Abstract
The simultaneous overexpression of multiple copies of Mn superoxide dismutase (SOD) and ectopic catalase (mtCat) transgenes in the mitochondria of the fruit fly, Drosophila melanogaster, was shown previously to diminish the life span. The hypothesis tested in the present study was that this effect was due primarily to the presence of one or the other transgene. An alternative hypothesis was that both transgenes have additive, negative effects. Crosses were performed between five pairs of transgenic lines containing single-copy insertions of either mtCat, Mn SOD, or P element vector control transgenes at unique loci, and the life spans of progeny containing two mtCat, Mn SOD or vector insertions were determined. Increasing amounts of mitochondrial catalase activity tended to be associated with decreases in mean life span. Overexpression of two copies of the genomic Mn SOD transgene had no effect on life span. The results do not support the hypothesis that enhanced mitochondrial SOD or catalase activity promotes longevity in flies.
Keywords: aging, antioxidant, catalase, superoxide dismutase, life span
INTRODUCTION
The oxidative stress hypothesis of aging postulates that deleterious, age-related phenomena arise in part from an imbalance between the rate of pro-oxidant production, antioxidant defenses and repair processes [1–3]. A straightforward prediction based on this hypothesis is that enhancement of antioxidant defenses would decrease oxidative damage and increase longevity. Augmentation of antioxidant activities in mitochondria, which are a major site of generation of superoxide anion radicals (O2•−) and hydrogen peroxide (H2O2), progenitors of a variety of other reactive oxygen species (ROS), would be expected to exert particularly pronounced, beneficial effects [4].
A large number of studies have been performed to test the hypothesis that the life spans of experimental animals can be extended by increasing the activities of antioxidant enzymes, with emphasis on superoxide dismutase (SOD) and catalase, which detoxify O2•− and H2O2, respectively. It is generally agreed that increasing the activity of catalase in the cytosol or peroxisomes of the fruit fly, Drosophila melanogaster, or the mouse, Mus musculus, has little or no effect on life span [5–8]. In Drosophila, ectopic expression of a single copy of a catalase transgene in mitochondria had no beneficial effect on survivorship [9]. In contrast, ectopic expression of catalase in mitochondria was reported to extend the life span of Mus [7]. Although some initial doubt was cast on the reproducibility of these findings [10], the mice expressing mitochondrial catalase have subsequently been shown to have a decreased disease burden, particularly a decreased burden of malignant, nonhematopoietic neoplasms [11]. Concerning SOD, overexpression of the cytosolic form of the enzyme (Cu-Zn SOD) had no effect on survivorship in the mouse [12], but the results for the mitochondrial Mn SOD have been discordant [13,14]. Likewise, in Drosophila, various groups have reported that overexpression of Cu-Zn SOD or Mn SOD had either beneficial or neutral effects, depending on the spatial and temporal patterns of SOD overexpression, the use of genomic vs. cDNA constructs, and the genetic background [6,15–22].
The results of overexpression of combinations of SOD and catalase transgenes in the mitochondrial and extramitochondrial compartments of the cell have also been mixed. In general, simultaneous enhancement of Cu-Zn SOD, catalase and/or Mn SOD activities has been found to increase longevity substantially in short-lived strains of Drosophila, and to have little or no effect on longevity in longer-lived backgrounds [23,24]. However, simultaneous overexpression of two copies each of a genomic Mn SOD and an ectopic, mitochondrial catalase (mtCat) transgene had a pronounced life-shortening effect [25]. The flies expressing mtCat and overexpressing Mn SOD also exhibited increased resistance to severe oxidative stress, showing that effects of a given treatment on aging and stress resistance are not always positively related. These results are consistent with the current theory that ROS are primarily destructive at high levels, whereas lower levels of ROS promote survival through the destruction of pathogens, and through their effects on the phosphoinositide 3 kinase and other signal transduction pathways [26].
The objective of the present study was to determine whether the combined effect of Mn SOD and mtCat transgenes on life span was due primarily to overexpression of one enzyme or the other, or whether both were required to diminish the life span. Given the importance of ROS in cellular signalling and survival pathways [26], and the complete abolition of H2O2 release in mitochondria isolated from mtCat flies [27], it was predicted that the mtCat transgene would have the greatest negative impact on survivorship. The results confirmed that any deleterious effect was associated with mtCat expression, but neither mtCat nor Mn SOD transgene expression extended the life span.
MATERIALS AND METHODS
Fly strains
Flies expressing ectopic mitochondrial catalase, Mn SOD and control stocks used in this study were generated and described previously [9,19]. The mtCat transgene consisted of a 7 kb genomic DNA fragment encoding Drosophila catalase, with a 5′ insertion containing a 22 codon cassette encoding the putative mitochondrial presequence of Drosophila ornithine aminotransferase [27]. The Mn SOD transgene was a 9 kb genomic DNA fragment inserted into the pCaSpeR vector [19]. The unmodified vector was used to generate control (+) strains.
Life span
Virgin females containing one homozygous, autosomal transgene were crossed to males bearing the same transgene at a different locus, and male progeny heterozygous for both transgenes were collected for studies of life span and biochemical assays. In two independent experiments on life span, flies were maintained in 4 groups of 25/vial at 25°C under constant light. Fresh food vials were provided and mortality was scored every 1–2 days.
Biochemistry
Activities of superoxide dismutase and catalase were measured as described previously [28–30]. SOD activity was assayed spectrophotometrically, based on its inhibitory effect on the reduction of nitroblue tetrazolium by xanthine/xanthine oxidase, and the consequent change in absorbance at 560 nm [30]. Mn SOD activity was measured in whole body homogenates of 10-day-old flies in the presence of 5 mM NaCN. Total SOD was determined in the absence of NaCN, and Cu-Zn SOD activity was calculated as the difference between total and Mn SOD activities.
Catalase activity was measured in homogenates of 10-day-old flies, and in mitochondria isolated from flies 2–3 days after eclosion, based on the consumption of H2O2 and resulting decrease in absorbance at 240 nm.
Statistics
Enzymatic activities were compared between control and Mn SOD or mtCat genotypes using unpaired, two-sample t tests, except that one-sample t tests were used to compare the mitochondrial catalase activity of each mtCat transgene combination to a hypothesized null value of 0. The mean life spans of genotypes (Mn SOD vs. mtCat vs. control) within each experiment were compared by one-way analysis of variance (ANOVA), treating the flies with each pair of transgenes as a single replicate. Where differences were observed, Tukey tests were performed for pairwise comparisons among genotypes. Additionally, a combined two-way ANOVA was performed, with genotype and replicate experiment as factors, and individual transgene combinations nested within genotypes. Linear correlation analyses were also performed, to test the hypothesis that the life spans of flies with different pairs of transgenes were related to total or mitochondrial SOD or catalase activity. All comparisons were performed using Microsoft Excel and SYSTAT 12 software (San Jose, CA).
RESULTS
Overexpression of SOD and Catalase
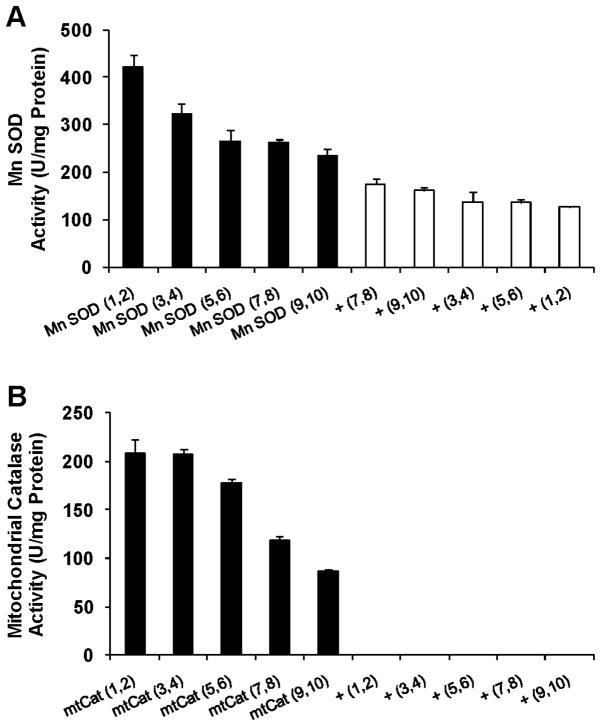
The total SOD activity of the five Mn SOD transgenic groups was increased by 32 ± 21% (mean ± S.D.), in comparison with the average of the five control groups (P = 0.02, Table 1). Mn SOD activity was increased by 104 ± 50% (P = 0.007, Figure 1A). The difference between total and Mn SOD, which represents Cu-Zn SOD activity, was essentially unchanged (−6 ± 46%, P = 0.8).
Table 1.
Enzyme Activities and Life Spans of Transgenic Flies
| Genotype | SOD Activity (U/mg) | Catalase Activity (U/mg) | Mean Life Span (Days) | |||
|---|---|---|---|---|---|---|
| Total | MnSOD | Total | Mito. | Expt. 1 | Expt. 2 | |
| Mn SOD (1,2) | 470 ± 23 | 422 ± 27 | n.d. | n.d. | 88.7 | 87.0 |
| Mn SOD (3,4) | 655 ± 16 | 322 ± 22 | n.d. | n.d. | 88.8 | 82.9 |
| Mn SOD (5,6) | 633 ± 59 | 264 ± 24 | n.d. | n.d. | 67.0 | 66.2 |
| Mn SOD (7,8) | 590 ± 40 | 263 ± 5 | n.d. | n.d. | 76.0 | 70.2 |
| Mn SOD (9,10) | 470 ± 28 | 235 ± 15 | n.d. | n.d. | 85.3 | 79.8 |
| Mean ± S.D. | 564 ± 89 | 301 ± 74 | n.d. | n.d. | 81.2 ± 9.5 | 77.2 ± 8.7 |
| mtCat(l,2) | n.d. | n.d. | 314± 12 | 208 ± 14 | 76.4 | 62.9 |
| mtCat (3,4) | n.d. | n.d. | 233± 4 | 207± 5 | 69.2 | 54.9 |
| mtCat (5,6) | n.d. | n.d. | 496 ± 21 | 178± 3 | 61.3 | 46.4 |
| mtCat (7,8) | n.d. | n.d. | 362± 3 | 118± 4 | 68.6 | 66.3 |
| mtCat(9,10) | n.d. | n.d. | 279± 2 | 87± 1 | 75.3 | 73.1 |
| Mean ± S.D. | n.d. | n.d. | 337±101 | 160 ± 55 | 70.2 ± 6.1 | 60.7±10.4 |
| + (1,2) | 428 ± 30 | 127 ± 2 | 200 ± 16 | 0 | 77.1 | 79.4 |
| + (3,4) | 389 ± 6 | 138 ± 20 | 183 ± 6 | 0 | 67.4 | 67.5 |
| + (5,6) | 375 ± 38 | 137 ± 7 | 135 ± 9 | 0 | 77.0 | 74.7 |
| + (7,8) | 450 ± 14 | 176 ±10 | 210 ± 7 | 0 | 74.5 | 72.3 |
| + (9,10) | 493 ± 57 | 161 ± 8 | 231 ± 10 | 0 | 83.5 | 77.4 |
| Mean ± S.D. | 427 ± 48 | 148 ± 20 | 192 ± 36 | 0 | 75.9 ± 5.8 | 74.3 ± 4.7 |
n = 119–126 flies per genotype per experiment, n.d. = not determined.
Figure 1.
Activities of Mn SOD (A) and mitochondrial catalase (B). Numbers in parentheses denote unique insertion sites of an Mn SOD, mtCat or vector control (+) transgene. Results are mean ± S.D. of 3–4 measurements.
The total catalase activity of the five mtCat groups was increased by 76 ± 52% (P = 0.03, Table 1). Mitochondria isolated from these flies expressed 87–208 units of catalase activity per mg protein (Figure 1B), whereas no catalase activity was detected in mitochondria from the control groups. The mitochondrial catalase activity in every mtCat group was significantly greater than 0 (P < 0.001).
Life spans of flies overexpressing multiple copies of Mn SOD or ectopic catalase transgenes
The life spans of flies heterozygous for either Mn SOD, mtCat or control transgenes at two different loci were determined in two separate experiments at 25°C (Figure 2, Table 1). In the first experiment, the mean life span of Mn SOD flies was 7% longer and the life span of mtCat flies was 8% shorter than the control life span, but the one-way ANOVA did not reveal a significant difference among any of the groups (P = 0.10). In the second experiment, the Mn SOD flies lived 4% longer and mtCat flies lived 18% shorter than controls (P = 0.02). Pairwise comparisons revealed differences between mtCat and Mn SOD groups (P = 0.02), a marginal difference between mtCat and controls (P = 0.058), but no difference between Mn SOD and control groups. A combined analysis of all of the data demonstrated significant differences between experiments (P = 0.003) and among genotypes (P < 0.0005), and a significant nesting effect within each genotype (P < 0.0005).
Figure 2.

Life spans of flies overexpressing Mn SOD or ectopic, mitochondrial catalase. Survivorship curves of experimental and control flies were obtained at 25°C in two independent experiments (A, B). Pooled results are shown in each panel for ~125 male flies per transgene combination for five combinations per genotype. Mean life spans of flies containing each combination of transgenes are shown in Table 1.
Owing to the wide variations in life span and enzymatic activity amongst groups within each genotype, the hypothesis of a linear correlation between these variables was tested. The life spans of the five mtCat and five control groups were negatively correlated with total catalase activity in the second experiment (r= −0.72, 0.01 < P < 0.02). Likewise, the correlation in the first experiment had a negative trend, although it did not reach the threshold of statistical significance (r = − 0.60, 0.05 < P < 0.10). There was no correlation between life span and total or Mn SOD or mitochondrial catalase activity.
DISCUSSION
The principal findings of this study were that overexpression of multiple copies of genomic Mn SOD transgenes had no effect on the life span of D. melanogaster, whereas mtCat transgenes had neutral or moderately negative effects in different experiments, depending in part on the insertion sites of the transgenes and the magnitude of catalase overexpression. These results suggest that mtCat was primarily responsible for the adverse effects observed previously in flies expressing both Mn SOD and mtCat transgenes simultaneously. Although Mn SOD might have exacerbated the effect mtCat, Mn SOD alone did not have a detrimental effect. These findings are consistent with the hypothesis that basal levels of ROS production are a physiological necessity, and that mtCat expression is more disruptive than Mn SOD overexpression.
In the current study, flies containing two copies of the mtCat transgene exhibited mitochondrial catalase activities of 87–208 U/mg protein, and their life span was diminished, on average, by 8–18% in comparison with the control groups. Previously, flies containing a single copy of the same transgene were reported to have mitochondrial catalase activities of 30–140 U/mg, associated with a 0–7% decrease in mean life span [9]. Flies containing two mtCat and two Mn SOD transgenes were found to have 194–243 U/mg catalase activity, and mean life spans 27–28% shorter than the control groups [25]. Thus, in a comparison among experiments performed at different times, there is a general trend toward a more pronounced decrease in life span as the total amount of mitochondrial catalase activity increases.
Within the present study, there was a negative correlation between the total catalase activity and life span of the individual mtCat and control groups, although it reached the threshold of statistical significnace in only one of the two experiments. The looseness of the association might result from the insertion of multiple transgenes at different loci in each group of flies, which could potentially interfere with the expression of genes at a different set of neighboring loci in each group.
Flies containing two copies of the Mn SOD transgene exhibited neither a significant increase nor a decrease in mean life span. However, the effects of both enzymes overexpressed together in a prior study were somewhat larger and more consistently negative than those for mitochondrial catalase alone. A possible explanation is that Mn SOD exerts an additional deleterious effect by facilitating the further removal of ROS, which are already insufficient in abundance in mitochondria expressing catalase.
The results of this study are consistent with some, but not all of the literature in the field. The lack of an effect of Mn SOD overexpression in a relatively long-lived Drosophila background in this study is consistent with past results from this laboratory, which showed that Mn SOD alone or in combination with Cu Zn SOD or cytosolic/peroxisomal catalase failed to extend the life span in similar genetic backgrounds [23]. Likewise, in comparatively long-lived mouse genetic backgrounds, overexpression of Mn SOD, Cu Zn SOD and catalase alone and in various combinations had no effect on life span [8]. In contrast, in both species, overexpression of SOD in substantially shorter-lived backgrounds was reported to increase survival times [13,24]. Considering only the results obtained in short-lived flies and long-lived mice, Jang and colleagues concluded recently that genetic manipulations have divergent effects on life span in invertebrate and mammalian systems [14]. In theory, such a situation could arise, because poikilotherms are susceptible to large changes in their metabolic rate, leading to substantial differences in life span [3,31]. In the case of SOD and cytosolic/peroxisomal catalase, however, the results in flies and mice are in fact strikingly similar.
Concerning mitochondrial catalase, life extension has been observed in a somewhat shorter-lived mouse model [7], whereas past and present studies from this laboratory demonstrated no effect (or a decrease with higher levels of mitochondrial catalase) in a longer-lived fly background. As early as 1971, Kohn reported that antioxidant supplementation can restore survival times of shorter-lived populations to optimum levels, but not beyond the longest median or maximum values for control animals of the same species [32]. The effect of mitochondrial catalase has yet to be investigated in a long-lived strain of mice. Such an experiment will be a key test of the hypothesis that expression of mitochondrial catalase slows the aging process in a mammal, or whether, alternatively, life extension by SOD and catalase is specific to shorter-lived strains in all cases.
Lastly, it should be noted that negative results in studies of antioxidant overexpression do not directly disprove the oxidative stress hypothesis of aging. In some instances, oxidants are generated and inflict damage at sites that are inaccessible to antioxidants [33]. Inferences based on the effects of antioxidants on life spans are further complicated by the fact that oxidants are intricately linked to the regulation of gene expression. Beneficial effects of antioxidants on the rate of aging could therefore be masked by unrelated, detrimental effects on cellular or organismal homeostasis.
Acknowledgments
This research was supported by grant RO1 AG7657 from the National Institutes of Health – National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 3.Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 4.Harman D. The biological clock: The mitochondria? J Amer Geriat Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 5.Orr WC, Sohal RS. The effects of catalase gene overexpression on life span and resistance to oxidative stress in transgenicDrosophila melanogaster. Arch Biochem Biophys. 1992;297:35–41. doi: 10.1016/0003-9861(92)90637-c. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol Cell Biol. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 8.Peréz VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mockett RJ, Bayne A-CV, Kwong LK, Orr WC, Sohal RS. Ectopic expression of catalase in Drosophila mitochondria increases stress resistance but not longevity. Free Radic Biol Med. 2003;34:207–217. doi: 10.1016/s0891-5849(02)01190-5. [DOI] [PubMed] [Google Scholar]
- 10.Choi CQ. Old mice hard to replicate. The Scientist. 2007;21:64. [Google Scholar]
- 11.Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM, Rabinovitch PS, Ladiges WC. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol Biol Sci. 2008;63A:813–824. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- 12.Huang T-T, Carlson EJ, Gillespie AM, Shi Y, Epstein CJ. Ubiquitous overexpression of CuZn superoxide dismutase does not extend life span in mice. J Gerontol Biol Sci. 2000;55A:B5–B9. doi: 10.1093/gerona/55.1.b5. [DOI] [PubMed] [Google Scholar]
- 13.Hu D, Cao P, Thiels E, Chu CT, Wu G-y, Oury TD, Klann E. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiol Learn Mem. 2007;87:372–384. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang YC, Pérez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, Ikeno Y, Epstein CJ, Van Remmen H, Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol Biol Sci. 2009;64A:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seto NOL, Hayashi S, Tener GM. Overexpression of Cu-Zn superoxide dismutase in Drosophila does not affect life-span. Proc Natl Acad Sci USA. 1990;87:4270–4274. doi: 10.1073/pnas.87.11.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reveillaud I, Niedzwiecki A, Bensch KG, Fleming JE. Expression of bovine superoxide dismutase in Drosophila melanogaster augments resistance to oxidative stress. Mol Cell Biol. 1991;11:632–640. doi: 10.1128/mcb.11.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orr WC, Sohal RS. Effects of Cu-Zn superoxide dismutase overexpression on life span and resistance to oxidative stress in transgenic Drosophila melanogaster. Arch Biochem Biophys. 1993;301:34–40. doi: 10.1006/abbi.1993.1111. [DOI] [PubMed] [Google Scholar]
- 18.Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 19.Mockett RJ, Orr WC, Rahmandar JJ, Benes JJ, Radyuk SN, Klichko VI, Sohal RS. Overexpression of Mn-containing superoxide dismutase in trangenic Drosophila melanogaster. Arch Biochem Biophys. 1999;371:260–269. doi: 10.1006/abbi.1999.1460. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spencer CC, Howell CE, Wright AR, Promislow DEL. Testing an ‘aging gene’ in long-lived Drosophila strains: increased longevity depends on sex and genetic background. Aging Cell. 2003;2:123–130. doi: 10.1046/j.1474-9728.2003.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin I, Jones MA, Grotewiel M. Manipulation of Sod1 expression ubiquitously, but not in the nervous system or muscle, impacts age-related parameters in Drosophila. FEBS Lett. 2009;583:2308–2314. doi: 10.1016/j.febslet.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orr WC, Mockett RJ, Benes JJ, Sohal RS. Effects of overexpression of copper-zinc and manganese superoxide dismutases, catalase, and thioredoxin reductase genes on longevity in Drosophila melanogaster. J Biol Chem. 2003;278:26418–26422. doi: 10.1074/jbc.M303095200. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Molitor J, Tower J. Effects of simultaneous over-expression of Cu/ZnSOD and MnSOD on Drosophila melanogaster life span. Mech Ageing Dev. 2004;125:341–349. doi: 10.1016/j.mad.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Bayne A-CV, Mockett RJ, Orr WC, Sohal RS. Enhanced catabolism of mitochondrial superoxide/hydrogen peroxide and aging in transgenic Drosophila. Biochem J. 2005;391:277–284. doi: 10.1042/BJ20041872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid Redox Signal. 2009;11:2655–2671. doi: 10.1089/ars.2009.2728. [DOI] [PubMed] [Google Scholar]
- 27.Kwong LK, Mockett RJ, Bayne A-CV, Orr WC, Sohal RS. Decreased mitochondrial hydrogen peroxide release in transgenic Drosophila melanogaster expressing intramitochondrial catalase. Arch Biochem Biophys. 2000;383:303–308. doi: 10.1006/abbi.2000.2093. [DOI] [PubMed] [Google Scholar]
- 28.Orr WC, Arnold LA, Sohal RS. Relationship between catalase activity, life span and some parameters associated with antioxidant defenses in Drosophila melanogaster. Mech Ageing Dev. 1992;63:287–296. doi: 10.1016/0047-6374(92)90006-y. [DOI] [PubMed] [Google Scholar]
- 29.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 30.Mockett RJ, Bayne A-CV, Sohal BH, Sohal RS. Biochemical assay of superoxide dismutase activity in Drosophila. Methods Enzymol. 2002;349:287–292. doi: 10.1016/s0076-6879(02)49343-3. [DOI] [PubMed] [Google Scholar]
- 31.Pearl R. The rate of living. New York: Alfred A. Knopf, Inc; 1928. [Google Scholar]
- 32.Kohn RR. Effect of antioxidants on life-span of C57BL mice. J Gerontol. 1971;26:378–380. doi: 10.1093/geronj/26.3.378. [DOI] [PubMed] [Google Scholar]
- 33.Climent I, Levine RL. Oxidation of the active site of glutamine synthetase: conversion of arginine-344 to γ-glutamyl semialdehyde. Arch Biochem Biophys. 1991;289:371–375. doi: 10.1016/0003-9861(91)90425-i. [DOI] [PubMed] [Google Scholar]