Abstract
Objective
Previous evidence suggests that responses to social stressors may play a mechanistic role in the behavioral and physiological changes associated with affective disorders such as depression. Prairie voles (Microtus ochrogaster) are socially monogamous rodents that share features of social behavior with humans, and therefore might provide a useful model for examining social regulation of behaviors and physiological responses related to depression. In the present study we hypothesized that social isolation in female prairie voles would induce depression-relevant behaviors and altered neuroendocrine responses to an acute social stressor.
Methods
Twenty adult female prairie voles were exposed to either 60 days of social isolation or paired (control) housing, and tested for a depression-like behavior (anhedonia), numbers of corticotropin-releasing factor- and oxytocin-immunoreactive cells in the paraventricular nucleus of the hypothalamus, and circulating levels of hormones and peptide in response to an acute social stressor (resident-intruder test).
Results
Chronic social isolation produced anhedonia, measured by a reduction in sucrose intake and sucrose preference relative to paired animals. Compared to paired animals, isolated prairie voles displayed increased plasma hormone and peptide levels (oxytocin, arginine vasopressin, and corticosterone) following a 5-minute resident-intruder test, mirrored by an increased number of oxytocin- and corticotropin-releasing factor-immunoreactive cells in the hypothalamic paraventricular nucleus.
Conclusions
These findings suggest that isolation in a socially monogamous rodent model induces both behavioral and neuroendocrine changes that are relevant to depression, and may provide insight into the mechanisms that underlie the development and/or maintenance of depressive disorders in humans.
Keywords: Affective disorders, Corticotropin-releasing factor, Hypothalamic-pituitary-adrenal axis, Oxytocin, Paraventricular nucleus, Stress
Introduction
Evidence from animal research, including humans, documents an important overlap of affective disorders and physiological dysfunction. Disorders of negative affect, such as depression, are associated with several neuroendocrine and autonomic alterations including dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis and activation of the sympathetic nervous system (1-6). For instance, alterations in corticotropin-releasing factor (CRF) are found in the cerebrospinal fluid (7) and hypothalamus (2) of depressed patients, and central CRF administration induces several depression-like effects in rodents and primates, including decreased food intake and sexual activity, disturbed sleep, altered motor behavior, and impaired learning (reviewed in 8). Also, cortisol may be hypersecreted in many depressed patients (9). Chronic activation of the HPA axis leads to activation of the sympathetic nervous system. To this end, previous research from our laboratory has demonstrated elevated sympathetic tone to the heart in a rodent model of depression (5,10). These physiological changes associated with affective disorders are not unlike those accompanying exposure to stressors.
It is widely recognized that exposure to and increased reactivity to environmental stressors are both associated with affective disorders (see 11-14). Furthermore, there is a growing body of literature that discusses the importance of the social environment in the development of behavioral and neuroendocrine dysfunction associated with affective disorders, as well as the role of positive social interactions in buffering against detrimental or prolonged behavioral and physiological responses to stressors (e.g. 15-19). For instance, a recent study involving middle-aged individuals found that perceived loneliness (including social isolation) is directly related to symptoms of depression and cardiovascular responses to a mental stressor (15). Also, a combination of oxytocin treatment and social support reduces cortisol responses and subjective anxiety reactions following a social stressor in men (17). Early life trauma (such as abuse) is associated with elevated adrenocorticotropic hormone (ACTH) and cortisol responses in adult female depressed patients (20). Similarly, rats that are subjected to maternal separation as pups exhibit activation of the HPA axis (21) and exaggerated stressor-induced corticosterone responses in adulthood (22), versus control groups. These previous findings suggest that behavioral and physiological responsiveness to acute stressors may be an important mechanism underlying depressive signs and symptoms.
The mechanisms of behavioral and physiological characteristics related to affective disorders will be best understood through knowledge of the underlying neurobiological processes, and therefore an integrative research program involving animal models is useful. The prairie vole (Microtus ochrogaster) is a unique rodent species that provides a valuable model for studying the mechanisms of psychiatric conditions and the role of social experiences in regulating behavior and physiology (see 23). This species exhibits traits of social monogamy that are similar to humans and some other primates, including an active engagement in and reliance on their social environment, the formation of adult pair bonds, engaging in biparental care, and living in family groups (19,24).
The present study utilized the prairie vole model to examine potential mechanisms that underlie behavioral and physiological responses related to depression. Our laboratory has previously demonstrated depression-like behaviors and neuroendocrine disturbances in a rodent model of depression that involves exposure to environmental and social stressors (5,6,10). Furthermore, previous evidence suggests that behavioral and physiological reactivity to stressors may play a role in mediating signs and symptoms of depression (15,17,22). Therefore, in the current study we investigated specifically behavioral and neuroendocrine responses to chronic social isolation in adult female prairie voles, with a focus on depression-like behaviors and acute stressor responsiveness. We hypothesized that prairie voles exposed to chronic social isolation would display anhedonia, a common behavioral sign of depression characterized by the reduced responsiveness to a pleasurable stimulus. Furthermore, we predicted that isolated prairie voles would display exaggerated neuroendocrine activation following an acute social stressor (resident-intruder test), including elevated HPA axis and oxytocin levels, and that these peripheral changes would be mirrored by increases in stressor-associated hormone and peptide levels in the hypothalamic paraventricular nucleus (PVN).
Methods
Animals
Twenty adult, reproductively naïve female prairie voles (35-45 grams) were used for the experimental procedures. Animals were descendants of a wild stock originally caught near Champaign, Illinois. Animals were maintained on a 14/10 h light/dark cycle (lights on at 0600 h), with a temperature of 25 ± 1° C and relative humidity of 21 ± 4 g/m3. All animals were allowed food (Purina rabbit chow) and water ad libitum, unless otherwise specified. Offspring were housed with breeding pairs in large polycarbonate cages (25×45×60 cm) with cotton nesting material until 21 days of age, at which time they were removed and housed in same-sex sibling pairs in smaller cages (12×18×28 cm). All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Illinois at Chicago Animal Care and Use Committee.
Female prairie voles were used as our model system for several reasons. First, depression is more common in women than in men (25), yet female rodents are an understudied group both in behavioral and physiological investigations relating to depression (see discussions in 26,27). Furthermore, female prairie voles have been studied extensively for their social behavior in our laboratory and they may be especially sensitive to the effects of social stressors (e.g. 28-30). Also, female prairie voles do not show a spontaneous puberty or estrous cycle; in this species the ovaries remain inactive until the female has physical contact with a male (31), allowing for the use of reproductively intact animals without the need for controlling the estrous cycle.
Social Isolation
Animals were randomly divided into paired (control; n = 10) or isolated (n = 10) conditions. Animals were subjected to social isolation after living with a female sibling in a standard-sized cage since weaning; the isolation period began when the animals were between 60-120 days of age (modal age = 90 days), and was carried out for 60 days. Isolation involved removing the experimental animal from the home cage and placing it into an isolated cage of the same size as the initial home cage. Paired (control) animals were also moved into new cages at the same time as the isolated animals, and then were continually housed with the siblings for the length of the respective isolation period. Handling and cage changing throughout the isolation period were matched between the two groups.
Fluid Intake
Following 50 days of social isolation, an acute fluid intake test was conducted during the light period (approximately 4 hours after lights onset) to operationally define anhedonia, using a modification of procedures described elsewhere (see 5). Anhedonia was operationally defined as reduced absolute sucrose intake and sucrose preference, relative to control values. All animals were allowed ad libitum access to 1% sucrose, along with food and water, for 1 week before beginning any experimental procedures to allow for adaptation to the taste of the sucrose. Food and water were removed from the cage for a period of 20 hours prior to the sucrose preference test. One hour before beginning the test, all animals were moved into clean, individual cages so as to ensure accurate fluid intake measurements of paired animals. Both groups (paired and isolated animals) were moved into clean cages, thus avoiding potentially differential responses to a novel environment in the two groups. Tap water and 1% sucrose were placed on the cages in premeasured bottles, and fluid intake was monitored for 1 hour. All animals were returned to the home cages immediately following the test.
Resident-Intruder Test
Following 60 days of isolation, all animals (both paired and isolated groups) participated in a resident-intruder test during the light period (approximately 3-5 hours after light onset), consisting of placing the paired or isolated animal (intruder) into the cage of an unrelated and unfamiliar female (resident) for 5 minutes. Residents and intruders had no prior contact before the test, and did not share parentage. The resident-intruder paradigm was used here because it has previously been demonstrated to be a stressor in female rodents (32,33). The time period employed here was shorter than previous 10-minute time periods reported for rats and mice (34,35) to ensure the acute nature of the stressor in prairie voles. Aggressive behavior of the intruder was scored during the 5-minute paradigm by an experimentally-blind observer, and was defined as aggressive grooming or posture, swatting, biting, thrusting, pulling, and/or attack behavior directed toward the other animal (34). An overall score of aggressive behavior was determined for each animal by summing the number of episodes of each behavior. The results are reported as mean number of aggressive episodes and the percentage of animals displaying aggressive behaviors during the 5-minute test.
Immediately following completion of the test, all animals (paired and isolated groups) were placed into individual cages to facilitate post-stressor observations and avoid possible effects of reuniting the paired animals with the siblings. Behavior of the animal was monitored by an experimentally-blind observer at 5 minutes following the test, for a total of 1 minute (immediately prior to sacrificing). Signs of behavioral agitation were recorded, defined as running around the perimeter of the cage, jumping, repeated self-grooming, and/or performing repeated, stereotypie-like behaviors. An overall score of behavioral agitation was determined for each animal by summing the number of episodes of each behavior. The results are reported as mean number of agitated behaviors and the percentage of animals displaying agitated behaviors during the 1-minute observation period following the resident-intruder test.
Collection of Plasma
Ten minutes following the completion of the resident-intruder test (4 minutes following the 1-minute post-stressor observation period), animals were anesthetized with a mixture of ketamine (67 mg/kg, sc; NLS Animal Health, Owings Mills, MD) and xylazine (13.33 mg/kg, sc; NLS Animal Health, Owings Mills, MD). To avoid potential anesthesia-induced hormone alterations, blood was sampled within 2 minutes of the anesthetic injection, from the retroorbital sinus via a heparanized capillary tube, and was collected during a period not exceeding 1 minute. The blood was placed immediately on ice, and then centrifuged at 4° C, 3500 rpm, for 15 minutes to obtain plasma. Plasma aliquots were stored at -80° C until assayed for circulating hormones and peptides.
Circulating Hormone and Peptide Analysis
Plasma levels of oxytocin and arginine vasopressin (AVP) were determined using commercially available enzyme-linked immunosorbent assay kits (Assay Designs, Ann Arbor, MI), which have been validated previously by our laboratory for use in prairie voles (36). Inter- and intra-assay coefficients of variation for oxytocin are 19.2% and 2.9%, respectively, and for AVP are 5.7% and 2.5%, respectively. The minimum detection limits for the assays are 4.68 pg/ml for oxytocin and 3.39 pg/ml for AVP. The antibodies have negligible cross-reactivity (<0.001%) with similar mammalian peptides.
Plasma levels of ACTH were determined by radioimmunoassay according to procedures described elsewhere (37). The inter- and intra-assay coefficients of variation are 14.6% and 4.2%, respectively. The sensitivity of this assay is 0.25 pg/tube.
Plasma levels of corticosterone were determined using a commercially available radioimmunoassay kit (MP Biomedicals, Irvine, CA). The plasma was diluted in assay buffer as necessary (1:2000) to give results reliably within the linear portion of the standard curve. The inter- and intra-assay coefficients of variation for corticosterone are less than 5%, and cross reactivity with other steroids is less than 1%. The minimum detectable dose for this assay is 7.7 ng/ml.
Collection of Tissue
Immediately following blood collection, anesthetized animals were sacrificed via cervical dislocation. Brains were carefully removed from the skulls and were processed with a spin immersion technique (38). Brains were immersed in a fixative solution consisting of 4% paraformaldehyde containing 5% acrolein (pH 8.6) for a total of 4 hours. Brains were first spun for 10 minutes in the fixative, and then removed and blocked, exposing the lateral ventricles. After blocking, brains were placed back in the fixative and spun gently for 1 hour and 50 minutes, at which time the fixative was replaced with fresh solution, and brains were gently spun for an additional 2 hours. Brains were postfixed for 24 hours in 4% paraformaldehyde, and sunk in 25% sucrose. Tissue was stored in 25% sucrose at 4° C until it was sectioned at 40 μm on a freezing sliding microtome. Sliced serial sections were stored in wells in cryoprotectant antifreeze solution at -20° C until assayed for CRF and oxytocin using standard avidin:biotinylated enzyme complex immunocytochemistry.
Immunocytochemistry
Free-floating sections were rinsed 6 times during a 1 hour period with potassium phosphate buffered saline (KPBS) to remove the cryoprotectant. Sections were then incubated in 1% sodium borohydride for 20 minutes at room temperature. After multiple washes in KPBS, sections were incubated in 0.014% phenylhydrazine for 15 minutes at room temperature. Tissue was rinsed 6 times during a period of 1 hour in KPBS. Sections were then incubated in primary antibody for either CRF or oxytocin (anti-CRF, 1:50,000, generously provided by Dr. Ann-Judith Silverman; anti-oxytocin, 1:200,000, generously provided by Dr. Mariana Morris; both antibodies were generated in rabbit) diluted in KPBS + 0.4% Triton X-100 for 1 hour at room temperature, and then incubated for 42 hours at 4° C. Following this incubation period, sections were rinsed 10 times during a period of 1 hour with KPBS. Sections were then incubated in anti-rabbit IgG (BA-1000; Vector Laboratories, Burlingame, CA; 1:600) for 1 hour at room temperature. Sections were rinsed 5 times during a period of 50 minutes with KPBS, and then incubated in A/B solution (Vectastain Elite PK-6100; Vector Laboratories, Burlingame, CA; 45 μl A, 45 μl B per 10 ml KPBS + 0.4% Triton X-100) for 1 hour in room temperature. Sections were rinsed 3 times in KPBS and then 3 times in either 0.175 M sodium acetate (for CRF) or Tris buffered saline (for oxytocin). CRF was visualized by incubation in nickel-diaminobenzadine (DAB) solution, dissolved in 0.175 M sodium acetate, for 15 minutes at room temperature, and then sections were rinsed 3 times with 0.175 M sodium acetate and 3 times with KPBS. Oxytocin was visualized by incubation in DAB dissolved in Tris buffered saline, for 15 minutes at room temperature, and then sections were rinsed 3 times with Tris buffered saline and 3 times with KPBS.
Stained sections were mounted on gelatin coated slides, air-dried, dehydrated in a series of ethanol dilutions, cleared with Histoclear (National Diagnostics, Atlanta, GA), and then coverslipped using Histomount mounting medium (National Diagnostics, Atlanta, GA).
Image Analysis
Brain sections were matched across subjects, captured using a Nikon Eclipse E 800 microscope, Sensi-cam camera, and IP Lab Software (Scanalytics, Inc., Fairfax, VA), and scored by a trained, experimentally-blind observer. The numbers of oxytocin- and CRF-immunoreactive cell bodies were manually counted bilaterally in the PVN and averaged. Only cell bodies were counted; stained fibers were excluded from analysis.
Data Analysis
All data are presented as means ± (or +) standard error of the mean (SEM). All data were analyzed using single factor analyses of variance (ANOVA) and a priori Student's t-tests. The parameters recorded during and following the resident-intruder test also were analyzed with tests for a significant difference between two proportions (z test). A Bonferroni correction was used for any multiple comparisons, and a probability value of p < 0.05 was considered to be statistically significant.
Sample sizes of the analyses varied slightly due to a few animals being excluded from specified analyses based on a priori criteria. One animal from the isolated group was excluded from the resident-intruder test and all subsequent analyses due to an apparent illness prior to the test (this animal was painlessly euthanized according to Animal Care and Use Committee guidelines). One animal from the isolated group was excluded from the plasma and tissue analyses due to problems with handling prior to the anesthetic injection. If blood was not collected within 3 minutes following the anesthetic injection, plasma hormones and peptides were not analyzed to avoid potential handling- and/or anesthetic-induced stressor responses (2 paired animals were excluded for this reason). Finally, if there was an insufficient number of intact brain slices from the PVN following the immunohistochemical assays, these data were excluded (1 paired and 2 isolated animals were excluded for this reason).
Results
Fluid Intake
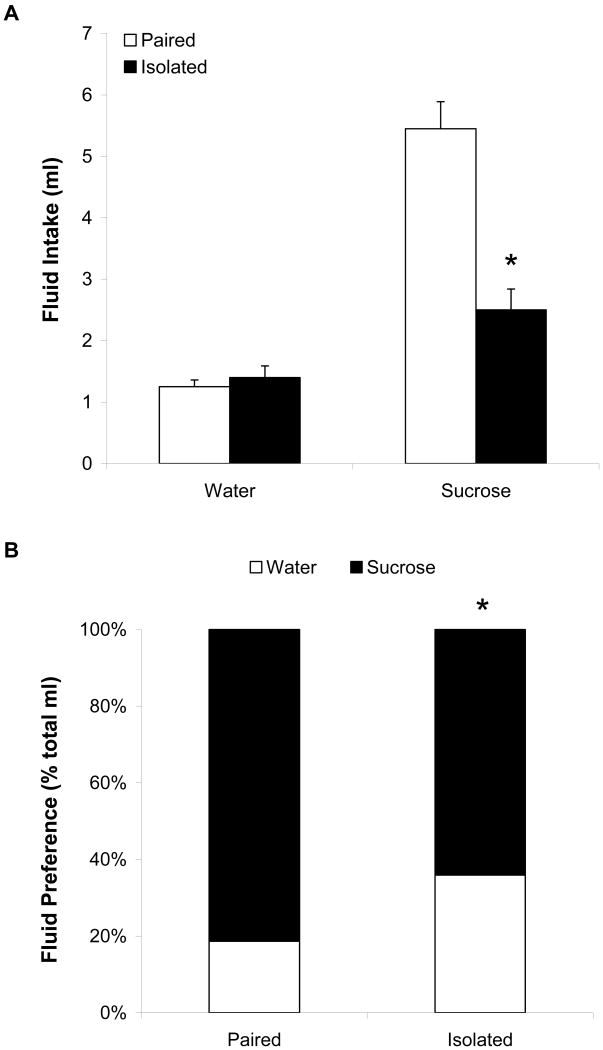
Figure 1 (Panel A) displays fluid intake in the paired and isolated groups following the isolation period. Single factor ANOVAs were performed on water and sucrose intake separately. The ANOVA performed on water intake was not significant (p > 0.05), indicating that there was no difference in water intake between the paired and isolated groups; no follow-up tests were performed. The ANOVA performed on sucrose intake yielded a significant main effect [F(1,18) = 27.75, p < 0.05]. The isolated group drank significantly less sucrose than did the paired group following the isolation period [t(18) = 5.27, p < 0.05].
Figure 1.
Mean (+ SEM) absolute water and sucrose intake (Panel A) and mean sucrose preference (Panel B) in paired and isolated prairie voles following 50 days of social isolation. *P < 0.05 versus respective paired value.
Social isolation also led to a reduction in sucrose preference, relative to social pairing. Figure 1 (Panel B) shows the preference for sucrose, relative to total fluid intake, in paired and isolated groups following the isolation period. The ANOVA yielded a significant main effect [F(1,18) = 12.04, p < 0.05]. The preference for sucrose was significantly lower in the isolated group versus the paired group [t(18) = 3.45, p < 0.05].
Resident-Intruder Test
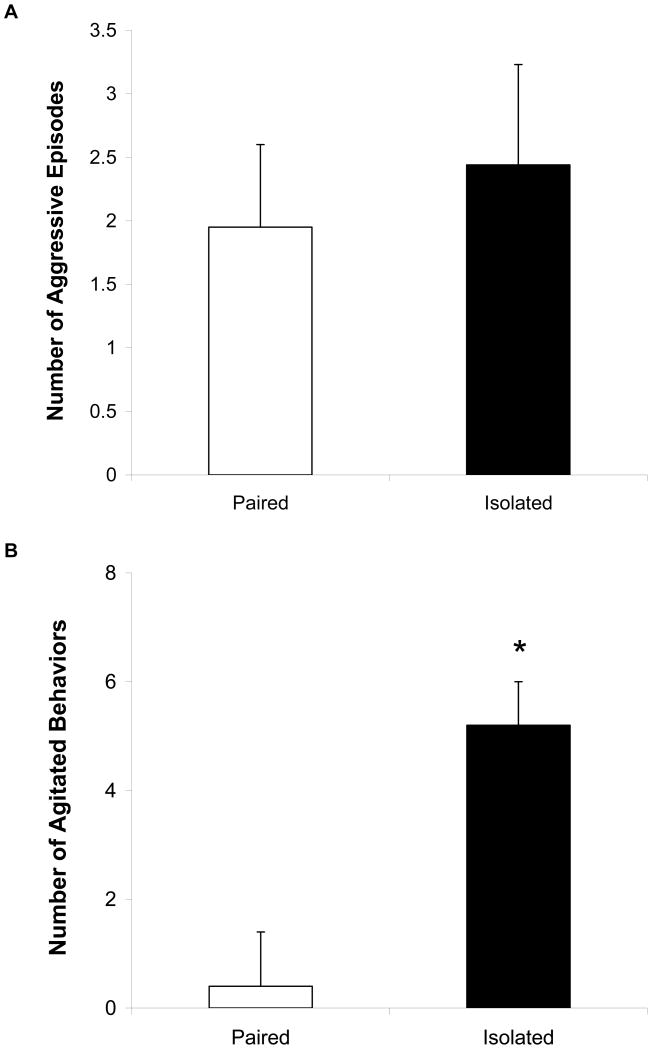
During the resident-intruder test (5-minute test period), there was no significant difference in the number of aggressive behaviors exhibited between paired and isolated intruders (Figure 2A; p > 0.05). Furthermore, the percentage of animals in each group displaying aggressive behaviors during the test was not significantly different (60% and 67% in paired and isolated groups, respectively; p > 0.05).
Figure 2.
Mean (+ SEM) number of aggressive episodes during the resident-intruder test (Panel A) and number of agitated behaviors following the resident-intruder test (Panel B) in paired and isolated groups. Note the scale differences in Panels A and B. *P < 0.05 vs. paired value.
Following the resident-intruder test (1-minute post-stressor observation period), the isolated group displayed significantly greater numbers of agitated behaviors versus the paired group [Figure 2B; t(17) = 6.12; p < 0.05]. Also, isolated animals were significantly more likely to display agitated behaviors compared to paired animals (22% and 89% in paired and isolated groups, respectively; z = 3.00, p < 0.05).
Circulating Hormone and Peptide Levels
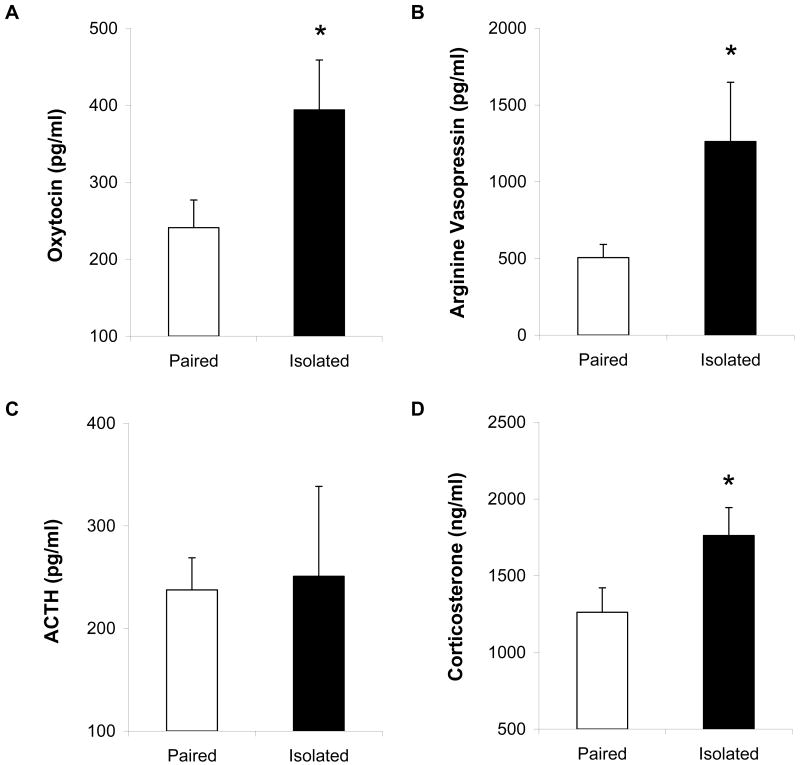
Figure 3 shows the circulating levels of oxytocin (Panel A), AVP (Panel B), ACTH (Panel C) and corticosterone (Panel D) in paired and isolated animals 10 minutes following the end of the resident-intruder paradigm. Compared with paired animals, socially isolated animals displayed significantly elevated oxytocin [t(14) = 2.01, p < 0.05], AVP [t(8) = 2.18, p < 0.05; these data were compared with a t-test assuming unequal variances], and corticosterone [t(14) = 2.00, p < 0.05]. There were no significant differences in ACTH levels following the resident-intruder test (p > 0.05; these data were compared with a t-test assuming unequal variances).
Figure 3.
Mean (+ SEM) circulating levels of oxytocin (Panel A), AVP (Panel B), ACTH (Panel C), and corticosterone (Panel D) in paired and isolated prairie voles at 10 minutes following a 5-minute resident-intruder test. Note the scale differences among the 4 panels *P < 0.05 versus paired value.
Tissue Hormone and Peptide Levels
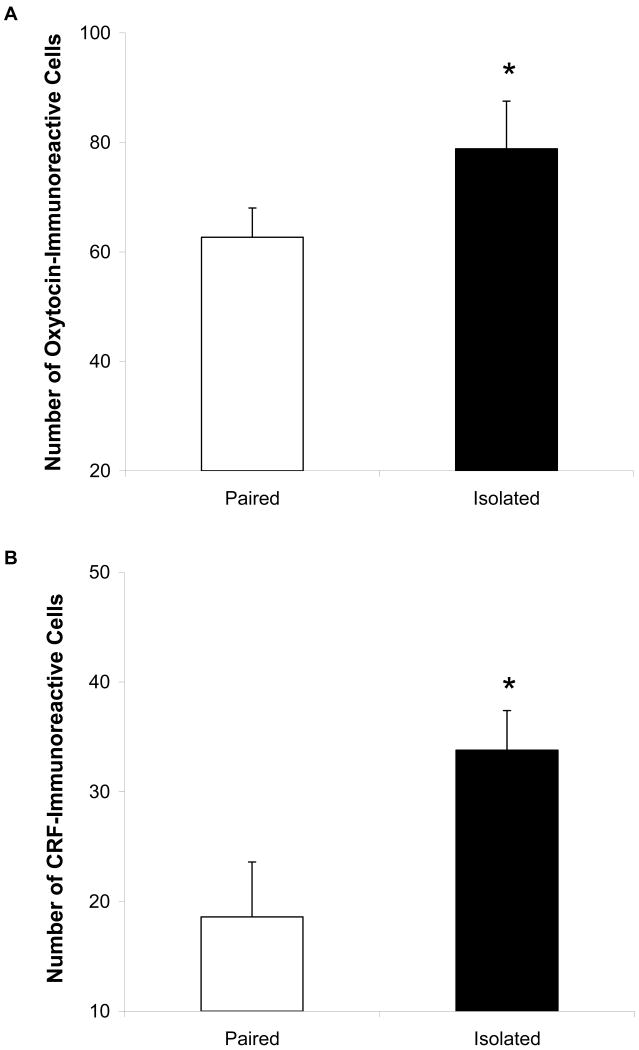
Figure 4 shows the oxytocin- (Panels A and B) and CRF-immunoreactivity (Panels C and D) distribution in the PVN of the hypothalamus in representative paired and isolated prairie voles, and Figure 5 displays the mean number of oxytocin- (Panel A) and CRF-immunoreactive (Panel B) cells in the PVN. The isolated group displayed significantly greater numbers of oxytocin-immunoreactive cells [t(13) = 1.78, p < 0.05] and CRF-immunoreactive cells [t(13) = 2.55, p < 0.05] in the PVN, versus the paired group
Figure 4.
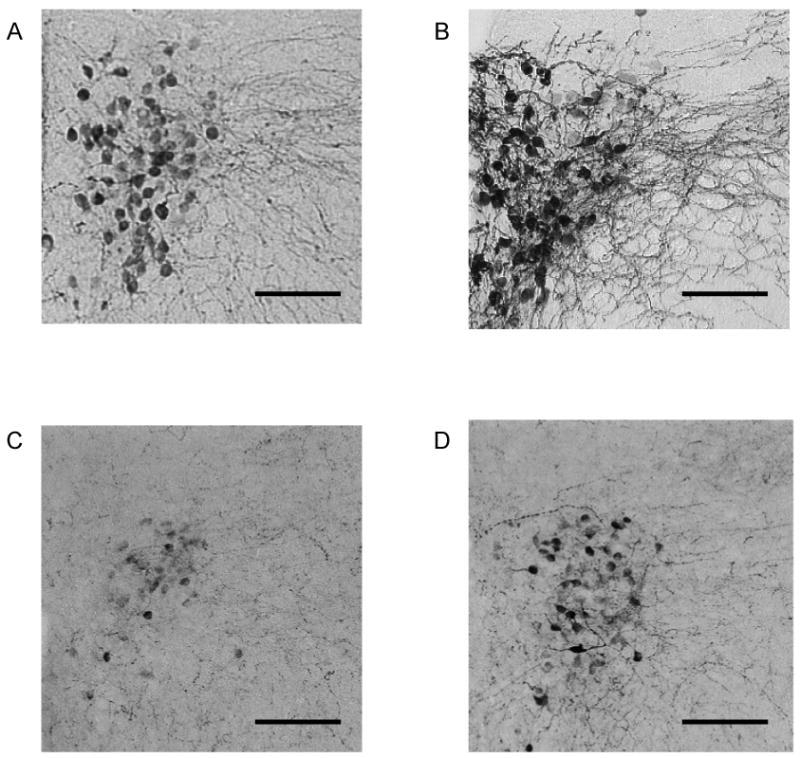
Brain sections (40 μm) showing oxytocin- (Panels A and B) and CRF-immunoreactivity (Panels C and D) in representative paired (Panels A and C) and isolated (Panels B and D) prairie voles in the hypothalamic PVN. Pictures are shown at 100× magnification. Scale bars = 100 μm. Stained fibers were excluded from analysis.
Figure 5.
Mean (+ SEM) number of oxytocin- (Panel A) and CRF-immunoreactive (Panel B) cells in the hypothalamic PVN in paired and isolated prairie voles. Note the scale differences in Panels A and B. *P < 0.05 versus paired value. Stained fibers were excluded from analysis.
Discussion
The present study investigated the role of the social environment in mediating behavioral and physiological processes relating to depression. The results suggest that social isolation induces anhedonia and increased levels of stress-related hormones and peptides in the PVN. Furthermore, social isolation is associated with increased behavioral and neuroendocrine reactivity to an acute social stressor (resident-intruder paradigm). These results extend previous findings from our laboratory showing that chronic exposure to environmental and social stressors in rats induces depression-relevant behaviors and neuroendocrine dysfunction (e.g. 5,6,10), and provide further insight into mechanisms that may underlie the development of behavioral and physiological signs of affective disorders.
Socially isolated prairie voles displayed a reduction in sucrose intake and sucrose preference versus paired prairie voles, indicative of anhedonia. Anhedonia a characteristic sign of depression, and may be present in approximately 95% of depressed patients (39). The sucrose deficit in isolated prairie voles represents a specific hedonic deficit rather than a generalized attenuation of fluid ingestion, as water intake was unaffected by the isolation procedure. Also, the reduced preference for sucrose in the isolated group was due entirely to a reduction in sucrose consumption, rather than an increase in water consumption. These data are consistent with previous studies, from our laboratory and others, that have examined hedonic behaviors in animal models of depression (5,10,27,40,41). To our knowledge, this is the first demonstration of a depression-like behavior following chronic social isolation in prairie voles using a validated, observable behavioral index. It is possible that a shorter period of isolation may be sufficient to induce anhedonia in prairie voles, as previous research suggests that 28 days of chronic mild stress induces anhedonia in rats (see for instance 5), and 3 days of isolation alters forced swim test behavior in male prairie voles that have been separated from a pair-bonded female (41).
Exposure to an acute social stressor increased circulating oxytocin, AVP, and corticosterone in socially isolated prairie voles, indicative of stressor-induced neuroendocrine activation (however, ACTH was not altered, which may be due to the high variability of responses in the isolated group; refer to Figure 3C). Consistent with these results is the observation that isolated prairie voles, versus paired animals, displayed a greater number of agitated behaviors following the acute stressor. Paired and isolated animals displayed the same number of aggressive behaviors during the resident-intruder test, indicating that generalized activity level and aggression did not lead to the increased agitation and neuroendocrine responses following this stressor. It is possible that the threatening situation (i.e., being the intruder) led to the behavioral and physiological responses to this stressor in isolated animals. The findings described here are in agreement with previous results from our laboratory showing elevated corticosterone in the chronic mild stress model of depression (6), as well as previous reports of peripheral oxytocin release in response to stressful stimuli in male and female rodents (42-44). Additionally, these changes mirror those observed in patients with affective disorders and in individuals who have experienced social stressors (7,17,20,45). However, AVP responses to stressors have been inconsistent in rodents (42,43,46), and may deserve further experimental attention.
The increased circulating oxytocin levels observed in the current study may be indicative of a compensatory response whereby this peptide is secreted to counteract the excess corticosterone and AVP. It is possible that corticosterone and AVP levels, while initially increased, would be reduced at a later time point following the resident-intruder stressor, suggesting that oxytocin levels are increased in a compensatory manner to lead to a subsequent reduction in the HPA axis response. Indeed, oxytocin can suppress the activity of the HPA axis in natural and experimental settings in humans (47,48). Recently, it was shown that intracerebroventricular oxytocin treatment attenuated central and peripheral responses to restraint stress in rats (49). Neumann and colleagues (50,51) have reviewed evidence for the role of oxytocin in mediating the physiological stress response, suggesting that its involvement is both brain region- and stressor-specific.
Social isolation in prairie voles is associated with increased circulating stressor-reactive hormones and peptides in the presence of an acute stressor, mirrored by elevated oxytocin- and CRF-immunoreactivity in the PVN. These findings are in agreement with Bosch et al. (32), who demonstrated increased oxytocin release in the PVN in female rats (lactating residents and virgin intruders) during a 10-minute resident-intruder test. Increases in oxytocin and CRF levels in the hypothalamus may represent long-term responses to social isolation, and may be linked to the release of peripheral oxytocin and/or activation of the HPA axis in response to an acute stressor. Acute stressors in both male and female rats have been shown to induce the release of oxytocin in the amygdala (52) and hypothalamus (32,43,53,54), measured via microdialysis and in situ hybridization, and therefore it will be important to investigate whether an acute social stressor can lead to differential changes in oxytocin, CRF, and AVP release in the central nervous system in isolated versus paired prairie voles. Furthermore, in previous studies, social isolation has been shown to elevate circulating corticosterone and tissue CRF levels (but not circulating or tissue oxytocin levels) of juvenile prairie voles (23; M. Ruscio unpublished observations), and reduce neurogenesis in the hypothalamus of adult prairie voles (55). Considering these findings, it may be possible for chronic social isolation alone to induce basal changes in some circulating or central factors in prairie voles. However, the elevated levels of CRF and oxytocin cells observed in the PVN may shed some light on this issue, as the time course of the acute stressor employed here (5-minute stressor with tissue collection 10 minutes following the stressor) may not be sufficient to produce changes in the number of oxytocin- and CRF-producing cells in the central nervous system. Future research should investigate basal changes in stressor-reactive hormones and peptides following isolation.
Specific limitations of this research may have had an impact on the present findings. It is possible that some results could have been influence by differential age-related responses. However, while not all animals were the same age in the current study (modal age 90 days), the age range used here has been used in a number of studies examining the behavior, stress responses, and physiological function in prairie voles and produced reliable differences (56,57,30), and therefore we feel that using this age range was acceptable. A second limitation in studies of social interactions is that the experimental design may add uncontrolled variables associated with temporary isolation experienced by the paired animals. However, if this was that case, differences between paired and isolated groups may have been less pronounced or absent; whereas the present results suggest several robust differences in both behavior and physiology between paired and isolated animals. A third limitation involves the difficulty in studying female rodents as a model for understanding processes that mediate behavior and physiology in women. The lack of spontaneous puberty and estrous cycle in female prairie voles (31) allows for conducting experiments without controlling for female-specific hormonal influences on the dependent variables in question, but also limits the translation of results to human conditions during which the menstrual cycle cannot be controlled (i.e., naturalistic settings in women). Future research might investigate the effects of social stressors in reproductively-primed prairie voles (or other rodents that show a spontaneous estrous cycle, such as rats or mice). To this end, a previous study from our laboratory suggests that exposure to chronic environmental and social stressors induces anhedonia and a disruption of the estrous cycle in adult female rats (27).
The current study demonstrates that neuroendocrine dysfunction and behavioral alterations can result from exposure to a combination of chronic and acute social stressors. These experiments, which include measurement of behavioral and neuroendocrine responses in the same animal, are relevant to understanding human mood disorders. Prairie voles may be especially valuable for understanding mechanisms underlying depression because this species demonstrates social behaviors, including social bonds, and physiological parameters, including high levels of parasympathetic activity, that mirror those of humans (19,23,24,30). Also, behavioral and physiological reactivity to acute stressors appears to be an important mediator of depressive signs and symptoms (for instance 15,17,22), and therefore the results described here may provide insight into relevant processes that underlie mood disorders. The prairie vole may be a useful model for preclinical investigation of novel treatments for affective disorders, such as pharmacological treatments that focus on CRF, AVP, oxytocin, or glucocorticoid systems. An increased understanding of the behavioral and neurobiological processes that underlie affective disorders can lead to the development of comprehensive treatments designed at targeting mechanisms, rather than symptoms, of these important mental disorders.
Acknowledgments
This research was funded by National Institute of Mental Health MH 73233 (AJG) and MH 01992 (BSC), and National Institute of Child Health and Human Development HD 48390 (CSC). The authors would like to thank Dr. Mariana Morris and Dr. Ann-Judith Silverman for generously donating the antibodies used in these experiments. The authors are grateful to Ms. Francisca Garcia for help with the ACTH assay, and Ms. Davida Gerena, Ms. Narmda Kumar, Ms. Lisa Sanzenbacher, and Mr. Raj Ughreja for technical assistance.
Acronyms
- ACTH
Adrenocorticotropic hormone
- ANOVA
analysis of variance
- AVP
arginine vasopressin
- CRF
Corticotropin releasing factor
- DAB
diaminobenzadine
- HPA
hypothalamic-pituitary-adrenal
- KPBS
potassium phosphate buffered saline
- PVN
paraventricular nucleus
- SEM
standard error of the mean
References
- 1.Nemeroff CB, Widerlöv E, Bissette G, Walléus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 2.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJG, Tilders FJH, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 3.Glowa JR, Gold PW. Corticotropin releasing hormone produces profound anorexigenic effects in the rhesus monkey. Neuropeptides. 1991;18:55–61. doi: 10.1016/0143-4179(91)90164-e. [DOI] [PubMed] [Google Scholar]
- 4.Garlow SJ, Musselman DL, Nemeroff CB. The neurochemistry of mood disorders: clinical studies. In: Charney DS, Nestler EJ, Bunney BS, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 1999. pp. 348–364. [Google Scholar]
- 5.Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- 6.Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Banki CM, Karmacsi L, Bissette G, Nemeroff CB. CSF corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. Eur Neuropsychopharmacol. 1992;2:107–113. doi: 10.1016/0924-977x(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 8.van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:891–907. doi: 10.1016/j.pnpbp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Asnis GM, Halbreich U, Ryan ND, Rabinowicz H, Puig-Antich J, Nelson B, Novacenko H, Friedman JH. The relationship of the dexamethasone suppression test (1 mg and 2 mg) to basal plasma cortisol levels in endogenous depression. Psychoneuroendocrinology. 1987;12:295–301. doi: 10.1016/0306-4530(87)90054-0. [DOI] [PubMed] [Google Scholar]
- 10.Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 11.Tafet GE, Bernardini R. Psychoneuroendocrinological links between chronic stress and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:893–903. doi: 10.1016/S0278-5846(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 12.Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 13.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 14.Anisman H, Zacharko RM. Depression as a consequence of inadequate neurochemical adaptation in response to stressors. Br J Psychiatry. 1992;160(Suppl. 15):36–43. [PubMed] [Google Scholar]
- 15.Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 16.Ploog DW. The place of the Triune Brain in psychiatry. Physiol Behav. 2003;79:487–493. doi: 10.1016/s0031-9384(03)00154-9. [DOI] [PubMed] [Google Scholar]
- 17.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 18.Adams KB, Sanders S, Auth EA. Loneliness and depression in independent living retirement communities: risk and resilience factors. Aging Ment Health. 2004;8:475–85. doi: 10.1080/13607860410001725054. [DOI] [PubMed] [Google Scholar]
- 19.Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. Horm Brain Behav. 2002;1:299–337. [Google Scholar]
- 20.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 21.Pihoker C, Owens MJ, Kuhn CM, Schanberg SM, Nemeroff CB. Maternal separation in neonatal rats elicits activation of the hypothalamic-pituitary-adrenocortical axis: a putative role for corticotropin-releasing factor. Psychoneuroendocrinology. 1993;18:485–493. doi: 10.1016/0306-4530(93)90042-j. [DOI] [PubMed] [Google Scholar]
- 22.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim JW, Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biol Psychiatry. 1996;40:918–922. doi: 10.1016/0006-3223(95)00546-3. [DOI] [PubMed] [Google Scholar]
- 24.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 26.Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 27.Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology. 2005;179:769–780. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- 28.DeVries AC, DeVries MB, Taymans SE, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc Natl Acad Sci. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- 30.Grippo AJ, Carter CS, Porges SW. Social isolation induces depression-like behaviors and autonomic dysfunction in socially monogamous prairie voles. FASEB J. 2006 http://www.eb2006-online.com/pdfs/001126.PDF?PHPSESSID=f07543fef8761587d860af5e0239a3bf.
- 31.Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- 32.Bosch OJ, Krömer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Pardon MC, Gérardin P, Joubert C, Pérez-Diaz F, Cohen-Salmon C. Influence of prepartum chronic ultramild stress on maternal pup care behavior in mice. Biol Psychiatry. 2000;47:858–863. doi: 10.1016/s0006-3223(99)00253-x. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell PJ, Fairhall SJ, Fletcher A, Redfern PH. Effects of single and repeated electroconvulsive shock on the social and agonistic behaviour of resident rats. Neuropharmacology. 2003;44:911–925. doi: 10.1016/s0028-3908(03)00075-3. [DOI] [PubMed] [Google Scholar]
- 35.Cacho R, Fano E, Areso P, Garmendia L, Vegas O, Brain PF, Azpíroz A. Endocrine and lymphoproliferative response changes produced by social stress in mice. Physiol Behav. 2003;78:505–512. doi: 10.1016/s0031-9384(03)00018-0. [DOI] [PubMed] [Google Scholar]
- 36.Kramer KM, Cushing BS, Carter CS, Wu J, Ottinger MA. Sex and species differences in plasma oxytocin using an enzyme immunoassay. Can J Zool. 2004;82:1194–1200. [Google Scholar]
- 37.Li Q, Levy AD, Cabrera TM, Brownfield MS, Battaglia G, Van de Kar LD. Long-term fluoxetine, but not desipramine, inhibits the ACTH and oxytocin responses to the 5-HT1A agonist, 8-OH-DPAT, in male rats. Brain Res. 1993;630:148–156. doi: 10.1016/0006-8993(93)90652-4. [DOI] [PubMed] [Google Scholar]
- 38.Cushing BS, Klein D, Hoffman GE, Carter CS, Le WW, De Vries GJ. Comparison of fixation techniques: immersion versus perfusion. Horm Behav. 2001;39:329. [Google Scholar]
- 39.Keller MB, Klein DN, Hirschfeld RM, Kocsis JH, McCullough JP, Miller I, First MB, Holzer CP, Keitner GI, Marin DB. Results of the DSM-IV mood disorder field trial. Am J Psychiatry. 1995;152:843–849. doi: 10.1176/ajp.152.6.843. [DOI] [PubMed] [Google Scholar]
- 40.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 41.Bosch OJ, Nair HP, Neumann ID, Young LJ. Pair-bonded prairie voles display depression-like behavior after separation. Soc Neurosci Abstr. 2004 http://sfn.scholarone.com/itin2004/index.html.
- 42.Lang RE, Heil JW, Ganten D, Hermann K, Unger T, Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983;37:314–316. doi: 10.1159/000123566. [DOI] [PubMed] [Google Scholar]
- 43.Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- 44.Douglas AJ, Johnstone HA, Wigger A, Landgraf R, Russell JA, Neumann ID. The role of endogenous opioids in neurohypophysial and hypothalamo-pituitary-adrenal axis hormone secretory responses to stress in pregnant rats. J Endocrinol. 1998;158:285–293. doi: 10.1677/joe.0.1580285. [DOI] [PubMed] [Google Scholar]
- 45.Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- 46.Kasting NW. Simultaneous and independent release of vasopressin and oxytocin in the rat. Can J Physiol Pharmacol. 1988;66:22–26. doi: 10.1139/y88-004. [DOI] [PubMed] [Google Scholar]
- 47.Chiodera P, Salvarani C, Bacchi-Modena A, Spallanzani R, Cigarini C, Alboni A, Gardini E, Coiro V. Relationship between plasma profiles of oxytocin and adrenocorticotropic hormone during suckling or breast stimulation in women. Hormone Res. 1991;35:119–123. doi: 10.1159/000181886. [DOI] [PubMed] [Google Scholar]
- 48.Legros JJ, Chiodera P, Geenen V, von Frenckell R. Confirmation of the inhibitory influence of exogenous oxytocin in cortisol and ACTH in man: evidence of reproducibility. Acta Endocrinol. 1987;114:345–349. doi: 10.1530/acta.0.1140345. [DOI] [PubMed] [Google Scholar]
- 49.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neumann ID, Krömer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 51.Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 52.Ebner K, Bosch OJ, Krömer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- 53.Wotjak CT, Naruo T, Muraoka S, Simchen R, Landgraf R, Engelmann M. Forced swimming stimulates the expression of vasopressin and oxytocin in magnocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2001;13:2273–2281. doi: 10.1046/j.0953-816x.2001.01613.x. [DOI] [PubMed] [Google Scholar]
- 54.Wigger A, Neumann ID. Endogenous opioid regulation of stress-induced oxytocin release within the hypothalamic paraventricular nucleus is reversed in late pregnancy: a microdialysis study. Neuroscience. 2002;112:121–129. doi: 10.1016/s0306-4522(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 55.Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- 56.DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology. 2002;27:705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 57.Cushing BS, Mogekwu N, Le WW, Hoffman GE, Carter CS. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 2003;965:203–211. doi: 10.1016/s0006-8993(02)04199-9. [DOI] [PubMed] [Google Scholar]