Abstract
Converging evidence suggests that the short allele of the serotonin transporter gene polymorphism increases risk for a variety of psychological disorders, including depression, anxiety, and alcoholism. Thus, the short allele is typically considered the “risk” allele, and findings related to the long allele are rarely discussed. However, upon closer examination, findings associated with the long allele of the serotonin transporter gene share striking similarities with findings from studies of psychopathy. Here, the parallels between findings associated with the long/long genotype and findings associated with psychopathic traits in the areas of neuropsychology, psychophysiology, hormones, and brain imaging are reviewed. It is suggested that the long/long genotype may be a potential risk factor for the development of psychopathic traits.
1. Introduction
The human serotonin transporter gene (SLC6A4) is considered the most investigated genetic variant in psychiatry, psychology, and neuroscience (Caspi et al., 2010). A common polymorphism in the promoter region (5-HTTLPR) of this gene results in two variants: a short allele and a long allele. A growing body of research suggests that the short allele is associated with increased psychological sensitivity to stress, thus increasing risk for multiple mental health-related conditions, including depression, anxiety, suicide, and stress-related substance abuse (Caspi et al., 2010). The presence of one or two short alleles has been linked to a number of intermediate endophenotypes, including increased stress hormone responses (Way & Taylor, 2010) and increased amygdala responses to threatening stimuli (Hariri et al., 2002), demonstrating the pathways by which this allele may confer risk.
While much attention has been paid to the risks associated with the short allele, findings regarding the long allele are rarely discussed. Compared to carriers of the short allele (denoted here as ‘s-carriers’), findings from individuals homozygous for the long allele (denoted here as ‘l-homozygotes’) tend to reflect reduced stress reactivity and reduced emotional responding. Many of these findings are very similar to findings observed in psychopathic individuals. Psychopathy is a disorder characterized by reduced emotionality (e.g., shallow affect, fearlessness, callousness), abnormal interpersonal features (e.g., superficial charm, egocentricity, deceitfulness), and behavioral traits (e.g., impulsivity, risk-taking, aggressiveness) that lead to frequent engagement in antisocial behavior (Hare, 2003). In this article, I reexamine the body of literature regarding the variants of the serotonin transporter, turning the focus to the long allele. Based on the number of parallels between these findings and findings of psychopathy, I propose that the long allele of the serotonin transporter may confer risk for psychopathy.
Functionally, the short allele is associated with reduced transcriptional activity of the serotonin transporter (often termed ‘low-activity’) compared to the long allele (Lesch et al., 1996). However, recent research suggests that there is an additional A→G single nucleotide polymorphism within the long allele, and that the LG version functions similarly to the short allele (Hu et al., 2005; Zalsman et al., 2006). Since many studies do not yet distinguish between the two versions of the long allele, I will focus only on the long/short distinction. However, I would hypothesize that the LA version is most likely to be associated with increased risk for psychopathy.
2. Gene Variants, Endophenotypes, and Complex Disorders
In most studies of psychological disorders, researchers are accustomed to establishing a direct association between the disorder and a particular phenomenon (e.g., depression and increased cortisol responses). However, linking a single genetic polymorphism to a psychological disorder is often less practical, because psychological disorders are complex and are likely influenced by many genetic polymorphisms, as well as environmental factors (Canli & Lesch, 2007), making the influence of one polymorphism very small. It is likely that the effect is further diminished by the fact that the characteristics of many disorders, including psychopathy, exist on a continuum, whereas many commonly studied genetic polymorphisms are biallelic, resulting in only three possible conditions (e.g., ll, ls, ss). Thus, studies attempting to link a candidate gene often require very large sample sizes to detect an effect. Indeed, studies directly linking the short allele of the serotonin transporter to behavior report very small effects, often accounting for only 3–4% of the variance (Lesch et al., 1996).
Experts in imaging genetics suggest that a promising approach in bridging the gap between gene variants with small effects and complex disorders is the use of endophenotypes (Hariri, 2009). This involves identifying genetic polymorphisms that are associated with particular intermediate effects (e.g., brain structure and function, physiology, neuropsychological effects) that are known to exist in that disorder. By showing that a particular gene variant is associated with a set of endophenotypes that have been observed in a complex disorder, we can infer that the gene variant confers some risk for the disorder. In this article, I implement the approach of identifying endophenotypes that have been associated with the long allele of the serotonin transporter and with psychopathy.
It should be noted that many of the endophenotypes observed in l-homozygotes have been reported not only in psychopathic individuals, but also in healthy control subjects and are not by themselves indicators of psychopathy. In populations of European ancestry, the genotype frequencies of the 5-HTTLPR are distributed in Hardy-Weinberg equilibrium (LL = 0.36, LS = 0.48, SS = 0.16) (Gelernter et al., 1997), meaning that approximately 36% of this population are l-homozygotes. In contrast, the prevalence of categorical psychopathy is approximately 1% of the population. Thus, the majority of l-homozygotes are not high in psychopathic traits, just as the majority of s-carriers do not have clinical depression or anxiety. The hypothesis presented here is that homozygosity for the long allele may be one factor among many that may contribute to the development of psychopathic traits.
A few very recent studies have begun to explore the direct associations between psychopathic traits and variants in the serotonin transporter; however, results remain unclear. Herman et al. (in press) examined the relationship between the serotonin transporter genotypes and psychopathy in a sample of individuals with alcohol dependence. Psychopathy was assessed using the Socialization scale of the California Psychological Inventory, which has been shown to demonstrate relatively strong associations with interview-based measures of psychopathy (Hare, 1985), as well as more recent self-report measures (Chapman et al., 2003). Males homozygous for the long allele were higher in psychopathy than s-carriers. However, females homozygous for the short allele were higher in psychopathy than l-homozygotes. This suggests that gender may be a potential moderator of the effects. Two additional studies examined the relationship of these genotypes with psychopathic traits in adolescents but found contradictory results. In adolescents with attention deficit hyperactivity disorder (ADHD), Fowler et al. (2009) found higher total psychopathy scores in s-homozygotes than l-homozygotes. This effect was small and was driven by the ‘emotional dysfunction’ aspect of psychopathy. In contrast, Sadeh et al. (in press) found that callous-unemotional and narcissistic traits were increased in l-homozygotes, although only in individuals with low socioeconomic status (SES). The discrepancies within and between these studies emphasize the difficulties in establishing a direct link between gene variants and a complex disorder. However, studies such as these are extremely valuable in helping to uncover the ways in which particular genetic polymorphisms may interact with other genes or environmental factors such as SES in conferring risk for a disorder.
3. Evidence from Endophenotypes
Initial studies demonstrating links between the short allele of the serotonin transporter gene and depressive symptomology have spurred great interest testing the effects of this allele on a variety of intermediate biological and behavioral processes, including brain structure and function, psychophysiology, hormone processes, and neuropsychological indicators. In each of these domains, parallels exist between findings in l-homozygotes and findings from studies of psychopathic individuals. A summary of these similarities is listed in Table 1.
Table 1.
Parallels between Findings in Psychopathic Individuals and L-Homozygotes
| Psychopathic individuals relative to controls | Study | L-homozygotes relative to s-carriers | Study | |
|---|---|---|---|---|
|
Brain level | ||||
| Amygdala responsivity to negative stimuli | Reduced |
Kiehl et al. (2001) Glenn et al. (2009) Birbaumer et al. (2005) |
Reduced |
Hariri et al. (2002) Heinz et al. (2005) |
| Connectivity between amygdala and VMPFC | Reduced |
Marsh et al. (2008) Craig et al. (2009) |
Reduced |
Heinz et al. (2005) Pezawas et al. (2005) |
| Error processing in the prefrontal cortex | Reduced error-related negativity Reduced positive ERP amplitude (Pe) |
Von Borries et al. (in press) Brazil et al. (2009) |
Reduced error-related negativity Trend toward reduced positive ERP amplitude (Pe) |
Fallgatter et al. (2004) Fallgatter et al. (2004) |
|
Psychophysiology | ||||
| Heart rate (resting) | Reduced | Hansen et al. (2007) | Reduced | Crisan et al. (2009) |
| Heart rate variability | Increased | Hansen et al. (2007) | Increased | Crisan et al. (2009) |
| Fear potentiated startle | Reduced |
Flor et al. (2002) Others reviewed in Patrick (1994) |
Reduced |
Brocke et al. (2006) Lonsdorf et al. (2009) |
| Reduced skin conductance responding during fear conditioning | Reduced | Flor et al. (2002) | Reduced | Garpenstrand et al. (2001) |
|
Hormones & Neurotransmitters | ||||
| Cortisol response to the Trier Social Stress Test (TSST) | Reduced |
O’Leary, Loney & Eckel (2007) O’Leary, Taylor & Eckel (in press) |
Reduced |
Gotlib et al. (2008) Alexander et al. (2009) Way & Taylor (2010) |
| Central serotonin functioning (fenfluramine challenge) | Elevated | Dolan & Anderson (2003) | Elevated | Reist et al. (2001) |
|
Neuropsychology | ||||
| Attention to negative stimuli in the periphery | Reduced |
Glass & Newman (2009) Newman et al. (2009) |
Reduced |
Beevers et al. (2007) Osinsky et al. (2008) Fox et al. (2009) |
| Passive avoidance learning | Poor |
Newman & Kosson (1986) Blair et al. (2004) |
Poor | Finger et al. (2007) |
| Risk taking (Balloon Analog Risk Task; BART) | Increased risk-taking | Hunt, Hopko & Bare (2005) | Increased risk-taking | Crisan et al. (2009) |
| Decisions based on reward and punishment | Poor | Blair et al. (2006) | Poor | Roiser et al. (2006) |
3.1. Brain level
In one of the most prominent studies in imaging genetics, Hariri et al. (2002) used functional magnetic resonance imaging (fMRI) to explore the link between genetic variation in the serotonin transporter gene and brain activity. The authors compared activity in the amygdala in s-carriers relative to l-homozygotes while participants viewed pictures of angry and afraid faces. S-carriers demonstrated a large increase in amygdala activity (28%) compared to l-homozygotes. The authors suggest that this large increase in s-carriers may reflect a hyperresponsiveness to environmental stimuli, which may predispose an individual toward stress-related psychopathology. However, it is also worth noting that the response of the amygdala in l-homozygotes was nearly zero, as l-homozygotes exhibited only a 3% increase in activity. Whereas too much amygdala reactivity to threatening stimuli may predispose toward stress-related pathology, too little amygdala reactivity has also been shown to be detrimental, as observed in studies of psychopathy. It has been suggested that reduced amygdala functioning is a key biological factor in psychopathy (Blair, 2008) due to its role in aversive conditioning and general emotional responsivity. Several recent brain imaging studies have provided support for this hypothesis, finding reduced activity in the amygdala during tasks involving negative or emotionally aversive stimuli (Birbaumer et al., 2005; Glenn et al., 2009; Kiehl et al., 2001). One possibility is that the bias in amygdala reactivity associated with the long allele of the serotonin transporter gene may contribute to the reduced amygdala functioning and related effects observed in psychopathy.
Findings linking the genetic variants of the serotonin transporter gene to amygdala functioning were replicated and extended by Heinz et al. (2005), who showed pictures of negative, neutral, and positive visual stimuli. Differential activation for carriers of the long and short alleles was only observed in the negative stimuli condition, suggesting that the reduction in amygdala activity in l-homozygotes appears to be specific to negative, but not positive stimuli. This finding is in line with several studies of psychopathy demonstrating deficits in response to negative emotional stimuli (Blair et al., 2001; Levenston et al., 2000; Patrick et al., 1993), but not necessarily positive stimuli (Kimonis et al., 2006). Differences have also been observed in the level of cerebral blood flow in the amygdala while at rest (i.e., not engaged in any task), with l-homozygotes demonstrating a reduction in blood flow compared to s-homozygotes (Rao et al., 2007). To the author’s knowledge, resting cerebral blood flow has not yet been examined in relation to psychopathy, but future studies will be able to determine whether this is also reduced in psychopathic individuals.
In addition to reduced amygdala functioning, two neuroimaging studies have also demonstrated that the long allele of the serotonin transporter is associated with a reduction in the functional connectivity between the amygdala and the ventromedial prefrontal cortex (VMPFC) (Heinz et al., 2005; Pezawas et al., 2005), a region important in decision-making that is thought to integrate input from the amygdala to guide appropriate behavioral responses. This functional decoupling between the amygdala and VMPFC has also been observed in studies of psychopathy. Marsh et al. (2008) found reduced functional connectivity between the amygdala and VMPFC in youth with psychopathic traits. Structural abnormalities have also been observed in the white matter pathways connecting the amygdala and VMPFC in psychopaths (Craig et al., 2009). Together these studies suggest that genetically driven variation in the serotonin transporter may shape the functioning and connectivity of the amygdala, leading to reduced responsivity to emotional cues and impaired conditioning abilities, which are necessary for appropriate moral development and behavior.
Finally, there are also parallels between studies of psychopathy and the serotonin transporter gene that have examined error processing in the prefrontal cortex by measuring event related potentials (ERPs). One example is in measuring error-related negativity (Ne/ERN), which occurs when a subject commits an error in a cognitive task; this is thought to reflect functioning of the anterior cingulate cortex. The Ne/ERN is followed by a positive ERP, denoted Pe, which also originates in the prefrontal cortex. One study comparing s-carriers to l-homozygotes found significantly lower amplitudes of the Ne/ERN and a trend toward lower amplitudes of the Pe in l-homozygotes (Fallgatter et al., 2004). Similarly, a recent study of psychopathy observed reduced Pe amplitudes in psychopaths compared to controls (Brazil et al., 2009). In another study, diminished amplitudes of the ERN were observed in psychopathic individuals when participants made errors on a probabilistic learning task (von Borries et al., in press). Together these studies suggest that both psychopathic individuals and individuals homozygous for the long allele of the serotonin transporter have abnormal functioning in the prefrontal cortex during the processing of error information.
3.2. Psychophysiological level
Some of the first biological observations regarding psychopathy were made in the area of psychophysiology. Recent research on the effects of the serotonin transporter polymorphism mirrors many of these findings from psychopathy. For example, one of the best replicated findings in psychopathy research is impaired aversive conditioning. This has been observed using two types of measures – fear potentiated startle, which reflects a basic, affective level of fear conditioning that is largely independent of higher cognitive processes – and skin conductance responding, which incorporates cognitive contingency learning (Hamm & Weike, 2005). Reduced startle responding has been observed in several studies of psychopathy using different paradigms (reviewed by Patrick, 1994). In a study of fear conditioning, Flor et al. (2002) found that psychopathic individuals did not differentiate between the reinforced conditioned stimulus (CS+) and the nonreinforced conditioned stimulus (CS−) in the fear potentiated startle response. Similarly, in a study of genetic influences on fear potentiated startle implementing a very similar design, Lonsdorf et al. (2009) found that l-homozygotes failed to show distinguishing startle responses to the two types of conditioned stimuli. Reduced startle responding has additionally been observed across multiple valence conditions in l-homozygotes (Brocke et al., 2006).
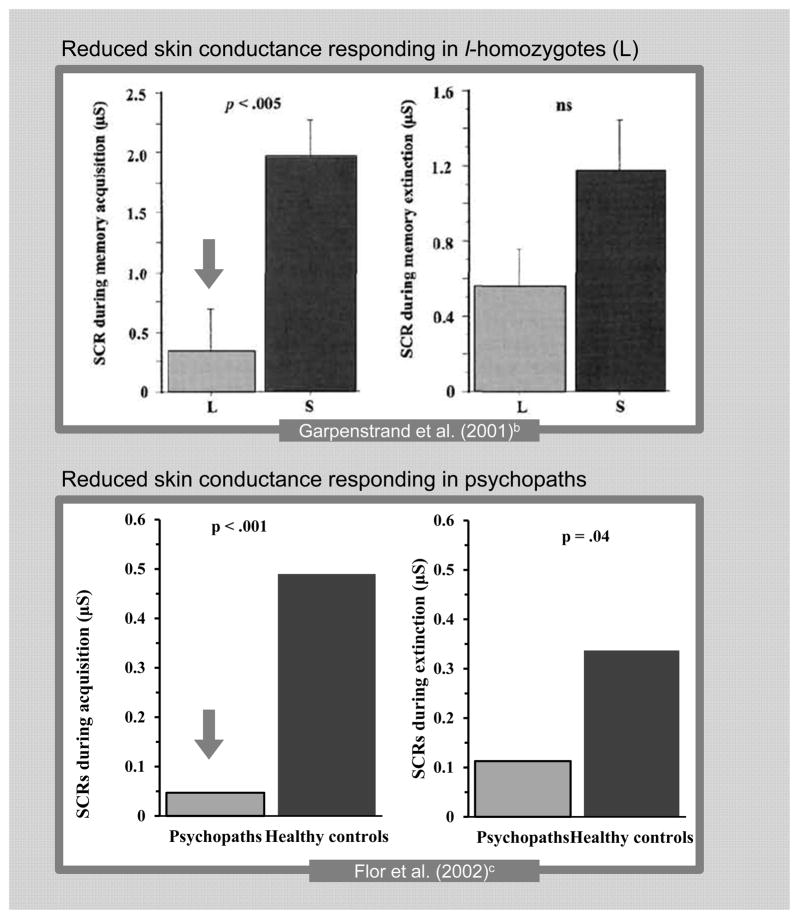
Measures of skin conductance responding during fear conditioning also reflect parallels. Reduced skin conductance responses during the acquisition phase of fear conditioning have been observed in l-homozygotes (Garpenstrand et al., 2001) and in psychopathic individuals (Flor et al., 2002), as depicted in Figure 1. In psychopathy research, it is suggested that such reductions reflect an inability to form associations between harmful transgressions and the distress of a victim (Blair, 2008).
Figure 1. Similar Reductions in Skin Conductance Responding during Fear Conditioninga in L-homozygotes and Psychopaths.
a The bars in both sets of graphs represent the mean difference between responses to the reinforced conditioned stimulus (CS+) compared to the nonreinforced conditioned stimulus (CS−). Differences in scale are due to the log transformation in Flor et al. (2002).
b Reprint of Figure 2 in “Human Fear Conditioning is Related to Domaminergic and Serotonergic Biological Markers” by Garpenstrand et al., 2001, Behavioral Neuroscience, 115, 358–364. Copyright 2001 by APA.
c With permission of the authors, data are adapted from Figure 2 in “Aversive Pavlovian Conditioning in Psychopaths: Peripheral and Central Correlates,” by Flor et al., 2002, Psychophysiology, 39, 505–518. Copyright 2002, Society for Psychophysiological Research.
In another domain of psychophysiology, male prisoners scoring higher in psychopathy have been found to have lower resting heart rate and increased heart rate variability (Hansen et al., 2007). Similarly, l-homozygotes have lower resting heart rate and increased heart rate variability compared to s-carriers (Crisan et al., 2009). Increased heart rate variability has previously been associated with low anxiety (Thayer et al., 1996) and with aggression (Scarpa et al., 1999). Lower resting heart rate has also been associated with antisocial behavior; it has been suggested that it may reflect a lack of fear or autonomic underarousal, which may increase the tendency toward stimulation-seeking (Raine, 1993), a characteristic of psychopathy.
3.3. Hormone and neurotransmitter level
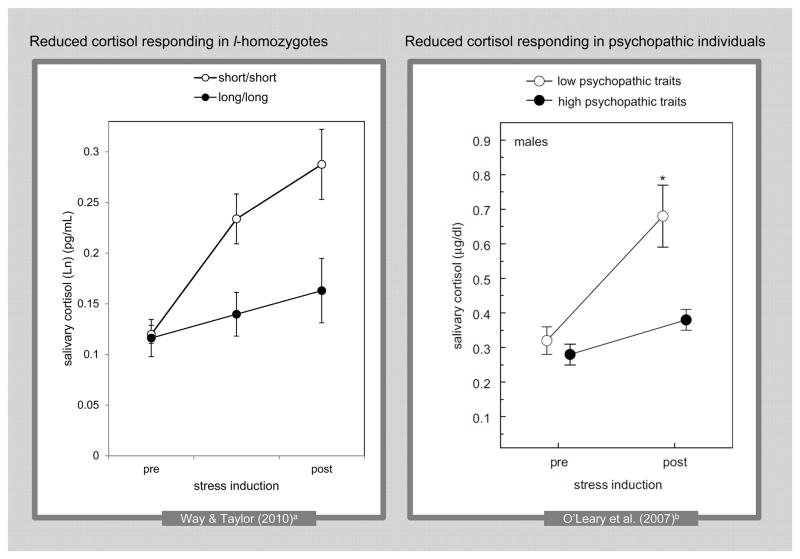
Similar findings have been observed regarding the release of the hormone cortisol in response to a stressor. Using the Trier Social Stress Test (TSST), a widely used laboratory stress challenge involving mental arithmetic and delivering a speech in front of a critically evaluative audience, Way & Taylor (2010) found that individuals homozygous for the short allele of the serotonin transporter demonstrated a significant cortisol response to the stressor, whereas l-homozygotes did not. This effect has also been observed in other studies of the cortisol response to stressors (Alexander et al., 2009; Gotlib et al., 2008). Way & Taylor (2010) focus their discussion on individuals with the short/short genotype, suggesting that these individuals might be especially vulnerable to social threat, which may put them at greater risk for psychological problems such as depression, anxiety, and suicide. However, with respect to the long/long genotype, these findings may also suggest that l-homozygotes may be relatively unresponsive to cues of social threat, which may predispose toward psychopathy.
Indeed, using the same task, O’Leary et al. (2007) found that males scoring low in psychopathy displayed a significant cortisol response, whereas males scoring higher in psychopathy did not. A comparison of these results with those of Way & Taylor (2010) is depicted in Figure 2. In addition, O’Leary et al. (2010) recently replicated this finding in males and extended it to women when accounting for progesterone levels. In this study, a reduced cortisol stress response was also observed in response to a stressor involving social rejection. Together these findings suggest that the long/long genotype may be one of the precursors to the reduced stress responsivity observed in psychopathic individuals. Such individuals may be more likely to be unresponsive to cues in the environment that may be important for guiding appropriate moral behavior.
Figure 2. Similar Reductions in Cortisol Reactivity to the Trier Social Stress Test (TSST) in L-homozygotes and Psychopathic Individuals.
a Data represent average cortisol values during the negative evaluative condition of the TSST for the 0-, 20-, and 40-minute time intervals. Data were provided by the authors of “The Serotonin Transporter Promoter Polymorphism Is Associated with Cortisol Response to Psychosocial Stress,” by B.M. Way & S.E. Taylor, 2010, Biological Psychiatry, 67, 487–492. Copyright 2009, Society of Biological Psychiatry.
b Reprint of Figure 1 (males) in “Gender Differences in the Association between Psychopathic Personality Traits and Cortisol Response to Induced Stress,” by M.M. O’Leary, B.R. Loney, & L.A. Eckel, 2007, Psychoneuroendocrinology, 32, 183–191. Copyright 2007, Elsevier, Ltd. Data represent average cortisol values during the TSST for the 0- and 20-minute time intervals.
At the neurotransmitter level, although the short and long alleles of the serotonin transporter gene have been associated with differential transcriptional efficiency of the serotonin transporter (Lesch et al., 1996), it is still unclear whether this is translated into differences in levels of serotonin or the serotonin transporter (Jedema et al., 2010). However, Reist et al. (2001) compared the central serotonin functioning of l-homozygotes and s-carriers by administering D-fenfluramine, a challenge drug that has proven to be effective in measuring overall serotonin activity. They found that the fenfluramine-induced prolactin release was greater in l-homozygotes, indicating elevated central serotonin functioning. Using this same fenfluramine challenge, Dolan & Anderson (2003) found that the interpersonal aspects of psychopathy (e.g., arrogant, deceitful) were associated with increased central serotonin functioning. Future research will be needed to clarify these processes before more concrete interpretations can be provided.
3.4. Neuropsychological level
Several neuropsychologial effects have been observed in l-homozygotes that are similar to those in psychopathy. For example, three studies have shown that, unlike s-carriers, l-homozygotes fail to show an attentional bias toward negative stimuli that is presented as peripheral, or unrelated, to the assigned task (Beevers et al., 2007; Fox et al., 2009; Osinsky et al., 2008). In contrast to s-carriers, who may be especially vigilant for threat-related information, l-homozygotes may fail to observe cues in the environment that signal potential threat. Similarly, a recent study showed that psychopaths failed to demonstrate the commonly observed memory bias for emotional over neutral words when the words were not the primary focus of attention (Glass & Newman, 2009). In another study by Newman et al. (2009), psychopathic prisoners failed to respond to cues of threat when presented as peripheral to the assigned task. This may contribute to the fearlessness observed in psychopathy.
Another neuropsychological finding, which has been replicated several times, is poor passive avoidance learning in psychopathic individuals (Blair et al., 2004; Newman & Kosson, 1986). It is suggested that this deficit may be related to the amygdala impairment and poor aversive conditioning observed in psychopathy (Blair et al., 2004). Using the same passive avoidance task as these studies, Finger et al. (2007) found that l-homozygotes also demonstrate impairments in passive avoidance learning; they are slower to learn to avoid punishment stimuli than s-carriers (Finger et al., 2007).
In addition to poor passive avoidance learning, there is also some indication of poor response reversal in both psychopathic individuals (Budhani & Blair, 2005; Mitchell et al., 2002) and l-homozygotes (Finger et al., 2007). However, findings regarding l-homozygotes were only observed during tryptophan depletion, and thus should be interpreted cautiously (this comparison is not listed in Table 1). Both psychopathic individuals and l-homozygotes under tryptophan depletion exhibit a greater number of overall errors on a response reversal task and are more likely to maintain a previously incorrect response.
L-homozygotes also demonstrate increased risk taking, which is commonly observed in psychopathy. In two separate studies, l-homozygotes and psychopathic individuals have shown increased risk taking on the Balloon Analog Risk Task (BART; Lejuez et al., 2002), a measure of risk taking in which participants can earn financial rewards by pumping balloons presented on a screen (Crisan et al., 2009; Hunt et al., 2005). On a risky choice task, l-homozygotes have also been found to attend less to the probabilities of winning versus losing compared to s-carriers (Roiser et al., 2006). The authors speculate that the affective responses elicited by cues signaling the probability of receiving punishment or reward may be greater in s-carriers than l-homozygotes, and refer to the differences that have been observed in functioning of regions such as the amygdala that are important in decision-making. Psychopathic individuals have also demonstrated impaired decision-making on the basis of reward and punishment information. One study found that individuals with psychopathy show significant impairment when choosing between objects associated with differential levels of reward and punishment (Blair et al., 2006).
It should be noted that the findings on measures of risk taking for both l-homozygotes and psychopathic individuals are variable, depending on the task. For example, using the Iowa Gambling Task, a common measure of risk-taking, Ha et al. (2009) found that the relationship between the serotonin transporter genotype and performance on the task is dependent on the allele of the dopamine receptor. Findings regarding the Iowa Gambling Task in psychopathic individuals have also been mixed (Mitchell et al., 2002; Schmitt et al., 1999), with one study finding impairments only in psychopathic individuals with low attention (Losel & Schmucker, 2004).
3.5. Inconsistent findings
Although multiple findings in l-homozygotes appear to reflect findings from the study of psychopathy, this literature is not without inconsistencies. For example, Pezawas et al. (2005) observed larger gray matter volumes in the amygdala of l-homozygotes, which is the opposite of recent findings of reduced gray matter volume of the amygdala in psychopathic individuals (2009). However, Pezawas et al. did not observe a significant correlation between amygdala volume and the amygdala reactivity measured in the group’s previous study (Hariri et al., 2002), suggesting that the genotype differences in structure and function of the amygdala may not necessarily be related. Furthermore, Rao et al. (2007) did not observe differences in amygdala volume between l-homozygotes and s-homozygotes.
Another discrepancy is between two studies examining the structural integrity of the uncinate fasciculus (UF), a white matter tract connecting the amygdala and the medial/orbitofrontal cortex. In a study of psychopathic individuals, the structural integrity of this pathway was found to be reduced (Craig et al., 2009). However, Pacheco et al. (2009) found that the long-allele of the serotonin transporter to be associated with increased structural integrity of the UF. The interpretation of these findings with respect to the functioning of this pathway remains unclear, since the long-allele has also been associated with reduced functional connectivity between these regions (Heinz et al., 2005; Pezawas et al., 2005).
Undoubtedly, there are several other findings that are inconsistent between research on psychopathy and on the long allele of the serotonin transporter gene. Moreover, there are a number of findings that are inconsistent within these fields that remain to be clarified, making it difficult in some cases to make comparisons. However, given the number of findings that do correspond, it may be beneficial to consider the possibility that the long allele may also be a risk factor for psychopathology – in the domain of psychopathy. In the seventeen studies reviewed here examining the effects of the serotonin transporter gene polymorphism, nearly all discuss the results primarily in terms of depression, anxiety, or stress-related disorders. However, a focus on findings associated with the long allele reveals many similarities to findings from brain imaging, psychophysiology, hormones, and neuropsychology in the field of psychopathy. As research on the serotonin transporter continues, it will be interesting to see whether findings continue to resemble those observed in psychopathy.
4. Aggression and the Serotonin Transporter
One issue in particular that may seem contradictory to the hypothesis presented here is that violence and aggression have consistently been associated with the short allele of the serotonin transporter and other indicators of reduced serotonin availability (Frankle et al., 2005; Retz et al., 2004). However, aggression is heterogeneous, and can roughly be dichotomized into two subtypes: a reactive-hostile-affective subtype (reactive aggression) and a controlled proactive-instrumental-predatory subtype (instrumental aggression) (Reif et al., 2007; Vitiello & Stoff, 1997). Whereas most psychological disorders involving violence are primarily associated with reactive aggression, psychopathy is unique in that it is associated with elevated levels of both reactive and instrumental aggression. Increased instrumental aggression is often a feature that distinguishes psychopathic criminals from nonpsychopathic criminals (Porter & Woodworth, 2006).
Most studies investigating the association between the serotonin transporter genotype and aggression either have not differentiated between the two types of aggression, or have primarily examined reactive, impulsive aggression (Reif et al., 2007). It is suggested that these types of aggression likely have distinct neurobiological underpinnings. Reactive aggression is relatively spontaneous and is associated with a low threshold for activating negative emotions such as fear and anxiety, which become accentuated, leading to retaliatory, aggressive behavior (Reif et al., 2007). Thus, it makes sense that the short allele, which is associated with hyperresponsivity to environmental stressors or cues of threat, may predispose individuals toward reacting violently. In contrast, the instrumental violence observed in psychopathic individuals likely stems from the opposite effect – a genetic insensitivity to environmental cues. Psychopathic individuals demonstrate insensitivity to cues of punishment, as well as insensitivity to cues that others may be in distress (Blair et al., 2001), leaving them undeterred from committing acts of violence. I propose that this type of aggression is more likely to be associated with the long allele of the serotonin transporter, which, as outlined, is associated with hyporesponsivity to environmental cues.
5. Potential Mechanisms
The exact mechanism whereby serotonin transporter gene polymorphisms mediate variability in brain functioning and behavior remains unknown, although a few possibilities have been suggested. In vitro studies have suggested that the long allele is associated with relatively increased serotonin transporter gene transcription, resulting in increased transporter levels and more rapid serotonin uptake (Greenberg et al., 1999; Lesch et al., 1996), which would imply that there may be reductions in extracellular serotonin in l-homozygotes. However, several in vivo studies have not observed such differences in serotonin transporter levels (e.g., Parsey et al., 2006; Willeit et al., 2001).
Another possible mechanism of action is the effect of the polymorphism on binding at pre- and post-synaptic autoreceptors, which has been found to be decreased in s-carriers (David et al., 2005). These autoreceptors are involved in regulating the excitability of neurons, and thus could alter the functioning of brain regions such as the amygdala that are densely innervated by serotonin neurons (Hariri & Holmes, 2006). With respect to psychopathy, it may be that homozygosity for the long allele results in hyporeactivity of neurons in the amygdala or other regions that have been implicated in psychopathy.
A final potential mechanism is based on a study of rhesus macaques that suggests that the variation in genotype may act through effects on cortical development, rather than via static differences in serotonergic signaling mechanisms such as serotonin transporter binding (Jedema et al., 2010). With respect to psychopathy, one hypothesis is that the long allele affects the development of one or more of the brain regions that have been implicated in psychopathy such as the amygdala or orbitofrontal cortex, thereby contributing to the development of psychopathic traits. Such developmental morphological differences may also lead to differences in the connectivity between regions. Future research is needed to determine the precise molecular and cellular mechanisms by which homozygosity for the long allele may confer risk for psychopathic traits.
6. Future Directions
Numerous studies could be conducted to further examine whether the endophenotypes associated with the long allele of the serotonin transporter gene correspond to the endophenotypes observed in psychopathy, as there are many findings in psychopathy that have not been examined in relation to the serotonin transporter, and vice versa. In addition, research is needed to determine the mechanisms by which the serotonin transporter gene polymorphism influences brain functioning in order to clarify how it may confer risk for psychopathy. As discussed above, currently it is unclear whether brain and behavioral differences associated with the serotonin transporter genotype act primarily through alterations in serotonin levels, action of serotonin transporters, binding at autoreceptors, through developmental morphological changes, or by some other mechanism or a combination of mechanisms. Elucidating this, as well as identifying which brain regions may be most affected and in what ways, will help researchers to develop hypotheses regarding how homozygosity for the long allele may confer risk for psychopathic traits. The use of well validated animal models, such as that used in the study by Jedema et al. (2010) may have great potential for furthering our understanding of the effects of the serotonin transporter genotype in humans. Such a model may be particularly useful in examining transient alterations in serotonin or the serotonin transporter that may occur after particular events such as a stressor – an event in which psychopathic individuals have been found to have clear differences in responding.
Additional studies examining the direct association between the serotonin transporter genotype and psychopathy will also be important. As more studies are conducted, meta-analytic techniques may become useful in integrating the data to gain a clearer picture of the relationship. In studies examining a direct link between the genotype and psychopathy, it will be beneficial to simultaneously consider additional genetic variants, gender, and environmental factors, since genes do not operate independently, but function against a background of other essential factors. The importance of environmental factors is emphasized by research linking the short allele to depression and anxiety, in which numerous studies demonstrate interactions with environmental stressors (Caspi et al., 2010). Similarly, in the study by Sadeh et al. (in press) linking the long allele to psychopathic traits in adolescents, an interaction was also observed with an environmental factor. This is in line with behavioral genetics studies which suggest that psychopathic traits are heritable, but that also find significant effects of nonshared environmental factors (Blonigen et al., 2003; Larsson et al., 2006). One possibility for future research might be to explore how the two serotonin transporter alleles interact differently with environmental factors to predispose toward different disorders.
The importance of examining additional genetic factors has also been demonstrated; several studies have found interactions between the serotonin genotypes and other genetic polymorphisms in predicting various disorders and behaviors (Benjamin et al., 2000; Ha et al., 2009; Skowronek et al., 2006). One polymorphism that may be of particular interest is a second polymorphism of the serotonin transporter gene that exists within intron 2 (Stin2). Stin2 comprises 9, 10, or 12 copies of a 16–17 base pair repeat (Battersby et al., 1996). Although Stin 2 has most often been studied separately from the linked promoter region polymorphism discussed in this review, recent research suggests that these two polymorphisms are likely to act on the same signaling pathway, acting in concert to modulate serotonin transporter gene expression (Ali et al., 2010; Hranilovic et al., 2004). These two polymorphisms have been found to differentially affect gene expression and to interact in a non-additive way. The interaction of these two polymorphisms may be most pronounced during development, potentially disrupting normal maturation of neuronal networks and thus increasing vulnerability for psychiatric disorders (Ali et al., 2010). It is suggested that the combined examination of these two polymorphisms may be able to better account for variation in brain functioning and behavior, making Stin2 an important factor to consider in future research on psychopathy.
Future studies investigating genetic polymorhpisms associated with serotonin and other neurotransmitters may help to clarify the role of these systems in the development of psychopathy. Indeed, some evidence from psychopathy research provides initial indication that gene-gene interactions are likely involved. Soderstrom et al. (2001; 2003) found that psychopathy scores were associated with the ratio between metabolites of dopamine and serotonin.
7. Conclusions
In the midst of a growing number of studies uncovering biological abnormalities that are associated with psychopathy, an important question is how these abnormalities may arise. By identifying candidate genes that contribute to variability in biological processes, we begin to shed light on this issue. For example, evidence suggests that serotonergic neurons are among the first neurons to be generated, and that serotonin plays an important role in cortical development (Gaspar et al., 2003). Thus, serotonin may affect the development of the brain regions and circuits that have been implicated in psychopathy, depression, and other disorders. Understanding the link between genes, brain, and behavior will be crucial in understanding how different disorders develop.
Another advantage of identifying candidate genes is that they can help to uncover the specific disease process that takes place in an individual (Hariri, 2009). Since there are often multiple pathways that lead to a particular disorder, testing for specific genetic polymorhpisms may provide information about the processes that may be contributing to psychopathology within a single individual. Such information could then be used in the development of personalized treatments (Hariri, 2009). It could also be useful in establishing predictive risk markers (Hariri, 2009); if it is known that a genotype interacts with a particular environmental factor, preventative measures could be developed to alter the environment in a way that risk for psychopathology could be reduced.
Overall, studies in several domains suggest that the presence of two long alleles may lead to an increased vulnerability for reduced emotional responding and psychopathic traits in the context of additional genetic and environmental factors. These findings suggest a potential causal mechanism for the impaired emotional neural circuitry observed in psychopathy. The study of such genetic mechanisms may help to further our understanding of the neurodevelopmental processes that may alter the neural circuitry implicated in psychiatric disorders.
References
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: Implications for the etiology of depression. Psychoneuroendocrinology. 2009;34:1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Ali FR, Visiliou SA, Haddley K, Paredes UM, Roberts JC, Miyajima F, et al. Combinatorial interaction between two human serotonin transporter gene variable number tandem repeats and their regulation by CTCF. Journal of Neurochemistry. 2010;112:296–306. doi: 10.1111/j.1471-4159.2009.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby S, Ogilvie AD, Smith CA, Blackwood DH, Muir WJ, Quinn JP, et al. Structure of a variable number tandem repeat of the serotonin transporter gene and association with affective disorder. Psychiatric Genetics. 1996;6:177–181. doi: 10.1097/00041444-199624000-00001. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. J Abnorm Psychol. 2007;116:208–212. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Osher Y, Lichtenberg P, Bachner-Melman R, Gritsenko I, Kotler M, et al. An interaction between the catechol O-methyltransferase and serotonin transporter promoter region polymorphisms contributes to tridimensional personality questionnaire persistence scores in normal subjects. Neuropsychobiology. 2000;41:48–53. doi: 10.1159/000026632. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Viet R, Lotze M, Erb M, Hermann C, Grodd W, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair KS, Morton J, Leonard A, Blair RJ. Impaired decision-making on the basis of both reward and punishment information in individuals with psychopathy. Pers Individ Dif. 2006;41:155–165. [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2008;363:2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DGV. A selective impairment in the processing of sad and fearful facial expressions in children with psychopathic tendencies. J Abnorm Child Psychol. 2001;29:491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Mitchell DGV, Leonard A, Budhani S, Peschardt KS, Newman C. Passive avoidance learning in individuals with psychopathy: modulation by reward but not by punishment. Pers Individ Dif. 2004;37:1179–1192. [Google Scholar]
- Blonigen DM, Carlson SR, Krueger RF, Patrick CJ. A twin study of self-reported psychopathic personality traits. Pers Individ Dif. 2003;35:179–197. [Google Scholar]
- Brazil IA, Bruijn ERA, Bulten BH, Von Borries AKL, van Lankveld JJDM, Buitelaar JK, et al. Early and late components of error monitoring in violent offenders with psychopathy. Biol Psychiatry. 2009;65:137–143. doi: 10.1016/j.biopsych.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Brocke B, Armbruster D, Muller JL, Hensch T, Jacob CP, Lesch KP, et al. Serotonin transporter gene variation impacts innate fear processing: acoustic startle response and emotional startle. Molecular Psychiatry. 2006;11:1106–1112. doi: 10.1038/sj.mp.4001908. [DOI] [PubMed] [Google Scholar]
- Budhani S, Blair RJ. Response reversal and children with psychopathic tendencies: success is a function of salience of contingency change. J Child Psychol Psychiatry. 2005;46:972–981. doi: 10.1111/j.1469-7610.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AL, Gremore TM, Farmer RF. Psychometric analysis of the Psychopathic Personality Inventory (PPI) with female inmates. Journal of Personality Assessment. 2003;80:164–172. doi: 10.1207/S15327752JPA8002_05. [DOI] [PubMed] [Google Scholar]
- Craig MC, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, et al. Altered connections on the road to psychopathy. Molecular Psychiatry. 2009;14:946–953. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Crisan LG, Pana S, Vulturar R, Heilman RM, Szekely R, Druga B, et al. Genetic contributions of the serotonin transporter to social learning of fear and economic decision making. Social Cognitive and Affective Neuroscience. 2009;4:399–408. doi: 10.1093/scan/nsp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Murthy NV, Rabiner EA, Munafo MR, Johnstone EC, Jacob R, et al. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. Journal of Neuroscience. 2005;25:2586–2590. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan MC, Anderson IM. The relationship between serotonergic function and the Psychopathy Checklist: Screening Version. Journal of Pharmacology. 2003;17:216–222. doi: 10.1177/0269881103017002011. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Herrmann MJ, Roemmler J, Ehlis AC, Wagener A, Heidrich A, et al. Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology. 2004;29:1506–1511. doi: 10.1038/sj.npp.1300409. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Buzas B, Kamel N, Rhodes R, Vythilingham M, et al. The impact of tryptophan depletion and 5_HTTLPR genotype on passive avoidance and response reversal instrumental learning tasks. Neuropsychopharmacology. 2007;32:206–215. doi: 10.1038/sj.npp.1301182. [DOI] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Hermann C, Ziegler S, Patrick CJ. Aversive Pavlovian conditioning in psychopaths: Peripheral and central correlates. Psychophysiology. 2002;39:505–518. doi: 10.1017.S0048577202394046. [DOI] [PubMed] [Google Scholar]
- Fowler T, Langley K, Rice F, van de Bree MBM, Ross K, Wilkinson LS, et al. Psychopathy trait scores in adolescents with childhood ADHD: the contribution of genotypes affecting MAOA, 5HTT and COMT activity. Psychiatric Genetics. 2009;19:312–319. doi: 10.1097/YPG.0b013e3283328df4. [DOI] [PubMed] [Google Scholar]
- Fox E, Ridgewell A, Ashwin C. Looking on the bright side: biased attention and the human serotonin transporter gene. Proceedings of the Royal Society B. 2009;276:1747–1751. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle WG, Lombardo I, New AS, Goodman M, Talbot PS, Huang Y, et al. Brain serotonin transporter distribution in subjects with impulsive aggressivity: A positron emission study with [11c]McN 5652. Am J Psychiatry. 2005;162:915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- Garpenstrand H, Annas P, Ekblom J, Oreland L, Fredrikson M. Human fear conditioning is related to dopaminergic and serotonergic biological markers. Behav Neurosci. 2001;115:358–364. [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nature Reviews Neuroscience. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in Afrian- and European-American and Japanese populations and in alcohol-dependent subjects. Human Genetics. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Glass SJ, Newman JP. Emotion processing in the criminal psychopath: The role of attention in emotion-facilitated memory. J Abnorm Psychol. 2009;118:229–234. doi: 10.1037/a0014866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Molecular Psychiatry. 2009;14:5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Gotlib I, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 1999;88:83–87. [PubMed] [Google Scholar]
- Ha RY, Namkoong K, Kang JI, Kim YT, Kim SJ. Interaction between serotonin transporter promoter and dopamine receptor D4 polymorphisms on decision making. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33:1217–1222. doi: 10.1016/j.pnpbp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. The international Journal of Psychophysiology. 2005;57:5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thornton D, Waage L, Thayer JF. Facets of psychopathy, heart rate variability and cognitive function. J Personal Disord. 2007;21:568–582. doi: 10.1521/pedi.2007.21.5.568. [DOI] [PubMed] [Google Scholar]
- Hare RD. Comparison of Procedures for the Assessment of Psychopathy. Journal of Consulting and Clinical Psychology. 1985;53:7–16. doi: 10.1037//0022-006x.53.1.7. [DOI] [PubMed] [Google Scholar]
- Hare RD. Hare Psychopathy Checklist-Revised (PCL-R) 2. Toronto: Multi-Health Systems, Inc; 2003. [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay V, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, et al. Amygdala-prefrontal coupling depends on genetic variation of the serotonin transporter. Nature Neuroscience. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Herman AI, Conner TS, Anton RF, Gelernter J, Kranzler HR, Covault J. Variation in the gene encoding the serotonin transporter is associated with a measure of sociopathy in alcoholics. Addiction Biology. doi: 10.1111/j.1369-1600.2009.00197.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hranilovic D, Stefulj J, Schwab S, Borrmann-Hassenbach M, Albus M, Jernej B, et al. Serotonin transporter promoter and intron 2 polymorphisms: Relationship between allelic variants and gene expression. Biol Psychiatry. 2004;55:1090–1094. doi: 10.1016/j.biopsych.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism, Clinical and Experimental Research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hunt MK, Hopko DR, Bare R. Construct validity of the Balloon Analog Risk Task (BART) Assessment. 2005;12:416–428. doi: 10.1177/1073191105278740. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, et al. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Molecular Psychiatry. 2010;15:512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Fazekas H, Loney BR. Psychopathy, aggression, and the processing of emotional stimuli in non-referred boys and girls. Behav Sci Law. 2006;24:21–37. doi: 10.1002/bsl.668. [DOI] [PubMed] [Google Scholar]
- Larsson H, Andershed H, Lichtenstein P. A genetic factor explains most of the variation in psychopathic personality. J Abnorm Psychol. 2006;115:221–230. doi: 10.1037/0021-843X.115.2.221. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk taking: The Balloon Analog Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg B, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as an observer: Emotion and attention in picture processing. J Abnorm Psychol. 2000;109:373–386. [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: Possible implications for gene-environment interaction in anxiety disorder. Psychol Sci. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Losel F, Schmucker M. Psychopathy, risk taking, and attention: A differentiated test of the somatic marker hypothesis. J Abnorm Psychol. 2004;113:522–529. doi: 10.1037/0021-843X.113.4.522. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Colledge E, Leonard A, Blair RJR. Risky decisions and response reversal: is there evidence of orbitofrontal cortex dysfunction in psychopathic individuals? Neuropsychologica. 2002;40:2013–2022. doi: 10.1016/s0028-3932(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Newman JP, Curtin JJ, Bertsch JD, Baskin-Sommers AR. Attention moderates the fearlessness of psychopathic offenders. Biol Psychiatry. 2009;67:66–70. doi: 10.1016/j.biopsych.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J Abnorm Psychol. 1986;95:252–256. [PubMed] [Google Scholar]
- O’Leary MM, Loney BR, Eckel LA. Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology. 2007;32:183–191. doi: 10.1016/j.psyneuen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- O’Leary MM, Taylor J, Eckel LA. Psychopathic personality traits and cortisol response to stress: The role of sex, type of stressor, and menstrual phase. Horm Behav. 2010;58:250–256. doi: 10.1016/j.yhbeh.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Osinsky R, Reuter M, Kupper Y, Schmitz A, Kozyra E, Alexander N, et al. Variation in the serotonin transporter gene modulates selective attention to threat. Emotion. 2008;8:584–588. doi: 10.1037/a0012826. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary JE, Stice E, Schnyer DM. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. Journal of Neuroscience. 2009;29:6229–6233. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY, et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: Startling new insights. Psychophysiology. 1994;31:319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: Startle reflex modulation. J Abnorm Psychol. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski B, Munoz KE, Kolachana B, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Porter S, Woodworth M. Psychopathy and aggression. In: Patrick CJ, editor. Handbook of Psychopathy. New York: Guilford Press; 2006. pp. 481–494. [Google Scholar]
- Raine A. The psychopathology of crime: Criminal behavior as a clinical disorder. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Rao H, Gillihan SJ, Wang J, Korczykowski M, Sankoorikal GMV, Kaercher KA, et al. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry. 2007;62:600–606. doi: 10.1016/j.biopsych.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Reif A, Rosler M, Freitag CM, Schneider M, Eujen A, Kissling C, et al. Nature and nurture predispose to violent behavior: Serotonergic genes and adverse childhood environment. Neuropsychopharmacology. 2007;32:2375–2383. doi: 10.1038/sj.npp.1301359. [DOI] [PubMed] [Google Scholar]
- Reist C, Mazzanti C, Vu R, Tran D, Goldman D. Serotonin transporter promoter polymorphism is associated with attentuated prolactin response to fenfluramine. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2001;105:363–368. doi: 10.1002/ajmg.1360. [DOI] [PubMed] [Google Scholar]
- Retz W, Retz-Junginger P, Supprian T, Thome J, Rosler M. Association of serotonin transporter promoter gene polymorphism with violence: Relation with personality disorders, impulsivity, and childhood ADHD psychopathology. Behav Sci Law. 2004;22:415–425. doi: 10.1002/bsl.589. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Rogers RD, Cook LJ, Sahakian BJ. The effect of polymorphism at the serotonin transporter gene on decision-making, memory and executive function in ecstasy users and controls. Psychopharmacology. 2006;188:213–227. doi: 10.1007/s00213-006-0495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Javdani S, Jackson JJ, Reynolds EK, Potenza MN, Gelernter J, et al. Serotonin transporter gene associations with psychopathic traits in youth vary as a function of socioeconomic resources. J Abnorm Psychol. doi: 10.1037/a0019709. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A, Romero N, Fikretoglu D, Bowser FM, Wilson JW. Community violence exposure and aggression: Biosocial interactions. Toronto, Canada: The American Society of Criminology; 1999. [Google Scholar]
- Schmitt WA, Brinkley CA, Newman JP. Testing Damasio’s somatic marker hypothesis with psychopathic individuals: Risk takers or risk averse? J Abnorm Psychol. 1999;108:538–543. doi: 10.1037//0021-843x.108.3.538. [DOI] [PubMed] [Google Scholar]
- Skowronek MH, Laucht M, Hohm E, Becker K, Schmidt MH. Interaction between the dopamine D4 receptor and the serotonin transporter promoter polymorhpisms in alcohol and tobacco use among 15-year-olds. Neurogenetics. 2006;7:239–246. doi: 10.1007/s10048-006-0050-4. [DOI] [PubMed] [Google Scholar]
- Soderstrom H, Blennow K, Manhem A, Forsman A. CSF studies in violent offenders. I. 5-HIAA as a negative and HVA as a positive predictor of psychopathy. J Neural Transm. 2001;108:869–878. doi: 10.1007/s007020170036. [DOI] [PubMed] [Google Scholar]
- Soderstrom H, Blennow K, Sjodin AK, Forsman A. New evidence for an association between the CSF HVA:5-HIAA ratio and psychopathic traits. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:918–921. doi: 10.1136/jnnp.74.7.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of general anxiety disorder and worry. Biol Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Stoff D. Subtypes of aggression and their relevance to child psychiatry. Journal of American Academy of Child & Adolescent Psychiatry. 1997;36:307–315. doi: 10.1097/00004583-199703000-00008. [DOI] [PubMed] [Google Scholar]
- von Borries AKL, Brazil IA, Bulten BH, Buitelaar JK, Verkes RJ, Bruijn ERA. Neural correlates of error-related learning deficits in individuals with psychopathy. Psychological Medicine. doi: 10.1017/S0033291709992017. in press. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biol Psychiatry. 2010;67:487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit M, Stastny J, Pirker W, Praschak-Rieder N, Neumeister A, Asenbaum S, et al. No evidence for in vivo regulation of midbrain serotonin transporter availability by serotonin transporter promoter gene polymorphism. Biol Psychiatry. 2001;50:8–12. doi: 10.1016/s0006-3223(00)01123-9. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Arch Gen Psychiatry. 2009;66:986–994. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu X, Brent DA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]