Abstract
Purpose
This paper describes an ongoing randomized controlled trial designed to assess the impact of genetic and environmental risk assessment (GERA) on colorectal cancer (CRC) screening.
Methods
The trial includes asymptomatic patients who are 50-79 years and are not up-to-date with CRC screening guidelines. Patients who responded to a baseline telephone survey are randomized to a GERA or Control group. GERA Group participants meet with a nurse, decide whether to have a GERA blood test (a combination of genetic polymorphism and folate), and, if tested, receive GERA feedback. Follow-up telephone surveys are conducted at one and six months. A chart audit is performed at six months.
Results
Of 2,223 eligible patients, 562 (25%) have enrolled. Patients who enrolled in the study were significantly younger than those who did not (p<0.001). Participants tended to be 50-59 years (64%), female (58%), white (52%), married (51%), and have more than a high school education (67%). At baseline, most participants had some knowledge of CRC screening and GERA, viewed CRC screening favorably, and reported that they had decided to do screening. Almost half had worries and concerns about CRC.
Conclusions
One in four eligible primary care patients enrolled in the study. Age was negatively associated with enrollment. Prospective analyses using data for all participants will provide more definitive information on GERA uptake and the impact of GERA feedback.
Keywords: decision making, colorectal neoplasms, mass screening, risk assessment, genetic screening
1. Introduction
Colorectal cancer (CRC) is the third most common cancer and third leading cause of cancer death. [1] Screening is an effective method to detect, and in many cases, prevent the development of CRC. National screening goals for adults over age 50 include: (a) 50% should have had a screening fecal occult blood test (FOBT) in the preceding two years, or (b) 50% should have ever had screening endoscopy. [2] The 2008 Behavioral Risk Factor Surveillance System (BRFSS) data show that 21% of Americans had a blood stool test within the past two years and 62% have ever had an endoscopy. [3] The need to boost CRC screening remains a public health priority in the face of cancer health disparities. [4]
Most patient-oriented approaches (e.g., public education, targeted and tailored contacts, and reminders) for increasing CRC screening have had only modest impact on uptake. [5-14] Relatively little research has been reported on the effect of personal risk information on CRC screening use. This availability of genetic and environmental risk information is a potential offspring of the Human Genome Project. That is, traditional “average risk” populations could be subdivided into different risk strata based on an individual’s genetic characteristics, modified by measurable environmental exposures. [15, 16] The use of personalized feedback regarding cancer risk, either by itself or in conjunction with more traditional behavioral interventions, may provide a new means to improve CRC screening uptake.
Currently, we know little about patient interest in genetic and environmental risk assessment, referred to here as GERA, in the primary care setting. Little is known about how GERA should be offered, how much patients understand about and GERA results, and, importantly, what effect GERA might have on target outcomes such as screening participation. [17] To address these issues, we selected CRC as a candidate disease for GERA in primary care. We chose CRC, because it is common, preventable, and has widely accepted screening recommendations. Further, there is emerging evidence that the combination of selected Methylene TetraHydroFolate Reductase (MTHFR) genetic polymorphisms and serum folate level are associated with CRC risk. [18] Joint assessment of these factors appears to stratify personal CRC risk. [19-21] Because of the sheer numbers of individuals at risk for CRC, the paradigm of trained genetic counselors directing the provision of GERA-related counseling and results disclosure may not be feasible. For practical purposes, GERA testing will likely take place in primary care settings, and results disclosure will be accomplished by existing office staff.
We are conducting a National Cancer Institute (NCI)-funded research study to gain insights into the process of integrating GERA into primary care, the uptake of GERA by average-risk adults, and the impact of receiving GERA results. Our main objective is to determine the impact of GERA feedback versus Usual Care (UC) on CRC screening utilization. Secondary objectives(s) are to evaluate the impact of GERA feedback and UC on psychological distress; determine the impact of GERA feedback versus UC on intention to have CRC screening, perceived CRC risk, CRC knowledge, salience and coherence of CRC screening, and support for CRC screening; and identify factors that moderate the impact of GERA feedback on CRC screening utilization. This report provides information on study design, demographic variables associated with study participation, and background characteristics of participants enrolled in the study.
2. Methods
This study includes patients from two large, urban primary care practices who are 50 to 79 years of age, at average risk for CRC, and eligible for CRC screening. The 2008 statement of the United States Preventive Services Taskforce (USPSTF) recommends annual stool blood testing, flexible sigmoidoscopy every five years with stool blood testing every three years, or colonoscopy every 10 years. [22] The participating primary care practices recorded more than 70,000 office visits annually by a diverse patient population. This study was reviewed and approved by institutional review boards at Fox Chase Cancer Center (FCCC) and Thomas Jefferson University (TJU).
2.1. Participant enrollment
Potentially eligible patients are routinely identified by using a software program to query the electronic billing and scheduling data bases of participating primary care practices. This program examines records to identify age-eligible patients who have no evidence of having a personal history of CRC, colorectal polyps, or inflammatory bowel disease, and no evidence of having had recent CRC screening. For identified patients, the program also determines whether individuals do or do not have an appointment for non-acute care.
Initially, recruitment efforts targeted patients with a scheduled appointment. Given a slower than expected pace of accrual, the research team decided to include patients without appointments. Patients with a scheduled office visit are allocated daily to a “study contact cohort” file keyed to the office visit date. In addition, a random sample of patients from the same practices without scheduled office visits is also assigned to each study contact cohort.
The research team mails prepared study materials to all study patients. This mailing includes a personalized invitation to participate, a description of the study, information how to opt out of future study contacts, and a postage-paid return envelope. Recipients can opt out by calling a toll-free telephone number or by returning an enclosed opt-out card in a postage-paid return envelope.
Trained survey interviewers make telephone contact with patients who do not opt out of the study within two weeks of the study invitation mailing. This contact is intended to verify study eligibility, obtain verbal consent for study participation, and administer a baseline survey. Survey respondents are then randomly assigned to intervention or control groups.
2.2. Baseline telephone survey
Items included on the Baseline Telephone Survey reflect constructs drawn from the Preventive Health Model (PHM) and the Precaution Adoption Process Model (PAPM). [23, 24] PHM constructs include sociodemographic background factors (i.e., age, race, ethnicity, marital status, education level and health insurance status), knowledge about GERA and CRC and CRC screening, and perceptions surrounding CRC screening. The PAPM guided the collection of data on screening decision stage and informed the development of the decision counseling approach, described below, that is being used to facilitate participant decision making about having a GERA blood test. Data were collected in accordance with study aims outlined earlier.
Participant knowledge about CRC screening and GERA is assessed by 10 face-valid items developed by the research team to assess participant understanding of CRC screening and GERA. Examples related to CRC screening include, “People can have colon cancer and polyps and not have any symptoms.” and “Screening can find colon cancer early, when cure is likely.” GERA knowledge is assessed by the other brief survey. Items such as the following were included: “A blood test can measure different types of vitamins in my body,” and “A blood test can show different types of genes I have.” For all items, respondents are asked to answer “true”, “false,” or “don’t know.”
Items that measure perceptions related to CRC screening were adapted from our earlier research. [25] These items are perceived salience and coherence (four items, α = 0.77), personal susceptibility to colorectal adenomas and cancer (four items, α = 0.82), response-efficacy (two items, α = 0.67), worries and concerns about being diagnosed with colorectal polyps or CRC (two items, α = 0.77), and social support and influence related to screening (four items, α = 0.60). Response to each item is measured on a five-point Likert scale and will be averaged to obtain factor scores. These scales are valid across population subgroups, as defined in terms of gender and race. We also include items that elicit CRC screening test decision stage (“decided against”=0, “never heard of”=1, “undecided”=2, or “decided to do”=3) for FOBT and colonoscopy. These items were adopted from our work in informed decision making. [26] Staging items are not included for other screening options, because providers in the practices reported that they rarely recommended or performed either flexible sigmoidoscopy or screening with double contrast barium enema x-ray for CRC screening.
In addition, the baseline survey includes items from the Impact of Event Scale (IES) to measure perceived emotional distress. [27] The IES includes two subscales that assess intrusive worries and avoidant thoughts and concerns about a stressor. We adapted this validated scale (15 items, α = 0.86) to assess CRC-specific psychological distress in the two-week period prior to survey completion.
2.3. Sample size and power estimates
Patients who complete a baseline survey are randomized either to a usual care Control (UC) Group (target n=650) or to an Intervention (GERA) Group (target n=1,300). Study participants are assigned to study groups according to a 1:2 ratio, respectively, in order to allow for 80% power to detect significant intervention effects (p≤0.05). Specifically, we posited that an absolute increase in screening uptake of 10 percentage points in participants at elevated risk (based on GERA) compared to no elevated risk (based on GERA) represented a clinically important difference.
2.4. Study contacts after randomization
Following random assignment, the research team mails each participant a notice that verifies consent. Control Group participants with or without a scheduled office visit proceed to receive usual care, as directed by their primary care physicians. In contrast, a study nurse educator (NE) contacts all Intervention Group participants by telephone to arrange a face to face meeting. This meeting is coordinated with scheduled office visits or a mutually convenient time is identified.
When meeting with participants, the NE follows a structured decision counseling protocol to facilitate decision making about GERA. In brief, the NE initially gives each participant a GERA informational pamphlet and reviews its content. Then, the NE guides the participant through a preference clarification process. This process involves eliciting factors that the individual feels are likely to influence the decision about whether or not to have GERA (“decision factors”) and assigning weights related to factor influence on the decision. The NE then enters the identified factors and designated weights into a hand-held computer and uses a software program to compute a GERA preference score. Preference scores, which range from 0.000-1.000, are related to preference categories as follows: prefer not to have GERA (0.000-0.454), no preference (0.455-0.544), and prefer to have GERA (0.545-1.000). The NE shares the categorical results with the participant, assesses whether the participant agrees that the result is accurate, and determines whether the participant does or does not want to have GERA. A written summary of the session results is provided to each participant. For participants who decide against testing, the NE closes the session.
Written consent for the GERA blood draw is obtained from those participants who decide to be tested. For GERA testing, two small tubes of blood are drawn for MTHFR polymorphism and serum folate analyses and are labeled to identify the participant. One tube is sent to a research laboratory for MTHFR genotyping, while the other tube is sent to a commercial laboratory for serum folate analysis. Typically, the time required to complete the decision counseling session, obtain the blood draw consent, and perform the blood sample is approximately 30 minutes. Participants are given $25 to defray the costs of travel and parking associated with the office visit. Within two weeks of the GERA blood draw, GERA results are returned to the NE for disclosure. The NE contacts the participants by telephone to disclose test results according to a structured protocol. Participants are given the opportunity to refuse being informed of the results.
Recipients with a normal GERA result (i.e., those with a serum folate above the 25th percentile for their age with any MTHFR polymorphism or a 677/1298 MTHFR genotype of the less common CC/CC or TT/AA type, regardless of folate level) are informed that their risk for CRC is comparable to other persons who are 50 or more years of age. They are also told established CRC screening guidelines for persons 50 years or older indicate that they should have CRC screening. Persons who have an abnormal GERA result (i.e., a serum folate level below the 25th percentile for age, along with a 677/1298 MTHFR genotype of CC/AA, CC/AC, CT/AA or CT/AC combination) are informed that the result indicates their risk for CRC is greater than other individuals who are 50 years of age and older. They receive the same message about CRC screening guidelines. The GERA results disclosure call is normally completed in 5 to 10 minutes. A letter summarizing the GERA results and the CRC screening recommendation is subsequently sent to the participant.
2.5. Follow-up survey
Approximately one month after the baseline survey, telephone interviewers attempt to complete a follow-up survey with all participants. This survey again measures participant knowledge, attitudes and beliefs regarding CRC screening, using items included on the baseline survey, including perceived personal risk associated with CRC; and feelings associated with CRC (using the PHM). Emotional distress related to cancer-related distress is measured using the IES. In addition, the Multidimensional Inventory of Cancer Risk Assessment (MICRA) is administered. [28] The MICRA is a 19-item scale (α = 0.75 to 0.86), with 3 subscales: distress (6 items), uncertainty (9 items), and positive experiences (4 items).
2.6. CRC screening kit mailing
The research team mails all Control Group and Intervention Group participants a CRC screening kit at about six weeks after the baseline survey. The screening kit includes a stool blood test (fecal immunochemical test or FIT). For those participants preferring endoscopy, information regarding how to schedule a screening colonoscopy at an eligible provider is also included.
2.7. Endpoint survey
A survey interviewer attempts to contact all study participants by telephone at six months after the office visit in order to administer the endpoint survey. As in the earlier surveys, participants are asked about their knowledge, attitudes and beliefs related to CRC screening. Participants are also asked about their multivitamin use. Intervention Group participants are also asked about receiving the diet and gene blood test, followed by questions similar to the four week survey related to concerns about genetic testing.
2.8. Measurement of CRC screening use
For this study, CRC screening participation is defined as having had a documented, at home stool blood test or a self-reported or documented flexible sigmoidoscopy, colonoscopy, or barium enema x-ray during the 6-month observation period following randomization. We will use data from multiple sources to assess screening use. Study research assistants will perform an endpoint chart audit for each participant to determine if there is evidence in the chart of CRC screening with stool blood test, flexible sigmoidoscopy, colonoscopy, or barium enema x-ray during the observation period. In addition, the project programmer analyst will inspect the electronic billing database, TJU endoscopy electronic billing records, and a stool blood test laboratory database to determine if there is evidence of screening. Finally, the research team will review endpoint telephone survey responses to items that asked about screening use (test performance and test date). As the study is ongoing, we have not yet determined screening outcomes.
3. Results
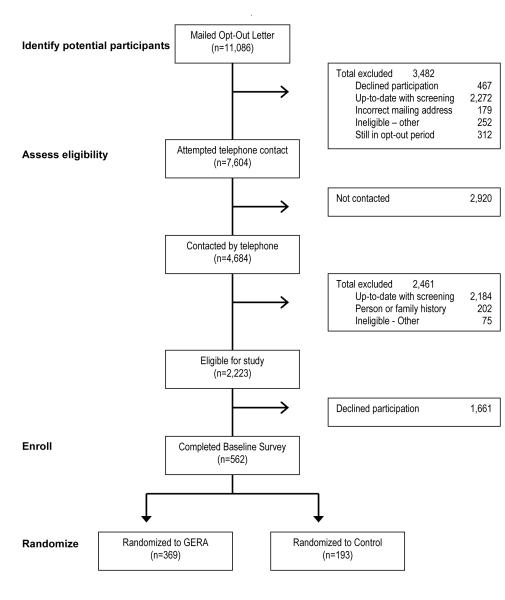
Figure 1 summarizes the flow of patients through the study from the point of being identified in the sampling frame to being available for telephone contact, and through the steps of study enrollment and random assignment. Individuals included in the figure represent individuals who were identified as potentially eligible for the study. By March 2010, a total of 10,764 patients were mailed an invitation to participate, along with instructions how to opt out. During a 4-week period, recipients were able to use a pre-addressed, postage-paid envelope to return an opt-out card if they either did not want or if they were ineligible to participate in the study. Alternatively, recipients could call a toll-free number to opt of further study-related contacts. A total of 3,482 individuals were excluded from further contact for the following reasons: up-to-date with CRC screening (N=2,272), declined participation (n=467), incorrect mailing address (n=179), and other reasons (n=252).
Figure 1.
Participant Recruitment and Random Assignment
There were a total of 7,604 patients who remained potentially eligible for the study and were available for telephone contact by the research team. We successfully contacted 4,684 of these individuals, but were unable to contact 2,920 patients. Information obtained in the calls resulted in the exclusion of 2,461 patients who were ineligible for the study due to the following reasons: up-to-date with CRC screening (n=2,184), personal or family history of colorectal neoplasia (n=202), or other reasons (n=75). Of the 2,223 patients who were found to be eligible for the study, 562 (25%) agreed to participate and 1,661 (75%) declined.
Comparisons were made between study participants and decliners based on available information on age (50 to 59 versus ≥ 60 years), race (white versus nonwhite), and gender. We determined that there were no significant gender or race differences between individuals who declined and those who agreed to participate. However, we found that individuals who agreed to participate in the study were significantly younger than those who declined participation (p<0.001).
Using data from the baseline survey, we were able to characterize study participants in term of sociodemographic background, knowledge about CRC screening and GERA, perceptions about CRC screening, CRC screening decision stage, and supplement use. Participants tended to be 50-59 years (64%), female (58%), white (52%), married (51%, and have more than a high school education (67%). We determined that participants provided correct responses to about two-thirds of CRC screening and GERA knowledge questions (67% and 63%, respectively). In addition, 97% of participants viewed CRC screening as important; 92% thought screening is efficacious; 83% felt they had social support for CRC screening; 47% were concerned about screening; and, 20% thought they were at risk for CRC. In terms of screening decision stage, 7% reported that they were not aware of the two most common CRC screening tests (i.e., stool blood test and colonoscopy); 17% were undecided about screening; and 76% had decided to have screening. Finally, 49% of participants reported taking vitamins regularly, while only 9% said that they took a folate supplement.
4. Discussion and Conclusion
4.1 Discussion
This report describes the study design and characteristics of participants in a project evaluating the impact of a genetic and environmental risk assessment on CRC screening uptake. The results of this ongoing trial to-date suggest that it is feasible to identify and recruit primary practice patients into research projects of this type; and, study procedures can be integrated into primary care settings. It is important to note that we have developed procedures that allow for contacts with patients who have a scheduled appointment and for patients who do not regularly visit practices. Thus, we have established a process that reaches all patients in participating practices who are potentially eligible for GERA and CRC screening.
Among eligible patients who could be contacted prior to a scheduled office visit, 25% agreed to participate. This level of participation is comparable to reported participation in a primary care-based study of genetic counseling for women about breast cancer. [29] The fact that three-quarters of patients did not participate, however, suggests the need to learn more about factors that encourage and discourage participation in studies of this type. It is also reasonable to consider the use of alternative recruitment strategies (e.g., internet-based enrollment).
It is likely that participation is influenced not only by perceptions related to risk assessment, but also by the fact that such testing is being offered as part of a research study. In this regard, the observed acceptance rate is lower than that reported for some studies evaluating the psychological impact of highly predictive genetic testing, but is higher than others. [30, 31] There is clearly variability in participation previously reported in studies evaluating the psychological and behavioral impact of genetic testing for BRCA 1/BRCA 2 mutations among patients whose personal or family histories bespeak a significantly elevated risk for cancer. [32] Arguably, a heightened perception of potential risk may be a more potent motivator than the modest risk elevation associated with combined MTHFR and folate. In addition, such studies have employed different recruitment methods that traditionally involve referral by an oncologist or other health professional, rather than the unannounced mailing approach employed here.
It is encouraging to note there were no differences between individuals who participated in and those who declined to participate in the study with regard to gender and race. However, the data suggest that study participants were younger than study decliners. These findings differ from earlier studies of genetic testing uptake, many of which have been conducted in relation to breast cancer, in that older age was positively associated with interest in testing. [33, 34] It may be the case that today there is increasing awareness and acceptance of the of genetic testing for disease risk and of participation in studies related to such testing conducted among individuals in the younger segment of the population at risk for CRC.
Overall, the levels of understanding about CRC and the GERA test were both relatively high. These findings are surprising, given the fact that all participants were either overdue for screening or had never screened, and that GERA is a novel test. We also found that while study participants generally had very positive attitudes, a surprising number expressed worries and concerns about CRC screening. Nonetheless, three-quarters of the sample reported that they had decided to do CRC screening. For the most part, these findings are comparable to findings reported in other CRC screening research studies that have involved primary care practice patients. [35, 36] However, the level of worries and concerns about screening among participants are higher than reported by participants in other studies. Interestingly, participants in this study also reported low levels of intrusive thoughts and ideation related to CRC.
4.2 Conclusion
Our initial findings suggest that a research study evaluating GERA can be integrated into primary care and that a significant subset of primary care patients is interested in the study. Regarding operational aspects of the study, our experience suggests that the type of study described here requires the dedication of substantial time, resources, and coordination to achieve recruitment goals. We have also learned that study participants tend to be younger than non-participants. These findings are worth considering in the design of future research studies. Going forward, we will focus analyses of study outcomes on actual GERA uptake and related predictors and on determining the impact of GERA results on CRC screening.
Table 1.
Characteristics of Patients by Participation Status
| Variables | Response categories |
Total N=2223 |
Participants N=369 |
Non-Participants N=193 |
p-value |
|---|---|---|---|---|---|
| Age [n, (%)] | <0.01 | ||||
| 50 to 59 years | 1169 (53) | 364 (65) | 805 (48) | ||
| 60 to 79 years | 1054 (47) | 198 (35) | 856 (52) | ||
| Race [n, (%)] | 0.08 | ||||
| White | 955 (43) | 257 (46) | 698 (42) | ||
| Nonwhite* | 835 (38) | 213 (38) | 622 (37) | ||
| Unknown | 433 (19) | 92 (16) | 341 (21) | ||
| Gender [n, (%)] | 0.73 | ||||
| Male | 914 (41) | 235 (42) | 679 (41) | ||
| Female | 1309 (59) | 327 (58) | 982 (59) |
Total Nonwhite participants: 689 African Americans, 116 Asians, 5 Native Americans, and 25 Other.
Table 2.
Characteristics of Study Participants
| Variables | Response categories |
Total N=562 |
|---|---|---|
| Sociodemographic Background | ||
| Age [n, (%)] | ||
| 50 to 59 years | 358 (64) | |
| 60 to 79 years | 202 (36) | |
| Race [n, (%)] | ||
| White | 291 (52) | |
| Nonwhite* | 267 (48) | |
| Gender [n, (%)] | ||
| Male | 235 (42) | |
| Female | 325 (58) | |
| Education [n, (%)] | ||
| ≤ high school grad | 183 (33) | |
| > high school grad | 376 (67) | |
| Marital Status [n, (%)] | ||
| married | 284 (51) | |
| not married | 274 (49) | |
| Perceptions of CRC Screening | ||
| Salience & Coherence [n, (%)] | ||
| ≤ 3 | 19 (3) | |
| > 3 | 543 (97) | |
| Susceptibility [n, (%)] | ||
| ≤ 3 | 448 (80) | |
| > 3 | 113 (20) | |
| Worries & Concerns [n, (%)] | ||
| ≤ 3 | 300 (53) | |
| > 3 | 262 (47) | |
| Response Efficacy [n, (%)] | ||
| ≤ 3 | 45 (8) | |
| > 3 | 516 (92) | |
| Social Influence [n, (%)] | ||
| ≤ 3 | 94 (17) | |
| > 3 | 467 (83) | |
| Regular Supplement Use | ||
| Vitamins [n, (%)] | ||
| Yes | 273 (49) | |
| No | 287 (51) | |
| Folate [n, (%)] | ||
| Yes | 50 (9) | |
| No | 511 (91) | |
| Decision Stage [n, (%)] | ||
| Never heard of | 38 (7) | |
| Undecided | 94 (17) | |
| Decided to screen | 430 (76) | |
| Patient Knowledge (percent correct) | ||
| CRC screening [mean, (std)] | 67.0 (16.5) | |
| GERA [mean, (std)] | 63.1 (13.9) | |
| Impact of Events Scale (0=never, 5=often) | ||
| IES scale [mean, (std)] | 0.39 (0.73) | |
Total Nonwhite participants: 238 African Americans, 11 Asians, 2 Native Americans, and 16 Others.
Acknowledgments
The GERA study funding is provided by NIH/NCI grant R01 CA11230-01A2.
Abbreviations
- CRC
colorectal cancer
- FOBT
fecal occult blood test
- FCCC
Fox Chase Cancer Center
- GERA
genetic and environmental risk assessment
- IES
Impact of Event Scale
- MICRA
Multidimensional Inventory of Cancer Risk Assessment
- MTHFR
Methylene TetraHydroFolate Reductase
- NE
nurse educator
- PHM
Preventive Health Mode
- PAPM
Precaution Adoption Process Model
- TJU
Thomas Jefferson University
- UC
Usual Care
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- [1].American Cancer Society . Cancer facts and figures 2008. American Cancer Society; Atlanta, GA: 2008. pp. 10–3. [Google Scholar]
- [2].U.S. Department of Health and Human Services [cited 2009 Sep 1];Cancer. U.S. Government Printing Office; Washington, DC: Healthy people 2010: Objectives for improving health. 2000 :3–24. Available from: http://www.healthypeople.gov/Document/HTML/Volume1/03Cancer.htm.
- [3].National Center for Chronic Disease Prevention & Health Promotion [cited 2009 April 30];Behavioral Risk Factor Surveillance System. BRFSS Prevalence and Trends Data. Colorectal Cancer Screening - 2008. Last reviewed May 15, 2009. Available from: http://www.cdc.gov/brfss/
- [4].Mitka M. Colorectal cancer screening rates still fall far short of recommended levels. JAMA. 2008;299:622. doi: 10.1001/jama.299.6.622. [DOI] [PubMed] [Google Scholar]
- [5].Myers RE, Ross EA, Wolf TA, Balshem A, Jepson C, Millner L. Behavioral interventions to increase adherence in colorectal cancer screening. Medical Care. 1991;29:1039–50. doi: 10.1097/00005650-199110000-00009. [DOI] [PubMed] [Google Scholar]
- [6].Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, Wolf T, Andrel J, Wender R. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110:2083–91. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- [7].Basch CE, Wolf RL, Brouse CH, Shmukler C, Neugut A, DeCarlo LT, Shea S. Telephone outreach to increase colorectal cancer screening in an urban minority population. Am J Public Health. 2006;96:2246–53. doi: 10.2105/AJPH.2005.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stone EG, Morton SC, Hulscher ME, Maglione MA, Roth EA, Grimshaw JM, Mittman BS, Rubenstein LV, Rubenstein LZ, Shekelle PG. Interventions that increase use of adult immunization and cancer screening services: A meta-analysis. Ann Intern Med. 2002;136:641–51. doi: 10.7326/0003-4819-136-9-200205070-00006. [DOI] [PubMed] [Google Scholar]
- [9].Vernon SW. Participation in colorectal cancer screening: A review. Journal of the National Cancer Institute. 1997;89:1406–22. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- [10].Church TR, Yeazel MW, Jones RM, Kochevar LK, Watt GD, Mongin SJ, Cordes JE, Engelhard D. A randomized trial of direct mailing of fecal occult blood tests to increase colorectal cancer screening. J Natl Cancer Inst. 2004;96:770–80. doi: 10.1093/jnci/djh134. [DOI] [PubMed] [Google Scholar]
- [11].Segnan N, Senore C, Andreoni B, Arrigoni A, Bisanti L, Cardelli A, Castiglione G, Crosta C, DiPlacido R, Ferrari A, Ferraris R, Ferrero F, Fracchia M, Gasperoni S, Malfitana G, Recchia S, Risio M, Rizzetto M, Saracco G, Spandre M, Turco D, Turco P, Zappa M. SCORE2 Working Group-Italy. Randomized trial of different screening strategies for colorectal cancer: Patient response and detection rates. J Natl Cancer Inst. 2005;97:347–57. doi: 10.1093/jnci/dji050. [DOI] [PubMed] [Google Scholar]
- [12].Ling BS, Schoen RE, Trauth JM, Wahed AS, Eury T, Simak DM, Solano FX, Weissfeld JL. Physicians encouraging colorectal screening: A randomized controlled trial of enhanced office and patient management on compliance with colorectal cancer screening. Arch Intern Med. 2009;169:47–55. doi: 10.1001/archinternmed.2008.519. [DOI] [PubMed] [Google Scholar]
- [13].Baron RC, Rimer BK, Coates RJ, Kerner J, Kalra GP, Melillo S, Habarta N, Wilson KM, Chattopadhyay S, Leeks K. Task Force on Community Preventive Services. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening: A systematic review. Am J Prev Med. 2008;35:S56–66. doi: 10.1016/j.amepre.2008.04.001. [DOI] [PubMed] [Google Scholar]
- [14].Sabatino SA, Habarta N, Baron RC, Coates RJ, Rimer BK, Kerner J, Coughlin SS, Kalra GP, Chattopadhyay S. Task Force on Community Preventive Services. Interventions to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers: Systematic reviews of provider assessment and feedback and provider incentives. Am J Prev Med. 2008;35:S67–74. doi: 10.1016/j.amepre.2008.04.008. [DOI] [PubMed] [Google Scholar]
- [15].Collins FS. Shattuck lecture--medical and societal consequences of the human genome project. New England Journal of Medicine. 1999;341:28–37. doi: 10.1056/NEJM199907013410106. [DOI] [PubMed] [Google Scholar]
- [16].Khoury MJ, Yang Q, Gwinn M, Little J, Flanders W Dana. An epidemiologic assessment of genomic profiling for measuring susceptibility to common diseases and targeting interventions. Genetics in Medicine. 2004;6:38–47. doi: 10.1097/01.gim.0000105751.71430.79. [DOI] [PubMed] [Google Scholar]
- [17].Marteau TM, Lerman C. Genetic risk and behavioural change. BMJ. 2001;322:1056–9. doi: 10.1136/bmj.322.7293.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, Speizer FE, Willett WC. Multivitamin use, folate, and colon cancer in women in the nurses’ health study. Ann Intern Med. 1998;129:517–24. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- [19].Keku T, Millikan R, Worley K, Winkel S, Eaton A, Biscocho L, Martin C, Sandler R. 5,10-methylenetetrahydrofolate reductase codon 677 and 1298 polymorphisms and colon cancer in African Americans and Whites. Cancer Epidemiol Biomarkers Prev. 2002;11:1611–21. [PubMed] [Google Scholar]
- [20].Chen J, Ma J, Stampfer MJ, Palomeque C, Selhub J, Hunter DJ. Linkage disequilibrium between the 677C > T and 1298A > C polymorphisms in human methylene tetrahydrofolate reductase gene and their contributions to risk of colorectal cancer. Pharmacogenetics. 2002;12:339–42. doi: 10.1097/00008571-200206000-00011. [DOI] [PubMed] [Google Scholar]
- [21].Chen J, Giovannucci EL, Hunter DJ. MTHFR polymorphism, methyl-replete diets and the risk of colorectal carcinoma and adenoma among U.S. men and women: An example of gene-environment interactions in colorectal tumorigenesis. J Nutr. 1999;129:560S–4S. doi: 10.1093/jn/129.2.560S. [DOI] [PubMed] [Google Scholar]
- [22].Screening for colorectal cancer: U.S. preventive services task force recommendation statement. Agency for Health Research and Quality; Rockville, MD: [cited 2009 Sep 1]. Oct, 2008. Available from: http://www.ahrq.gov/clinic/uspstf08/colocancer/colors.htm. [Google Scholar]
- [23].Vernon SW, Myers RE, Tilley BC. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiology, Biomarkers & Prevention. 1997;6:825–32. [PubMed] [Google Scholar]
- [24].Weinstein ND. The precaution adoption process. Health Psychology. 1988;7:355–86. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- [25].Tiro JA, Vernon SW, Hyslop T, Myers RE. Factorial validity and invariance of a survey measuring psychosocial correlates of colorectal cancer screening among african americans and caucasians. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:2855–61. doi: 10.1158/1055-9965.EPI-05-0217. [DOI] [PubMed] [Google Scholar]
- [26].Liberatore M, Nydick R, Daskalakis C, Kunkel E, Cocroft J, Myers R. Helping men decide about scheduling a prostate cancer screening exam. Interfaces. 2009;39:209–17. [Google Scholar]
- [27].Horowitz M, Wilner N, Alvarez W. Impact of event scale: A measure of subjective stress. Psychosomatic Medicine. 1979;41:209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- [28].Cella D, Hughes C, Peterman A, Chang C, Peshkin BN, Schwartz MD, Wenzel L, Lemke A, Marcus AC, Lerman C. A brief assessment of concerns associated with genetic testing for cancer: The multidimensional impact of cancer risk assessment (MICRA) questionnaire. Health Psychology. 2002;21:564–72. [PubMed] [Google Scholar]
- [29].Helmes AW, Bowen DJ, Bowden R, Bengel J. Predictors of participation in genetic research in a primary care physician network. Cancer Epidemiol Biomarkers Prev. 2000;9:1377–9. [PubMed] [Google Scholar]
- [30].Schwartz MD, Lerman C, Brogan B, Peshkin BN, Halbert CH, DeMarco T, Lawrence W, Main D, Finch C, Magnant C, Pennanen M, Tsangaris T, Willey S, Isaacs C. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol. 2004;22:1823–9. doi: 10.1200/JCO.2004.04.086. [DOI] [PubMed] [Google Scholar]
- [31].Schwartz MD, Tercyak KP, Peshkin BN, Valdimarsdottir H. Can a computer-based system be used to educate women on genetic testing for breast cancer susceptibility? Nat Clin Pract Oncol. 2005;2:24–5. doi: 10.1038/ncponc0055. [DOI] [PubMed] [Google Scholar]
- [32].Jacobsen PB, Valdimarsdottier HB, Brown KL, Offit K. Decision-making about genetic testing among women at familial risk for breast cancer. Psychosom Med. 1997;59:459–66. doi: 10.1097/00006842-199709000-00001. [DOI] [PubMed] [Google Scholar]
- [33].Armstrong K, Calzone K, Stopfer J, Fitzgerald G, Coyne J, Weber B. Factors associated with decisions about clinical BRCA1/2 testing. Cancer Epidemiol Biomarkers Prev. 2000;9:1251–4. [PubMed] [Google Scholar]
- [34].Biesecker BB, Ishibe N, Hadley DW, Giambarresi TR, Kase RG, Lerman C, Struewing JP. Psychosocial factors predicting BRCA1/BRCA2 testing decisions in members of hereditary breast and ovarian cancer families. Am J Med Genet. 2000;93:257–63. doi: 10.1002/1096-8628(20000814)93:4<257::aid-ajmg1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- [35].Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, Wolf T, Andrel J, Wender R. A Randomized Controlled Trial of Targeted and Tailored Intervention Impact on Colorectal Cancer Screening. Cancer. 2007;110:2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- [36].Myers RE, Hyslop T, Sifri R, Bittner-Fagan H, Katurakes NC, Cocroft J, DiCarlo M, Wolf T. Tailored Navigation in Colorectal Cancer Screening. Med Care. 2008;46((9) Supplement 1):S123–131. doi: 10.1097/MLR.0b013e31817fdf46. [DOI] [PubMed] [Google Scholar]