence within our data using Mx [33]. We utilized a liability threshold model to estimate the genetic and environmental influence on each of the three derived SE scales. This model assumes an underlying normal liability, with the number of thresholds corresponding to the number of categories.
Univariate
The contribution of familial factors to observed variation in a trait is traditionally decomposed into genetic influences that include the summed influence of many genes acting additively (A) and the effects of two-alleles within the same gene interacting (D). Environmental influences are those shared by all siblings (C), environmental influences shared only by twins (T), and non-shared or individual specific (E). The magnitude of genetic and environmental influences can be inferred initially by comparing the extent siblings of different genetic relatedness correlate, with genetic influences suggested when the MZ twin correlation exceeds that of the DZ twins or FS [34].
Models were to allow for the ordinal character of the positive and negative experience scales using the raw maximum-likelihood estimation option in Mx [33]. The significance of model parameters was evaluated by a comparison of the twice log-likelihood (-2LL) for full and restricted models, with the difference distributed as a chi-square distribution and the degrees of freedom being equal to the difference between the number of parameters estimated. A non-significant chi-square difference between two models indicates that the parameters dropped are not significantly different from zero. We used two fit indices to determine the best-fitting model: the Akaike Information Criteria (AIC) [35] and the Bayesian Information Criterion (BIC) [36]. Though we used BIC values in determining the best fitting model, both lower AIC and BIC values indicate a better fit to the data.
Multivariate
Based on evidence that positive and negative SE are weakly related [10-15] we fit a bivariate Cholesky Decomposition model [34] to test the extent that the genetic and environmental influences on positive SE to a drug overlap with those influences on negative SE to the same drug. From this model we obtained the genetic and environmental correlations.
To determine the structure of genetic and environmental influences on the covariation of effects to alcohol, tobacco, and marijuana we fit two models: independent pathways (Figure 1a) and common pathways (Figure 1b). Unlike univariate genetic analysis that examines the latent influences on variation in a single variable, these two models decompose the pattern of covariation among three or more variables into genetic and environmental influences that are common as well as unique to the different measures. The independent and common pathways models differ, however, in their hypothesis in that the common pathways model tests whether the covariation of positive and negative SE is due to a single latent or underlying sensitivity factor that is itself influenced by genes and environments. A more direct contribution of genes and environments to SE is hypothesized by the independent pathways model. As in our univariate models, raw ordinal data were fit with thresholds freely estimated. Our full model was refined by comparing the fit of a series of nested sub-models that equated particular parameters to zero.
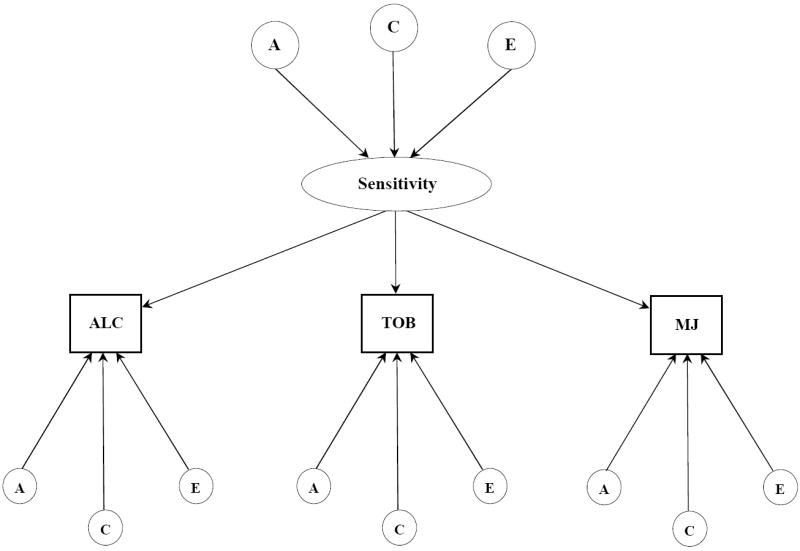
Figure 1.
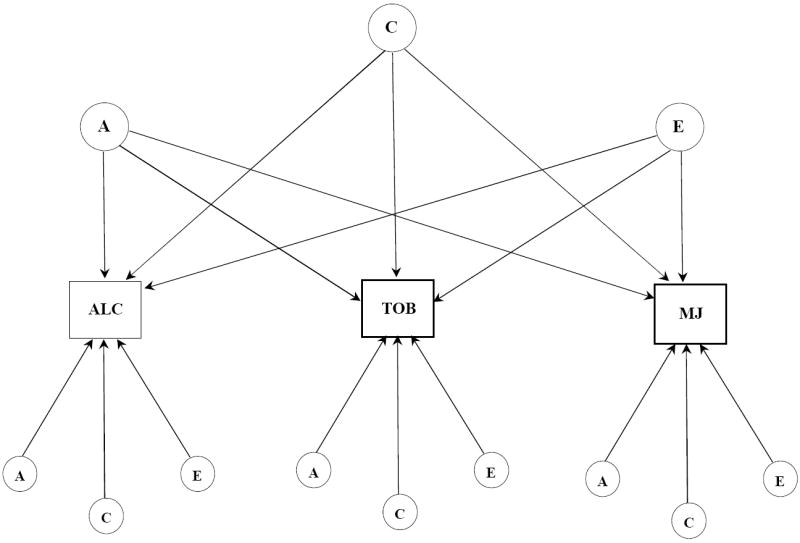
a. Independent pathway model for subjective effects to alcohol, tobacco, and marijuana. Latent variables are depicted in circles, observed variables in rectangles. Single headed arrows represent the partial regression of an observed variable on the latent factor. The variance for each observed variable was standardized to 1.0. A, indicates additive genetic influences; C, indicates shared environmental influences; E, indicates individual-specific environmental influences and includes measurement error.
b. Common pathway model for subjective effects to alcohol, tobacco, and marijuana. Latent variables are depicted in circles, observed variables in rectangles. Single headed arrows represent the partial regression of an observed variable on the latent factor. The variance for each observed variable was standardized to 1.0. A, indicates additive genetic influences; C, indicates shared environmental influences; E, indicates individual-specific environmental influences and includes measurement error.
Results
Sibling correlations were calculated for an initial characterization of the etiological influences on subjective effects (Supplementary Figures 1 and 2). In general, the MZ correlations are higher than the DZ and sibling correlations, suggesting genetic influences. The MZ twin correlations are relatively low, suggesting substantial non-shared environmental influences. The relatively similar same-sex MZ and DZ twin correlations for subjective effects to alcohol as compared with tobacco and marijuana suggest that twins may share more alcohol-specific environmental influences in common. Although, higher same-sex than opposite-sex twin and sibling correlations indicate that it is not likely that broad environmental influences such as neighborhood or familial setting would influence subjective effects. Non-additive genetic influences are suggested by the low to negative correlations for non-MZ siblings.
Univariate Models
For each drug, the fit of a full model that included A, C, and E influences that were estimated separately for each sex was compared to the fit of a model that include D instead of C influences. Sex-limited genetic influences (a′) and twin (T) environmental effects were also included. For both positive and negative effects across all three drugs, results indicated that there was not a statistically significant difference in model fit. Therefore, for each drug our baseline model included A, C, and E influences that were different for males and females, sex-limited genetic (a′), and twin environmental (T) effects. For both positive and negative effects, setting sex-limited genetic and twin environmental effects to zero and equating A, C, and E effects across sex did not result in a significant deterioration of model fit for any drug (Models 1-4, Supplementary Tables 1 and 2, respectively). Across both types of effects, models that equated either A or C influences to zero resulted in a further improvement in model fit, with models that equated C influences to zero offering the most parsimonious explanation of the data for tobacco and marijuana (Model 7). For positive subjective effects to alcohol, dropping A resulted in a significant deterioration in model fit, and judging by BIC values, an AE model (Model 7) offered the best fit to the data. An AE model of the negative effects to alcohol was also chosen as the best-fitting model. Parameter estimates from our baseline and best-fitting univariate models of positive and negative SE to alcohol, tobacco, and marijuana are shown in Tables 1 and 2, respectively.
Table 1.
Sources of Variance on POSITIVE Subjective Effects to Alcohol, Tobacco and Marijuana use, %, (95% CI).
| Model | A | C | E | T | |
|---|---|---|---|---|---|
| ALCOHOL | |||||
| Full | Males | 0.59 (0.32-0.69) | 0.00 (0.00-0.29) | 0.41 (0.31-0.55) | 0.00 (0.00-0.67) |
| Females | 0.40 (0.00-0.64) | 0.12 (0.00-0.49) | 0.48 (0.36-0.67) | ||
| Final | (Males and Females set equal) | 0.56 (0.56-0.64) | -- | 0.44 (0.36-0.54) | -- |
| TOBACCO | |||||
| Full | Males | 0.03 (0.00-0.34) | 0.00 (0.00-0.18) | 0.91 (0.65-0.99) | 0.06 (0.00-0.16) |
| Females | 0.15 (0.00-0.48) | 0.00 (0.00-0.33) | 0.79 (0.52-0.99) | ||
| Final | (Males and Females set equal) | 0.16 (0.01-0.31) | -- | 0.84 (0.69-0.99) | -- |
| MARIJUANA | |||||
| Full | Males | 0.25 (0.00-0.43) | 0.00 (0.00-0.00) | 0.75 (0.57-0.95) | 0.00 (0.00-0.17) |
| Females | 0.29 (0.00-0.51) | 0.00 (0.00-0.34) | 0.71 (0.49-0.99) | ||
| Final | (Males and Females set equal) | 0.27 (0.11-0.41) | -- | 0.73 (0.59-0.89) | -- |
Note: CI, confidence interval; A, additive genetic; D, non-additive genetic; C, shared environment, E: non-shared environment; T, twin influences.
Table 2.
Sources of Variance on NEGATIVE Subjective Effects to Alcohol, Tobacco and Marijuana use, %, (95% CI).
| Model | A | C | E | T | |
|---|---|---|---|---|---|
| ALCOHOL | |||||
| Full | Males | 0.24 (0.00-0.42) | 0.00 (0.00-0.22) | 0.73 (0.58-0.88) | 0.04 (0.00-0.20) |
| Females | 0.26 (0.00-0.48) | 0.03 (0.00-0.35) | 0.67 (0.52-0.84) | ||
| Final | (Males and Females set equal) | 0.31 (0.21-0.41) | -- | 0.69 (0.59-0.79) | -- |
| TOBACCO | |||||
| Full | Males | 0.07 (0.00-0.48) | 0.00 (0.00-0.30) | 0.76 (0.51-0.94) | 0.00 (0.00-0.28) |
| Females | 0.18 (0.00-0.55) | 0.00 (0.00-0.30) | 0.32 (0.55-0.99) | ||
| Final | (Males and Females set equal) | 0.26 (0.08-0.42) | -- | 0.74 (0.58-0.92) | -- |
| MARIJUANA | |||||
| Full | Males | 0.23 (0.00-0.41) | 0.00 (0.00-0.00) | 0.77 (0.59-0.98) | 0.00 (0.00-0.21) |
| Females | 0.27 (0.00-0.62) | 0.16 (0.00-0.48) | 0.77 (0.59-0.81) | ||
| Final | (Males and Females set equal) | 0.32 (0.17-0.46) | -- | 0.68 (0.54-0.73) | -- |
Note: CI, confidence interval; A, additive genetic; D, non-additive genetic; C, shared environment, E: non-shared environment; T, twin influences.
Multivariate Models
Our bivariate results suggested that positive and negative SE share few etiological influence in common (Supplementary Results). There fore, multivariate models included latent A and E factors and examined positive and negative SE separately. For positive SE, a comparison of the -2LL for the independent pathways and common pathways models revealed no significant differences (Δχ2 = 0.50, Δdf = 2, p = 0.77). This indicated that the covariation of positive SE to these drugs can be explained by a common underlying sensitivity. Tests of whether common A influences could be equated to zero resulted in a deterioration in model fit (Δχ2 = 9.68, Δdf = 1, p = 0.00, AIC = 7.68, BIC = -10567.97). Residual A influences on positive effects to these three drugs were also significant sources of variation (Δχ2 = 31.96, Δdf = 3, p = 0.00, AIC = 25.96, BIC = -10563.99) and could not be dropped.
The best-fitting model of positive subjective effects (Figure 2) indicated that similar effects across three different drugs were due to an underlying phenotype that was moderately heritable. Factor loadings of this latent sensitivity phenotype on each of the three drugs were largest for alcohol and moderate for tobacco and marijuana. Residual A and E influences, in general, were small to moderate, though positive effects to tobacco evidenced strong individual-specific environmental effects. Though such influences also contain measurement error, this result suggested the possibility that positive SE to tobacco are highly influenced by the context in which the drug was taken. The total heritable contributions for alcohol [(0.52)2 · (0.77)2 + (0.39)2 = 0.31], tobacco [(0.52)2 · (0.48)2 + (0.37)2 = 0.27], and marijuana [(0.52)2 · (0.55)2 + (0.46)2 = 0.29] were similar to those obtained from our univariate analyses which indicated that our model had sufficient power.
Figure 2.
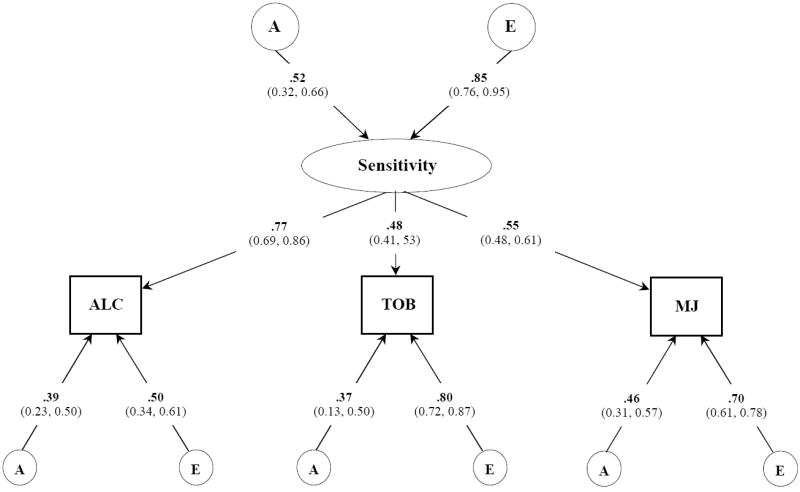
Best fitting common pathway model for POSITIVE subjective effects to alcohol, tobacco, and marijuana. Variance component estimates (95% confidence intervals) are shown for the latent common factor and unstandardized path coefficients for each observed variable. A, indicates additive genetic influences; E, indicates non-additive genetic influences and includes measurement error;
For negative SE, we did not detect a significant difference between the fit of the independent pathways model and the common pathways model (Δχ2 = 3.71, Δdf = 2, p = 0.15). This indicated that the covariation of negative effects across alcohol, tobacco, and marijuana was influenced by a similar underlying sensitivity to all three drugs. A significant reduction in model fit was observed when common A influences were equated to zero (Δχ2 = 10.35, Δdf = 1, p = 0.01, AIC = 8.35, BIC = -11657.82). Similarly, tests of the residual A influences suggested that they were an important source of variation (Δχ2 = 20.57, Δdf = 3, p = 0.00, AIC = 14.57, BIC = 11659.89) and could not be dropped.
Figure 3 describes the best-fitting model of the genetic and environmental contributions to the covariation of negative SE to three drugs. Unstandardized parameter estimates are presented. As shown, this final model implicated a moderately heritable latent sensitivity factor that was best indexed by the effects to alcohol. Drug-specific variation in negative SE was more strongly influenced by individual-specific environmental experiences than by genetic contributions. In total, the heritable factors accounted for alcohol [(0.52)2 · (0.80)2 + (0.33)2 = 0.28], tobacco [(0.52)2 · (0.49)2 + (0.45)2 = 0.27], and marijuana [(0.52)2 · (0.60)2 + (0.41)2 = 0.27] were similar to those obtained from our univariate analyses.
Figure 3.
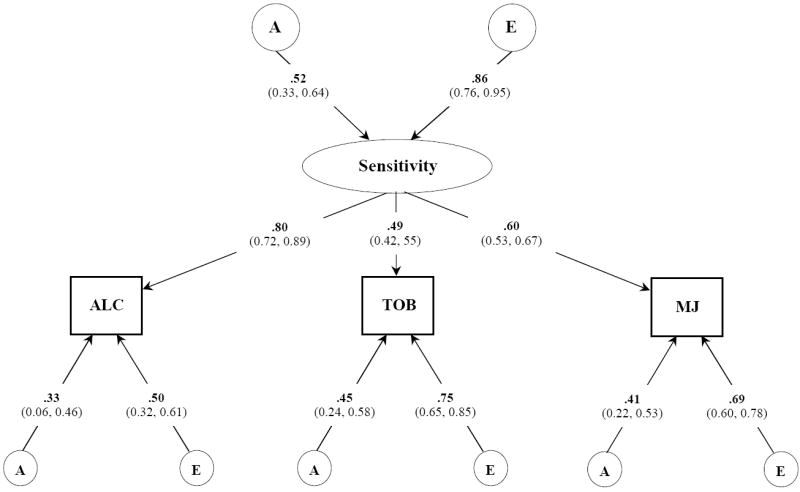
Best fitting common pathway model for NEGATIVE subjective effects to alcohol, tobacco, and marijuana. Variance component estimates (95% confidence intervals) are shown for the latent common factor and unstandardized path coefficients for each observed variable. A, indicates additive genetic influences; E, indicates non-additive genetic influences and includes measurement error;
Discussion
The current report details findings from a genetically informative study of subjective effects in a sample of adolescents and young adults. We showed that heritable and individual-specific environmental influences were important contributors to observed variation in the effects to three drugs. Moreover, we found little evidence for sex differences in these latent influences or that the genetic and environmental influences were similar for positive and negative effects. Lastly, our results suggest that there are shared or common sensitivity factor that underlie similar subjective effects to alcohol, tobacco, and marijuana.
Etiology of subjective effects
Heritable and environmental influences on a variety of drug use behaviors have been established. In general, environmental influences that are shared have greater influence on the initiation of substance use than on chronic and disinhibited patterns of use for which genetic and individual-specific influences are greatest. As there have been few considerations of the role genetic and environmental effects have on how drugs are experienced, we sought to thoroughly characterize the extent and nature of these effects on the SE to alcohol, tobacco, and marijuana. By comparison with simpler models, we obtained estimates of the additive genetic and three types of environmental influences. Irrespective of drug class or type of effect, heritable factors contributed moderately to observed variation while individual-specific environmental experiences contributed strongly; replicating previous estimates [25, 26]. The absence of shared environmental influences is consistent with previous reports for marijuana [25] and alcohol [26] and further elaborates the type of environmental influences suggested by other genetically informative studies of SE [14, 24]. That the genetic and environmental influences are the same for males and females suggests that sex differences observed for other drug use behaviors such as PSU [2, 37-39] are not related to the SE following drug exposure.
Structure of genetic and environmental contributions to subjective effects
As most individuals endorse having experienced both positive and negative effects and drugs of different classes impact similar brain regions and structures, we examined the etiological influences on the covariation of SE within a drug and across different drugs. Understanding this has at least two potential benefits. First, given the differentially predictive relationships between positive and negative effects and the subsequent use and misuse of the same or other drugs, separate etiologies may help to refine or focus prevention and treatment approaches to PSU. Second, genetic approaches may potentially offer insight into whether a similar response to multiple drugs shares a common liability and why the effects to different drugs vary instead of being a consistent response.
For all but alcohol, the phenotypic relationship between positive and negative effects is typically low to near zero [10-15, 30]. This suggests that there are few if any overlapping or shared etiological influences. In these data, the covariation of positive and negative effects to tobacco and marijuana was solely due to similar individual-specific environmental experiences, which includes measurement error. A different picture emerged, however, for the effects of alcohol, where we observed substantial genetic and small environmental overlap. While this suggested that the environmental influences on the effects to alcohol are largely different, it also indicated that knowing the genetic influences on positive effects does not provide a complete picture of the genetic influences on negative effects. Further our results suggest that understanding the environmental contributions to negative effects may be informative for prevention and treatment programs as those efforts would also be expected to impact positive effects which are strongly predictive of problematic use.
Previous studies suggest that individual characteristics and experiences are important influences on a general vulnerability to licit and illicit drug use (40-45). Findings from those studies also highlight the influences of drug-specific genetic and individual-specific environmental factors. In these data, we found differences in these latent influences across substances, with the majority of genetic influences on alcohol coming from the common factor for both positive and negative effects. For positive effects to tobacco, we identified roughly equal contributions from common and drug-specific genetic factors. For negative effects to tobacco and both effects to marijuana, drug-specific heritable influences contributed more than genetic influences common to all three drugs. Taken together these patterns of influence have important implications for treatment and molecular genetic studies. First, though the subjective effects to tobacco and marijuana share heritable influences with alcohol, they are more genetically heterogeneous. This suggests that the effects of alcohol may not serve as a proxy for the genetic influences on the effects to the other two drugs. Similarly, individual-specific environments are also heterogeneous which suggests measured environmental influences may need to be examined both globally as well as in a more directed manner.
Limitations
Though the findings here are consistent with converging evidence from neurobiological, genetic, and pharmacological studies, a number of limitations should be considered. First, SE were collected retrospectively and among participants with different use histories. Differences in recall among siblings would introduce measurement error, thereby lowering our ability to detect genetic influences as well as increasing the overall proportion of variance due to individual-specific environments. The impact of expectancies, as related to the effects of one drug on the subsequent effects of other drugs, could also be a relevant contributor to increased sibling differences. Second, the SE to tobacco were collected only if participants used every day for 30 days, whereas for alcohol and marijuana subjective responses were collected from those who reported using these drugs six or more times. This may have added heterogeneity to our sample and potentially biased the extent of environmental influences. Third, we examined SE to different drugs individually and did not take into account the potential synergistic impact that different combinations may have on the effects of a single drug. Lastly, we could not control for between-subject differences in the quality of the drug used, depth of inhalation, and dosage [46-50]. Individual differences in these are particularly relevant to SE, though it is unclear how to account for their influences outside a controlled laboratory environment.
Supplementary Material
Acknowledgments
BCH, JSZ were supported by grant HD031921. RPC, CJH, MCS, SHR, JKH was supported by grant 2P60DZ011015. CJH was also supported by grants 1RO1DA021913, 5RO1DZ015522.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
Literature Cited
- 1.Palmer RHC, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, et al. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: evidence of generalized risk. Drug & Alcohol Dependence. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- 3.Duhig AM, Cavallo DA, McKee SA, George TP, Krishnan-Sarin S. Daily patterns of alcohol, cigarette, and marijuana use in adolescent smokers and nonsmokers. Addictive Behaviors. 2005;30:271–283. doi: 10.1016/j.addbeh.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLOS Medicine. 2009;5:1053–1067. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pape H, Rossow I, Storvoll EE. Under double influence: Assessment of simultaneous alcohol and cannabis use in general youth populations. Drug & Alcohol Dependence. 2009;101:69–73. doi: 10.1016/j.drugalcdep.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Timberlake DS, Haberstick BC, Hopfer CJ, Bricker J, Sakai JT, Lessem JM, Hewitt JK. Progression from marijuana use to daily smoking and nicotine dependence in a national sample of U.S. adolescents. Drug & Alcohol Dependence. 2007;11:272–281. doi: 10.1016/j.drugalcdep.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Lewinsohn PM, Rohde P, Brown RA. Level of current and past adolescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction. 1999;94:913–921. doi: 10.1046/j.1360-0443.1999.94691313.x. [DOI] [PubMed] [Google Scholar]
- 8.Peters EN, Hughes JR. Daily marijuana users with past alcohol problems increase alcohol consumption during marijuana abstinence. Drug & Alcohol Dependence. 2009 doi: 10.1016/j.drugalcdep.2009.07.027. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addition: From additions to habit to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 10.Perkins KA, Jetton C, Keenan J. Common factors across acute subjective effects of nicotine. Nicotine & Tobacco Research. 2003;6:869–875. doi: 10.1080/14622200310001614629. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez D, Audrian-McGovern J. Construct validity analysis of the early smoking experience questionnaire for adolescents. Addictive Behaviors. 2004;29:1053–1057. doi: 10.1016/j.addbeh.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- 13.Grant JD, Scherrer JF, Lyons MJ, Tsuang M, True WR, Bucholz KK. Subjective reactions to cocaine and marijuana are associated with abuse and dependence. Addictive Behaviors. 2005;30:1574–1586. doi: 10.1016/j.addbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Scherrer JF, Grant JD, Duncan AE, Sartor CE, Haber JR, Jacob T, et al. Subjective effects to cannabis are associated with use, abuse and dependence after adjusting for genetic and environmental influences. Drug & Alcohol Dependence. 2009;105:76–83. doi: 10.1016/j.drugalcdep.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson ES, Schenk S. Variability in subjective responses to marijuana: initial experiences of college students. Addictive Behaviors. 1994;19:531–538. doi: 10.1016/0306-4603(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 16.Le Strat Y, Ramoz N, Horwood J, Falissard B, Hassler C, Romo L, et al. First positive reactions to cannabis constitute a priority risk factor for cannabis dependence. Addiction. 2009;104:1710–1717. doi: 10.1111/j.1360-0443.2009.02680.x. [DOI] [PubMed] [Google Scholar]
- 17.Fergusson DM, Horwood LJ, Lynskey MT, Madden MAF. Early reactions to cannabis predict later dependence. Archives of General Psychiatry. 2003;60:1033–1039. doi: 10.1001/archpsyc.60.10.1033. [DOI] [PubMed] [Google Scholar]
- 18.Morean ME, Corbin WR. Subjective alcohol effects and drinking behavior: the relative influence of early response and acquired tolerance. Addictive Behaviors. 2008;33:1306–1313. doi: 10.1016/j.addbeh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Chung T, Martin CS. Subjective stimulant and sedative effects of alcohol during early drinking experiences predict alcohol involvement in treated adolescents. Journal of Studies on Alcohol & Drugs. 2009;70:660–667. doi: 10.15288/jsad.2009.70.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pomerleau CS, Marks JL, Pomerleau OF, Snedecor SM. Relationship between early experiences with tobacco and early experiences with alcohol. Addictive Behaviors. 2004;29:1245–1251. doi: 10.1016/j.addbeh.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Hu MC, Muthen B, Schaffran C, Griesler PC, Kandel DB. Developmental trajectories of criteria of nicotine dependence in adolescence. Drug & Alcohol Dependence. 2008;98:94–104. doi: 10.1016/j.drugalcdep.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Stacy A, Zheng H, Shan J, Spruijt-Metz D, Unger JB, et al. Sensations from initial exposure to nicotine predicting adolescent smoking in China: A potential measure of vulnerability to nicotine. Nicotine & Tobacco Research. 2003;5:455–463. doi: 10.1080/14622200307239. [DOI] [PubMed] [Google Scholar]
- 23.Perkins KA, Coddington SB, Karelitz JL, Jetton C, Scott JA, Wilson AS, et al. Variability in initial nicotine sensitivity due to sex, history of other drug use, and parental smoking. Drug & Alcohol Dependence. 2009;99:47–57. doi: 10.1016/j.drugalcdep.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomerleau OF, Pomerleau CS, Snedecor SM, Gaulrapp S, Kardia SLR. Heterogeneity in phenotypes based on smoking status in the Great Lakes Smoker Sibling Registry. Addictive Behaviors. 2004;30:607–611. doi: 10.1016/j.addbeh.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 25.Lyons MJ, Toomey R, Meyer JM, Green AI, Eisen SA, Goldberg J, et al. How do genes influence marijuana use? The role of subjective effects. Addiction. 1997;92:409–417. [PubMed] [Google Scholar]
- 26.Schuckit MA, Smith TL, Danko G, Kupermann S, Bierut LJ, Hesselbrock V. Correlations among first-degree relatives for responses on the Self-Rating of the Effects of Alcohol Questionnaire in teenagers. Journal of Studies on Alcohol & Drugs. 2005;66:62–65. doi: 10.15288/jsa.2005.66.62. [DOI] [PubMed] [Google Scholar]
- 27.Rhea SA, Gross AA, Haberstick BC, Corley RP. Colorado Twin Registry. Twin Research and Human Genetics. 2006;9:941–949. doi: 10.1375/183242706779462895. [DOI] [PubMed] [Google Scholar]
- 28.Petrill S, Plomin R, DeFries JC, Hewitt JK, editors. Nature, nurture, and the transition to adolescence. Oxford University Press; New York: 2003. [Google Scholar]
- 29.Stallings MC, Corley RP, Dennehey B, Hewitt JK, Krauter KS, Lessem JM, et al. A genome-wide search for quantitative trait loci that influence antisocial drug dependence in adolescence. Archives of General Psychiatry. 2005;62:1042–1051. doi: 10.1001/archpsyc.62.9.1042. [DOI] [PubMed] [Google Scholar]
- 30.Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, et al. Subjective responses to marijuana associated with marijuana use. doi: 10.1016/j.drugalcdep.2009.12.026. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartman CA, Gelhorn H, Crowley TJ, Sakai JT, Stallings MC, Young SE, et al. Item response theory analysis of DSM-IV cannabis abuse and dependence criteria in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:165–173. doi: 10.1097/chi.0b013e31815cd9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mokken RJ. Nonparametric models for dichotomous responses. In: van der Linden J, Hambleton, editors. Handbook of modern item response theory. New York: Springer; 1997. [Google Scholar]
- 33.Neale MC. Mx: Statistical Modeling. Box 126 MCV, Richmond, VA 23298: Department of Psychiatry; 2004. [Google Scholar]
- 34.Neale MC, Cardon LR. Methodology for Genetic Study of Twins and Families. Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 35.Akaike H. Factor analyses and AIC. Psychometrika. 1987;52:317–322. [Google Scholar]
- 36.Raftery AE. Bayesian model selection in social research. Sociological Methodology. 1995;25:111–163. [Google Scholar]
- 37.Hardie TL, Moss HB, Lynch KG. Sex differences in the heritability of alcohol problems. American Journal of Addiction. 2008;17:319–327. doi: 10.1080/10550490802139010. [DOI] [PubMed] [Google Scholar]
- 38.Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescents and young adult twins. Behavior Genetics. 1999;29:383–393. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- 39.Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- 40.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. American Journal of Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 41.Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Archives of General Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- 42.Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: general or specific? Behavior Genetics. 2006 doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 43.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, et al. Co-occurrence of abuse of different drugs in men. Archives of General Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 44.Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Baher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neuroscience and Biobehavioral Reviews. 2003;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Sarton CE, Agrawal A, Lynskey MT, Bucholz KK, Madden PAF, Health AC. Common genetic influences on the timing of first use for alcohol, cigarettes, and cannabis in young African-American women. Drug & Alcohol Dependence. 2009;102:49–55. doi: 10.1016/j.drugalcdep.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brady SS, Song AV, Halpern-Felsher BL. Adolescents report both positive and negative consequences of experimentation with cigarette use. Preventative Medicine. 2008;46:585–590. doi: 10.1016/j.ypmed.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacology, Biochemistry, and Behavior. 1989;34:173–179. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- 48.Ilan AB, Gevins A, Coleman M, El Shohly MA, de Wit H. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behavioral Pharmacology. 2005;16:487–496. doi: 10.1097/00008877-200509000-00023. [DOI] [PubMed] [Google Scholar]
- 49.Addicott MA, Marsh-Richard DM, Mathias CW, Dougherty DM. The biphasic effects of alcohol: comparisons of subjective and objective measures of stimulation, sedation, and physical activity. Alcoholism Clinical and Experimental Research. 2007;31:1883–1890. doi: 10.1111/j.1530-0277.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 50.Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/ heavy and light social drinkers. Alcoholism Clinical and Experimental Research. 2000;24:789–794. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


