Abstract
Rationale and Objectives
The relationship between chronic obstructive pulmonary disease (COPD) and atherosclerosis has been suggested; this association may relate to systemic inflammation and endothelial dysfunction, which can lead to alteration of small pulmonary vessels. The relationship between atherosclerosis and small pulmonary vessel alteration, however, has not been assessed in COPD patients. We tested the hypothesis that the severity of thoracic aortic calcification measured by computed tomography (CT) would be associated with the total cross-sectional area of small pulmonary vessels (CSA) on CT images.
Materials and Methods
The study was approved by the institutional review board and was HIPAA compliant. Informed consent was waived. For 51 COPD patients enrolled in the NHBLI Lung Tissue Research Consortium (LTRC), we calculated the percentage of total CSAs of less than 5 mm2 for the total lung area (%CSA<5). Thoracic aortic calcification, quantified by modified Agatston score, was measured. The correlations between thoracic aortic calcification score and %CSA<5, pulmonary function, and extent of emphysema were evaluated. Multiple linear regression analysis using aortic calcification score as the dependent outcome was also performed.
Results
The %CSA<5 had a significant negative correlation with the thoracic aortic calcification score (r=−0.566, p<0.0001). Multiple linear regression analysis showed significant correlation between the aortic calcification score and %CSA<5 (p<0.0001) independent of age, pack-years, extent of emphysema, and FEV1%.
Conclusions
Atherosclerosis, assessed by aortic calcification, is associated with the small pulmonary vascular alteration in COPD. Systemic inflammation and endothelial dysfunction may cause the close relationship between atherosclerosis and small pulmonary vessel alteration.
Keywords: chronic obstructive, systemic inflammation, pulmonary artery, atherosclerosis, computed tomography
Introduction
Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disorder (1–4) in which inflammation may be associated to the development of cardiovascular diseases (5,6). Although the underlying mechanisms remain unknown, cardiovascular disease contributes significantly to morbidity and mortality in COPD (7,8). Atherosclerosis is the principal cause of cardiovascular diseases including coronary heart disease, stroke, and peripheral vascular disease (9,10), and it is thought to be associated with systemic inflammation and endothelial dysfunction (11–14). Prior investigations suggested that systemic inflammation in COPD may promote atherosclerosis (12), and recent studies have reported relationships between atherosclerosis and COPD (15–20).
The development of arterial calcification is an active process seen at all stages of atherosclerotic plaque development, and is closely associated with vascular injury (21,22). Many researchers have reported the relationship between aortic calcification and an increased risk of cardiovascular events (23–26). Patients with calcification in the thoracic aorta have 3.8 times the relative risk for obstructive coronary artery disease independent of age (26). In addition, a recent study showed that the severity of thoracic aortic calcification measured by computed tomography (CT) strongly correlates with inflammatory markers such as interleukin-6 (27).
Small pulmonary vascular alteration is a characteristic feature of COPD. Recent studies suggest that both pulmonary and extra-pulmonary vascular alterations in COPD patients closely relate to systemic inflammation and endothelial dysfunction (15–19, 28–30). Previously we have demonstrated that the total cross-sectional area of small pulmonary vessels (CSA) can provide clinically relevant data regarding pulmonary vascular disease in COPD (31, 32). We sought to determine if this same CT based measure of pulmonary vascular disease is related to accepted measures of arteriosclerosis in COPD. The principal purpose of this study was to evaluate the relationship between the severity of thoracic aortic calcification and CSA, each measured on CT images. To our knowledge, the relationship between systemic and pulmonary vascular alteration has not been assessed in COPD patients. Further, although correlations between atherosclerosis and the extent of emphysema and airflow obstruction have been reported (15–20), a relationship with thoracic aortic calcification has not been assessed. Thus, we also evaluated those relationships.
Methods
Subjects
We retrospectively evaluated CT scans and clinical data collected as part of the National Heart, Lung, and Blood Institute (NHBLI) Lung Tissue Research Consortium (LTRC). Further information on LTRC is available on the website www.ltrcpublic.com. This retrospective study was approved by the Institutional Review Board at our institution, and performed in compliance with the Health Insurance Portability and Accountability Act guidelines. All subjects gave written informed consent. Subjects were evaluated for inclusion in our study if they had both a diagnosis of COPD (a ratio of postbronchodilator forced expiratory volume in one second to forced expiratory vital capacity, FEV1/FVC < 0.7) and high resolution CT scans available for quantitative analysis. The LTRC cohort has CT images scanned in two different tube current protocol, subjects with the protocol using an automated dose modulation were excluded. The subjects with chronic heart failure, diabetes mellitus, or chronic renal failure were excluded from this study. Additional exclusion criteria included 1) obvious abnormal lung parenchymal lesions other than emphysema on the CT images used for analysis; 2) other potentially confounding abnormalities including pneumothorax, pleural effusion, cardiomegaly, findings suggestive of suggesting cardiac failure, or postoperative status; 3) excessive image noise preventing image analysis.
All subjects underwent standardized spirometric lung function measurements according to American Thoracic Society (ATS) guidelines (33). Post-bronchodilator forced expiratory volume in one second (FEV1) and forced expiratory vital capacity (FVC) were recorded in liters and expressed as percentages of predictive values (FEV1% predicted). Single-breath diffusing capacity for carbon monoxide was also obtained and expressed as percentages of predictive values (DLco% predicted).
Multislice CT scanning
All subjects were scanned with 16-detector CT (Light Speed 16 or LightSpeed Pro16, GE Medical Systems, Milwaukee, WI) at full inspiration, without receiving contrast medium. Images were obtained using 140 kV and 300 mA. Acquisition time was 1 second or less and the matrix size was 512 × 512 pixels. Images were reconstructed with bone algorithm at a slice thickness of 1.25 mm and interval of 0.625 mm.
CT measurement of small pulmonary vessels
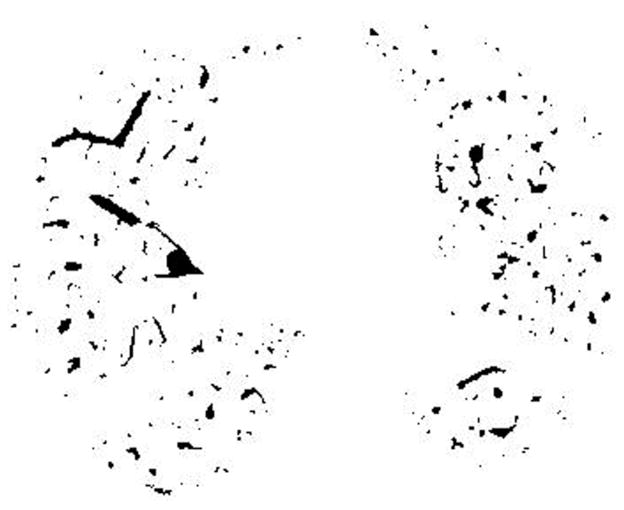
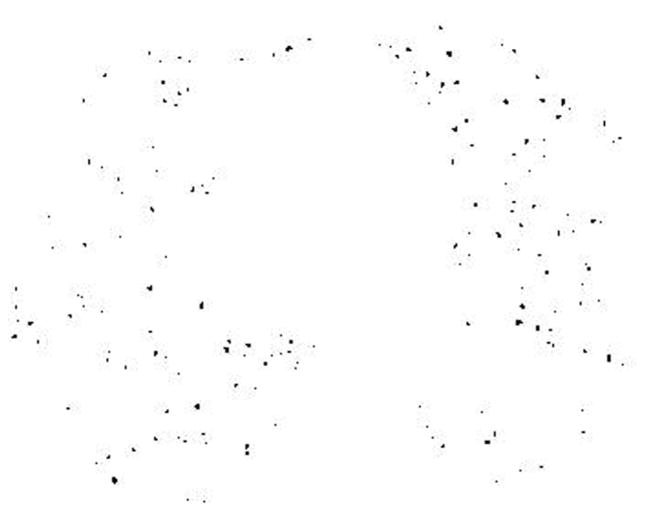
CT measurements of the pulmonary CSA has been described elsewhere (31,32). In brief, the following procedures were performed: First, the lung field was segmented using a threshold technique with all pixels between −500 and −1024 Hounsfield Units (HU) on each CT image (Figure 1A). Next, segmented images were converted into binary images with a window level of −720HU (Figure 1B). We measured CSA at sub-subsegmental level; the range of CSA of each vessel was defined as less than 5 mm2 at the sub-subsegmental level (34). After these settings, CSA of each vessel was calculated (Figure 1C). Finally, we totaled the CSA of vessels measured on each set of three CT slices, and those totals were abbreviated as follows: CSA<5 for the total cross sectional area of vessels that ranged less than 5mm2. The total area of the lung in the three selected slices was obtained using threshold values between −500HU and −1024HU, and the percentages of CSA<5 (%CSA<5) for the total area of the lung were calculated.
Figure 1.
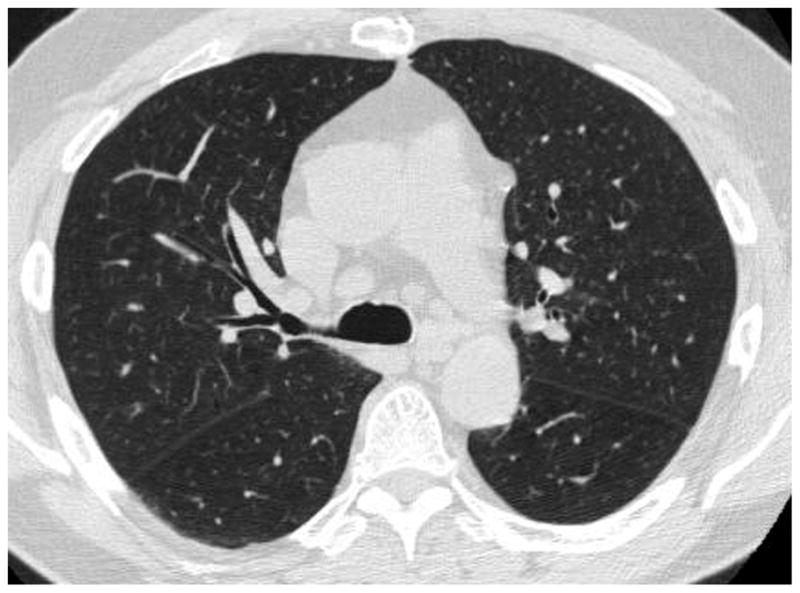
The method of measuring the cross sectional area of small pulmonary vessels using ImageJ software. (A) Computed tomography (CT) image of lung field segmented within the threshold values from −500 HU to −1024 HU, (B) Binary image converted from segmented image (A) with window level of −720 HU. Pulmonary vessels are displayed in black. (C) Mask image for particle analysis after setting vessel size parameters within 0 – 5 mm2, and the range of circularity within 0.9 – 1.0.
CT measurement of the thoracic aortic calcification
Reconstructed sagittal CT images were used for the assessment of thoracic aortic calcification. A calcified lesion in the thoracic aorta was defined as an area within an aortic wall with CT attenuation above a threshold of 90 HU (35). The thoracic aorta was defined as the region from the ascending aorta to the descending aorta at the level of the cardiac apex. Regions of interest around all lesions in each slice were placed by an experienced radiologist and were automatically analyzed by the OsiriX image processing software (Version 3.5.1; available at http://www.osirix-viewer.com). A modification of Agatston’s scoring method was applied, with a threshold of 90 HU instead of 130 HU (36, 37), and an attenuation factor for each lesion: 1 = 90 to 199 HU, 2 = 200 to 299 HU, 3 = 300 to 399 HU, and 4 = 400+ HU. An aortic calcification score for each region of interest was calculated automatically by multiplying the attenuation factor by the area. Calculated aortic calcification scores were adjusted for slice thickness as follows: original score × slice thickness/3.0 (38).
Statistical Analysis
For the main object of this study, the correlation between the total volume of thoracic aortic calcification and %CSA<5 was tested using Spearman’s rank correlation analysis. We also used Spearman correlation coefficients to analyze relationships between the total volume of thoracic aortic calcification and age, pack-years, body mass index (BMI), the extent of emphysema, as well as pulmonary function tests, including FEV1% predicted and DLco % predicted. The extent of emphysema was obtained by calculating the mean percentage of low attenuation values lower than −950 HU (%LAA-950) on CT images using ImageJ software (39).
Multiple linear regression analysis using the total volume of thoracic aortic calcification as the dependent outcome was also performed to evaluate the impact of measured CT values, lung function, pack-years, age, gender, and body mass index (BMI). Data were expressed as the mean ± standard deviation for all normally distributed variables. For all statistical analyses, the null hypothesis was rejected at the 5% level. All statistical analyses were performed using JMP 5.0 software (SAS Institute, Cary, NC).
Results
Subject characteristics, including the results of pulmonary function tests, are presented in Table 1. Seventy-nine patients were diagnosed with COPD and had CT data suitable for this study. Five patients were excluded due to having chronic heart failure, diabetes mellitus, or chronic renal failure. According to the CT exclusion criteria in this study, additional 23 subjects were excluded because of obvious abnormal lung parenchymal lesions other than emphysema on CT images that were used for analysis (n = 16), pneumothorax (n = 2), or image noise (n = 5). Thus, 51 subjects (mean age, 66 ± 9 years; range, 47 – 82 years; 22 women, 63 ± 9 years; 29 men, 68 ± 8 years) were included in this study.
Table 1.
Demographics, pulmonary function, and CT measurements (n=51)
| Mean | SD | Range | |
|---|---|---|---|
| Age (y) | 66 | 9 | 47 – 82 |
| Gender, % female (%) | 42.8 | ||
| BMI | 26.3 | 4.6 | 16.5 – 41.8 |
| Pack-Years | 48.0 | 30.8 | 1 – 128 |
| FEV1 (l) | 1.42 | 0.77 | 0.5 – 3.9 |
| FVC (l) | 3.0 | 1.1 | 0.9 – 5.8 |
| FEV1% predicted (%) | 50.4 | 23.7 | 16.0 – 93.0 |
| FEV1/FVC | 0.46 | 0.15 | 0.23 – 0.69 |
| DLco% predicted (%) | 54.4 | 21.9 | 22.0 – 98.0 |
| %CSA<5 (%) | 0.63 | 0.15 | 0.38 – 1.02 |
| %LAA-950 (%) | 20.9 | 15.1 | 0.4 – 47.9 |
Definition of abbreviations: SD = standard deviation; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; DLCO = diffusing capacity of the lung for carbon monoxide; BMI = body mass index; %CSA<5 = percentage of total lung area taken up by the cross sectional area of pulmonary vessels less than 5mm2; %LAA-950 = CT measurement of the percentage of low attenuation area less than −950 HU, defined as emphysema.
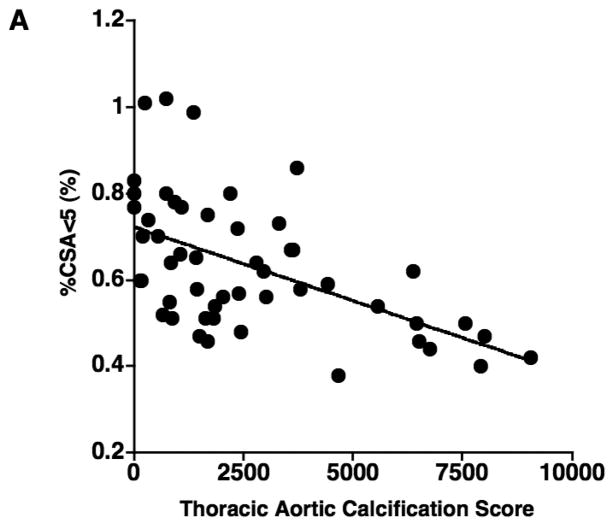
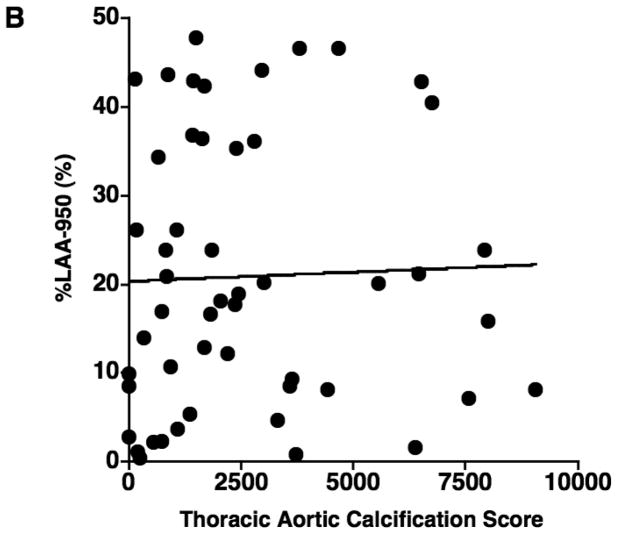
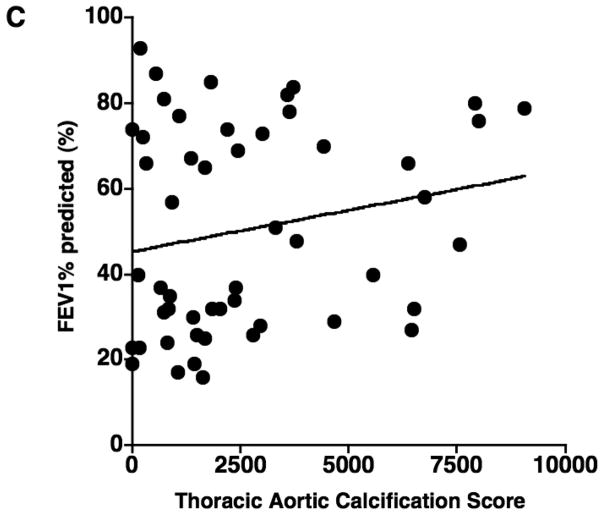
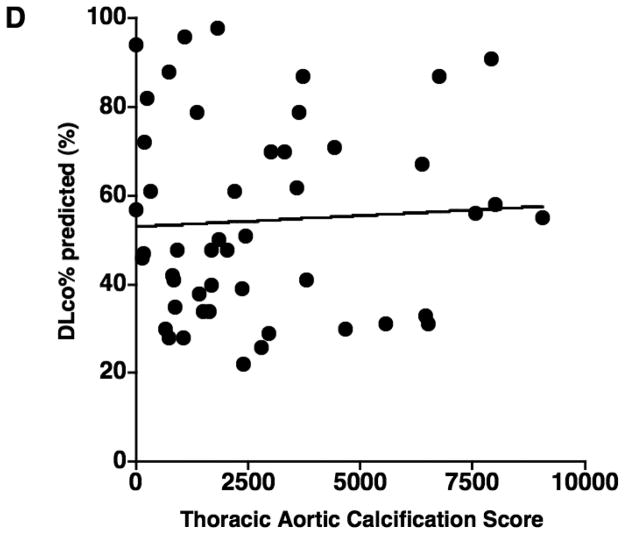
The results of correlations between aortic calcification scores and continuous variables are shown in Table 2. The mean aortic calcification score was 2660.0 ± 2437.6 (range: 0 – 9058), and the mean %CSA<5 was 0.63% ± 0.15 (range: 0.38 – 1.02). The aortic calcification score had a significant negative correlation with %CSA<5 (r = −0.566, p < 0.0001) (Figure 2), but not with %LAA-950, FEV1% predicted, and DLco% predicted (Figure 2). The aortic calcification score was significantly correlated to age (r = 0.508, p = 0.0001). No significant correlation was found between the aortic calcification score and either BMI or pack-years.
Table 2.
Correlations of Aortic Calcification Score with Continuous Variables (Spearman’s Rank Correlations) (n=51)
| Aortic Calcification Score |
||
|---|---|---|
| r | p | |
| Age | 0.508 | 0.0001 |
| BMI | −0.001 | 0.992 |
| Pack-Years | 0.183 | 0.197 |
| FEV1% predicted | 0.201 | 0.156 |
| DLco% predicted | 0.014 | 0.925 |
| %CSA<5 | −0.566 | <0.0001 |
| %LAA-950 | 0.119 | 0.405 |
Definition of abbreviations: SD = standard deviation; FEV1 = forced expiratory volume in 1 second; DLCO = diffusing capacity of the lung for carbon monoxide; BMI = body mass index; %CSA<5 = percentage of total lung area taken up by the cross sectional area of pulmonary vessels less than 5mm2; %LAA-950 = CT measurement of the percentage of low attenuation area less than −950 HU, defined as emphysema.
Figure 2.
The relationship between the thoracic aortic calcification score and (A) the percentage of the area taken up by the cross sectional area of pulmonary vessels smaller than 5 mm2 (%CSA<5), (B) %LAA-950, (C) FEV1% predicted, and (D) DLco% predicted. The thoracic aortic calcification score has a significant negative correlation with %CSA<5 (r = − 0.566, p < 0.0001), while there is no significant correlation of thoracic aortic calcification with %LAA-950 (r = 0.119, p = 0.405)., FEV1% predicted (r = 0.201, p = 0.156), and DLco% predicted (r = 0.014, p = 0.925).
In multiple linear regression analysis with aortic calcification score as the dependent variable, and age, gender, BMI, pack-years, FEV1% predicted, DLco% predicted, the total area of the lung, %LAA-950, and %CSA<5 as the independent variables, only the following two variables were independent predictors of aortic calcification score (r2 = 0.561): %CSA<5 (p < 0.0001) and age (p = 0.0049) (Table 3). The association between the aortic calcification score and %CSA<5 was independent of age, gender, BMI, pack-years, FEV1% predicted, DLCO% predicted, the total area of the lung, and %LAA-950.
Table 3.
Multivariate Analysis of Aortic Calcification Score (n=51)
| Partial Correlation Coefficient | p value | |
|---|---|---|
| Age | 0.351 | 0.0049 |
| Gender | −0.053 | 0.838 |
| BMI | −0.061 | 0.672 |
| Pack-years | 0.007 | 0.968 |
| FEV1% predicted | −0.044 | 0.886 |
| DLco% predicted | 0.084 | 0.601 |
| %CSA<5 | −0.564 | <0.0001 |
| Total Lung Area | −0.175 | 0.223 |
| %LAA-950 | −0.223 | 0.172 |
Definition of abbreviations: SD = standard deviation; FEV1 = forced expiratory volume in 1 second; DLCO = diffusing capacity of the lung for carbon monoxide; BMI = body mass index; %CSA<5 = percentage of total lung area taken up by the cross sectional area of pulmonary vessels less than 5mm2; %LAA-950 = CT measurement of the percentage of low attenuation area less than −950 HU, defined as emphysema.
Discussion
In the present study, we showed that the thoracic aortic calcification score shows an inverse correlation with %CSA<5 in COPD patients. This indicates that vascular alteration is present in both systemic and pulmonary vessels in COPD, and that those severities are correlated. Several studies demonstrate that both pulmonary and systemic vascular alterations in patients with COPD closely relate to endothelial dysfunction and inflammation (15–19, 28–30). A recent study shows the relationship between increased systemic inflammation and endothelial dysfunction in the systemic vasculature in COPD (18). Atherosclerosis is a representative systemic vascular alteration promoted by inflammation and endothelial dysfunction (11–14), and aortic calcification is one of its manifestations. Recently, Takasu and colleagues (27) showed the relationship between increased interleukin-6, a systemic inflammatory marker, and the presence and severity of thoracic aortic calcification. Meanwhile, small pulmonary arteries lose the ability to dilate due to endothelial dysfunction, which inhibits regulation of vascular tone and control of endothelium growth. Consequently, the cross-sectional area of small pulmonary vessels decreases, as observed in our study, and structural vascular alterations lead to functional impairments. Resent study also showed the role for interleukin −6 in the development of pulmonary hypertension in patients with COPD (40). Although we did not assess the relationship between endothelial dysfunction and systemic inflammation, and vascular abnormality in COPD is not restricted to the endothelial dysfunction (41), considering previous studies that assessed the correlation of vascular alteration and endothelial dysfunction and inflammation, the relationship between atherosclerosis of the aorta and small pulmonary vascular alteration we observed could be explained by systemic inflammation and endothelial dysfunction in COPD.
Recent evidence suggests that inflammation is present in all stages of COPD (1–6), and a correlation between systemic inflammation and increased risk of cardiovascular morbidity and mortality has been observed in patients with COPD (5,6). Even in mild to moderate COPD cases, the risk of cardiovascular morbidity and mortality is two to three times higher than in the general community (42–44). Likewise, pulmonary vascular alterations are not exclusive to advanced COPD, as they are present in patients with mild COPD and even in smokers with normal pulmonary function (30, 45–48). Pulmonary vascular alterations lead to functional impairments, including pulmonary hypertension and cardiac failure. Therefore, high prevalence of cardiovascular disease in COPD patients might be related to not only systemic vascular dysfunction, but also to pulmonary vascular impairments. The relationship between atherosclerosis and pulmonary vascular alteration might generate a synergistic effect to increase the risk of cardiovascular events.
The association between arterial stiffness and the extent of emphysema has been reported by McAllister and colleagues (16). Several studies also demonstrated the relationship between endothelial dysfunction and emphysema (15, 28, 29, 49, 50). Kasahara and colleagues (28) demonstrated the association of endothelial dysfunction with emphysema and pulmonary vascular alteration. Recently, Barr and colleagues (15) suggested a correlation between endothelial dysfunction measured by flow-mediated dilation in the brachial artery and an increase in the extent of emphysema in CT scans. In fact, in our study cohort, there was a significant correlation between %CSA<5 and the extent of emphysema (r = −0.605, p < 0.0001). Impaired flow-mediated dilation also has been reported to be strongly related to systemic inflammation (18). Thus, endothelial dysfunction and inflammation may be related to both emphysema and vascular alteration. However, we could not find a significant correlation between aortic calcification and the extent of emphysema. Although this discrepancy might be partially due to the different techniques for the evaluation of systemic vascular abnormalities or patient selection, it suggests that the relationship between atherosclerotic change and endothelial dysfunction and inflammation might be different in each stage of atherosclerosis.
Large studies have demonstrated that airflow limitation is an independent risk factor for cardiovascular events (42,43). The relationship between airflow limitation and atherosclerosis has been recognized (5). Recent studies showed that an increase in intima-media thickness measured by carotid ultrasonography was associated significantly with the decline of FEV1% predicted (19,20). The relationship between airflow limitation and atherosclerosis is also related to systemic inflammation and endothelial dysfunction (1,5,15,18). In contrast, we did not find a significant correlation between the severity of aortic calcification and FEV1% predicted. Although this discrepancy also might be due to different evaluation techniques, it may be that different pathological processes of atherosclerosis are involved in intima-media thickness and calcification. In fact, Schroeder and colleagues (50) reported that carotid plaque was not associated with FEV1. Recently, Iwamoto and colleagues (19) showed that the frequency of carotid plaques in smokers was independently associated with C-reactive protein (CRP) but not FEV1, whereas intima-media thickness showed a significant correlation with FEV1 but not CRP. Meanwhile, the relationship between atherosclerosis and morphological airway change such as airway wall thickness has not been accessed. COPD phenotypes might be related to atherosclerosis. Further studies will be required to clarify the complex relationship between the different pathological processes of atherosclerosis and airflow obstruction.
There are some limitations to this study. First, our sample size was relatively small and we did not have a control group. Thus, we cannot conclude that the relationship between aortic calcification and small pulmonary vascular bed alteration exists only in COPD patients. Second, the relationship between aortic calcification and small pulmonary vascular bed alteration is considered to be associated with systemic inflammation and endothelial dysfunction; however, we did not address the degree of systemic inflammation, such as CRP or endothelial dysfunction, and its relation with CT measurements. Third, we did not take into account some risk factors of cardiovascular disease such as hyperlipemia or hypertension, which might be influenced by those cardiovascular risk factors.
In conclusion, we found that the severity of aortic calcification, which is one of the pathological processes of atherosclerosis, is significantly correlated to decreases in the cross-sectional area of small pulmonary vessels. Although the underlying mechanisms are still unknown, systemic inflammation and endothelial dysfunction may promote both systemic and pulmonary vascular alteration, and may cause the close relationship between atherosclerosis and small pulmonary vascular alteration.
Acknowledgments
This work was supported by National Institutes of Health [Grants K23HL089353-01A1, U01 HL089856-02], and a grant from The Parker B. Francis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;128:1995–2004. doi: 10.1378/chest.128.4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto-Plata VM, Müllerova H, Toso JF, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61:23–28. doi: 10.1136/thx.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palange P, Testa U, Huertas A, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J. 2006;27:529–541. doi: 10.1183/09031936.06.00120604. [DOI] [PubMed] [Google Scholar]
- 5.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 6.Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–853. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthonisen NR, Connett JE, Enright PL, Manfreda J Lung Health Study Research Group. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med. 2002;166:333–339. doi: 10.1164/rccm.2110093. [DOI] [PubMed] [Google Scholar]
- 8.Calverley PM, Anderson JA, Celli B, et al. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 10.Faxon DP, Fuster V, Libby P, et al. Atherosclerotic vascular disease conference: writing group iii: pathophysiology. Circulation. 2004;109:2617–2625. doi: 10.1161/01.CIR.0000128520.37674.EF. [DOI] [PubMed] [Google Scholar]
- 11.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 12.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 13.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 14.Neunteufl T, Katzenschlager R, Hassan A, et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129:111–118. doi: 10.1016/s0021-9150(96)06018-2. [DOI] [PubMed] [Google Scholar]
- 15.Barr RG, Mesia-Vela S, Austin JH, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med. 2007;176:1200–1207. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAllister DA, Maclay JD, Mills NL, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:1208–1214. doi: 10.1164/rccm.200707-1080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills NL, Miller JJ, Anand A, et al. Increased arterial stiffness in patients with chronic obstructive pulmonary disease: a mechanism for increased cardiovascular risk. Thorax. 2008;63:306–311. doi: 10.1136/thx.2007.083493. [DOI] [PubMed] [Google Scholar]
- 18.Eickhoff P, Valipour A, Kiss D, et al. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:1211–1218. doi: 10.1164/rccm.200709-1412OC. [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto H, Yokoyama A, Kitahara Y, et al. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med. 2009;179:35–40. doi: 10.1164/rccm.200804-560OC. [DOI] [PubMed] [Google Scholar]
- 20.Sabit R, Bolton CE, Edwards PH, et al. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:1259–1265. doi: 10.1164/rccm.200701-067OC. [DOI] [PubMed] [Google Scholar]
- 21.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atheroscleroticlesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaque. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witteman JC, Kannel WB, Wolf PA, et al. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990 Nov 1;66(15):1060–4. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 24.Takasu J, Katz R, Nasir K, et al. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2008;155:765–771. doi: 10.1016/j.ahj.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisen A, Tenenbaum A, Koren-Morag N, et al. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation. 2008;118:1328–1334. doi: 10.1161/CIRCULATIONAHA.107.712141. [DOI] [PubMed] [Google Scholar]
- 26.Takasu J, Mao S, Budoff MJ. Aortic atherosclerosis detected with electron-beam CT as a predictor of obstructive coronary artery disease. Acad Radiol. 2003;10:631–637. doi: 10.1016/s1076-6332(03)80081-8. [DOI] [PubMed] [Google Scholar]
- 27.Takasu J, Katz R, Shavelle DM, et al. Inflammation and descending thoracic aortic calcification as detected by computed tomography: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2008;199:201–206. doi: 10.1016/j.atherosclerosis.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos S, Peinado VI, Ramirez J, et al. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1250–1256. doi: 10.1164/rccm.200210-1233OC. [DOI] [PubMed] [Google Scholar]
- 30.Peinado VI, Barberá JA, Abate P, Ramírez J, Roca J, Santos S, Rodriguez-Roisi R. Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1605–1611. doi: 10.1164/ajrccm.159.5.9807059. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka S, Washko GR, Dransfield MT, et al. Quantitative CT Measurement of Cross-sectional Area of Small Pulmonary Vessel in COPD: Correlations with Emphysema and Airflow Limitation. Acad Radiol. 2010;17:93–99. doi: 10.1016/j.acra.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuoka S, Washko GR, Yamashiro T, et al. Pulmonary Hypertension and CT Measurement of Small Pulmonary Vessels in Severe Emphysema. Am J Respir Crit Care Med. 2009 Oct 29; doi: 10.1164/rccm.200908-1189OC. (in press Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 34.Coche E, Pawlak S, Dechambre S, Maldague B. Peripheral pulmonary arteries: identification at multi-slice spiral CT with 3D reconstruction. Eur Radiol. 2003;13:815–822. doi: 10.1007/s00330-002-1734-2. [DOI] [PubMed] [Google Scholar]
- 35.Shemesh J, Apter S, Rozenman J, et al. Calcification of coronary arteries: detection and quantification with double-helix CT. Radiology. 1995;197:779–783. doi: 10.1148/radiology.197.3.7480756. [DOI] [PubMed] [Google Scholar]
- 36.Agaston AS, Janowitz WR, Hildner FJ, Zusmer M, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 37.Eisen A, Tenenbaum A, Koren-Morag N, et al. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation. 2008;118:1328–1334. doi: 10.1161/CIRCULATIONAHA.107.712141. [DOI] [PubMed] [Google Scholar]
- 38.Wu MH, Chern MS, Chen LC, et al. Electron beam computed tomography evidence of aortic calcification as an independent determinant of coronary artery calcification. J Chin Med Assoc. 2006;69:409–414. doi: 10.1016/S1726-4901(09)70283-7. [DOI] [PubMed] [Google Scholar]
- 39.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 40.Chaouat A, Savale L, Chouaid C, et al. Role for interleukin-6 in COPD-related pulmonary hypertension. Chest. 2009;136:678–687. doi: 10.1378/chest.08-2420. [DOI] [PubMed] [Google Scholar]
- 41.Maclay JD, McAllister DA, Mills NL, et al. Vascular dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:513–520. doi: 10.1164/rccm.200903-0414OC. [DOI] [PubMed] [Google Scholar]
- 42.Schünemann HJ, Dorn J, Grant BJ, Winkelstein W, Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118:656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 43.Bang KM, Gergen PJ, Kramer R, Cohen B. The effect of pulmonary impairment on all-cause mortality in a national cohort. Chest. 1993;103:536–540. doi: 10.1378/chest.103.2.536. [DOI] [PubMed] [Google Scholar]
- 44.Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1976;294:1071–1075. doi: 10.1056/NEJM197605132942001. [DOI] [PubMed] [Google Scholar]
- 45.Hale KA, Niewoehner DE, Cosio MG. Morphologic changes in the muscular pulmonary arteries: relationship to cigarette smoking, airway disease, and emphysema. Am Rev Respir Dis. 1980;122:273–278. doi: 10.1164/arrd.1980.122.2.273. [DOI] [PubMed] [Google Scholar]
- 46.Wright JL, Lawson L, Pare PD, et al. The structure and function of the pulmonary vasculature in mild chronic obstructive pulmonary disease. The effect of oxygen and exercise. Am Rev Respir Dis. 1983;128:702–707. doi: 10.1164/arrd.1983.128.4.702. [DOI] [PubMed] [Google Scholar]
- 47.Santos S, Peinado VI, Ramirez J, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–638. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- 48.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163:737–744. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- 49.Kanazawa H, Asai K, Hirata K, Yoshikawa J. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med. 2003;114:354–358. doi: 10.1016/s0002-9343(02)01562-0. [DOI] [PubMed] [Google Scholar]
- 50.Schroeder EB, Welch VL, Evans GW, Heiss G. Impaired lung function and subclinical atherosclerosis. The ARIC Study. Atherosclerosis. 2005;180:367–373. doi: 10.1016/j.atherosclerosis.2004.12.012. [DOI] [PubMed] [Google Scholar]