Abstract
DNA alkylating agents alone or with ionizing radiation have been the preferred conditioning treatment in allogeneic hematopoietic stem cell transplantation (allo-HSCT). In search of less toxic alternatives, we hypothesized that combination of busulfan (Bu), fludarabine (Flu) and clofarabine (Clo) would provide superior efficacy. At low concentrations, these drugs show synergistic cytotoxicity in Bu-resistant AML KBM3/Bu2506 cells. Similar molecular responses were observed in other AML cell lines and in primary explanted AML cells. The [Clo+Flu+Bu] combination activates an intense DNA damage response through the ATM pathway, leading to cell cycle checkpoint activation and apoptosis. Phosphorylations of SMC1 and SMC3, and methylations of histones 3 and 4, are much more pronounced in cells exposed to [Clo+Flu+Bu] than [Clo+Flu], suggesting their relevance in the efficacy of the triple-drug combination. A possible mechanism for these observed synergistic effects involves the capability of [Clo+Flu] to induce histone methylations and subsequent chromatin remodeling, which may render the genomic DNA more accessible to Bu alkylation. The Bu-mediated DNA cross-linking may provide a feedback loop which perpetuates the DNA damage response initiated by [Clo+Flu] and commits the cells to apoptosis. Our results provide a conceptual mechanistic basis for exploring this triple-drug combination in pretransplant conditioning therapy for allo-HSCT.
Keywords: clofarabine, fludarabine, busulfan, synergistic cytotoxicity, chromatin remodeling
1. Introduction
The rapid proliferation of leukemia cells heavily depends on salvage synthesis of nucleosides as substrates for DNA synthesis. This dependence provides a rationale for the design of nucleoside analogues as anti-leukemia drugs. Several nucleoside analogues have been used to treat neoplastic diseases [1]. The poor stability and/or low aqueous solubility of some of these analogues led to the design and clinical investigation of 2-chloro-2' arabino-fluoro-2' deoxyadenosine (clofarabine, Clo), one of the latest and most efficacious nucleoside analogues used in the treatment of leukemia [2]. Clo is cytotoxic to both proliferating and non-proliferating cells, resistant to phosphorylitic cleavage and stable under acidic conditions [3, 4].
Although similar in their chemical structures, nucleoside analogues differ in their mechanisms of action and clinical efficacies [5]. They are taken up by the cells via nucleoside transporters and phosphorylated by deoxynucleotide kinases [6]. In general, their incorporation into DNA during DNA synthesis causes termination of strand elongation and triggers a chain of events, including induction of DNA strand breaks and subsequent activation of pro-apoptotic pathways [7–9]. The attempted repair of these damaged DNA sites further facilitates the incorporation of nucleoside analogues, propagates DNA breaks and enhances their cytotoxic effects. This activity is also potentiated by their inhibition of ribonucleotide reductase (RR), which leads to decreased deoxynucleotide pools and preferential incorporation of the nucleoside triphosphate analogues into growing DNA strands [10].
Nucleoside analogues are more often effective for treating leukemia when used in combination with DNA alkylating agents [11, 12]. A previous study demonstrated how Clo and Flu inhibit DNA repair initiated by 4-hydroperoxycyclophosphamide in lymphocytes from CLL patients [13]. A subsequent multi-center phase I study confirmed that the combination of Clo with etoposide and cyclophosphamide was effective and well tolerated in pediatric patients with relapsed/refractory acute leukemia [14]. These observations rationalize the clinical use of mechanism-based therapies which combine nucleoside analogues and DNA alkylating agents to more effectively exploit the cytoreductive potential of such combinations. Identification of drug combinations with complementary rather than overlapping toxicities may also yield a greater therapeutic index and help to alleviate therapy-related adverse effects.
In hematopoietic stem cell transplantation (HSCT), nucleoside analogues and DNA alkylators are increasingly being used in myeloablative reduced-toxicity pre-transplant conditioning therapy. For example, a combination of intravenous busulfan (Bu) with Flu compared favorably with [intravenous Bu+cyclophosphamide] as a reduced-toxicity conditioning regimen for allogeneic HSCT in AML and MDS [12, 15, 16]. Whether the effective cytotoxicity and immunosuppressive properties of Clo [17] can replace or complement Flu in such combination therapy remains to be determined.
To evaluate the possibility of using combinations of Clo and/or Flu with Bu as a pre-HSCT conditioning regimen, we used a Bu-resistant AML cell line model to study the cytotoxicity of various combinations of these agents and identify their mechanisms of action. We observed synergistic cytotoxicity between Clo and Flu; their cytotoxicity was further enhanced when combined with Bu. We attribute this synergism to drug-mediated inhibition of DNA synthesis and repair and changes in chromatin structure. Since [Clo+Flu] and Bu belong to different classes of drugs and invoke different mechanisms of cytotoxicity, crosstalk between the resulting DNA damage and response to that damage possibly exacerbates genomic injury and consequently activates apoptosis.
2. Materials and Methods
2.1. Cell lines and drugs
The Bu-resistant KBM3/Bu2506 cells used in this study were established from the p53-negative human AML KBM3 cell line as described previously [18]. KBM3/Bu2506, HL60 and OCI-AML3 cell lines were cultured in RPMI 1640 medium (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) at 37°C in a humidified atmosphere of 5% CO2 in air. Bu and Flu (Sigma-Aldrich, St. Louis, MO) were both freshly dissolved in dimethyl sulfoxide (DMSO) immediately prior to cellular drug exposure(s). The final concentration of DMSO in all experiments did not exceed 0.08% by volume, a level that does not induce differentiation of these cell lines (data not shown). Clofarabine (Clolar®) was obtained from Genzyme Oncology, Cambridge, MA (1 mg/ml solution) and diluted in RPMI 1640 medium prior to use. Adenosine, cytidine, hydroxyurea and deferoxamine mesylate were obtained from Sigma-Aldrich.
2.2. Flow-cytometric analysis of cell cycle phase distribution
Cells in logarithmic growth phase (5 × 105 cells/ml) were incubated with the indicated concentrations of the drug(s) at 37°C for 24 hr (to determine immediate effects on cell cycle phase distribution) or 48 hr (later effects on apoptosis and protein level/modifications). The cells were centrifuged, resuspended in 70% ethanol in phosphate-buffered saline (PBS), and fixed at −20°C overnight. Fixed cells were pelleted at 3,000 × g at room temperature, washed with PBS, and treated with 0.25 ml of 500 U/ml RNAse A in PBS containing 1.12% sodium citrate at 37°C for 30 min. After addition of 0.25 ml propidium iodide (PI, 50 µg/ml) solution, the cells were kept in subdued light for at least 1 hr prior to analysis by flow cytometry. The cellular DNA content of at least 10,000 cells was analyzed using a BD FACS Calibur instrument (BD Biosciences, San Jose, CA) and the proportion of cells in the different phases of the cell cycle was determined using the CellQuest™ software (Becton Dickinson, Franklin Lakes, NJ). Histograms were analyzed using ModFit LT (Verity Software House, Topsham, ME).
2.3. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Actively growing KBM3/Bu2506 cells (5 × 105 cells/ml) were exposed to the indicated concentrations of the drug(s) at 37°C for 48 hr. Cells were analyzed for apoptosis using the Apo-Direct™ TUNEL assay kit (Millipore, Billerica, MA) according to the procedure provided by the manufacturer. TUNEL-positive cells were determined by analysis of at least 15,000 cells using a FACS Calibur instrument.
2.4. Cytotoxicity assay and graphical analysis
Cell suspensions were aliquoted (50 µl of 4 × 105 cells/ml) into 96-well plates in the presence of drug(s) or solvent alone and incubated as above at 37°C for 4 days to observe more pronounced drug effects on cell survival. The cells were analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay [19]. Graphical analyses including calculations of IC20 values (the concentration of drug required for 20% growth inhibition) were done using Prism 5 (GraphPad Software, San Diego, CA). Drug combination effects were estimated based on the combination index (CI) values [20] calculated using the CalcuSyn software (Biosoft, Ferguson, MO). This program was developed based on the median-effect method: CI < 1 indicates synergy, CI ≈ 1 is additive, and CI > 1 suggests antagonism.
2.5. Reversal of Clo or Flu effects using normal nucleosides
Cells were exposed to 0.06 µM Clo or 2.4 µM Flu as described above in the presence or absence of 100 µM adenosine or cytidine. Higher concentrations of Clo and Flu were used here to elicit a more dramatic cytotoxicity. After 48 hr, cells were harvested and analyzed by flow-cytometry for apoptosis as indicated by sub-G1 DNA content.
2.6. Exposure of cells to ribonucleotide reductase inhibitor
Cells were exposed to 0.02 µM Clo or 0.6 µM Flu in the presence or absence of 25 µM deferoxamine (DFO) for 48 hr and analyzed by flow cytometry for apoptosis as indicated by sub-G1 DNA content.
2.7. Western blot analysis
Cells were collected by centrifugation, washed with PBS and lysed with cell lysis buffer (Cell Signaling Technology, Danvers, MA) as recommended by the manufacturer. Total protein concentrations in the cell lysates were determined using a BCA Protein Assay kit (Thermo Fisher Scientific, Rockford, IL). Western blot analysis was done by separating protein extracts on polyacrylamide-SDS gels and blotting onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Immunoblot analyses by chemiluminescence were done using either the SNAP i.d.™ Protein Detection System or the Immobilon Western Chemiluminescent HRP Substrate (both from Millipore, Bedford, MA). All antibodies, their sources and other relevant information are listed in Table 1. The β-actin protein was used as an internal control.
Table 1.
List of primary antibodies, their sources and dilutions
| Antigen | Source/Cat. # | Clone type* | Dilution** |
|---|---|---|---|
| ATM | Santa Cruz Biotech/23921 | mAb | 750 |
| Caspase 9 | Cell Signaling/9502 | pAb | 1500 |
| CDC25A | Cell Signaling/3652 | pAb | 1500 |
| CDK2 | Santa Cruz Biotech/6248 | mAb | 700 |
| CHK1 | Cell Signlaing/2345 | pAb | 2000 |
| CHK2 | Cell Signlaing/2662 | pAb | 2500 |
| Cleaved Caspase 3 | Cell Signaling/9661 | pAb | 2500 |
| c-MYC | Cell Signaling/9402 | pAb | 2000 |
| Cyclin E2 | Cell Signaling/4132 | pAb | 3000 |
| Histone 3 | Abcam/1791 | pAb | 3000 |
| MCL-1 | Santa Cruz Biotech/819 | pAb | 500 |
| p21 | Upstate Biotech/05-345 | mAb | 1000 |
| PARP1 | Santa Cruz Biotech/8007 | mAb | 1000 |
| P-ATM (Ser1981) | Rockland/200-301-4000 | mAb | 2000 |
| P-ATR (Ser428) | Cell Signaling/2853 | pAb | 2000 |
| P-CDC25B (Ser187) | Santa Cruz Biotech/130184 | pAb | 1000 |
| CDC25B | Santa Cruz Biotech/56266 | mAb | 1000 |
| P-CDK2 (Thr160) | Santa Cruz Biotech/101656 | pAb | 500 |
| P-CHK1 (Ser317) | Cell Signaling/2344 | pAb | 2000 |
| P-CHK2 (Ser19) | Cell Signaling/2666 | pAb | 2500 |
| P-CHK2 (Ser33/35) | Cell Signaling/2665 | pAb | 2500 |
| P-SMC1 (Ser360) | Cell Signaling/4029 | pAb | 2500 |
| P-SMC1 (Ser957) | Novus Biolog/NB100-205 | pAb | 2000 |
| P-SMC1 (Ser966) | Abcam/1276 | pAb | 3000 |
| P-SMC3 (Ser1083) | Bethyl Lab, Inc/A300-480A | pAb | 2000 |
| PUMA | Cell Signaling/4976 | pAb | 2000 |
| RAD21 | Cell Signaling/4321 | pAb | 2500 |
| SMC1 | Cell Signaling/4802 | pAb | 2500 |
| SMC3 | Millipore/AB3914 | pAb | 4000 |
| STAG2 | Cell Signaling/4239 | pAb | 2500 |
| XIAP | BD Transduction Lab/610717 | mAb | 1500 |
| β-actin | Sigma/A5316 | mAb | 10000 |
| γ-H2AX | Upstate Biotech/05-636 | mAb | 3000 |
| 2MeH3K27 | Cell Signaling/9755 | pAb | 2000 |
| 2MeH3K36 | Cell Signaling/9758 | pAb | 2000 |
| 2MeH3K4 | Cell Signaling/9725 | mAb | 2000 |
| 2MeH3K79 | Cell Signaling/9757 | pAb | 2000 |
| 2MeH3K9 | Cell Signaling/9753 | pAb | 2000 |
| 2MeH4K20 | Cell Signaling/9759 | pAb | 2000 |
| 3MeH3K27 | Cell Signaling/9756 | pAb | 2500 |
| 3MeH3K4 | Cell Signaling/9751 | mAb | 2000 |
| 3MeH3K9 | Cell Signaling/9754 | pAb | 2000 |
pAb: polyclonal antibody; used anti-rabbit IgG (or anti-goat as indicated) for secondary antibody from Bio-Rad Lab
mAb: monoclonal antibody; used anti-mouse IgG for secondary antibody from Bio-Rad Lab
Fold dilution in PBS with 0.05% Tween 20
2.8. Immunofluorescent staining
Cells were exposed to the indicated concentrations of Clo, Flu and Bu for 48 hr, harvested and washed twice with PBS, and resuspended in 2% paraformaldehyde in PBS for 20 min at room temperature with occasional mixing. Cells were centrifuged using an IEC CL31R Multispeed centrifuge at 70 × g for 5 min at 4°C and washed 3 times with PBS. After the last wash, the cells were resuspended in PBS and a 40 µl aliquot was used to cytospin onto glass slides at 200 rpm for 3 min. Cells on slides were permeabilized with acetone at −20°C for 4 min and washed briefly with PBS at room temperature. To minimize fluorescence background, cells were incubated with blocking buffer (1% FBS, 3% BSA in PBS) for 1 hr in a humidified chamber kept at 37°C. This was followed by incubation of the cells in 1:100 diluted anti-γ-H2AX antibody (Millipore) or 1:200 anti-P-ATM antibody (Rockland, Gilbertsville, PA) under the same conditions for 3 hr. Cells were washed three times with gentle shaking in PBS for 20 min at room temperature, then incubated with 1:500 diluted anti-mouse IgG antibody conjugated to Alexa Fluor 488 (Invitrogen, Carlsbad, CA) for 1 hr. Cells were washed three times in PBS for 20 min at room temperature. Chromosomal DNA was counterstained with 300 nM 4',6-diamidino-2-phenylindole (DAPI; Invitrogen) for 2 min and washed three times with PBS for 10 min. Slides were kept in PBS at 4°C overnight prior to immunofluorescence imaging using an Olympus IX81 fluorescence microscope equipped with a spinning disk confocal attachment at 60 X magnification.
2.9. Cell permeabilization and exposure to micrococcal nuclease
Chromosomal DNA was analyzed according to the procedure described by Zaret [21] with modifications. Cells were exposed to the indicated concentration of the drugs for 24 hr, harvested, washed twice with PBS and resuspended in permeabilization solution (35 mM HEPES, pH 7.4, 150 mM sucrose, 80 mM KCl, 5 mM K2HPO4, 5 mM MgCl2, 0.5 mM CaCl2) at a cell density of 3 × 106 cells/ml. Cells (1.5 ml) were mixed first with 50 µl of 25 gel units/ml micrococcal nuclease (New England Biolabs, Ipswich, MA) then with 3 µl of 5 mg/ml lysolecithin (MP Biomedicals, Solon, OH). Permeabilization and nuclease treatment were done for 1 min at room temperature. Cells were centrifuged immediately for 6–8 seconds at 4,500 × g and washed with PBS. Cell pellets were kept on ice until ready for genomic DNA isolation which was done using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI). Genomic DNA was analyzed on a 0.8% agarose gel in TAE (40 mM Tris-Acetate, pH 8.3, 1 mM EDTA) buffer containing ethidium bromide.
2.10. Analysis of DNA cross-linking
The DNA alkylating agent mitomycin C (MMC), which is a more potent DNA cross-linker than Bu, was used as a probe to determine whether pre-exposure of cells to [Clo+Flu] would cause remodeling of chromatin and thereby increase its susceptibility to MMC-induced cross-linking reactions. Cells were exposed to [0.03 µM Clo and 1.2 µM Flu] for 24 hr, then incubated with 10 µg/ml MMC at 37°C for 2 hr. Cells were harvested and washed with ice-cold PBS. Genomic DNA was isolated using the Wizard Genomic DNA purification kit (Promega) and digested with the restriction enzyme Nsi I (New England Biolabs) overnight at 37°C. The digested DNA was extracted with phenol:chloroform and precipitated with 0.3 M sodium acetate and 70% ethanol at −80°C for 1 hr. The DNA pellet was washed with 70% ethanol, dried and dissolved in TE buffer. The DNA concentration was determined using picogreen (Life Technologies, Carlsbad, CA). The denaturation-renaturation, gel electrophoresis and transfer of DNA onto a nylon membrane were done as described by Matsumoto et al. [22]. Pre-hybridization, hybridization and detection were done using the ULTRAhyb hybridization buffer and BrightStar® BioDetect Nonisotopic Detection kit (Life Technologies). A 1 kb BamHI-EcoRI fragment of the 18S rDNA gene was labeled using BrightStar® Psoralen-Biotin Nonisotopic Labeling kit (Life Technologies) and used as a probe. Band signals on the autoradiogram were digitized using the UN-SCAN-IT software (Silk Scientific, Orem, UT). The degree of MMC-induced DNA cross-linking was assessed by calculating the % double-stranded (DS) DNA relative to the sum of single-stranded (SS) plus DS DNA.
2.11. Patient cell samples
Peripheral blood and bone marrow samples from leukemia patients were collected after obtaining written informed consent. Mononuclear cells were purified using lymphocyte separation medium (Mediatech). The purified cells were incubated in suspension in RPMI 1640 supplemented with 10% FBS at a density of 5 × 105 cells/ml with the indicated drugs. All studies were performed according to a protocol approved by the Institutional Review Board of the University of Texas M D Anderson Cancer Center, in accordance with the Declaration of Helsinki.
3. Results
3.1. Clo and Flu have synergistic inhibitory effects on the survival of KBM3/Bu2506 cells; addition of Bu further enhances these effects
Survival analysis by MTT assay of KBM3/Bu2506 cells exposed to the respective individual drug shows IC20 values of 20 µg/ml Bu, 0.018 µM Clo and 0.67 µM Flu for a 96-hr treatment (data not shown). We used values close to these concentrations to study the effects of the individual drugs and their combinations. The immediate effects of individual drugs on the cell cycle were determined after a 24-hr drug exposure. As expected, minimal cell death was observed after a 24-hr drug exposure. Analysis of the cell cycle phase distribution showed increase in both S-phase and G2-phase of cells exposed to 20 µg/ml Bu; the cell fraction in S-phase increased from 41 ± 3% to 60 ± 3% relative to the solvent control, and the cell fraction in G2-phase increased from 6 ± 2% to 19 ± 2% (Figure 1A). Exposure of cells to 0.015 µM Clo or 0.6 µM Flu for 24 hr increased S-phase without significant effects on the cell fraction in G2-phase. Clo and Flu increased the cell fraction in S-phase from 41 ± 3% to 59 ±2% and 52 ± 2%, respectively (Figure 1A). These results are consistent with previous reports on the effects of Bu and nucleoside analogues on the cell cycle [18, 23, 24].
Fig. 1.
Effects on cell cycle distribution and synergistic cytotoxicity of Clo, Flu and Bu in KBM3/Bu2506 cells. (A) Effects of individual drugs on cell cycle distribution after a 24-hr drug exposure. Cells were fixed with ethanol, stained with propidium iodide and analyzed by flow cytometry. (B) Cells were exposed to drugs, alone or in combination, for 48 hr and analyzed for apoptosis based on cell fractions in sub-G1. Typical cell cycle histograms are shown and % cells in sub-G1 (±SD) are indicated at the bottom. (C) Similarly treated cells were evaluated for apoptosis by determining the proportion of cells with fragmented DNA using the TUNEL assay. These experiments were repeated at least 4 times with very similar results. (D) The MTT assay was used to determine the synergistic effects of Clo and Flu on cell killing. Cells were incubated for 4 days with the indicated concentrations of Clo or Flu alone, or in combination. Values for cell survival (%) on the Y-axis (logarithmic scale) were calculated relative to control cells exposed to solvent alone. (E) A similar assay was done to determine the combined effects of 10 µg/ml Bu plus [0.01 µM Clo + 0.2 µM Flu]. All error bars indicate standard deviations (SD) which were calculated using the Microsoft Office Excel Program.
To determine the effects of these drugs on apoptosis, cells were exposed to the respective drugs for 48 hr. The cell fraction in sub-G1 or positive in the TUNEL assay were used as measures of apoptosis. Exposure of cells to Bu, Clo or Flu alone resulted in only a marginal increase in sub-G1 fraction from ~5% in solvent control to 6%–7% in drug-treated cells (Figure 1B). [Bu+Clo] and [Bu+Flu] combinations slightly increased the sub-G1 fraction to 8% and 10%, respectively. The combination of the two nucleoside analogues, Clo and Flu, resulted in ~20% cells in sub-G1, suggesting synergism. Addition of 20 µg/ml Bu to this [Clo+Flu] mixture further increased the sub-G1 fraction to ~36% (Figure 1B). These changes in the sub-G1 fractions were qualitatively consistent with the levels of apoptotic cells induced by these drug combinations as measured by the TUNEL assay (Figure 1C). Exposure of cells to Bu, Clo or Flu alone resulted in 0.4%, 0.2% and 0.3% TUNEL-positive cells, respectively, while the combinations of [Clo+Flu] or [Clo+Flu+Bu] increased these values to ~5% and ~15%, respectively.
To further assess the synergistic cytotoxicity of Clo and Flu, cells were exposed to various drug concentrations, alone or in combination, and cell survival was determined by the MTT assay. When values for cell survival (logarithmic scale) at three different concentrations of Clo were plotted against Flu concentration (linear scale), the slopes of the three lines increased with increasing Clo concentration (Figure 1D), further suggesting a synergistic effect of Clo and Flu. Analysis of the resulting cytotoxicity data using the median-effect method [20] supports the observed synergism. For example, a combination index of 0.5 was calculated when cells were exposed to [0.02 µM Clo+0.60 µM Flu].
The synergistic effects were more evident when Bu was added to the [Clo+Flu] combination and lower drug concentrations were used (Figure 1E). A cell survival of 85% was obtained when cells were exposed to 10 µg/ml Bu, and survival was 84% when cells were exposed to [0.01 µM Clo+0.2 µM Flu]. Survival significantly decreased to 59% when cells were simultaneously exposed to the three drugs at these same concentrations. Overall, these results suggest synergistic cytotoxicity when Clo and Flu were combined, and a pronounced increase in the cytotoxic effects was obtained when Bu was included in the combination.
3.2. Cytidine reverses the cytotoxic effect of Clo and Flu whereas deferoxamine enhances this effect
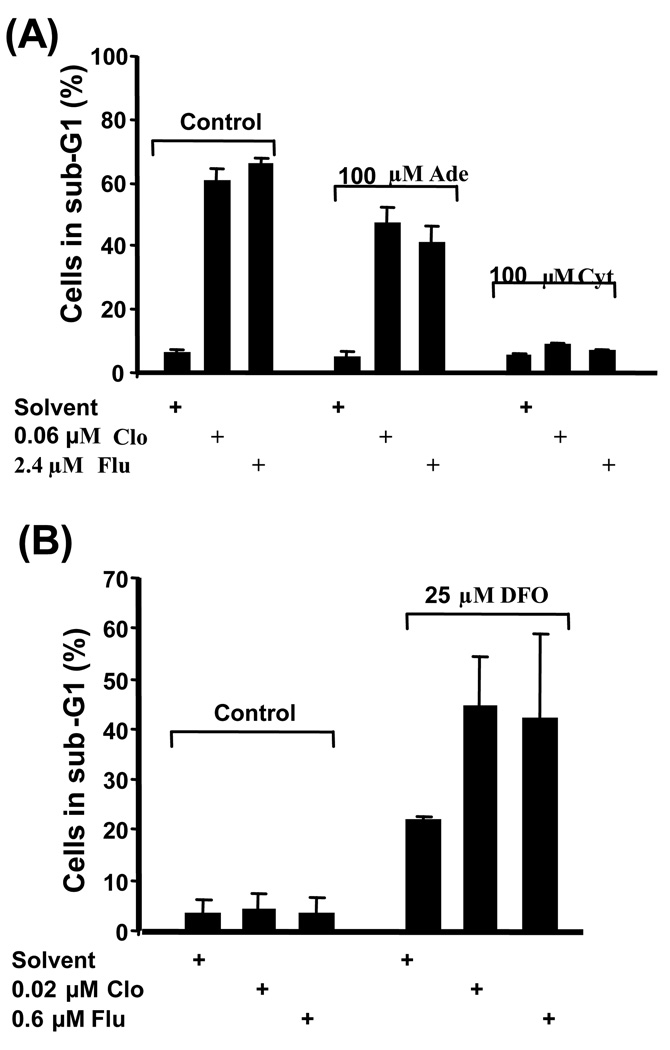
Both Clo and Flu are phosphorylated and thereby activated by deoxycytidine kinase (DCK) [25], which is feedback-inhibited by dCTP [6]. To determine indirectly if the same enzyme might mediate the activation of Clo and Flu in our AML cell line model, we examined the effects of cytidine on the cytotoxicity of these two nucleoside analogues. We hypothesized that the metabolism of cytidine to dCTP would lead to DCK inhibition and negate the effects of Clo and Flu. Indeed, Figure 2A shows an almost complete reversal of the cellular toxicity mediated by Clo and Flu. Exposure of cells to 0.06 µM Clo or 2.4 µM Flu for 48 hr resulted in 61% and 66% of the cells appearing in the sub-G1 compartment, respectively. In the presence of 100 µM cytidine and the same concentrations of the nucleoside analogues the sub-G1 population decreased to 7% – 9%, which is similar to the control. In the presence of 100 µM adenosine, cells exposed to 0.06 µM Clo or 2.4 µM Flu resulted in 47% and 41% cells in sub-G1, respectively, suggesting only weak reversal of the cytotoxicity of Clo and Flu. These results are consistent with the hypothesis that cytidine is metabolized to dCTP which inhibits DCK and consequently prevents the phosphorylation of Clo and Flu.
Fig. 2.
Effects of normal nucleosides and an inhibitor of ribonucleotide reductase on the cytotoxicity (apoptosis) of Clo and Flu. (A) Cells were exposed to 0.06 µM Clo or 2.4 µM Flu in the presence or absence of the indicated normal nucleosides (Ade: adenosine; Cyt: cytidine) for 48 hr and analyzed by flow cytometry for apoptosis based on sub-G1 DNA content. (B) Enhancement of the cytotoxicity of Clo or Flu in the presence of deferoxamine (DFO), an inhibitor of ribonucleotide reductase. All error bars indicate standard deviations (SD).
Clo and Flu have also been shown to inhibit ribonucleotide reductase (RR), the enzyme that converts nucleosides to deoxyribonucleosides [10]. This results in a decrease in deoxynucleotide pools and preferential incorporation of the analogues into DNA strands, leading to auto-potentiation of the drugs [10]. However, at the concentrations of Clo and Flu used here, RR inhibition will be relatively modest. Consequently, we hypothesized that inhibition of RR with deferoxamine (DFO) would potentiate the cytotoxic activity of Clo and Flu in our AML cell line. Figure 2B shows that 0.02 µM Clo or 0.6 µM Flu for 48 hr exerts negligible cytotoxicity whereas 25 µM DFO exposure results in 21% of the cells appearing in the sub-G1 compartment. Combination of 25 µM DFO with 0.02 µM Clo or 0.6 µM Flu resulted in ~40% cells in sub-G1, suggesting synergistic effects of DFO plus either nucleoside analogue. Similar results were obtained when Clo or Flu was combined with 100 µM hydroxyurea (data not shown), another inhibitor of RR.
3.3. The combinations of [Clo+Flu+Bu] activate the DNA damage response signaling pathway through ATM
Published reports have shown that nucleoside analogues, when incorporated into nascent DNA strands during replication, cause stalled replication forks and DNA strand breaks which activate DNA repair mechanisms [26]. A similar DNA damage response (DDR) is activated when cells attempt to repair DNA strands cross-linked by alkylators [27]. We therefore wished to characterize the DDR in AML cells treated with nucleoside analogues alone or in combination with a DNA alkylator at relatively low drug concentrations.
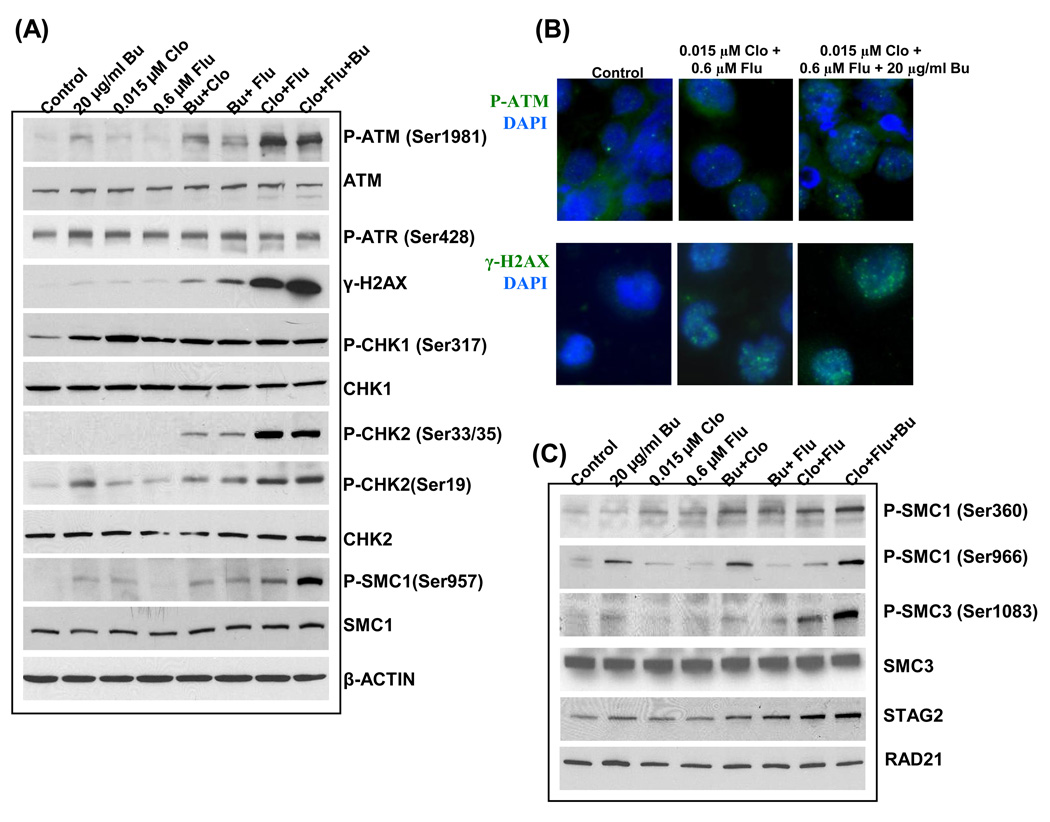
Activations of ATM (Ataxia Telangiectasia Mutated) and ATR (ATM and Rad3-related) are among the early steps associated with the cellular DDR. As shown in Figure 3A, ATR was not significantly activated by phosphorylation following exposure to any of the drugs either alone or in combination in our cell model. We therefore focused on the ATM signaling pathway. ATM is a protein serine-threonine kinase which phosphorylates various substrates following activation, including ATM itself (at Ser1981), p53, CHK1, CHK2, SMC1, NBS1, BRCA1 and RAD17 [28–30]. Using individual drugs at concentrations equivalent to their IC20, Clo, Flu and Bu marginally caused autophosphorylation of ATM at Ser1981 while combinations of Bu and Clo or Flu resulted in a higher degree of ATM phosphorylation (Figure 3A). A more dramatic result was obtained when cells were exposed to [0.015 µM Clo + 0.6 µM Flu] in the absence or presence of 20 µg/ml Bu (Figure 3A).
Fig. 3.
Combinations of Clo, Flu and Bu activate the DNA damage response. Cells were exposed to drugs alone, or in combination, for 48 hr and changes in protein expression or phosphorylation status were determined by immunoblot (A) or immunofluorescence (B) analysis. (C) Analysis of protein expression and phosphorylation status of the components of cohesion complex 1.
One of the early events mediated by ATM activation is the phosphorylation of histone 2AX (γ-H2AX), another widely-used indicator of DDR activation [31]. We observed a strong correlation between the formation of γ-H2AX and phosphorylation of ATM, with exposure of cells to the [Clo+Flu+Bu] combination providing the highest level of γ-H2AX (Figure 3A). We also used immunofluorescence staining to examine the level of ATM and H2AX phosphorylation in cells exposed to [Clo+Flu+Bu]. Figure 3B shows the drug-dependent increase in the level of P-ATM and γ-H2AX as indicated by the presence of green foci, which are known to localize with DNA double-strand breaks (DSBs) [32].
We next examined the activation status of several other proteins involved in the ATM signaling pathway. Because the KBM3/Bu2506 cell line is p53-negative, we focused on ATM substrates other than p53. Although Bu, Clo or Flu, when used alone, slightly increased the phosphorylation of CHK1 at Ser317, none of the drug combinations further enhanced CHK1 phosphorylation (Figure 3A). On the other hand, [Clo+Flu+Bu] combination caused increased phosphorylation of CHK2 at Ser33/35 and of SMC1 at Ser957 compared to the individual drugs. The [Clo+Flu] combination resulted in almost equal phosphorylation of CHK2 at Ser19 or Ser33/35 in the absence or presence of Bu (Figure 3A). However, the phosphorylation of SMC1 at Ser957 was greatly increased when all three drugs were combined, suggesting that phosphorylation of SMC1 plays a critical role in the cellular response to the triple drug combination when compared with [Clo+Flu].
SMC1 (Structural Maintenance of Chromosomes 1) together with SMC3, RAD21 and STAG2 forms the cohesin complex 1, which is involved in sister chromatid cohesion [33] and DNA recombination during DSB repair [34]. We decided to analyze the effects of [Clo+Flu+Bu] on the phosphorylation of SMC1 at other residues, as well as the expression of the other subunits of cohesion complex 1. Figure 3C shows that exposure to the triple drug combination resulted in the highest phosphorylation of SMC1 at Ser360 and Ser966, similar to the phosphorylation of Ser957 residue (Figure 3A). This drug combination also increased the phosphorylation of SMC3 at Ser1083. The drugs did not affect the level of expression of SMC3 and RAD21 but did increase the level of STAG2 (Figure 3C).
Overall, these results demonstrate that the DDR activated by [Clo+Flu+Bu] is transduced primarily through the ATM-CHK2 axis and suggest that phosphorylation of SMC1 and SMC3 may play a critical role in this response. These results are not unique to the KBM3/Bu2506 cell line model; similar molecular responses to [Bu+Clo], [Bu+Flu], [Clo+Flu] and [Clo+Flu+Bu] were observed in the HL60 and OCI-AML3 cell lines (data not shown).
3.4. [Clo+Flu+Bu] combinations activate cell cycle checkpoint proteins
Next, we sought to examine the effects of Clo/Flu/Bu combinations on the levels and activation status of downstream proteins that mediate the S-phase and G2/M checkpoints. As noted above, both [Clo+Flu] and [Clo+Flu+Bu] resulted in increased phosphorylation of CHK2, an ATM substrate involved in both the S-phase and G2/M checkpoints (Figure 3A). Activation of CHK2 may lead to phosphorylation of CDC25A, which consequently inactivates CDC25A phosphatase activity and leads to its degradation [35]; a dramatic decrease in the level of expression of CDC25A was indeed seen in cells exposed to [Clo+Flu+Bu] (Figure 4). Another factor that may contribute to decreased CDC25A expression is the observed down-regulation of c-MYC (Figure 4), a known positive transcription factor for the CDC25A gene [36, 37]. The observed down-regulation of CDC25A is most likely responsible for the increased level of P-CDK2 (Figure 4), which results in activation of the intra-S phase checkpoint [38]. This effect could be reinforced by the observed increase in the level of cyclin E2 (Figure 4), similar to a previous report on stabilized cyclin E-induced increased S-phase in response to replication fork stress caused by MMC or UV irradiation [39]. Another protein that could inactivate CDK2 is p21CIP1, a known CDK inhibitor that can also reinforce the S-phase checkpoint by inhibiting proliferating cell nuclear antigen (PCNA) [40]. Exposure of KBM3/Bu2506 cells to [Clo+Flu+Bu] resulted in a p53-independent up-regulation of p21CIP1, which might be attributed to the down-regulation of c-MYC (Figure 4) [41]. Another potential contributor to increased fraction in S-phase of cells exposed to [Clo+Flu+Bu] is the phosphorylation of SMC1 and SMC3 (Figure 3c) [42] described in the previous section.
Fig. 4.
Levels of expression and phosphorylation status of proteins involved in cell cycle checkpoints are affected by Clo ± Flu ± Bu. KBM3/Bu2506 cells were exposed to drugs alone, or in combination, for 48 hr and analyzed for their expression/phosphorylation of proteins involved in increased S-phase and G2/M.
The functions of CDC25A and SMC1 are not limited to the S-phase checkpoint. SMC1 has also been reported to be a critical factor in activating the G2/M checkpoint [43]. Its phosphorylation at Ser966 might be important for this function, as suggested by its induction in the presence of Bu alone (Figure 3C), a strong G2-phase arrest mediator (Figure 1A). Another phosphatase that regulates cell cycle is CDC25B, which is also a substrate of CHK2. A marked phosphorylation of CDC25B at Ser187, presumably due to activation of CHK2, was observed in cells exposed to [Clo+Flu+Bu] (Figure 4). This phosphorylation is known to inactivate CDC25B phosphatase activity and would consequently activate G2/M checkpoint [44]. Overall, the phosphorylation of SMC1, down-regulation of CDC25A, phosphorylation of CDC25B and up-regulation of p21CIP1 probably contribute to increased fraction of cells in G2 phase.
3.5. [Clo+Flu+Bu] combinations trigger apoptosis and down-regulate pro-survival genes
Cells of hematological origin typically undergo apoptosis when overwhelmed by DDR. The observed extensive activation of ATM and cell cycle checkpoint signaling (Figures 3 and 4) is strongly suggestive of such a response to high levels of complex genomic injury that may ultimately lead to apoptosis. Indeed, the [Clo+Flu+Bu] combination activated apoptosis to a much greater extent than [Clo+Flu], as indicated by cleavage of PARP1 (poly-ADP ribose polymerase-1), activation (by cleavage) of two key apoptotic proteins, caspases 3 and 9, cleavage of MCL1, and up-regulation of PUMA (Figure 5). The activation of caspase 3 correlates with the cleavage of one of its known substrates, ANP32B (Figure 5) [45], a histone chaperone [46]. The activation of these pro-apoptotic genes/proteins is consistent with the increase in the fraction of cells with sub-G1 DNA content or that are TUNEL-positive (Figures 1B and C) in the presence of [Clo+Flu+Bu].
Fig. 5.
Analysis of proteins involved in apoptosis and cell survival. Cells were exposed to the various drugs alone, or in combination, for 48 hr and analyzed for the indicated proteins by Western blotting.
The cytotoxicity of [Clo+Flu+Bu] may not only be explained by the upregulation/activation of apoptotic proteins; the observed down-regulation of the pro-survival proteins c-MYC and XIAP in cells exposed to [Clo+Flu+Bu] (Figures 4 and 5) may also contribute to the cytotoxicity of the three-drug combination. Since c-MYC is a ubiquitous transcription factor [37], its down-regulation would lead to deregulation of the expression of genes related to cell cycle and growth. Examples are shown by the down-regulation of CDC25A and up-regulation of p21CIP1 (Figure 4), as discussed above.
3.6. [Clo+Flu+Bu] combinations result in methylations of histone 3
To further understand the cellular responses underlying the synergistic cytotoxicity of [Clo+Flu+Bu], we examined several markers of chromatin remodeling related to covalent histone modifications [47]. Immunostaining analysis of extracts from cells exposed to [Clo+Flu] for 48 hr shows greatly increased methylations of lysine residues of histone 3 at positions 4, 9, 27, 36 and 79 (Figure 6A). Methylation was also observed for Lys-20 of histone 4 (Figure 6A). All methylations of histones 3 and 4 were further increased when Bu was combined with Clo and Flu.
Fig. 6.
Combinations of Clo, Flu and Bu cause methylations of histones and chromatin remodeling. (A) KBM3/Bu2506 cells were exposed to drugs alone, or in combination, for 48 hr and analyzed for methylation of histones 3 and 4. (B) Cells were exposed to solvent, 0.015 µM Clo + 0.6 µM Flu ([Clo+Flu]-1), or 0.03 µM Clo + 1.2 µM Flu ([Clo+Flu]-2) for 24 hr, permeabilized with lysolecithin (Lyso) in the presence of micrococcal nuclease (Nuc), and the genomic DNA was isolated and analyzed on an agarose gel. The first 3 lanes contain DNA samples from non-permeabilized cells. (c) Cells were similarly exposed to DMSO solvent or [Clo+Flu]-2 for 24 hr followed by 10 µg/ml MMC for 2 hr prior to isolation of gDNA and Southern blot analysis of non-denatured (lane 1) and denatured (lanes 2–4) DNA. Double stranded (DS) and single-stranded (SS) DNA signals were analyzed quantitatively using UN-SCAN-IT software.
These changes in chromatin structure may well contribute to the increased efficacy of [Clo+Flu+Bu] compared with [Clo+Flu]. One possibility is that [Clo+Flu] initiates chromatin remodeling and exposes the genomic DNA to alkylation by Bu. If this is correct, then the genomic DNA should also be more susceptible to nucleases. We, therefore, permeabilized cells treated with [Clo+Flu] and exposed them to micrococcal nuclease. Figure 6B shows a dose-dependent susceptibility of the genomic DNA to nuclease in cells exposed to these drugs as indicated by an increase in DNA laddering. The first three lanes in Figure 6b are genomic DNA isolated from cells exposed to solvent alone (control) or increasing concentrations of [Clo+Flu], but the cells were not treated with permeabilizing agent and micrococcal nuclease prior to DNA isolation. The presence of low amounts of degraded DNA in lanes 2 and 3 may be attributed to apoptosis occurring during the drug exposure, consistent with the results shown in Figures 1 and 5. The level of degraded DNA increased dramatically, relative to the control, when permeabilized cells were exposed to nuclease (Figure 6b, lanes 5 and 6).
The [Clo+Flu]-mediated reorganization of chromatin is further suggested by an increase in MMC-mediated DNA cross-linking observed in cells pre-exposed to [Clo+Flu] (Figure 6C). When Nsi I-digested genomic DNA from cells exposed to DMSO solvent alone was denatured with 0.1 N NaOH (Figure 6C), almost all of the DNA fragments were converted to single strand (SS, lane 2) which migrates in an agarose gel much faster than the non-denatured sample (lane 1). When the DNA from control cells treated with DMSO and MMC was denatured with NaOH, cross-linked and renatured DNA migrated at the same position as the DS DNA (lane 3). Pre-exposure of cells to [Clo+Flu] for 24 hr followed by treatment with 10 µg/ml MMC resulted in a 2.2-fold increase in cross-linking (lane 5) when compared to cells exposed to MMC alone (lane 3). Together, these results are consistent with our hypothesis that [Clo+Flu] causes histone 3 methylations, opens up chromatin and makes the genomic DNA more accessible to alkylation and cross-link formation by drugs such as MMC and, by extension, Bu.
3.7. [Clo+Flu+Bu] combinations have similar synergistic effects in patient leukemia cells
To assess the potential clinical implications of our observations, we isolated mononuclear cells (MNC) from peripheral blood and bone marrow of patients with active AML and exposed the cells to various drug combinations. Figure 7 (upper panel) shows the characteristics of the AML patients whose MNCs were used in this study. We generally observed a lower degree of drug sensitivity of the primary leukemia cells and therefore used 0.03 µM Clo, 1.2 µM Flu and 20 µg/ml Bu for this study. Exposure of the isolated MNC to [Clo+Flu] for 48 hr increased the phosphorylation of histone 2AX and SMC1, decreased c-MYC expression and increased methylation of histone 3 at Lys-9 and Lys-27 relative to the control (Figure 7). Addition of Bu to [Clo+Flu] generally enhanced these effects on protein expression or modifications (Figure 7). Although the data are not perfect (e.g., the unanticipated decrease in total SMC1 seen in patient 2), these results are reasonably consistent with those obtained in the cell line model.
Fig. 7.
Effects of [Clo+Flu±Bu] combinations on mononuclear cells isolated from AML patients. The table shows the characteristics of the patients. The cell samples were exposed to solvent (Cont), 0.03 µM Clo + 1.2 µM Flu (CF) or 0.03 µM Clo + 1.2 µM Flu + 20 µg/ml Bu (CFBu) for 48 hr and analyzed by Western blot as indicated (lower panel).
4. Discussion
Our report presents evidence of a strong synergistic cytotoxicity of [Clo+Flu+Bu] combinations at low drug concentrations almost equivalent to their IC20, the concentrations that cause 20% inhibition of cell survival when used individually. This synergism was observed in the AML cell lines KBM3/Bu2506, HL60, and OCI-AML3, and similar events at the molecular level were seen in mononuclear cells isolated from AML patients, suggesting a general cytotoxic efficacy of [Clo+Flu+Bu] combinations. The cytotoxic activity of these drug combinations may be attributed to at least four interrelated mechanisms involving crosstalk between different types of DNA damage and cellular responses to that damage: (1) inhibition of DNA synthesis and DNA repair responses [9, 10, 26, 48], (2) hyperactivation of cell cycle checkpoint signaling, (3) extensive remodeling of chromatin, and (4) induction of apoptosis.
Our results show that [Clo+Flu+Bu] strongly activate components of the DDR pathway when these drugs are combined even at concentrations as low as their IC20. When used individually, much higher concentrations of these drugs are required to elicit similar responses. These DDR signals are primarily transduced through activation of the ATM-CHK2 axis. Autophosphorylation of ATM at Ser1981 correlates with the phosphorylation of CHK2, SMC1 and SMC3. SMC1 is a chromosomal protein that heterodimerizes with SMC3 to form a cohesin complex whose major function is to enable sister chromatid cohesion to occur after DNA replication [49]. Recent reports also suggest a role for this cohesion complex in DNA repair [50, 51] and, as with SMC1, SMC3 phosphorylation has been shown to be involved in the DDR [52]. SMC1 is phosphorylated in an ATM-dependent and -independent manner in response to DNA damaging agents, resulting in activation of the intra-S-phase cell cycle checkpoint [30, 42]. Our results are consistent with ATM-dependent phosphorylation of SMC1, although they do not rule out the involvement of an ATM-independent pathway.
There are, however, some notable differences in the response to the various drug combinations. Thus, the [Clo+Flu] combination seems to be more strongly associated with activation of the CHK2 pathway, whereas the [Clo+Flu+Bu] combination appears to preferentially invoke the SMC1/SMC3 signaling cascade. Figure 3A shows a dramatic phosphorylation of CHK2 at Ser33/35 in the presence of [Clo+Flu] relative to each individual drug, but addition of Bu does not further enhance this effect. On the other hand, phosphorylations of SMC1 and SMC3 are more evident with the three-drug combination (Figure 3C). The activation of CHK2, SMC1 and SMC3 is transmitted to the cell cycle via the S-phase and G2/M checkpoint machinery as shown by the down-regulation of CDC25A, stabilization of cyclin E2 and phosphorylation of CDK2 and CDC25B (Figure 4).
The preferential phosphorylations of SMC1 and SMC3 in cells treated with [Clo+Flu+Bu] presumably reflects the above-mentioned interactions between the various elements of the DDR invoked in response to the combined genomic injury caused by the nucleoside analogues and alkylating agent that results in the increased levels of cell death/apoptosis. The inhibitory effects of Clo and Flu on DNA synthesis are well established. These nucleoside analogues inhibit DNA polymerases-α and -ε [10] and their incorporation into growing DNA strands causes replication forks to stall, in addition to strand breakage [26]. In contrast, DNA alkylators, exemplified by Bu, cause DNA crosslinks and their repair also induces replication forks to stall [53, 54]. The combinations of Clo/Flu/Bu are, therefore, expected to exert a more significant inhibition of DNA synthesis as well as unique types of DNA damage. Indeed, SMC1 has been shown to be phosphorylated at the sites of DSB, and it is believed to be involved in DNA recombination steps during repair [50, 51]. Based on our data and published reports, the incorporation of the nucleoside analogues Clo and Flu into the nascent DNA strands and the Bu-mediated cross-linking of DNA may have activated DNA repair and created DSBs. DNA damage checkpoint activation complexes, which include the ATM and SMC proteins, could then be recruited to the sites of these complex DSB-generating lesions.
The robust activation of the DDR by the combination of [Clo+Flu+Bu] clearly results in enhanced triggering of the apoptotic cascade (Figures 1B and 1C) as indicated by the cleavage of PARP1, caspase 3, caspase 9 and MCL1, by the up-regulation of the pro-apoptotic protein PUMA, and by the down-regulation of the anti-apoptotic XIAP and pro-survival c-MYC proteins (Figure 5). c-MYC, a ubiquitous transcription factor, appears to play an important role in the cell cycle checkpoint activation and apoptosis mediated by [Clo+Flu+Bu]. The down-regulation of c-MYC correlates with the down-regulation of CDC25A and induction of p21CIP1, proteins which assume critical roles in cell cycle control. It will be important to identify other c-MYC target genes relevant to the cytotoxicity of [Clo+Flu+Bu].
The above-mentioned mechanisms all converge at the level of the remodeling of chromatin, a process effected by post-translational modifications of histones. Analysis of the effects of [Clo+Flu+Bu] on these AML cells shows significant lysine methylations of histones 3 and 4, which are much more extensive when compared to the effects of [Clo+Flu] (Figure 6A). These histone modifications and their impact on chromatin structure consequently affect DNA-related cellular transactions such as transcription, replication and repair, as well as apoptosis [32, 47]. Histone 3 methylations at Lys-9 and Lys-27 are associated with repression of gene expression and might explain the observed down-regulation of the c-MYC gene (Figure 5).
The increased histone methylations may directly contribute to the increased cytotoxicity seen in cells exposed to [Clo+Flu+Bu] compared with [Clo+Flu]. By initiating these modifications, the nucleoside analogues may open the chromatin structure, making the DNA more accessible to Bu alkylation. This hypothesis is consistent with the observed susceptibility of the genomic DNA of [Clo+Flu]-treated cells to exogenous nuclease (Figure 6B) and with the increased MMC-mediated cross-linking of DNA seen in cells pre-treated with [Clo+Flu] (Figure 6C). The increase in damage levels and complexity may elicit a positive feedback loop that perpetuates histone methylations, DDR and DNA repair. Together with [Clo+Flu]-mediated DNA repair inhibition, this loop mechanism may be responsible for the observed massive DDR which, when overwhelmed, causes the synergistic potentiation of cytotoxicity of the [Clo+Flu+Bu] combination by committing the cells to apoptosis.
Similar molecular responses to [Clo+Flu+Bu] were observed when we used mononuclear cells isolated from AML patients. Notably, phosphorylation of SMC1, down-regulation of c-MYC and histone 3 methylations at Lys-9 and Lys-27 were enhanced in cells exposed to [Clo+Flu+Bu] versus [Clo+Flu]. These results suggest that [Clo+Flu+Bu] combinations have similar mechanisms of action in established cell lines and primary leukemia cells. Furthermore, our results form a basis for exploring combinations not only of one nucleoside analogue with an alkylating agent but also of two nucleoside analogues followed by a DNA alkylating agent. This approach would be expected to be most advantageous where the administered doses of both the nucleoside analogues and DNA alkylating agents can be escalated without concerns for any possible irreversible damage to normal hematopoiesis. Based on these results, we have initiated a clinical trial on the safety and efficacy of Clo±Flu plus intravenous Bu as a part of myeloablative pretransplant conditioning therapy for allogeneic HSCT patients who are at high risk of recurrent leukemia post-transplantation and/or who have active (chemotherapy-unresponsive) leukemia at the start of treatment.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (P01 CA055164 and CCSG Core CA16672), and The Stephen L. and Lavinia Boyd Fund for Leukemia Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
No conflict of interest.
Contributor Information
Benigno C. Valdez, Email: bvaldez@mdanderson.org.
Yang Li, Email: Yangli@mdanderson.org.
David Murray, Email: David.Murray5@albertahealthservices.ca.
Richard E. Champlin, Email: rchampli@mdanderson.org.
Borje S. Andersson, Email: bandersson@mdanderson.org.
References
- 1.Korycka A, Lech-Miranda E, Robak T. Novel purine nucleoside analogues for hematological malignancies. Recent Pat Anticancer Drug Discov. 2008;3:123–136. doi: 10.2174/157489208784638811. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, Jeha S, Gandhi V, Wess M, Faderl S. Clofarabine: past, present and future. Leuk Lymphoma. 2007;48:1922–1930. doi: 10.1080/10428190701545644. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery JA, Shortnacy-Fowler AT, Clayton SD, Riordan JM, Secrist JA., 3rd Synthesis and biologic activity of 2’-fluoro-2-halo derivatives of 9-beta-D-arabinofuranosyladenine. J Med Chem. 1992;35:397–401. doi: 10.1021/jm00080a029. [DOI] [PubMed] [Google Scholar]
- 4.Carson DA, Wasson DB, Esparza LM, Carrera CJ, Kipps TJ, Cottam HB. Oral antilymphocyte activity and induction of apoptosis by 2-chloro-2’-arabino-fluoro-2’deoxyadenosine. Proc Natl Acad Sci USA. 1992;89:2970–2974. doi: 10.1073/pnas.89.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plunkett W, Gandhi V. Purine and pyrimidine nucleoside analogs. Cancer Chemother Biol Response Modif. 2001;19:21–45. [PubMed] [Google Scholar]
- 6.Arner ESJ, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmac Ther. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- 7.Huang P, Chubb S, Plunkett W. Termination of DNA synthesis by 9-beta-D-arabinofuranosyl-2-fluoroadenine. A mechanism for cytotoxicity. J Biol Chem. 1990;265:16617–16625. [PubMed] [Google Scholar]
- 8.Iwasaki H, Huang P, Keating MJ, Plunkett W. Differential incorporation of ara-C, gemcitabine, and fludarabine into replicating and repairing DNA in proliferating human leukemia cells. Blood. 1997;90:270–278. [PubMed] [Google Scholar]
- 9.Zhenchuk A, Lotfi K, Juliusson G, Albertioni F. Mechanisms of anti-cancer action and pharmacology of clofarabine. Biochem Pharm. 2009;78:1351–1359. doi: 10.1016/j.bcp.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 10.Parker WB, Shaddix SC, Chang CH, White EL, Rose LM, Brockman RW, et al. Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5’-triphosphate. Cancer Res. 1991;51:2386–2394. [PubMed] [Google Scholar]
- 11.Russell JA, Savoie ML, Balogh A, Turner AR, Larratt L, Chaudhry MA, et al. Allogeneic transplantation for adult acute leukemia in first and second remission with a novel regimen incorporating daily intravenous busulfan, fludarabine, 400 cGy total-body irradiation, and thymoglobulin. Biol Blood Marrow Transplant. 2007;13:814–821. doi: 10.1016/j.bbmt.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14:672–684. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamauchi T, Nowak BJ, Keating MJ, Plunkett W. DNA repair initiated in chronic lymphocytic leukemia lymphocytes by 4-hydroperoxycyclophosphamide is inhibited by fludarabine and clofarabine. Clin Cancer Res. 2001;11:3580–3589. [PubMed] [Google Scholar]
- 14.Hijiya N, Gaynon P, Barry E, Silverman L, Thomson B, Chu R, et al. A multi-center phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia. 2009;23:2259–2264. doi: 10.1038/leu.2009.185. [DOI] [PubMed] [Google Scholar]
- 15.de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeoloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 16.Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:523–536. doi: 10.1016/j.bbmt.2008.12.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas X, Raffoux E, Elhamri M, Lobe I, Cannas G, Dombret H. Clofarabine for the treatment of adult acute myeloid leukemia. Future Oncol. 2009;5:1197–1210. doi: 10.2217/fon.09.105. [DOI] [PubMed] [Google Scholar]
- 18.Valdez BC, Murray D, Ramdas L, de Lima M, Jones R, Kornblau S, et al. Altered gene expression in busulfan-resistant human myeloid leukemia. Leuk Res. 2008;32:1684–1697. doi: 10.1016/j.leukres.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Zaret K. Current Protocols in Molecular Biology. 2007. Chromatin assembly and analysis; pp. 21.0.1–21.0.3. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto A, Vos J-MH, Hanawalt PC. Repair analysis of mitomycin C-induced DNA crosslinking in ribosomal RNA genes in lymphoblastoid cells from Fanconi’s anemia patients. Mut Res. 1989;217:185–192. doi: 10.1016/0921-8777(89)90070-0. [DOI] [PubMed] [Google Scholar]
- 23.Shi Z, Azuma A, Sampath D, Li YX, Huang P, Plunkett W. S-phase arrest by nucleoside analogues and abrogation of survival without cell cycle progression by 7-hydroxystaurosporine. Cancer Res. 2001;61:1065–1072. [PubMed] [Google Scholar]
- 24.Hassan Z, Hassan M, Hellstrom-Lindberg E. The pharmacodynamic effect of busulfan in the P39 myeloid cell line in vitro. Leukemia. 2001;15:1240–1247. doi: 10.1038/sj.leu.2402193. [DOI] [PubMed] [Google Scholar]
- 25.Masson E, Flordal E, Liliemark J, Spasokoukotskaja T, Elford H, Lagercrantz S, et al. Down-regulation of deoxycytidine kinase in human leukemic cell lines resistant to cladribine and clofarabine and increased ribonucleotide reductase activity contributes to fludarabine resistance. Biochem Pharmacol. 2003;65:237–247. doi: 10.1016/s0006-2952(02)01484-3. [DOI] [PubMed] [Google Scholar]
- 26.Ewald B, Sampath D, Plunkett W. Nucleoside analogs: molecular mechanisms signaling cell death. Oncogene. 2008;27:6522–6537. doi: 10.1038/onc.2008.316. [DOI] [PubMed] [Google Scholar]
- 27.Drabløs F, Feyzi E, Aas PA, Vaagbø CB, Kavli B, Bratlie MS, et al. Alkylation damage in DNA and RNA--repair mechanisms and medical significance. DNA Repair (Amst) 2004;3:1389–1407. doi: 10.1016/j.dnarep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- 29.Zhou BB, Elledge SJ. The DNA damage response: Putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 30.Kim S-T, Xu B, Kastan MB. Involvement of the cohesion protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev. 2002;16:560–570. doi: 10.1101/gad.970602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 32.van Attikum H, Gasser SM. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 2009;19:207–217. doi: 10.1016/j.tcb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Hirano T. SMC-mediated chromosome mechanics: A conserved scheme from bacteria to vertebrates? Genes Dev. 1999;13:11–19. doi: 10.1101/gad.13.1.11. [DOI] [PubMed] [Google Scholar]
- 34.Stursberg S, Riwar B, Jessberger R. Cloning and characterization of mammalian SMC1 and SMC3 genes and proteins, components of the DNA recombination complexes RC-1. Gene. 1999;228:1–12. doi: 10.1016/s0378-1119(99)00021-9. [DOI] [PubMed] [Google Scholar]
- 35.Karlsson-Rosenthal C, Millar JB. Cdc25: mechanisms of checkpoint inhibition and recovery. Trends Cell Biol. 2006;16:285–292. doi: 10.1016/j.tcb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-Myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 37.Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- 38.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 39.Lu X, Liu J, Legerski RJ. Cyclin E is stabilized in response to replication fork barriers leading to prolonged S phase arrest. J Biol Chem. 2009;284:35325–35337. doi: 10.1074/jbc.M109.035949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fotedar R, Bendjennat M, Fotedar A. Role of p21WAF1 in the cellular response to UV. Cell Cycle. 2004;3:134–137. [PubMed] [Google Scholar]
- 41.Coller HA, Grandori C, Tamayo P, Colbert T, Lander ES, Eisenman RN, et al. Expression analysis with oligonucleotide microarrays reveals MYC regulates genes involved in growth, cell cycle, signaling and adhesion. Proc Natl Acad Sci USA. 2000;97:3260–3265. doi: 10.1073/pnas.97.7.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yazdi PT, Wang Y, Zhao S, Patel N, Lee E, Qin J. SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev. 2002;16:571–582. doi: 10.1101/gad.970702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watrin E, Peters JM. The cohesion complex is required for the DNA damage-induced G2/M checkpoint in mammalian cells. EMBO J. 2009;28:2625–2635. doi: 10.1038/emboj.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol. 2006;18:185–191. doi: 10.1016/j.ceb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Shen SM, Yu Y, Wu YL, Cheng JK, Wang LS, Chen GQ. Down-regulation of ANP32B, a novel substrate of caspase-3, enhances caspase-3 activation and apoptosis induction in myeloid leukemic cells. Carcinogenesis. 2009;31:419–426. doi: 10.1093/carcin/bgp320. [DOI] [PubMed] [Google Scholar]
- 46.Munemasa Y, Suzuki T, Aizawa K, Miyamoto S, Imai Y, Matsumura T, Horikoshi M, et al. Promoter region-specific histone incorporation by the novel histone chaperone ANP32B and DNA-binding factor KLF5. Mol Cell Biol. 2008;28:1171–1181. doi: 10.1128/MCB.01396-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang GG, Allis CD, Chi P. Chromatin remodeling and cancer, part I: covalent histone modifications. Trends Mol Med. 2007;13:363–372. doi: 10.1016/j.molmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Willis N, Rhind N. Regulation of DNA replication by the S-phase DNA damage checkpoint. Cell Div. 2009;4:13. doi: 10.1186/1747-1028-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michaelis C, Ciosk R, Nasmyth K. Cohesins : chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- 50.Sjogren C, Nasmyth K. Sister chromatid cohesion is required for postreplicative double-strand break repair in Saccharomyces cerevisiae. Curr Biol. 2001;11:991–995. doi: 10.1016/s0960-9822(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 51.Bauerschmidt C, Arrichiello C, Burdak-Rothkamm S, Woodcock M, Hill MA, Stevens DL, et al. Cohesin promotes the repair of ionizing radiation-induced DNA double-strand breaks in replicated chromatin. Nucleic Acids Res. 2010;38:477–487. doi: 10.1093/nar/gkp976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo H, Li Y, Mu JJ, Zhang J, Tonaka T, Hamamori Y, et al. Regulation of intra-S phase checkpoint by ionizing radiation (IR)-dependent and IR-independent phosphorylation of SMC3. J Biol Chem. 2008;283:19176–19183. doi: 10.1074/jbc.M802299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponti M, Souhami RL, Fox BW, Hartley JA. DNA interstrand crosslinking and sequence selectivity of dimethanesulphonates. Br J Cancer. 1991;63:743–747. doi: 10.1038/bjc.1991.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stokes MP, Michael WM. DNA damage-induced replication arrest in Xenopus egg extracts. J Cell Biol. 2003;163:245–255. doi: 10.1083/jcb.200306006. [DOI] [PMC free article] [PubMed] [Google Scholar]