Abstract
The elevated plus and zero mazes (Plus and Zero, respectively) are used to assess behavior related to anxiety in rodents but direct comparisons of the two tests are lacking for rats. We compared the two methods in adult male Sprague-Dawley rats. Untreated rats in the Zero spent more time in open zones and exhibited more head dips than in the Plus whereas start latency and closed area entries were lower in the Zero than in the Plus. Diazepam (1 mg/kg) exposure increased time in the open in both mazes. Restraint (60 min prior to testing), yohimbine (2.5 mg/kg), and caffeine (100 mg/kg) had the opposite effect, significantly decreasing time spent in open zones in both mazes. No sexual dimorphism in behavior was seen in either maze in untreated rats. Although more open area time was evident in untreated animals in the Zero, after drug challenge both mazes detected anxiolytic and anxiogenic effects equally. Zero maze data can be analyzed directly because no center region exists; otherwise the two methods appear comparable following challenge.
Keywords: elevated plus maze, elevated zero maze, anxiety, diazepam, yohimbine, caffeine, nicotine, restraint stress
1. Introduction
Novel environments evoke conflict in rodents between exploratory and fear/defensive behavior. When allowed to make a choice between two novel areas, closed areas are preferred (Montgomery 1955;Pellow et al. 1985;Shepherd et al. 1994). For example, rats spend more time in the closed arm of a T-maze than the open arm (Montgomery 1955). The distribution of behavior in conflict tests is the most widely accepted index of anxiety (Montgomery 1955). There are a number of such tests, among which are the elevated plus and elevated zero mazes.
The elevated plus maze (Plus), with two adjacent closed arms perpendicular to two open arms (Figure 1A), has been validated by use of pharmacological (Lister 1987;Pellow et al. 1985;Pellow and File 1986) and behavioral (Lister 1987;Pellow et al. 1985) manipulations in rats and mice. Time spent in open areas is frequently reported as a percentage of time compared to the total time spent in the closed and open areas, thereby removing time spent in the center region from anxiety analysis (Hogg 1996). This adjustment is not be trivial considering that rats in some experiments spend upwards of 30% of the time in the center area, which reduces the time available to explore the arms (Rodgers and Dalvi 1997). Time spent in the center has been used by some investigators to assess non-anxiety behavior such as risk-assessment (Carobrez and Bertoglio 2005;Hogg 1996;Rodgers and Dalvi 1997) but this practice is less common.
Figure 1.
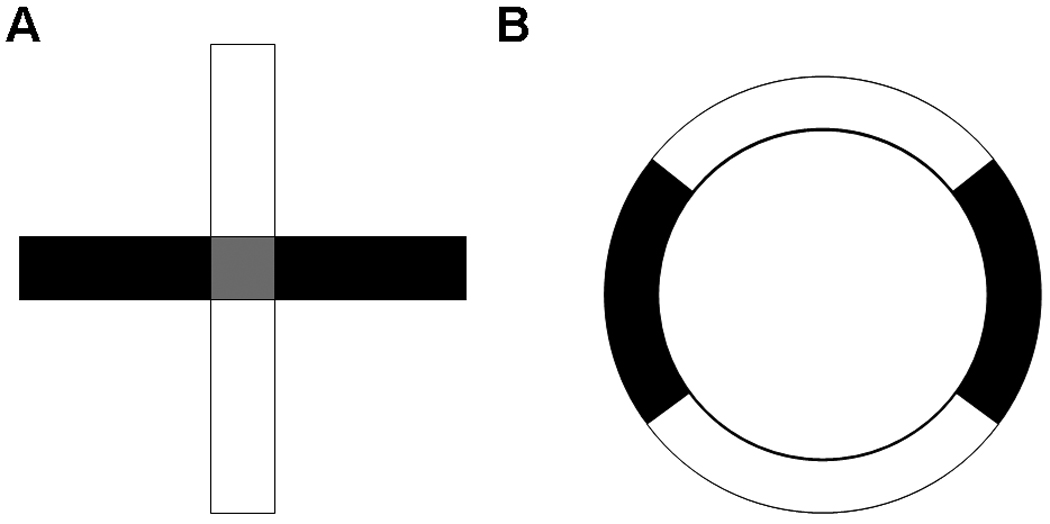
(A) Elevated Plus Maze. (B) Elevated Zero Maze. Black indicates enclosed areas, white indicates open areas, and gray represents center region in the Plus.
The elevated zero maze (Zero) was created to eliminate the center region of the Plus, and has also been pharmacologically validated with anxiolytic drugs (Shepherd et al. 1994). Unfortunately, no direct comparison of these two mazes has been reported in rats. The Zero is an elevated ring-shaped runway with the same amount of area devoted to adjacent open and closed quadrants (Figure 1B). The Zero has not been used as extensively as the Plus but has seen increased use in recent years. In mice, a recent study found the Zero was more sensitive to benzodiazepines than the Plus however no adjustment for center time in the Plus was made (Kulkarni et al. 2007). Whether a similar conclusion is possible in rats is unknown. Hence, there remains a gap in how these tests compare in rats with center time adjusted for in the Plus. Accordingly, the purpose of this experiment was to compare the two mazes using diazepam, the standard anxyiolytic used to validate this test, whereas several anxiogenic drugs were used as these have more variable effects.
Percent time spent in open areas is the most accepted scoring variable used to reflect anxiety (Lister 1987;Montgomery 1955;Pellow et al. 1985;Pellow and File 1986;Rodgers et al. 1997;Rodgers and Dalvi 1997;Shepherd et al. 1994). Increases in time in open areas is interpreted as decreased anxiety (Montgomery 1955). Time to leave the first closed area (start latency), is also used (Rodgers et al. 1997). Longer start latencies suggest higher anxiety. The number of head dips is another index of anxiety (Cole and Rodgers 1995;Cruz et al. 1994;Fernandes and File 1996;Rodgers and Johnson 1995). As head dips increase, anxiety is regarded as decreased. The number of closed entries is generally used as an index of general activity (Rodgers and Dalvi 1997).
Diazepam has been used in both mazes previously and therefore was used here (Pellow et al. 1985;Shepherd et al. 1994). Anxiogenic treatments are more variable, therefore we tested four: nicotine, yohimbine, caffeine, and restraint (Bhattacharya et al. 1997;Gulati et al. 2007;Irvine et al. 2001;Jain et al. 2005;Johnston and File 1989). Restraint was included in order to assess one non-pharmacological treatment (Lister 1990). Male and female untreated groups were separately compared to determine whether there were sex differences without regard to drug effects. After maze testing, animals were placed in a photocell-based locomotor test to independently assess activity levels. Diazepam at higher doses reduces locomotor activity (Nishino et al. 2008), therefore, we selected a dose previously shown not to affect locomotion.
2. Materials and Methods
2.1 Animals
Adult male (351–460 g) and female (180–225 g) Sprague-Dawley rats (Charles River, Raleigh, NC) 60 to 80 days old were tested once in either the Plus or Zero balanced for time of day. Each animal was used only once, in one maze or the other and then tested for locomotor activity. Testing occurred between 1300 h and 1800 h. Animals were acclimated to the vivarium for at least 1 week prior to testing and only handled during cage cleaning in order to minimize handling effects. Animals were pair-housed in polycarbonate cages (46 × 24 × 20 cm) containing woodchip bedding until 3 days prior to testing, then housed singly in identically sized cages, so that cage mates were not disrupted during the testing period. Animals had free access to food and water and were housed in an environmentally controlled vivarium (21 ± 1°C) with a 14 h light-dark cycle (lights on 600 h). The experimental protocol was approved by the Institutional Animal Care and Use Committee and the vivarium is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
2.2 Treatments
Males were used in drug and restraint experiments. The dose of each drug was based on published reports (Table 1). Diazepam (NLS Animal Health, Pittsburgh, PA), (−)-nicotine hydrogen tartrate (Sigma, Poole, UK), yohimbine hydrochloride (Sigma, Poole, UK), caffeine (Sigma, Poole, UK) or saline (SAL) were injected subcutaneously in a volume of 3 ml/kg 30 min prior to testing. Diazepam was obtained in injectable form (each ml contained 5 mg diazepam dissolved in 40% propylene glycol, 10% alcohol, 5% sodium benzoate and benzoic acid, and 1.5% benzyl alcohol (Hospira, Lake Forest, IL)) and diluted to the proper concentration in physiological saline. Other drugs were dissolved in physiological saline. Restraint was by placing the animal in semi-circular acrylic holders (13 × 5 × 8.3 cm) for 60 min prior to testing. Following drug or restraint treatment, each animal was assigned to one maze for testing. Testing was balanced for treatment and maze within and across days. Alternating groups of 4 animals were tested in the Plus, the next 4 in the Zero, balanced for treatment, successively. Animals were randomized for treatment within each testing block.
Table 1.
Dose and Rodent Half-Life of Pharmacological Treatments
| Treatment | Dose (mg/kg) | Rodent Half-Life |
|---|---|---|
| Diazepam | 1.0 | 1.4 h |
| Nicotine | 0.1 | 52 min |
| Yohimbine | 2.5 | 7–8 h |
| Caffeine | 100.0 | 3 h |
2.3 Apparatus and Procedures
2.3.1 Elevated Plus Maze (Figure 1A)
The maze was constructed of black polyethylene and elevated 50 cm above the floor. It consisted of two open arms 10 cm wide and 50 cm in length connected perpendicular to two closed arms of equal dimensions with a 10 cm square center region. The closed arms had black walls 30 cm in height (AB Plastics, Milford, OH). The open arms had 1 cm curbs along the edges to prevent falls. A dimly lit halogen lamp (average 11.7 lux at the center of the maze) in one corner of the testing room was used as the lighting source during testing. Animals were started in the center region facing a closed arm and allowed to explore for 5 min. Dependent measures were start latency (time the animal took to leave the beginning closed arm), head dips, percent time in the open arms, number of closed arm entries, and percent time in the center region. An overhead camera attached to a DVD recorder recorded the location of the rats and scoring was completed later using ODLog software (Macropod Software, Armidale, Australia). Between trials, the maze was cleaned with 70% ethanol.
2.3.2 Elevated Zero Maze (Figure 1B)
The maze was constructed of black acrylic in a circular track 10 cm wide, 105 cm in diameter, and elevated 72 cm from the floor (San Diego Instruments, San Diego, CA). The maze was divided in four quadrants of equal length with two opposing open quadrants with 1 cm high clear acrylic curbs to prevent falls and two opposing closed quadrants with black acrylic walls 28 cm in height. A 5 min trial under the same lighting conditions as in the Plus began with the animal placed in the center of a closed quadrant. Dependent measures were the same as for the Plus except that there was no center region. Between trials, the maze was cleaned with 70% ethanol.
2.4 Locomotor activity
For treated animals, a test of locomotor activity was conducted immediately following maze testing. Locomotor activity was measured for 30 min in 41 × 41 × 30 cm monitors equipped with 16 pairs of photodetector-LED sources along the x and y axes (Accuscan Instruments, Columbus, OH). The chambers were cleaned with 70% ethanol between animals. Total distance traveled was recorded in 5 min intervals.
2.5 Statistical Procedures
Data were analyzed using general linear model analyses of variance (ANOVA) (Proc GLM, SAS Institute, Cary, NC). Treatment and maze were between-subject factors in the analyses. Where both sexes were tested, sex was used as the between factor. Significant interactions were further analyzed using simple-effect ANOVAs. For locomotor data, mixed linear model ANOVAs were used with treatment as the between factor and interval as the within factor. In order to account for possible differences in locomotor activity, activity was used as a covariate in the analysis of percent time in the open in the mazes by analysis of covariance (ANCOVA). Two ANCOVAs were performed, one using locomotor activity during the entire 30 min test session and one using the first 5 min. Percent time animals spent in the center region of the Plus or for comparisons where no drug treatment was used were analyzed by two-tailed t-tests. Significance was set at p ≤ 0.05.
3. Results
Prior to analyses for treatment effects, control groups were compared. SAL controls from the nicotine and diazepam tested in the same timeframe were not significantly different and were therefore combined (Zero n = 16; Plus n = 18). SAL controls from the yohimbine and caffeine experiments were also not significantly different and were therefore combined (n = 6/maze) but were not combined with the two prior control groups because the latter experiments were conducted a year later. The untreated males from the untreated condition and for the restraint experiment (also untreated) were not significantly different from one another, were conducted in the same timeframe, and were therefore combined (Zero n = 21; Plus n = 22).
Using either 5 or 30 min locomotor activity as covariates did not alter differences for percentage of time in open for either maze.
3.1 Untreated rats
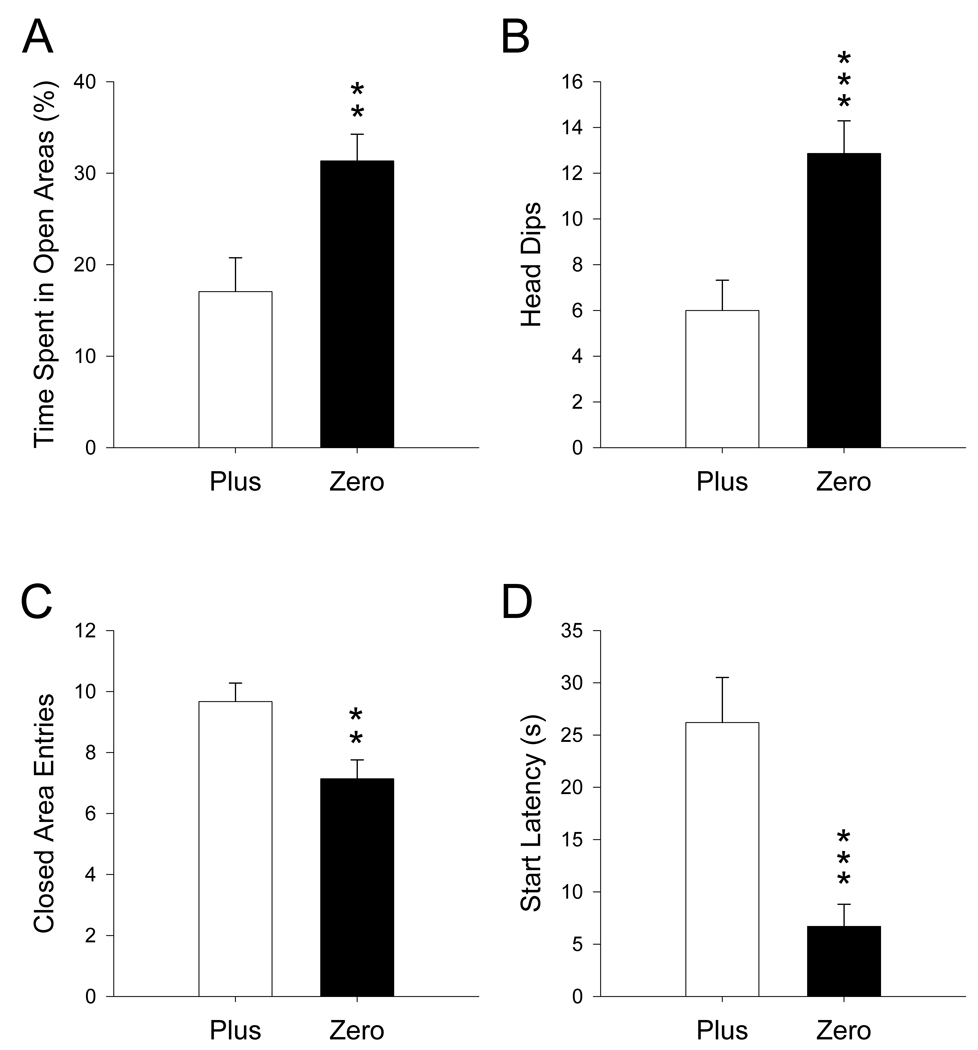
Untreated animals in the Zero had higher percent time in open areas (t(41) = 3.01, p ≤ 0.01; Figure 2A) and exhibited more head dips (t(41) = 3.54, p ≤ 0.001; Figure 2B) compared to animals in the Plus. Animals tested in the Plus had more closed area entries (t(31) = −2.91, p ≤ 0.01; Figure 2C; reduced N because this variable was not measured in the first set of animals tested in this experiment) and significantly longer start latencies compared to animals tested in the Zero (t(41) = −4.06, p ≤ 0.001; Figure 2D).
Figure 2.
Maze comparison in untreated male rats: Untreated rats tested in the Zero spent a larger percentage of time in the open (A) and exhibited more head dips (B) than rats in the Plus. Rats tested in the Plus had increased closed area entries (C) and start latencies (D) compared to rats tested in the Zero. **p ≤ 0.01; ***p ≤ 0.001. Zero N = 21; Plus N = 22.
3.2 Diazepam
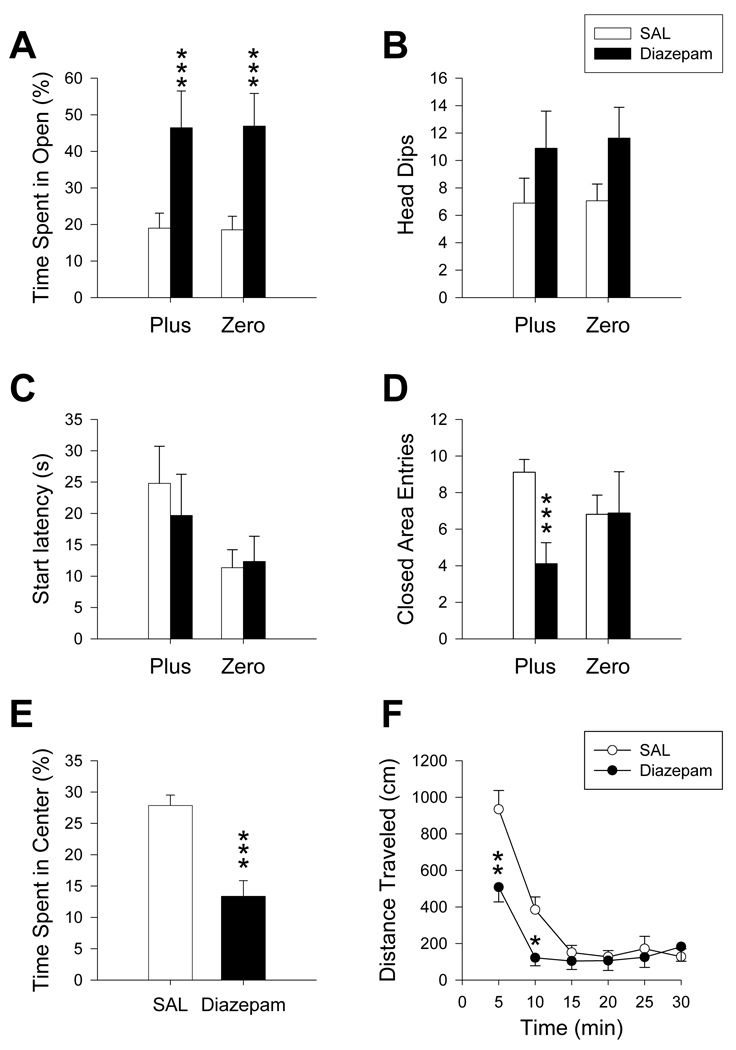
Diazepam-treated animals (Zero n = 8; Plus n = 9) spent a significantly greater percentage of time in the open areas compared to SAL controls (F(1, 47) = 19.66, p ≤ 0.001; Figure 3A), regardless of maze. Neither number of head dips nor start latency were significantly altered by diazepam in either maze (Figure 3B, C). However, the number of closed area entries was decreased in response to diazepam treatment (F(1, 47) = 4.6, p ≤ 0.05; Figure 3D), which was only significant in the Plus, i.e., for closed area entries there was a treatment x maze interaction (F(1, 25) = 15.20, p ≤ 0.001). No other maze effects or interactions were obtained. Center region time was examined in the Plus. The percent time spent in the center was decreased in diazepam-treated animals compared to controls (t(24) = 4.84, p ≤ 0.001; Figure 3E). In the locomotor chambers, diazepam-treated animals showed reduced distance traveled but the effect was not significant (main effect, F(1, 45) = 3.86; p ≤ 0.06); however there was a treatment x time interaction (F(5, 225) = 5.09; p ≤ 0.01; Figure 3F). Further analysis showed that at the 5 and 10 min intervals diazepam-treated animals had decreased distance relative to SAL-treated animals. Diazepam treatment did not cause a difference in time spent in the center or peripheral regions during activity testing.
Figure 3.
Maze comparison after diazepam treatment: Regardless of maze, diazepam increased percent time in open (A), but did not alter the number of head dips (B) or start latency (C) compared to SAL-treated controls. Number of closed area entries (D) was decreased in animals treated with diazepam in the Plus, but not the Zero, compared to controls. In the Plus, diazepam decreased the percent time animals spent in the center region of the maze (E). Total distance traveled in the open-field locomotor test (F) decreased after diazepam treatment during the first 10 min. ANCOVA revealed that maze findings were unaltered using open-field locomotion as a covariate. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. Diazepam: Zero N = 8; Plus N = 9; SAL: Zero N = 16; Plus N = 18.
3.3 Nicotine
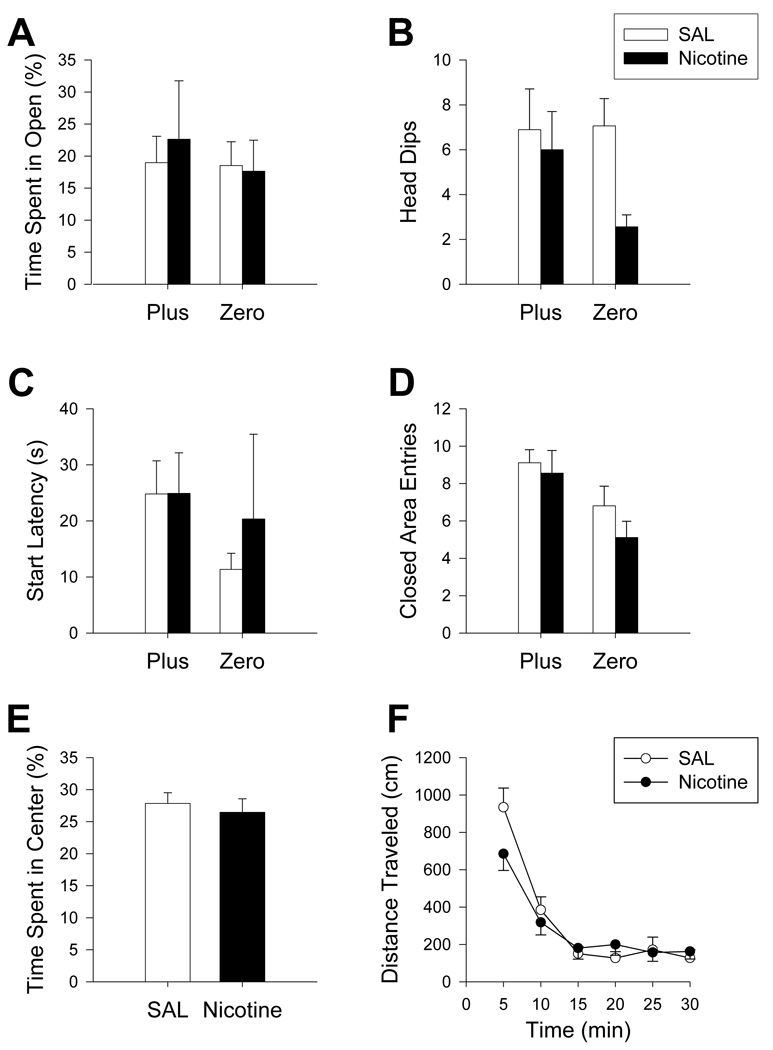
Nicotine treatment (n = 9/maze) had no significant effect on either maze or any endpoint (ex. Percent time in open: F(1, 48) = 0.01, p = 0.9; Figure 4A–E). In the locomotor chambers, distance traveled was unaffected by nicotine (Figure 4F). When the first 5 min of testing was separately analyzed for center and peripheral distance, no differences were found.
Figure 4.
Maze comparison after nicotine treatment: Regardless of maze, percentage of time animals spent in the open areas (A), number of head dips (B), start latency (C) and number of closed area entries (D) were not altered by nicotine treatment. In the Plus, no difference was seen in the percentage of time animals spent in the center region (E) between nicotine and SAL treatment. Nicotine treatment did not produce differences in distance traveled (F) in the open-field. Nicotine: N = 9/maze; Zero N = 16; Plus N = 18.
3.4 Yohimbine
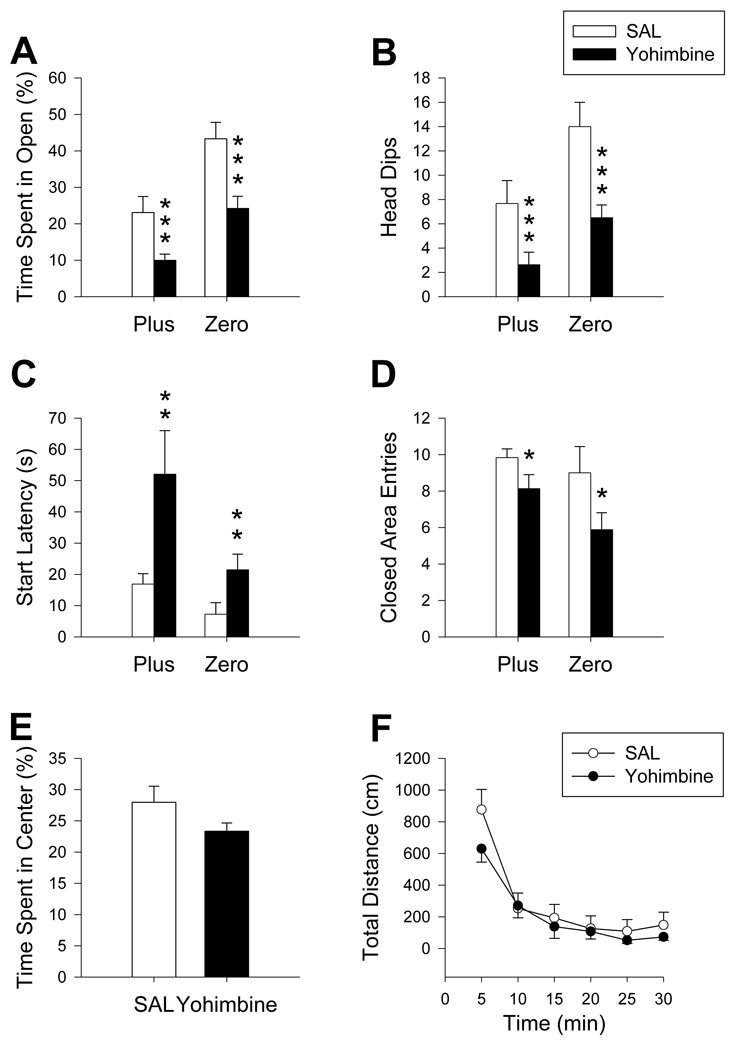
Exposure to yohimbine (n= 8/maze) significantly decreased the percent time spent in open of both mazes compared to SAL (F(1, 24) = 21.55, p ≤ 0.001; Figure 5A). In both mazes, yohimbine reduced the number of head dips (F(1, 24) = 18.53, p ≤ 0.001; Figure 5B) while start latencies were increased (F(1, 24) = 7.69, p ≤ 0.01; Figure 5C) compared to SAL. The number of closed area entries was also reduced in yohimbine-treated animals in both mazes (F(1, 24) = 6.38, p ≤ 0.05, Figure 5D). A tendency toward reductions in time spent in the center region of the Plus was observed in the yohimbine-treated animals but was not significant (t(12) = 3.02, p ≤ 0.10; Figure 5E). No other main significant effects or interactions were obtained. In the locomotor chambers, yohimbine did not alter distance traveled (Figure 5F). Neither activity in the center or peripheral regions differed as a function of yohimbine treatment during activity testing.
Figure 5.
Maze comparison after yohimbine treatment: In both mazes, percentage of time animals spent in the open areas (A) and number of head dips (B) were decreased following yohimbine exposure. Start latencies (C) were increased in yohimbine-treated animals compared to SAL controls. The number of closed area entries (D) in both mazes was reduced after yohimbine treatment compared to controls. In the Plus, yohimbine-treated animals exhibited a trend towards significance in percentage of time spent in the center region (E). No difference in distance traveled (F) in the open-field was observed. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. Yohimbine: N = 8/maze; SAL: N = 6/maze.
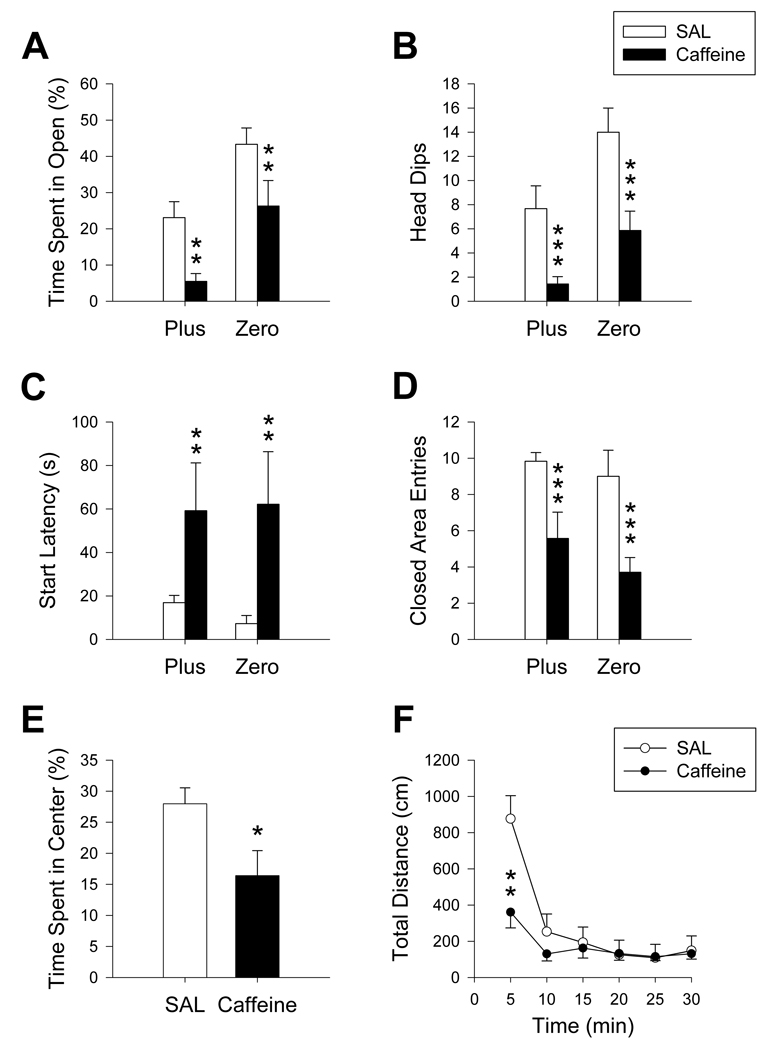
3.5 Caffeine
Caffeine exposure (n= 7/maze) significantly decreased both the percent time in open (F(1, 22) = 12.28, p ≤ 0.01; Figure 6A) and number of head dips (F(1, 22) = 21.01; Figure 6B), and increased start latencies (F(1, 22) = 7.38, p ≤ 0.01; Figure 6C) compared to SAL-treated controls. Caffeine also reduced the number of closed area entries in both mazes (F(1, 22) = 17.61, p ≤ 0.001; Figure 6D) compared to SAL. Percent time in the center region (Plus) was decreased in caffeine-treated animals compared to SAL-treated animals (t(11) = 5.42, p ≤ 0.05; Figure 6E). No other significant main effects or interactions were seen. In the locomotor chambers, no main effect of caffeine was seen, however there was a treatment x time interaction (F(5, 24) = 12.34, p ≤ 0.001; Figure 6F). This was attributable to the first 5 min during which caffeine-treated animals had decreased distance. The reported maze outcomes remained significant using locomotor activity as a covariate indicating activity levels did not alter the anxiety profile in the mazes. Separate analyses of 5 and 30 min central and peripheral activity showed significant decreases for both regions (center: F(1, 22) = 6.44, p ≤ 0.05; periphery: F(1, 22) = 12.05, p ≤ 0.01), consistent with what was seen for total distance.
Figure 6.
Maze comparison after caffeine treatment: Decreases in percent time spent in open areas (A) and number of head dips (B) were seen following caffeine treatment. Start latencies (C) were increased in caffeine-treated animals in both mazes compared to controls while the number of closed area entries (D) decreased. In the Plus, caffeine-treated animals spent less time in the center region (E) than controls. Caffeine decreased distance traveled (F) in the first 5 min of open-field testing. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. Caffeine: N= 7/maze; SAL: N = 6/maze.
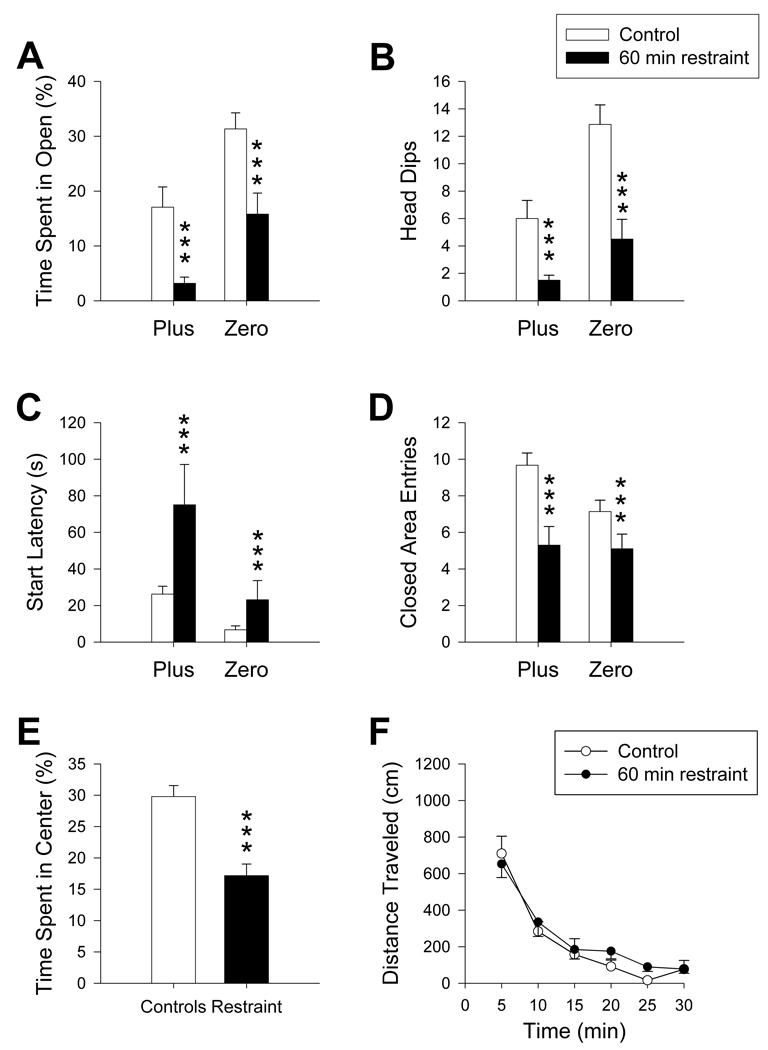
3.6 Restraint
Preliminary data showed that restraint for 30 min did not affect performance in either maze (not shown). Restraint for 60 min (n= 10/maze) produced decreases in the percent time animals spent in open of both mazes relative to controls (F(1, 59) = 15.30, p ≤ 0.001; Figure 7A). In both mazes, the number of head dips was reduced (F(1, 59) = 17.95, p ≤ 0.001; Figure 7B), and start latencies were increased compared to controls (F(1, 59) = 13.34, p ≤ 0.001; Figure 7C). Restraint-treated animals also had a decrease in number of closed area entries, regardless of maze, compared to controls (F(1, 49) = 15.77, p ≤ 0.001; Figure 7D). In the Plus, restraint decreased the amount of time animals spent in the center region relative to controls (t(31) = 4.91, p ≤ 0.001; Figure 7E). No other significant main effects or interactions were seen. In the locomotor chambers, no difference was seen in distance traveled between restraint-treated animals and controls (Figure 7F). Activity levels in the central and peripheral regions were similarly not significantly different.
Figure 7.
Maze comparison after restraint stress: 60 min restraint decreased both percent time spent in open areas (A) and number of head dips (B) compared to controls regardless of maze. Start latency (C) increased after restraint stress compared to controls in both mazes. Animals under restraint stress decreased the number of closed area entries (D) made compared to controls. In the Plus, restraint stress decreased the percentage of time animals spent in the center region of the maze (E). Distance traveled (F) in the open-field was not altered from restraint stress. ***p ≤ 0.001. Restraint stress: N = 10/maze; Control: Zero N= 21; Plus N = 22.
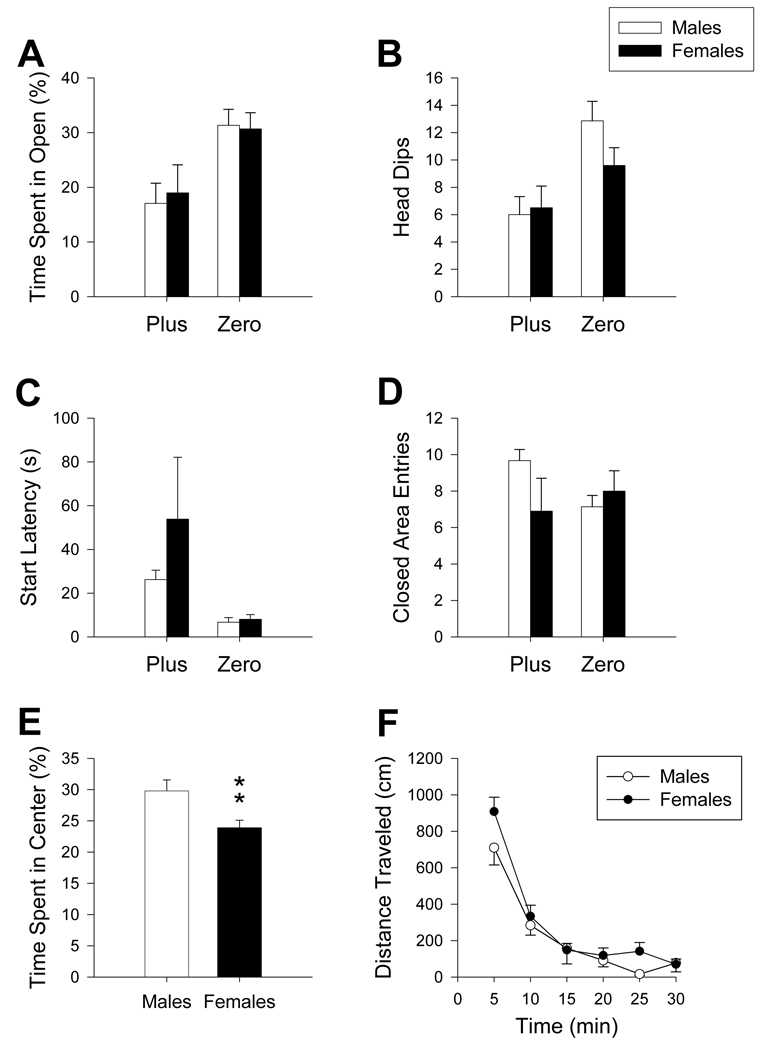
3.7 Sexual dimorphism
Analysis of untreated males and females (n= 10/maze) showed no sex differences in percent time in open, head dips, start latency, or closed area entries (Figure 8A–D). In the Plus, females spent less time in the center than males (t(31) = 2.76, p ≤ 0.01; Figure 8E). In the locomotor chambers, no difference in distance traveled between sexes was seen (Figure 8F).
Figure 8.
Maze comparison between sexes: No difference between males and females was observed in percent time animals spent in the open (A), number of head dips made (B), latency to start (C), or number of closed area entries (D) regardless of maze. Untreated females spent less time in the center platform (E) than untreated males did in the Plus. No difference in total distance traveled (F) in locomotor testing was observed. **p ≤ 0.01. Females: N=10/maze; Males: Zero N= 21; Plus N = 22.
4. Discussion
We compared the Plus and Zero mazes in adult Sprague-Dawley rats to determine if there are differences in their detection of behaviors associated with approach-avoidance conflict and as a function of treatments documented to change such behavior in opposite directions. Both mazes were constructed according to published dimensions (Pellow et al. 1985;Shepherd et al. 1994). Open area curbs were not present in the original Pellow et al. Plus design but were subsequently shown to contribute to open area exploration (Fernandes and File 1996); curb height was standardized in our mazes. While the elevation of our mazes differed, this has been shown to have no significant effect on open time (Treit et al. 1993). Both the Plus and Zero were equally effective at reflecting the anxiogenic effects of restraint, yohimbine, and caffeine and the anxiolytic effects of diazepam. The only discrepant finding between mazes following challenge was that diazepam-treated groups in the Plus showed a decrease in closed area entries not seen in the Zero. However, all indices related to anxiety were identical between mazes and no detection of effect differences were observed.
Several treatments (pharmacological and behavioral) were included to increase the generality of the findings (Pellow et al. 1985). In line with approach-avoidance concepts, the percentage of time spent in the open areas of both mazes was independent of the level of overall locomotor movement, and rats spent more time in closed than open areas, consistent with the idea that open areas evoke anxiety resulting in avoidance behavior (Lister 1987;Montgomery 1955;Pellow et al. 1985;Rodgers et al. 1997;Rodgers and Dalvi 1997;Shepherd et al. 1994). In this experiment, each treatment, except for nicotine, altered percentage time spent in open areas in the expected direction in both mazes; that is, decreasing it after yohimbine, caffeine, or restraint exposure and increasing it after diazepam treatment. These data support the use of percent time in open areas (or open time in the Zero) as the principal index anxiety-related behavior. The number of head dips has also been suggested to be an index of anxiety in rats (Cole and Rodgers 1995;Cruz et al. 1994;Fernandes and File 1996;Rodgers and Johnson 1995), and in agreement with this, restraint, yohimbine, and caffeine all produced significant reductions in head dips.
Nicotine, at the dosage used here, had no effect. An identical dose of nicotine has previously been shown to increase anxiety in the Plus, although there were several differences between that study and ours that could have contributed to this, including handling prior to maze testing in the previous study, lighting differences within the testing room, materials used to construct the mazes, and maze cleaning procedures (Hogg 1996;Irvine et al. 2001). Difference in rat strain and age between studies could also have been contributing factors (Hogg 1996;Imhof et al. 1993). All of these were controlled within the present experiment so that comparisons across mazes could be made, and no main effect of maze was shown following the treatments used here.
In addition, both male and female untreated rats performed similarly in the two mazes. Lister female rats (60–70 days old) have previously been shown to spend increased percent time in open areas of the Plus (Johnston and File 1991). While it has been suggested that, in the Plus, females are more active and exploratory as opposed to being less anxious (Fernandes et al. 1999), other studies have not shown significant differences in male and female performance in this test in naïve animals or following prior testing (Doremus-Fitzwater et al. 2009a;Doremus-Fitzwater et al. 2009b;Wilson et al. 2004). This difference between studies on the effect of sex could be due to strain or age differences as well as estrous cycle in females (Imhof et al. 1993;Reddy and Kulkarni 1999;Rodgers and Cole 1993;Trullas and Skolnick 1993). Care should be taken with regard to these factors in future studies. The use of females here was not to determine so much whether sex was a significant variable within mazes, but whether there were differential patterns between mazes. The data showed no such differential effect.
In untreated rats, the Zero produced higher exploration in open areas than the Plus, suggesting that some factor in the Plus may inhibit exploration of open areas more than in the Zero, perhaps because of the time spent in the central region in the Plus. Examination of Figure 2D, suggests that latency to first open entry was also increased in the Plus and this may explain some of the differences in open area exploration observed between mazes. The difference in these baseline patterns may make the Zero more sensitive to some treatments than the Plus but this will require further investigation to prove. We also note that SAL-treated animals across experiments showed differences in time in open in the range of 18–45%. By contrast, the SAL-treated animals in the Plus showed time in open in the range of 16–25% which was more consistent across experiments. However, when testing drug effects, the data showed that the mazes were comparable at detecting differences.
The relative sensitivity of these two mazes to detect alterations in anxiety through dose-response experiments was not included here. While our experiment was in progress, we identified two studies that compared the Plus and Zero in mice using anxiolytic and anxiogenic drugs at multiple doses (Kulkarni et al. 2007;Kulkarni et al. 2008). While Kulkarni et al. found the Zero to be more sensitive to benzodiazepines (i.e., the Plus showed significant drug effects at more dose levels of each drug), they did not report the time spent in open areas of the Plus as a percentage, nor did they include the amount of time mice spent in the center region, making cross-maze comparisons difficult. However, mice tested in either the Plus or Zero were shown to have altered anxiety profiles following pharmacological manipulation, implying that the testing of additional doses in rats is unlikely to change our conclusions.
To account for possible locomotor activity differences in addition to measuring closed area entries, locomotor activity was assessed in locomotor chambers for 30 min immediately following maze testing. With the exception of nicotine, the time span for locomotor testing was within the half-life of each drug, whereas for nicotine testing the first 5 min was within its half-life (Banna et al. 2010;Ghosheh et al. 1999;Lau et al. 1995;Loscher and Schwark 1985). Nicotine treatment did not alter the number of closed area entries in either maze or distance traveled in the locomotor chambers. Restrained animals as well as caffeine- and yohimbine-treated animals had no overall difference from controls in locomotor activity, but in both mazes had decreased entries into the closed areas, suggesting some reduction in activity at short time intervals after drug administration that appeared during maze testing but was absent during locomotor activity testing. Consistent with diazepam-induced hypoactivity previously reported (Nishino et al. 2008), we found diazepam-induced decreases in closed area entries in the Plus, but no changes in the Zero. Only diazepam-treated animals showed a decrease in distance traveled in the activity chambers compared to SAL-treated controls, but ANCOVA showed this hypoactivity did not correlate with anxiety measures. The ANCOVA was performed for each treatment using either the first 5 min or the entire 30 min of locomotor testing as the covariate. The first 5 min interval of locomotor testing is the most immediate to maze testing and the most likely to reflect general drug effects although the half-life for each drug, with the exception of nicotine, fell within the 65 min total testing time (30 min from injection to maze start, 5 min Plus or Zero maze testing, 30 min activity testing). Neither ANCOVA on the first 5 min nor 30 min altered the difference in percentage of time spent in the open for any group supporting the efficacy of both mazes in separating anxiety from activity. Without the locomotor test, the anxiogenic effect of caffeine might have been attributed to an activity difference because of the decreased number of closed area entries. This could support the use of an independent measure of activity in addition to determining the number of closed area entries for compounds that are likely to induce some level of locomotor change. An alternative approach to using a separate index of activity is counting the number of line crossings, photocell interruptions, or path length using tracking devices made by animals within both regions of the mazes. Others have used these methods in lieu of closed area entries thereby removing anxiety measures from activity scores (Jacobson and Cryan 2008) and it appears that simultaneous measurements of this kind are preferable. This was not possible in this study due to the camera angle of our video recording system using a one camera arrangement. Therefore, utilizing locomotor activity in situations where within zone activity measurements are not possible may provide some useful information.
We also analyzed the first 5 min of locomotor activity testing for time spent in the periphery versus center region, which is often used as an index of anxiety. Only caffeine exhibited an altered activity in the center region, however peripheral activity was also decreased, hence, the effect was non-specific. These results were consistent with the overall locomotor findings as opposed to the maze findings; indicating that central zone activity in the apparatus we used may not be as sensitive as the Plus and Zero mazes for assessing anxiety when given after maze testing. Being handled and tested in either the Plus or Zero prior to open field testing could potentially have altered performance on the latter test. Since maze and locomotor testing order was not counterbalanced exact determination of order effects cannot be obtained from these data.
The main difference in the Zero is that it removes the center region found in the Plus. Depending on treatment, animals tested here in the Plus spent 13% to 30% of their test time in the center, thereby decreasing the time spent in open or closed areas by 39–90 s of the total 300 s test session. Differing levels of anxiety could present themselves based not on the amount of time spent in an open area, but in the amount of time spent in the center, thereby increasing the percentage of time spent in the open areas. The Zero allows time spent in the open areas to be expressed in direct proportion to total test time without the need to convert it to percentage open time. Since time spent in open is the measure of principal interest, it is important that Plus data be expressed this way: unfortunately, this is not always the case (Drapier et al. 2007;Frye et al. 2008;Koks et al. 2001;Kompagne et al. 2008;Kulkarni et al. 2008;McDermott and Kelly 2008;Nosek et al. 2008). This more direct measure of time in open, therefore, represents an advantage of the Zero maze.
This is the first experiment to compare the elevated Plus and Zero mazes in rats. The main advantage of the Plus is the historical data available. The present results indicate that when observing pharmacological or behavioral manipulations on anxiety, if time spent in the center region in the Plus is excluded such that time in the open is represented as a percentage, the results from both mazes are essentially equal for the independent variables evaluated here. The slight advantage in the Zero with higher percent time in the open may be important in some contexts since in many experiments detection sensitivity is a function of the baseline level of performance relative to control groups. Since the Zero often showed a higher baseline, detecting change in both directions may be easier in the Zero such as when comparing basal levels in transgenic models.
Acknowledgements
This work was supported by NIH grant DA021394, ES015689, and DA006733, and training grant T32 ES07051. Preliminary data from this paper have been presented at the 2008 Annual Meeting of the Society for Neuroscience, Washington, D.C. and the 2008 Annual Meeting of the International Behavioral Neuroscience Society meeting, St. Thomas, VI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration
The authors report no conflicts of interest in this research. The authors alone are responsible for the content and writing of this paper.
Reference List
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav. Brain Res. 2010;208:144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya SK, Satyan KS, Chakrabarti A. Anxiogenic action of caffeine: an experimental study in rats. J. Psychopharmacol. 1997;11:219–224. doi: 10.1177/026988119701100304. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cole JC, Rodgers RJ. Ethological comparison of the effects of diazepam and acute/chronic imipramine on the behaviour of mice in the elevated plus-maze. Pharmacol. Biochem. Behav. 1995;52:473–478. doi: 10.1016/0091-3057(95)00163-q. [DOI] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol. Biochem. Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Effects of pretest manipulation on elevated plus-maze behavior in adolescent and adult male and female Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2009a;92:413–423. doi: 10.1016/j.pbb.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009b;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier D, Bentue-Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, Reymann JM. Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav. Brain Res. 2007;176:202–209. doi: 10.1016/j.bbr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol. Biochem. Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- Fernandes C, Gonzalez MI, Wilson CA, File SE. Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol. Biochem. Behav. 1999;64:731–738. doi: 10.1016/s0091-3057(99)00139-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Estrogen is necessary for 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) infusion to the ventral tegmental area to facilitate social and sexual, but neither exploratory nor affective behavior of ovariectomized rats. Pharmacol. Biochem. Behav. 2008;91:261–270. doi: 10.1016/j.pbb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosheh O, Dwoskin LP, Li WK, Crooks PA. Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2'-(14)C]nicotine. Drug Metab Dispos. 1999;27:1448–1455. [PubMed] [Google Scholar]
- Gulati K, Chakraborti A, Ray A. Modulation of stress-induced neurobehavioral changes and brain oxidative injury by nitric oxide (NO) mimetics in rats. Behav. Brain Res. 2007;183:226–230. doi: 10.1016/j.bbr.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav. Brain Res. 1993;56:177–180. doi: 10.1016/0166-4328(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Tolerance to nicotine's effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol. Biochem. Behav. 2001;68:319–325. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents. Neuropharmacology. 2008;54:854–862. doi: 10.1016/j.neuropharm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Jain NS, Hirani K, Chopde CT. Reversal of caffeine-induced anxiety by neurosteroid 3-alpha-hydroxy-5-alpha-pregnane-20-one in rats. Neuropharmacology. 2005;48:627–638. doi: 10.1016/j.neuropharm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Yohimbine's anxiogenic action: evidence for noradrenergic and dopaminergic sites. Pharmacol. Biochem. Behav. 1989;32:151–156. doi: 10.1016/0091-3057(89)90225-6. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Koks S, Beljajev S, Koovit I, Abramov U, Bourin M, Vasar E. 8-OH-DPAT, but not deramciclane, antagonizes the anxiogenic-like action of paroxetine in an elevated plus-maze. Psychopharmacology (Berl) 2001;153:365–372. doi: 10.1007/s002130000594. [DOI] [PubMed] [Google Scholar]
- Kompagne H, Bardos G, Szenasi G, Gacsalyi I, Harsing LG, Levay G. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav. Brain Res. 2008;193:311–314. doi: 10.1016/j.bbr.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Singh K, Bishnoi M. Elevated zero maze: a paradigm to evaluate antianxiety effects of drugs. Methods Find. Exp. Clin. Pharmacol. 2007;29:343–348. doi: 10.1358/mf.2007.29.5.1117557. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Singh K, Bishnoi M. Comparative behavioural profile of newer antianxiety drugs on different mazes. Indian J. Exp. Biol. 2008;46:633–638. [PubMed] [Google Scholar]
- Lau CE, Ma F, Falk JL. Oral and IP caffeine pharmacokinetics under a chronic food-limitation condition. Pharmacol. Biochem. Behav. 1995;50:245–252. doi: 10.1016/0091-3057(94)00306-4. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol. Ther. 1990;46:321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- Loscher W, Schwark WS. Development of tolerance to the anticonvulsant effect of diazepam in amygdala-kindled rats. Exp. Neurol. 1985;90:373–384. doi: 10.1016/0014-4886(85)90026-3. [DOI] [PubMed] [Google Scholar]
- McDermott C, Kelly JP. Comparison of the behavioural pharmacology of the Lister-Hooded with 2 commonly utilised albino rat strains. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1816–1823. doi: 10.1016/j.pnpbp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J. Comp Physiol Psychol. 1955;48:254–260. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- Nishino T, Takeuchi T, Takechi K, Kamei C. Evaluation of anxiolytic-like effects of some short-acting benzodiazepine hypnotics in mice. J. Pharmacol. Sci. 2008;107:349–354. doi: 10.1254/jphs.08107fp. [DOI] [PubMed] [Google Scholar]
- Nosek K, Dennis K, Andrus BM, Ahmadiyeh N, Baum AE, Woods LC, Redei EE. Context and strain-dependent behavioral response to stress. Behav. Brain Funct. 2008;4:23. doi: 10.1186/1744-9081-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Sex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behaviors in rats. Pharmacol. Biochem. Behav. 1999;62:53–60. doi: 10.1016/s0091-3057(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cao BJ, Dalvi A, Holmes A. Animal models of anxiety: an ethological perspective. Braz. J. Med. Biol. Res. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC. Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiol Behav. 1993;54:729–736. doi: 10.1016/0031-9384(93)90084-s. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol. Biochem. Behav. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Treit D, Menard J, Royan C. Anxiogenic stimuli in the elevated plus-maze. Pharmacol. Biochem. Behav. 1993;44:463–469. doi: 10.1016/0091-3057(93)90492-c. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl) 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD. Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacol. Biochem. Behav. 2004;78:445–458. doi: 10.1016/j.pbb.2004.04.017. [DOI] [PubMed] [Google Scholar]