Abstract
The asymmetric synthesis of all four of the known natural phlegmarines and one synthetic derivative has been accomplished in 19 to 22 steps from 4-methoxy-3-(triisopropylsilyl)pyridine. Chiral N-acylpyridinium salt chemistry was used twice to set the stereocenters at the C-9 and C-2′ positions of the phlegmarine skeleton. Key reactions include the use of a mixed Grignard reagent for the second N-acylpyridinium salt addition, zinc/acetic acid reduction of a complex dihydropyridone, and a von Braun cyanogen bromide N-demethylation of a late intermediate. These syntheses confirmed the absolute stereochemistry of all the known phlegmarines.
Introduction
The lycopodiaceous plants have produced numerous and structurally interesting alkaloids which have proven to be challenging targets for total synthesis.1 One of the Lycopodium alkaloids, huperzine A, is a potential therapeutic agent for treatment of Alzheimer’s disease.2 This medicinally important compound has spurred the isolation of several new Lycopodium alkaloids having various biological activities including cytotoxicity.3 The discovery of significant biological activities among the Lycopodium alkaloids has prompted renewed interest in the development of new synthetic strategies for their preparation.4 As part of our natural product synthesis program,5,6 we have examined approaches to the phlegmarine alkaloids.
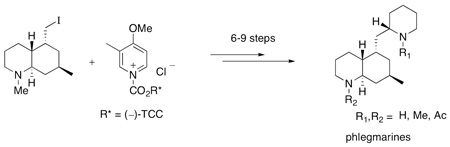
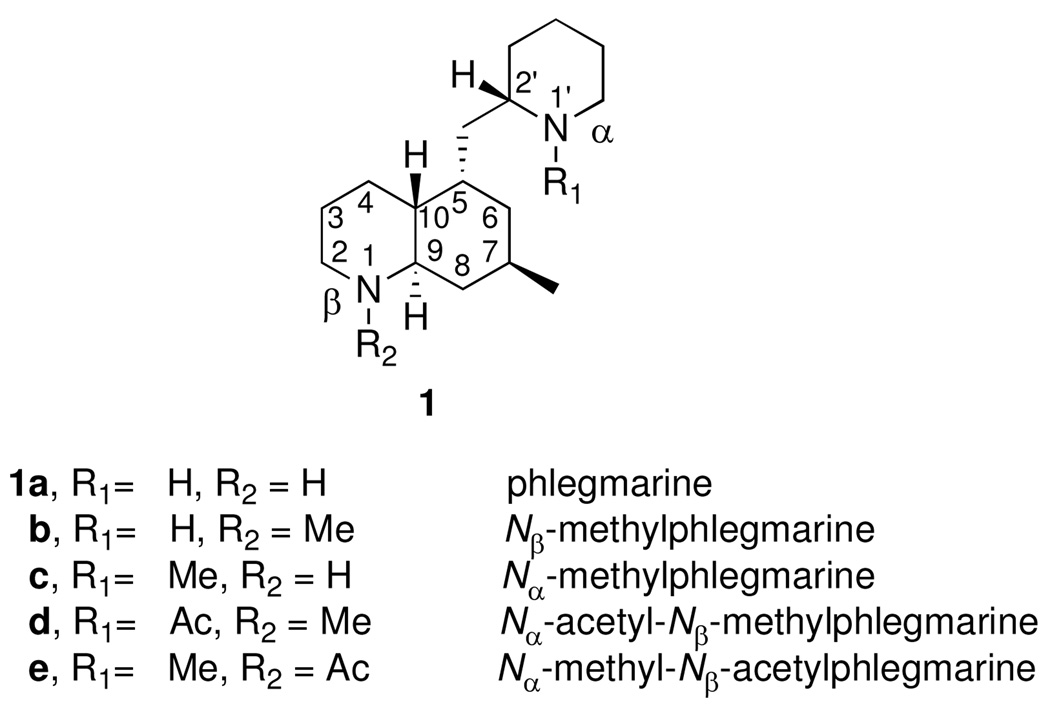
The phlegmarines are a C16N2 skeletal group of Lycopodium alkaloids discovered by Braekman and coworkers in 1978.7a All four naturally occurring members of this group (1a–d) possess the same skeleton and differ only by their nitrogen atom substituents.7b Synthesis and spectroscopic structure studies carried out by Braekman’s group7a determined the basic carbon skeleton, but it was not until the work of MacLean8 and co-workers that the relative stereochemistry of all five stereogenic centers of the phlegmarines was defined. The absolute stereochemistry of these alkaloids was established in our laboratories through the asymmetric total synthesis of (−)-Nα-acetyl-N-β-methylphlegmarine (1d).4c We report herein the total synthesis of all the known phlegmarine alkaloids 1a–d, and the Nβ-acetyl derivative 1e.
Results and Discussion
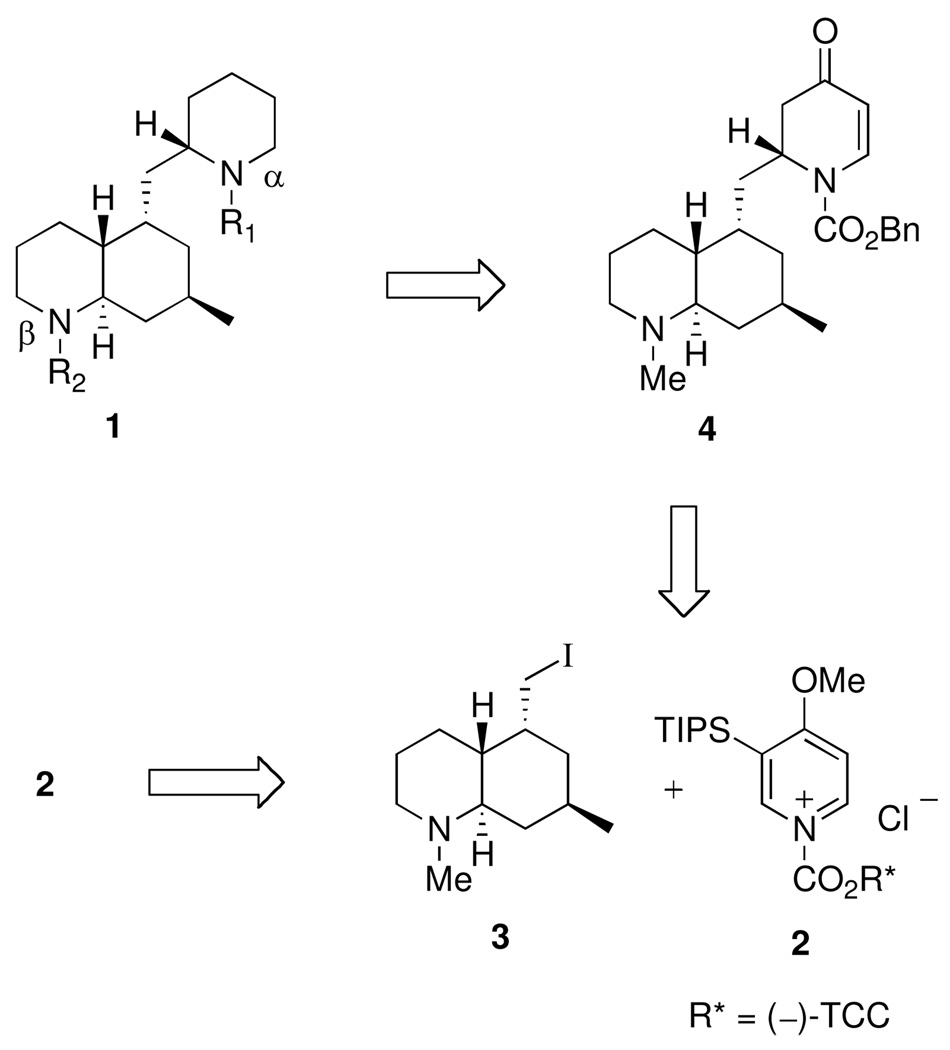
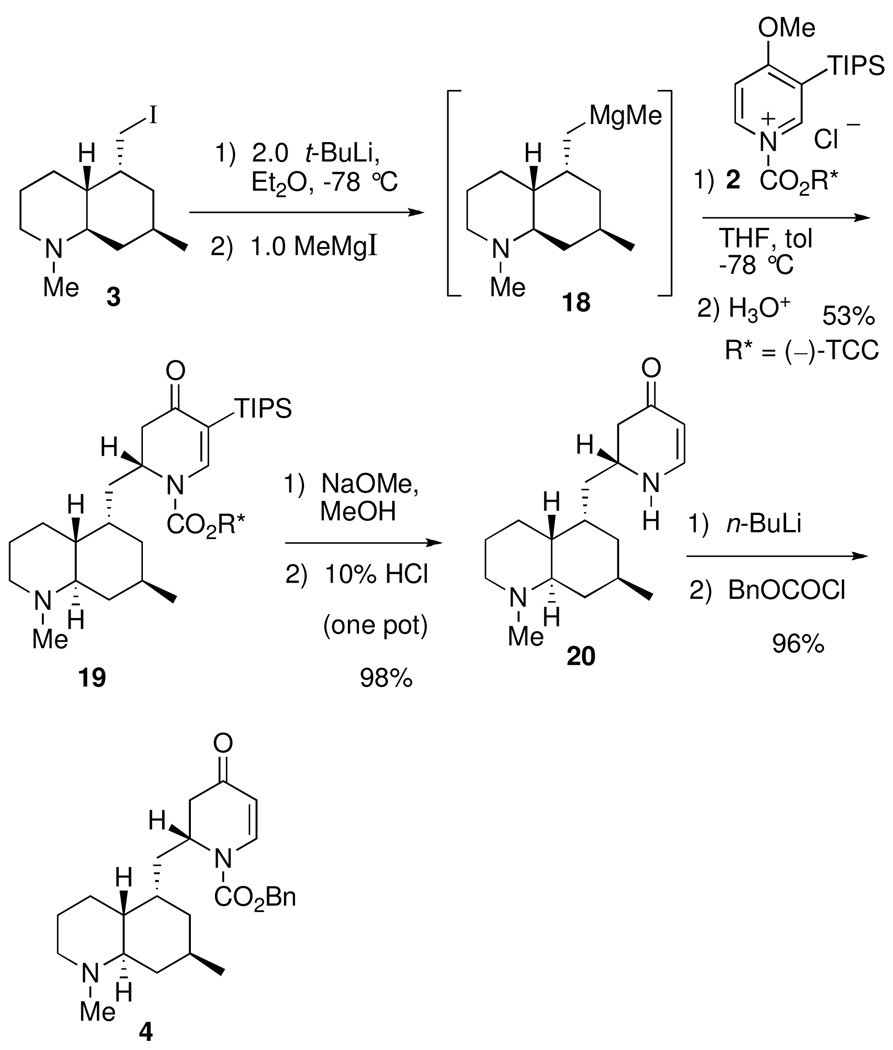
Our strategy for synthesizing the phlegmarines is depicted in Scheme 1. All of the alkaloid targets were to be prepared from the common dihydropyridone intermediate 4, which would arise from the key fragment 3 and chiral 1-acylpyridinium salt 2. Fragment 3 would also be prepared from the same antipode of 2 by a modification of our published procedure.4c
SCHEME 1.
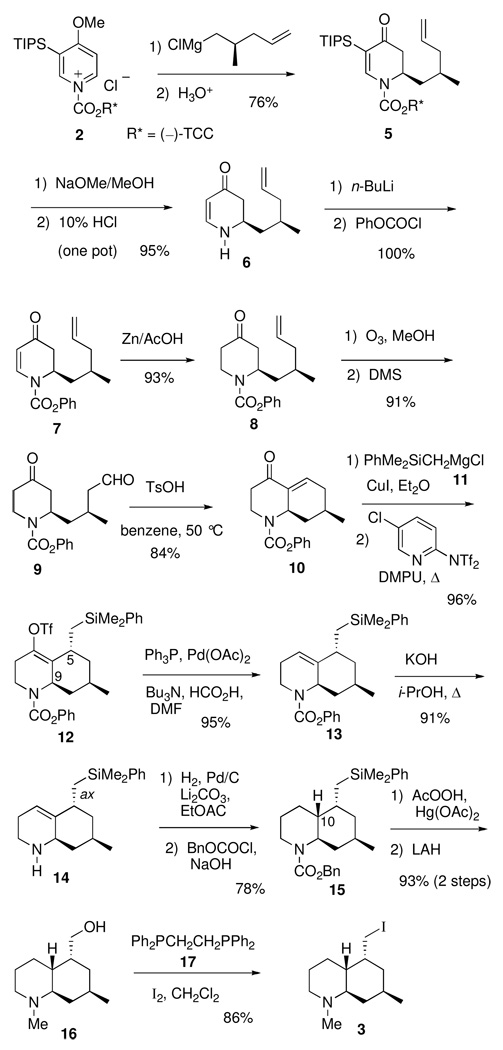
The Grignard of (R)-5-chloro-4-methylpentene9 was added to chiral N-acylpyridinium salt 2, prepared in situ from 4-methoxy-3-(triisopropylsilyl)pyridine10 and the chloroformate of (−)-trans-2-(α-cumyl)cyclohexanol (TCC),11 to give the crude N-acyldihydropyridone 5 in 90% yield and 88% de (Scheme 2). Purification by recrystallization from ethanol provided a 76% yield of the major diastereomer 5 as an isomerically pure white solid. A one-pot reaction of 5 with NaOMe/MeOH followed by aqueous 10% HCl furnished dihydropyridone 6 in 95% yield with 95% recovery of the chiral auxiliary, (−)-TCC. N-Acylation of 6 with n-BuLi and phenyl chloroformate gave a quantitative yield of enantiopure carbamate 7. Conjugate reduction of 7 can be effected with L-Selectride/BF3•OEt2 4c (86% yield) or more conveniently with Zn/AcOH12 to give 8 in 93% yield. Ozonolysis of the terminal alkene of 8 provided a high yield of aldehyde 9, which on acid-mediated cyclization13 was converted efficiently to bicyclic enone 10.
SCHEME 2.
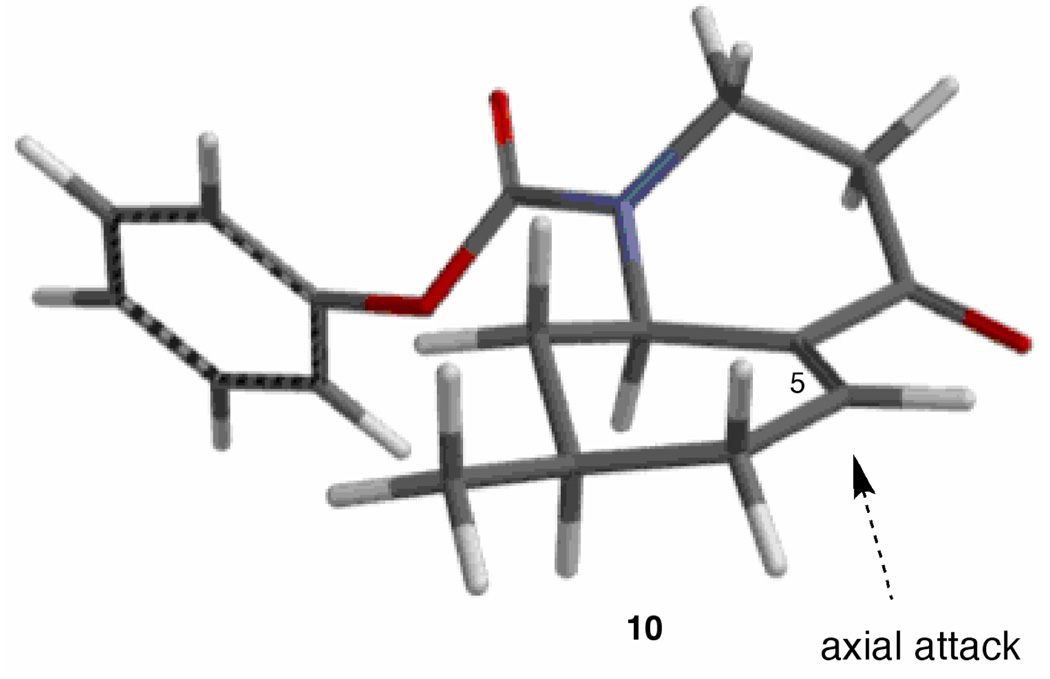
The next step required a facial selective conjugate addition of a nucleophile containing the latent functionality of a hydroxymethyl group. Based on our earlier model studies,14 (dimethylphenylsilyl)methylmagnesium chloride was chosen for use in a copper-mediated 1,4-addition to enone 10. The resulting facial selectivity was anticipated to be high based on conformational and stereoelectronic arguments. Due to A(1,3) strain,15 the reactive conformation is concave as shown in Figure 2. Stereoelectronically controlled axial attack of the nucleophile at C-5 would afford the desired stereochemical outcome. In the presence of copper iodide, addition of Grignard 11 to 10 and trapping of the resulting enolate with N-(5-(chloro-2-pyridyl)triflimide16 provided a 96% yield of vinyl triflate 12. Since protonation of the enolate leads to the cis-fused ring juncture,14 in situ vinyl triflate formation was necessary to set up the eventual incorporation of the required stereochemistry at C-10. The vinyl triflate function would not survive the subsequent hydrolysis of the carbamate group, so 12 was cleanly reduced to the more stable alkene 13 using Cacchi’s conditions.17
FIGURE 2.
Calculated lowest energy conformation of 10 (MMFF).
Carbamate hydrolysis using KOH/2-propanol at reflux gave a high yield of the secondary amine 14. The use of 2-propanol was essential to obtaining a clean product, for the analogous reaction with KOH/ethanol gave a significant amount of the corresponding ethyl carbamate via carbamate exchange. Catalytic hydrogenation of 14 provided an 89/11 mixture of crude amines that were converted to Cbz carbamate 15 (78%) and the corresponding cis C-10 epimer. The trans selectivity can be attributed to significant shielding of the bottom face of the alkene in 14 by the axial (phenyldimethylsilyl)methyl group. Oxidation using Fleming’s conditions18 and subsequent lithium aluminum hydride reduction gave amino alcohol 16 in high yield. Conversion to iodide 3 was effected using 1,2-bis-(triphenylphosphino)ethane (17) and I2.19 This method proved superior to the more common procedure using triphenylphosphine/I2 due to ease of product purification.
The key intermediate 4 was prepared as shown in Scheme 3. The mixed Grignard reagent 18 was prepared from iodide 3, by lithium-halogen exchange and addition of methylmagnesium iodide, and treated with 2 equiv of N-acylpyridinium salt 2 to give dihydropyridone 19 in 53% yield. The five stereocenters were correctly installed as determined by single-crystal X-ray analysis.4c The TIPS group and TCC auxiliary were removed from 19 in one step using our standard procedure to afford 20, which was converted to key intermediate 4 in high yield on lithiation and treatment with benzyl chloroformate. With the intermediate 4 in hand, the five target alkaloids were prepared as described below.
SCHEME 3.
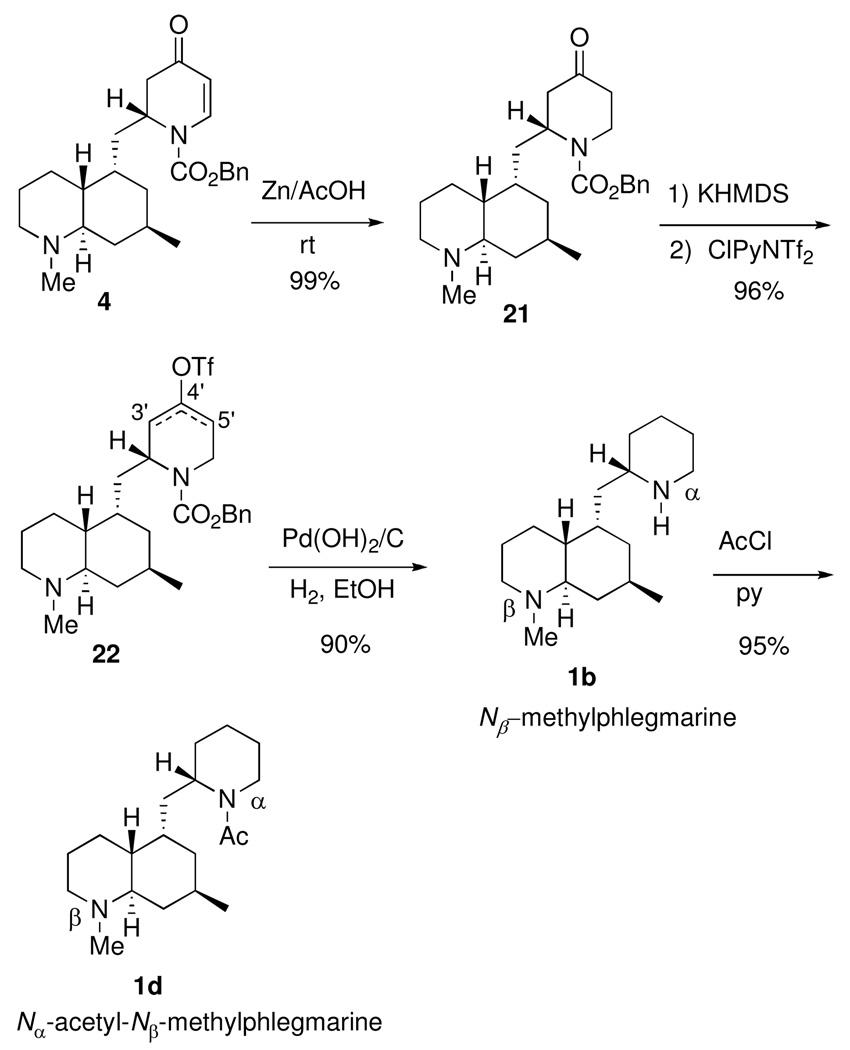
Nβ-Methylphlegmarine (1b) and N α-acetyl-N β-methylphlegmarine (1d)
Conjugate reduction of 4 with zinc in acetic acid gave piperidone 21 in near quantitative yield (Scheme 4). Deprotonation with KHMDS and trapping with N-(5-chloro-2-pyridyl)triflimide provided the vinyl triflates 22 in a 3:1 ratio favoring olefin formation at the 4,′5′ position. Catalytic hydrogenation over Pearlman’s catalyst afforded the natural product 1b, which exhibited spectral data and optical rotation in agreement with its assigned structure.
SCHEME 4.
The alkaloid Nα-acetyl-Nβ-methylphlegmarine (1d) was prepared in one step from 1b by simple acetylation. Although the rotation of 1d was higher than that previously reported for the natural product, all spectral data were in agreement with literature values.7a
Phlegmarine (1a), N α-methylphlegmarine (1c) and unnatural Nα-methyl-Nβ-acetyl-phlegmarine (1e)
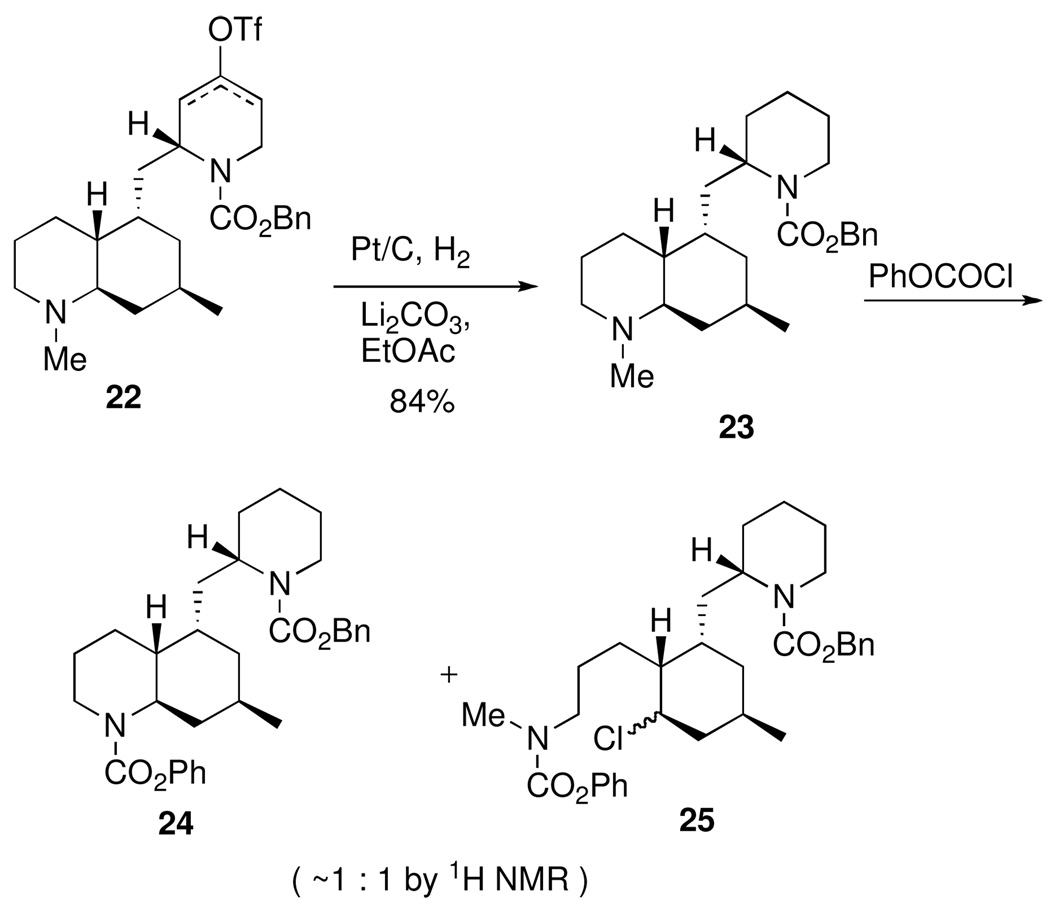
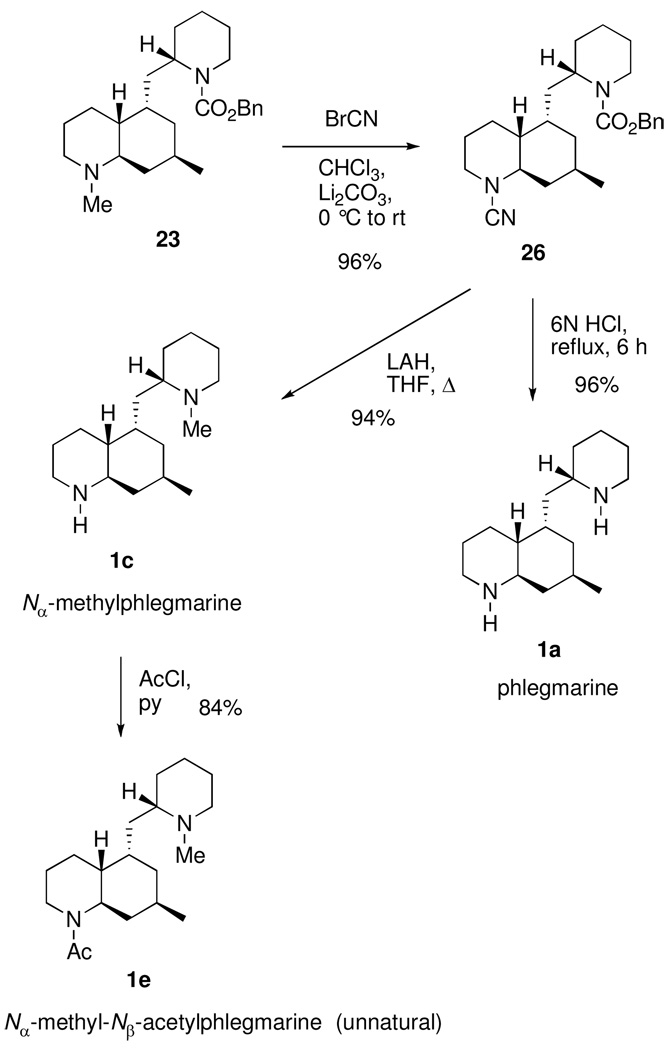
In order to prepare the remaining two natural phlegmarines (1a,c) and the known synthetic derivative 1e from intermediate 4, a demethylation of the beta nitrogen would be required toward the end of the synthesis. The vinyl triflate mixture 22 was reduced selectively via hydrogenation over platinum on carbon to afford the benzyl carbamate 23 (Scheme 5). Initial attempts to N-demethylate 23 with phenyl chloroformate proved problematic giving an inseparable mixture of dicarbamate 24 and the ring-opened product 25 as determined by 1H NMR and MS analysis. The use of other chloroformates20 led to similar results with a poor ratio of products regardless of temperature, solvent, or the addition of additives (LiBr, LiCl). The von Braun demethylation reaction using cyanogen bromide21 was examined next. To our delight, treatment of tertiary amine 23 with cyanogen bromide at rt gave a near quantitative conversion to cyanamide 26 (Scheme 6). When 26 was treated with dilute HCl at reflux, both the cyanamide and Cbz groups were hydrolyzed to the secondary amines providing natural phlegmarine (1a) in high yield. Nα-Methylphlegmarine (1c) was also prepared in excellent yield from 26 in one step by concomitant reduction of the cyanamide and carbamate groups with LAH. N-Acylation of 1c afforded Nα-methyl-Nβ-acetylphlegmarine (1e) in good yield. All three of our synthetic phlegmarines (1a,c,e) exhibited characterization data in agreement with literature values for the known compounds.
SCHEME 5.
SCHEME 6.
In summary, all four of the known phlegmarine alkaloids and one previoiusly reported synthetic derivative have been synthesized enantiopure from key dihydropyridone intermediate 4. These syntheses have confirmed the absolute stereochemistry of the phlegmarines as 2′S, 5S, 7R, 9R, 10R. Our chiral N-acylpyridinium salt chemistry was used twice during the synthetic route to set the stereocenter at C-9 and the remote center at C-2′ of the phlegmarines. The syntheses ranged from 19 to 22 steps and were accomplished with excellent stereocontrol.
Experimental Section
The experimentals for compounds 5–10 and 12–17 have been previously published.4c
2S-2-[(4aR,5S,7R,8aR)-1,2,3,4a,5,6,7,8,8a-Decahydroquinolin-1,7-dimethyl-5-ylmethyl)-1-[(benzyloxy)carbonyl]-2,3-dihydro-4-pyridone (4)
To a cooled (−78 °C) solution of dihydropyridone 20 (58.2 mg, 211 µmol) in THF (5.0 mL) was added n-BuLi (84 µL, 230 µmol, 1.1 equiv) dropwise. The solution immediately turned bright yellow and became heterogeneous. After 10 min the anion was rapidly quenched with freshly distilled benzyl chloroformate (60 µL, 420 µmol, 2.0 equiv). The solution was allowed to stir for an additional 2.5 h and then a 50% saturated aqueous solution of NaHCO3 (2.0 mL) was added. The aqueous phase was extracted with EtOAc (4 × 3.0 mL). The combined organic phases were washed with brine (5 mL), dried (MgSO4), filtered (Celite) and concentrated in vacuo. The crude oil was purified by radial PLC (silica gel, 75% EtOAc/hexanes, 2% TEA) to give 4 (82.7 mg, 96%) as a colorless oil. [α]D24 + 6.0 (c 0.81, MeOH); IR (film, NaCl) 2922, 1725 (C=O), 1672 (O-C=O), 1603 (C=C), 1327, 1192 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.79 (d, J = 6.3 Hz, 1H) 7.40-7.37 (m, 5H), 5.35-5.31 (m, 2H), 5.21 (d, J = 11.7 Hz, 1H), 4.55 (br s, 1H), 2.81-2.71 (m, 2H), 2.45 (d, J = 16.5 Hz, 1H), 2.21 (s, 3H), 1.98-1.89 (m, 2H), 1.79 (m, 1H), 1.63-128 (m, 9H), 1.13-0.655 (m, 6H); 13C NMR (75 MHz, CDCl3) δ 192.9, 152.5, 141.9, 135.0, 129.1, 129.0, 128.8, 107.2, 69.3, 62.7, 57.7, 52.2, 44.3, 43.0, 39.9, 38.3, 37.3, 34.5, 28.9, 26.6, 26.0, 25.6, 22.9; HRMS calcd for C25H34N2O3(M+H)+ 411.2648, found 411.2653.
(2R)-2-[(R)-2-methyl-4-pentenyl]-1-(phenoxycarbonyl)-2,3,5,6-tetrahydro-4-pyridone (8)4c. Improved procedure
To a rapidly stirred solution of dihydropyridone 7 (1.25 g, 4.18 mmol) in glacial acetic acid (38 mL) was added zinc powder (5.5 g, 84 mmol, 20 equiv) in small portions, to prevent the zinc from clumping. The mixture was allowed to stir for 17 h, filtered (Celite) and then concentrated in vacuo. The heterogeneous oil was purified by radial PLC (silica gel, 10–20% EtOAc/ hexanes) to provide piperidone 8 (1.17 g, 93%) as a colorless oil. Spectral data is identical to that previously reported.
2S-2-[(4aR,5S,7R,8aR)-1,2,3,4a,5,6,7,8,8a-Decahydroquinolin-1,7-dimethyl-5-ylmethyl)-1-[(1R,2S]-2-((1-methyl-1-phenyl)ethyl)-cyclohexyloxyl-oxycarbonyl]-5-(triisopropylsilanyl)-2,3-dihydro-4-pyridone (19).4c Improved procedure
The chiral N-acylpyridinium salt was prepared in situ by adding (−)-TCC chloroformate (253 mg, 901 µmol, 2.1 equiv) to a cooled (−45 °C) solution of 3-(triisopropylsilyl)-4-methoxypyridine (241mg, 901 µmol, 2.1 equiv) in toluene (27 mL). The solution was allowed to stir at −45 °C for 45 min followed by cooling to −78 °C before addition of the organometallic. The organometallic was prepared by dropwise addition of t-BuLi (559 µL, 944 µmol, 1.7 M in pentane, 2.2 equiv) to a cooled (−78 °C) solution of (4aR,5S,7R,8aR)-5-iodomethyl-1,7-dimethyl-1,2,3,4a,5,6,7,8,8a- decahydroquinoline4c (17) (132 mg, 429 µmol) in ether (9.0 mL). The solution was stirred for 45 min at −78 °C and then warmed to −42 °C (dry ice/acetonitrile) for 45 min. To the solution was added methylmagnesium iodide (154 µL, 429 µmol, 2.85M in ether, 1.0 equiv), and the solution was allowed to stir for an additional 10 min. This solution was added dropwise via cannula over 3 min to the cooled (−78 °C) N-acylpyridinium salt solution. This mixture was allowed to stir for 5 h at −78 °C followed by addition of 10% HCl(aq) (4 mL). The mixture was allowed to warm to rt and stirred for an additional 30 min. The aqueous phase was made basic with solid K2 CO3 and then extracted with EtOAc (5 × 3 mL). The combined organic phases were washed with brine (5 mL), dried (K2CO3), filtered (Celite), and concentrated in vacuo. The crude oil was purified by radial PLC (SiO2, 50% EtOAc/hexanes, 1% TEA) providing 19 (198 mg, 69%) as a mixture of isomers at the newly generated stereocenter; diasteriomeric excess was found to be 84% by HPLC. 1H NMR (400 MHz, CDCl3) δ 7.72 (s, 1H), 7.32-7.28 (m, 4H), 7.12 (t, J = 6.8 Hz, 1H), 4.89 (dt, J = 10.8, 4.4 Hz, 1H), 2.76 (d, J = 11.6 Hz, 1H), 2.64 (m,1H), 2.31 (dd, J = 15.6, 6.0 Hz, 1H), 2.19 (s, 3H), 2.05-1.99 (m, 4H), 1.88-1.74 (m, 3H), 1.61-1.19 (m, 23H), 1.06-1.01 (m, 24H), 071 (q, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 196.8, 152.8, 152.2, 147.6, 128.3, 125.2, 125.1, 110.5, 77.8, 62.8, 57.7, 51.4, 50.7, 44.1, 43.0, 39.8, 39.5, 39.1, 37.5, 34.3, 33.8, 31.0, 28.9, 26.9, 26.8, 26.2, 26.0, 24.8, 23.0, 21.6, 19.0, 18.9, 11.3;
2S-2-[(4aR,5S,7R,8aR)-1,2,3,4a,5,6,7,8,8a-Decahydroquinolin-1,7-dimethyl-5-ylmethyl)-2,3-dihydro-4-pyridone (20).4c Improved procedure
Sodium methoxide (734 µL, 3.2 mmol, 4.36 M in MeOH, 10 equiv) was added to a suspension of 19 (217 mg, 320 µmol) in methanol (20 mL). The mixture was brought to reflux affording a homogeneous solution. The solution was refluxed for 17 h, allowed to cool to rt, and concentrated in vacuo. The residual oil was taken up in THF (20 mL) and then 10% HCl(aq) (2.0 mL), was added. The mixture was allowed to stir for 3 h. The acidic mixture was carefully quenched with K2CO3(s) (~500 mg). Anhydrous K2CO3(s) (~5–7 g) was then added until the solution appeared to be dry. The clumps of K2CO3 were broken up with a glass rod, and the mixture was filtered through Celite. The filter pad was washed with hot EtOAc (5 × 20 mL), and the filtrate was concentrated in vacuo. The crude oil was purified by radial PLC (silica gel, 50–100% EtOAc/hexanes, 1% TEA) to give the dihydropyridone 20 (86.9 mg, 98%) as an oil that solidified upon standing, mp 141–142 °C (50% EtOAc/hexanes); 1H NMR (400 MHz, CDCl3) δ 7.16 (t, J = 7.6 Hz, 1H), 5.03 (d, J = 7.6 Hz, 1H), 4.72 (br s, 1H), 3.65 (m, 1H), 2.83 (br d, J = 11.2 Hz, 1H), 2.49 (dd, J = 16.0, 4.4 Hz, 1H), 2.54 (d, J = 16 Hz, 1H), 2.25 (s, 3H), 2.06 (m, 1H), 1.99 (m, 1H), 1.74-1.46 (m, 9H), 1.38 (m, 1H), 1.23 (m, 1H), 1.12 (dt, J = 13.0, 4.0 Hz, 1H), 0.91 (d, J = 6.4 Hz, 3H), 0.76 (q, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 192.9, 151.6, 98.7, 62.8, 57.6, 52.4, 44.2, 42.9, 41.5, 39.8, 38.5, 35.0, 32.1, 29.1, 26.0, 25.9, 22.9.
2S-2-[(4aR,5S,7R,8aR)-1,2,3,4a,5,6,7,8,8a-Decahydroquinolin-1,7-dimethyl-5-ylmethyl)-1-[(benzyloxy)carbonyl]-4-piperidone (21)
Zinc powder was added slowly to a rapidly stirred solution of 4 (24.6 mg, 60 µmol) in glacial acetic acid (1.0 mL). The reaction mixture was allowed to stir for 11 h, and then filtered through Celite, and the filter pad was washed with methanol (5 mL). The filtrate was concentrated in vacuo. The resulting amorphous solid was suspended in dichloromethane (5 mL) and passed through a plug of basic alumina (activity 2). The plug was washed with a 10% solution of methanol in dichloromethane (5 × 5 mL). The solution was concentrated in vacuo to provide a colorless oil. The crude material was purified by flash chromatography on silica gel (50% EtOAc/hexanes, 1% TEA) to provide 21 (24.6 mg, 99%) as a colorless oil. [α]D23 −49 (c 0.69 , CHCl3); IR (film, NaCl) 2925, 2775 (N-Me), 1721 (C=O), 1698 (O-C(=O)N), 1422, 1233, 1104, 1005 cm−1; 1H NMR at 50 °C (300 MHz, CDCl3) δ 7.37-7.31 (m, 5H), 5.24 (d, J = 9.0 Hz, 1H), 5.13 (d, J = 9.0 Hz, 1H), 4.59 (br s, 1H), 4.38 (dd, J = 9.6, 6.0 Hz, 1H), 3.33 (ddd J = 11.2, 8.5, 2.9 Hz, 1H), 2.81 (br d, J = 8.4 Hz, 1H), 2.60 (dd, J = 10.8, 4.8 Hz, 1H), 2.50 (ddd, J = 11.5, 8.5, 5.2 Hz, 1H), 2.34 (br d, J = 10.8 Hz, 1H), 2.22 (s, 3H), 2.04-1.94 (m, 2H), 1.64-1.42 (m, 9H), 1.30 (m, 1H), 1.20-1.12 (m, 1H), 1.03 (m, 1H), 0.83 (d, J = 4.2 Hz, 3H), 0.70 (q, J = 9.0 Hz, 1H); 13C NMR at 50 °C (100 MHz, CDCl3) δ 207.5, 155.5, 136.7, 128.8, 128.5, 128.4, 68.0, 63.1, 57.9, 52.3, 44.6 (2C, determined by 13C NMR at 70 °C in benzene-d6 δ 45.5, 44.3), 43.0, 40.8, 40.1, 39.4, 39.1, 35.7, 31.2, 29.3, 26.2 (2C, determined by 13C NMR at 70 °C (in benzene-d6 δ 26.9, 26.4), 22.9; HRMS calcd for C25H36N2O3 (M+H)+ 413.2804, found 413.2797.
Mixture of enol triflate isomers (22)
To a cooled (−78 °C) solution of piperidone 21 (10.8 mg, 26.3 µmol) and 2-(5-chloropyridyl)triflimide (33 mg, 84 µmol, 3.2 equiv) in THF (2.0 mL) was added potassium bis(trimethylsilyl)amide (3 × 56 µL, 84 µmol, 0.5M in toluene, 3 equiv) dropwise in three portions. The reaction mixture was allowed to stir at −78 °C for 30 min, saturated aqueous NaHCO3 (1 mL) was rapidly added, and the mixture was allowed to warm to rt. The aqueous phase was diluted with water until it became homogeneous (1 mL). The aqueous phase was extracted with EtOAc (5 × 1 mL). The combined organic phases were washed with brine (1 mL), dried (Na2SO4), filtered (Celite), and concentrated in vacuo. The crude oil was purified by flash column chromatography with basic alumina (activity 2, elution with 5% MeOH/CH2Cl2), and then a second column with silica gel (elution with 50% EtOAc/hexanes) to afford the product (13.8 mg, 96%) as an inseparable mixture of enol triflate isomers 22 (ratio of 3,4- to 4,5-enol triflate found to be 1.0 to 3.1 by 1H NMR). 1H NMR at 50 °C (400 MHz, CDCl3) δ 7.33 (br s, 5H, mixture of isomers), 5.81 (s, 1H, minor isomer), 5.75 (s, 1H, major isomer), 5.25-5.07 (m, 2H, mixture of isomers), 4.57-4.38 ( m, 2 H, mixture of isomers), 3.08 (m, 1H, minor isomer), 2.80 (d, J = 10 Hz, major isomer), 2.74-2.61 (m, 1H, mixture of isomers), 2.22-2.22 (m, 7H, mixture of isomers), 2.01-1.94 (m, 4H, mixture of isomers), 1.65-1.02 (m, 22H, mixture of isomers), 0.88-0.67 (m, 7H, mixture of isomers); HRMS calcd for C26H35F3N2O5S(M+H)+ 545.2297, found 545.2310.
2S-2-[(4aR,5S,7R,8aR)-1,2,3,4a,5,6,7,8,8a-Decahydroquinolin-1,7-dimethyl-5-ylmethyl)-1-[(benzyloxy)carbonyl]piperidine (23)
A solution of the mixed triflates 22 (13.8 mg, 25 µmol), 5% platinum on carbon (14 mg) and lithium carbonate (18 mg, 240 µmol) in EtOAc was stirred under an atmosphere of hydrogen gas for 5.75 h. The solution was filtered through a plug of Celite and then concentrated in vacuo. The crude oil was purified by flash column chromatography (silica gel, 50% EtOAc/hexanes, 1% TEA) providing piperidine 23 (8.3 mg, 84%) as a colorless oil. [α]D24 –20.6 (c 0.65, MeOH ); IR (film, NaCl) 2930, 1694 (C=O), 1422, 1259 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.35-7.25 (m, 5H), 5.18 (d, J = 12.3 Hz, 1H), 5.07 (d, J = 12.3 Hz, 1H), 4.23 (br s, 1H), 4.06 (br d, J = 13.2 Hz, 1H), 2.23-2.80 (m, 2H), 2.23 (s, 3H), 2.03-1.95 (m, 2H), 1.75-1.22 (m, 17H), 0.97 (dt, J = 13.4, 3.9 Hz, 1H), 0.82 (d, J = 6.0 Hz, 3H), 2.75 (q, J = 11.9 Hz, 1H); 13C NMR at 50 °C (75 MHz, CDCl3) δ 155.7, 137.2, 128.7, 128.2, 128.1, 67.2, 63.0, 57.8, 49.9, 44.7, 43.1, 40.1, 39.6, 38.5, 35.6, 29.2, 27.2, 27.0, 26.3, 26.0, 25.7, 23.1, 18.8; TLC Rf = 0.26 (30% EtOAc/hexanes, basic alumina); HRMS calcd for C25H38N2O2 (M+H)+ 399.3012, found 399.3010.
2S-2-[(4aR,5S,7R,8aR)-1,2,3,4a,5,6,7,8,8a-Decahydroquinolin-1-cyano-7-methyl-5-ylmethyl)-1-[(benzyloxy)carbonyl]piperidine (26)
To a cooled (0 °C) solution of 23 (8.3 mg, 21 µmol) and lithium carbonate (2.1 mg, 21 µmol, 1 equiv) in chloroform (1.0 mL) was added cyanogen bromide (10 µL, 31 µmol, 3M solution in CH2Cl2, 1.5 equiv). The solution was allowed to stir at 0 °C for 30 min, warmed to rt, and concentrated in vacuo. The crude oil was purified by flash column chromatography (silica gel, 20% EtOAc/hexanes) to give 26 (8.2 mg, 96%) as a colorless oil. [α]D23 –30 (c 0.79, CHCl3); IR (film, NaCl) 2929, 2205 (C≡N), 1693 (CO), 1454, 1422, 1262 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38-7.26 (m, 5H), 5.19 (d, J = 12.0 Hz, 1H), 3.05 (d, J = 12.0 Hz, 1H), 4.22 (br s, 1H), 4.07 (br d, J = 13.2 Hz, 1H), 3.41 (br d, J = 12.8 Hz, 1H), 2.95 (td, J = 11.4, 3.6 Hz, 1H), 2.85 (t, J = 12.8 Hz, 1H), 2.69 (br s, 1H), 2.01 (br d, J = 9.2 Hz, 1H), 1.67-1.25 (m, 16H), 1.07-0.85 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 155.6, 137.0, 128.7, 128.3 (br s, 2C), 117.1, 67.3, 57.1, 51.3, 49.7, 43.9, 39.6, 39.2, 38.4, 35.2, 27.9, 26.9, 26.6, 26.0, 25.6, 25.2, 22.5, 18.8; TLC Rf = 0.21 (30% EtOAc/hexane); HRMS calcd for C25H35N3O2 (M+H)+ 410.2808, found 410.2814.
Phlegmarine (1a)
A mixture 26 and 6M HCl(aq) (1.0 mL) was heated at reflux for 3 h. The solution was cooled to rt and then extracted with ether (3 × 0.5 mL). The organic phase was discarded and the aqueous phase was made basic by careful addition of solid K2CO3. More potassium carbonate was added until the solution became saturated. The aqueous phase was then extracted with EtOAc (14 × 1.0 mL) until the extracts no longer contained product by tlc. The crude solid was purified by flash chromatography (basic alumina (activity 2), MeOH) providing 1a as a solid. The solid was then taken up in hexanes (1.0 mL) and passed through a plug of cotton providing phlegmarine (1a) (4.8 mg, 96%) as a solid. [α]D24 -29 (c 0.39, CHCl3); IR (film, NaCl) 3230 (N-H), 2923, 2852, 1120, 743 cm−1; 1H NMR (400 MHz, CDCl3) δ 3.08-2.99 (m, 2H), 2.63 (dt, J = 11.8, 2.7 Hz, 1H), 2.56 (dt, J = 11.9, 2.5 Hz, 1H), 2.45-2.35 (m, 2H), 1.86-1.46 (m, 11H), 1.45-1.23 (m, 5H), 1.07 (tt, J = 11.2, 3.8 Hz, 1H), 1.05 (dt, J = 12.4, 4.0 Hz, 1H), 0.99-0.90 (m, 2H), 0.88 (d, J = 6.0 Hz, 3H), 0.80 (q, J = 13.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 56.1, 55.5, 47.5, 47.0, 46.1, 43.2, 39.7, 35.5, 35.2, 32.5, 28.9, 27.6, 27.0, 26.1, 25.0, 22.8; LRMS: 250 (M+, 7), 235 (5) 167 (12), 150 (14), 124 (6), 110 (9), 97 (24), 84 (100), 70 (90) 56 (10), 44 (80) m/z; HRMS calcd for C16H30N2 (M+H)+ 251.2487, found 251.2485.
Nβ-Methylphlegmarine (1b)
A solution of the mixed enol triflates 22 (17.7 mg, 32.5 µmol) and 20% palladium hydroxide on carbon (17.7 mg) in ethanol (1.0 mL) was stirred under an atmosphere of hydrogen gas for 6 h. The solution was filtered (Celite) and solid K2CO3 (100 mg) was added to the filtrate. The suspension was allowed to stir for 1 h, again filtered (Celite), and concentrated in vacuo. The residual oil was purified by flash chromatography (basic alumina (activity 2), 2% MeOH/CH2Cl2) to provide Nβ-methylphlegmarine (1b) (7.7 mg, 90 %) as a colorless oil. [α]D22 -65 (c 0.39 , CHCl3); IR (film, NaCl) 3276 (N-H), 2925, 2774 (N-Me), 1455, 1331, 1118, 1006 cm−1; 1H NMR (400 MHz, CDCl3) δ 3.06 (br d, J = 11.6 Hz, 1H) 2.82 (br d, J = 11.2 Hz, 1H), 2.63 (dt, J = 11.6, 2.8Hz, 1H), 2.41 (m, 1H), 2.24 (s, 3H), 2.06-2.00 (m, 2H), 1.80-1.58 (m, 9H), 1.45 (m, 8H), 1.04 (dt, J = 12.7, 4.6 Hz, 1H), 0.96 (m, 1H), 0.89 (d, J = 6.8 Hz, 3H), 0.72 (q, J = 11.9 Hz); 13C NMR (100 MHz, CDCl3) δ 63.0, 57.7, 56.1, 47.4, 44.6, 42.9, 39.9, 39.2, 35.5, 35.4, 32.3, 29.1, 26.8 (two carbons), 26.8, 24.9, 23.4; TLC Rf = 0.13 (5% MeOH/CH2Cl2, basic alumina); LRMS: 264 (M+, 20), 249 (22), 207 (16), 180 (12), 166 (55), 164 (44), 150 (10), 124 (40), 111 (38), 97 (28), 84 (100), 44 (68) m/z; HRMS calcd for C17H32N2 (M+H)+ 265.2644, found 265.2654.
Nα-Methylphlegmarine (1c)
Lithium aluminum hydride (400 µL, 400 µmol, 1.0 M in THF, 12 equiv) was added dropwise slowly into a solution of 26 (13.7 mg, 33.5 µmol) in THF (6.0 mL). The solution was then heated at reflux for 3 h, cooled to 0 °C, and water (10 µL) was added carefully followed by a 25% solution of NaOH (20 µL). The solution was warmed to rt and Celite (0.5 g) was added. After stirring for 1.5 h, the mixture was filtered through Celite, and the filter cake was washed with hot EtOAc (3 × 10 mL). Concentration of the filtrate gave a crude heterogeneous oil which was purified by flash column chromatography (basic alumina (activity 2), stepwise gradient from 75–100% EtOAc/hexanes then 5% MeOH/CH2Cl2) to afford Nα-methylphlegmarine (1c) (8.4 mg, 94%) as an oil. [α]D22 –77 (c 0.42, CHCl3); IR (film, NaCl) 3364 (N-H), 2926, 2777 (N-Me), 2852, 1455, 1371, 1026 cm−1; 1H NMR (300 MHz, CDCl3) δ 3.01 (br d, J = 12.2 Hz, 1H), 2.83 (br d, J = 11.4 Hz, 1H), 2.57 (dt, J = 12.2, 2.5 Hz, 1H), 2.42 (dt, J = 10.6, 3.6 Hz, 1H), 2.27 (s, 3H), 2.11 (dt, J = 11.4, 5.2 Hz, 1H), 1.84-1.4 (m, 15H), 1.39-1.05 (m, 4H), 1.00 (br t, J = 10 Hz, 1H), 0.89 (d, J = 6.4 Hz, 3H), 0.81 (q, J = 11.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 62.2, 57.3, 55.5, 47.1, 46.4, 43.4, 43.3, 38.4, 35.2, 30.7, 29.3, 28.9, 27.8, 26.2, 25.9, 24.3, 23.0; TLC Rf = 0.18 (5% MeOH/CH2Cl2, basic alumna); LRMS: 264 (M+, 0.07), 249 (0.5), 150 (5), 134 (1), 122(1), 110 (3), 98 (100), 82 (2), 70 (4), 54 (2), 44 (23) m/z; HRMS calcd for C17H32N2 (M+H)+ 265.2644, found 265.2643.
Nα-Acetyl-Nβ-methylphlegmarine (1d)
To a cooled solution (0 °C) of Nβ-methylphlegmarine (1b) (6.9 mg, 26 µmol) and pyridine (6.3 µL, 78 µmol) in dichloromethane (1.0 mL) was added acetyl chloride (5.6 µL, 78 µmol) dropwise. The solution was stirred at 0 °C for 30 min, warmed to rt for an additional 30 min, and then saturated NaHCO3 (0.5 mL) was added. The aqueous phase was extracted with EtOAc (5 × 0.5 mL), and then the combined organic phases were concentrated in vacuo to give a colorless oil. The crude material was purified by flash chromatography over basic alumina (activity 2, 2% MeOH/CH2Cl2) providing Nα-acetyl-Nβ-methylphlegmarine (1d) (7.6 mg, 95%) as a colorless oil. [α]D23 -75 (c 0.37, CHCl3); lit.7 [α]D - 11 (c 0.7, CHCl3) ; IR (film, NaCl) 2929, 2776 (NCH3), 1643 (C=O), 1424, 1263, 1005 cm−1; 1H NMR (300 MHz, CDCl3) δ 4.75 and 3.80 (2 br s due to rotamers, total 1H), 4.54 and 3.59 (2 br d due to rotamers, J = 13.0 Hz, total 1H), 3.15 and 2.63 (2 tt due to rotamers, J = 3.2, 13.2 Hz, total 1H) 2.83 (br d, J = 8.4 Hz, 1H), 2.25 and 2.23 (2 s due to rotamers, total 3H), 2.08 and 2.07 (2 s due to rotamers, total 3H), 2.02-1.90 (m, 3H), 1.68-1.24 (m, 18H), 1.09 and 1.02 (2 dt due to rotamers, J = 13.0, 3.8 Hz, total 1H), 0.92 (apparent t due to rotamers, J = 5.8 Hz, 3H), 0.75 and 0.71 (2 q due to rotamers, J = 11.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 169.0, 63.1 and 63.0 (doubled due to rotamers), 57.9 and 57.7 (doubled due to rotamers), 52.8 and 47.1 (doubled due to rotamers), 44.7 and 44.6 (doubled due to rotamers), 43.1, 42.3, 40.1 and 38.9 (doubled due to rotamers), 38.4 and 36.9 (doubled due to rotamers), 36.0 and 35.8 (doubled due to rotamers), 29.2, 27.3 and 27.2 (doubled due to rotamers), 26.8, 26.4 and 26.3 (doubled due to rotamers), 26.3 and 26.0 (doubled due to rotamers), 26.2 and 25.5 (doubled due to rotamers), 23.2 and 23.0 (doubled due to rotamers), 22.4 and 21.8 (doubled due to rotamers), 19.0 and 18.9 (doubled due to rotamers); TLC Rf = 0.23 (2.5% MeOH/CH2Cl2, basic alumina); LRMS: 306 (M+, 10), 291(7), 264 (1), 263 (4), 250 (2), 249 (11), 206 (3), 181 (1), 180 (6), 167 (13), 166 (100), 164 (7), 127 (3), 126 (32), 124 (5), 123 (4), 98 (2), 97 (10), 96 (8); HRMS calcd for C19H34N2O (M+H)+ 307.2749, found 307.2750.
Nα-Methyl-Nβ-acetylphlegmarine (1e)
To a cooled (0 °C) solution Nα-methylphlegmarine (1c) (8.4 mg, 32 µmol) and pyridine (8.6 µL, 110 µmol, 3.5 equiv) in dichloromethane (1.0 mL) was added acetyl chloride (7.5 µL, 110 µmol) dropwise. The solution was allowed to stir for 30 min at 0 °C and then warmed to rt for an additional 30 min. Saturated NaHCO3 (1.0 mL) was added and the phases were separated. The aqueous phase was extracted with dichloromethane (5 × 1 mL), and the combined organic phases were washed with brine (1.0 mL), dried (Na2SO4), filtered (Celite) and concentrated in vacuo. The crude oil was purified by flash column chromatography (basic alumina (activity 2), stepwise elution with 50–100% EtOAc/hexanes then 10% MeOH/CH2Cl2) providing Nα-methyl-Nβ-acetylphlegmarine (1e) (8.2 mg, 84%) as a colorless oil. [α]D23 –192 (c 0.40, CHCl3); IR (film, NaCl) 2928, 2777 (N-Me), 1640 (C=O), 1439, 1247, 1027 cm−1; 1H NMR at 50 °C (400 MHz, CDCl3) δ 3.71 (br m, 2H), 3.11 (br m, 1H), 2.82 (dt, J = 11.6, 3.4 Hz, 1H), 2.27 (s, 3H), 2.13 (dt, J = 11.6, 6.8 Hz, 1H), 2.05 (s, 3H), 2.03 (m, 1H), 1.92-1.55 (m, 13H), 1.42 (m, 1H), 1.35-1.02 (m, 6H), 0.94-0.85 (m, 1H), 0.91 (d, J = 6.4 Hz, 3H); 13C NMR at 50 °C (100 MHz, CDCl3) δ 169.9, 62.2, 56.8, 54.8, 43.2, 41.5, 39.6, 38.6, 37.9, 35.4, 30.7, 28.5, 26.8, 25.9, 24.1, 23.1, 23.0, 22.5, 22.1; TLC Rf = 0.29 (2.5% MeOH/CH2Cl2, basic alumina); LRMS: 306 (M+, 0.96), 291 (0.41), 263 (1.6), 207 (1.9), 192 (1.3), 150 (5.2), 110 (2.8), 98 (100), 70 (5.1), 55 (3.0), 44 (49) m/z; HRMS calcd for C19H34N2O (M+H)+ 307.2749, found 307.2747.
Supplementary Material
FIGURE 1.
Structures of the four known phlegmarine alkaloids and derivative 1e.
Acknowledgment
This work was supported in part by the National Institutes of Health (Grant No. GM 34442). NMR and mass spectra were obtained at NCSU instrumentation laboratories which were established by grants from the North Carolina Biotechnology Center and the National Science Foundation Grant (Grant No. CHE-0078253).
Footnotes
Supporting Information Available: Characterization data sheets for 1a,b and comparison data tables for 1c–e. NMR spectra for 1, 8, 19–23, and 26. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Ayer WA, Trifonov LS. In: The Alkaloids. Cordell GA, Brossi A, editors. Vol. 45. San Diego: Academic Press; 1994. pp. 233–274. [Google Scholar]; (b) Blumenkopf TA, Heathcock CH. In: Alkaloids: Chemical and Biological Perspectives. Pelletier SW, editor. Vol. 3. New York: Wiley; 1985. pp. 185–240. [Google Scholar]; (c) Ma X, Gang DR. Nat. Prod. Rep. 2004;21:752–772. doi: 10.1039/b409720n. [DOI] [PubMed] [Google Scholar]; (d) Kobayashi J, Morita H. In: The Alkaloids. Cordell GA, editor. Vol. 61. New York: Academic Press; 2005. p. 1. [Google Scholar]; (e) Hirasawa Y, Kobayashi J, Morita H. Heterocycles. 2009;77:679. [Google Scholar]
- 2.For pertinent reviews, see: Kozikowski AP, Tueckmantel W. Acc. Chem. Res. 1999;32:641. Ma X, Tan C, Zhu D, Gang DR, Xiao P. J. Ethnopharmacol. 2007;113:15–34. doi: 10.1016/j.jep.2007.05.030.
- 3.(a) Kobayashi J, Hirasawa Y, Yoshida N, Morita H. J. Org. Chem. 2001;66:5901. doi: 10.1021/jo0103874. [DOI] [PubMed] [Google Scholar]; (b) Kobayashi J, Hirasawa Y, Yoshida N, Morita H. Tetrahedron Lett. 2001;41:9069. [Google Scholar]; (c) Morita H, Arisaka M, Yoshida N, Kobayashi J. J. Org. Chem. 2000;65:6241. doi: 10.1021/jo000661e. [DOI] [PubMed] [Google Scholar]; (d) Tan C-H, Jiang S-H, Zhu D-Y. Tetrahedron Lett. 2000;41:5733. [Google Scholar]; (e) Gao W-Y, Li Y-M, Wang B-D, Zhu D-Y. Chin. Chem. Lett. 1999;10:463. [Google Scholar]; (f) Tan X-J, Wang H-Q, Jiang H-L, Zhu W-L, Jiang S-H, Zhu D-Y, Chen K-X, Ji R-Y. Huaxue Xeubao. 2000;58:1386. [Google Scholar]; (g) Wang B-D, Teng N-N, Zhu D-Y. Youji Huaxue. 2000;20:812. [Google Scholar]; (h) Gao W-Y, Wang B-D, Li Y-M, Jiang SH, Zhu D-Y. Chin. J. Chem. 2000;18:614. [Google Scholar]; (i) Gao W-Y, Li Y-M, Jiang S-H, Zhu D-Y. Planta Med. 2000;66:664. doi: 10.1055/s-2000-8630. [DOI] [PubMed] [Google Scholar]; (j) Morita H, Hirasawa Y, Shinzato T, Kobayashi J. Tetrahedron. 2004;60:7015–7023. [Google Scholar]
- 4.(a) Comins DL, Williams AL. Org. Lett. 2001;3:3217. doi: 10.1021/ol016556o. [DOI] [PubMed] [Google Scholar]; (b) Comins DL, Brooks CA, Al-awar RS, Goehring RR. Org. Lett. 1999;1:229. doi: 10.1021/ol990028j. [DOI] [PubMed] [Google Scholar]; (c) Comins DL, Libby AH, Al-awar RS, Foti CJ. J. Org. Chem. 1999;64:2184. doi: 10.1021/jo1019688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Comins DL, Joseph SP. In: Advances in Nitrogen Heterocycles. Moody CJ, editor. Vol. 2. Greenwich, CT: JAI Press Inc.; 1996. pp. 251–294. [Google Scholar]; (b) Comins DL, Joseph SP. In: Comprehensive Heterocyclic Chemistry. 2nd ed. McKillop A, editor. Vol. 5. Oxford, England: Pergamon Press; 1996. pp. 37–89. [Google Scholar]; (c) Comins DL. J. Heterocycl. Chem. 1999;36:1491. [Google Scholar]
- 6.For recent and leading references, see: Comins DL, Zhang Y. Y. Am. Chem. Soc. 1996;118:12248. Comins DL, Chen X, Morgan LA. J. Org. Chem. 1997;62:7435. doi: 10.1021/jo9711495. Comins DL, LaMunyon DH, Chen X. J. Org. Chem. 1997;62:8182. doi: 10.1021/jo971448u. Comins DL, Green GM. Tetrahedron Lett. 1999;40:217. Comins DL, Zhang Y, Joseph SP. Org. Lett. 1999;1:1941. doi: 10.1021/ol990738p. Kuethe JT, Comins DL. Org. Lett. 2000;2:855. doi: 10.1021/ol0056271. Huang S, Comins DL, Huang S, McArdle CL, Ingalls CL. Org. Lett. 2001;3:469. doi: 10.1021/ol0069709. Comins DL, Sandelier MJ, Abad Grillo T. J. Org. Chem. 2001;66:6829. doi: 10.1021/jo015834u.
- 7. Nyembo L, Goffin A, Hootele C, Braekman J-C. Can. J. Chem. 1978;56:851.. (b) For the recent isolation and synthesis of related phlegmarine-type Lycopodium alkaloids, see Tanaka T, Kogure N, Kitajima M, Takayama H. J. Org. Chem. 2009;74:8675. doi: 10.1021/jo9018182. and references cited therein.
- 8.(a) Leniewski A, Szychowski J, MacLean DB. Can. J. Chem. 1981;59:2479. [Google Scholar]; (b) Leniewski A, MacLean DB, Saunders JK. Ibid. 1981;59:2695. [Google Scholar]
- 9.The chloride was prepared (NCS, Ph3P, CH2Cl2) from the known enantiopure alcohol, see: Evans DA, Bender SL, Morris J. J. Am. Chem. Soc. 1988;110:2506.
- 10.Comins DL, Joseph SP, Goehring RR. J. Am. Chem. Soc. 1994;116:4719. [Google Scholar]
- 11. Comins DL, Salvador JM. J. Org. Chem. 1993;58:4656.. (b) The (+)- and (−)-TCC alcohols are available from Aldrich Chemical Co.
- 12.Comins DL, Brooks CA, Ingalls CL. J. Org. Chem. 2001;66:2181. doi: 10.1021/jo001609l. [DOI] [PubMed] [Google Scholar]
- 13.(a) Comins DL, Dehghani A. J. Org. Chem. 1995;60:794. [Google Scholar]; (b) Comins DL, Dehghani A. Chem. Comm. 1993:1838. [Google Scholar]
- 14.Comins DL, Al-awar RS. J. Org. Chem. 1995;60:711. [Google Scholar]
- 15.Rubiralta M, Giralt E, Diez A. Piperidine: Structure, Preparation, Reactivity and Synthetic Applications of Piperidine and its Derivatives. New York: Elsevier; 1991. Chapter 7. [Google Scholar]
- 16.(a) Comins DL, Dehghani A. Tetrahedron Lett. 1992;33:6299. [Google Scholar]; (b) Comins DL, Dehghani A, Foti CJ, Joseph SP. Org. Synth. 1996;74:77. [Google Scholar]
- 17.Cacchi S, Morera E, Ortar G. Tetrahedron Lett. 1984;25:4821. [Google Scholar]
- 18.Fleming I, Sanderson PE. Tetrahedron Lett. 1987;28:4229. [Google Scholar]
- 19.Schmidt SP, Brook DW. Tetrahedron Lett. 1987;48:1313. [Google Scholar]
- 20.For a review on amine dealkylations with acyl chlorides, see: Cooley JH, Evain EJ. Synthesis. 1989:1–7.
- 21.(a) von Braun J. Chem. Ber. 1900;33:1438. Review: [Google Scholar]; (b) Hageman HA. Org. React. 1953;7:198. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.