Abstract
Background
Cognitive control deficits are pervasive in individuals with schizophrenia and are reliable predictors of functional outcome, but the specificity of these deficits and their underlying neural mechanisms have not been fully elucidated. The objective of the present study was to determine the nature of response inhibition and response monitoring deficits in schizophrenia and their relationship to symptoms and social and occupational functioning using a behavioral paradigm that provides a translational approach to investigating cognitive control.
Methods
17 patients with schizophrenia and 16 demographically-matched healthy controls participated in a saccadic countermanding task. Performance on this task is approximated as a race between movement generation and inhibition processes; this race model provides an estimate of the time needed to cancel a planned movement. Response monitoring can be assessed by the reaction time (RT) adjustments based on trial history.
Results
Saccadic reaction time was normal, but patients required more time to inhibit a planned saccade. The latency of the inhibitory process was associated with the severity of negative symptoms and poorer occupational functioning. Both groups slowed down significantly following correctly cancelled and erroneously non-cancelled stop signal trials, but patients slowed down more than controls following correctly inhibited saccades.
Conclusion
These results suggest that schizophrenia is associated with a difficulty in inhibiting planned movements and an inflated response adjustment effect after inhibiting a saccade. Further, behavioral results are consistent with potential abnormalities in frontal and supplementary eye fields in patients with schizophrenia.
Keywords: schizophrenia, saccades, inhibition, response monitoring, cognitive control, stop signal
Introduction
Executive control and flexible modification of behavior based on feedback are essential to adaptive functioning in a dynamic environment. Schizophrenia is associated with impairments in a range of cognitive functions that underlie behavioral flexibility, including working memory (1), attention(2), and cognitive control (3). Cognitive deficits in schizophrenia predict functional outcome better than clinical symptoms(4) and are major targets for pharmacotherapy(5). The saccade countermanding paradigm(6) is an ideal measure of cognition in treatment studies, as it measures two key components of cognitive control, inhibition and response monitoring, that have been studied in humans and nonhuman primates under similar experimental conditions. Thus, it provides a translational bridge for understanding deficits in schizophrenia.
Response inhibition is the ability to deliberately inhibit actions(7). Although inhibitory deficits have been described in schizophrenia(8, 9), inhibition is not a unitary construct(10). Further, correlations among performance measures on tasks of response inhibition are typically low(11–13). Thus, there is utility in investigating patient performance on a variety of inhibition-related tasks. The present research focuses on inhibition in the saccadic countermanding task.
Response monitoring involves evaluation of actions via feedback to guide future performance and is commonly indexed by error detection and response time (RT) adjustments as a function of trial history. There is mixed evidence for deficits in immediate error-related performance adjustments in patients with schizophrenia(14–17).
The countermanding paradigm is used to investigate the ability to control the execution of a response(18, 19). In the saccadic countermanding task, subjects are instructed to make a saccade to a visual target, unless a stop signal appears at some delay following target presentation. On these trials, subjects are instructed to withhold the saccade. The time needed to cancel a movement, the stop signal reaction time (SSRT), can be estimated from the distribution of RTs on no-stop signal trials and the probability of making a saccade given that a stop signal occurred; it is hypothesized to be based on a race between STOP and GO processes (20). Neural activity in the frontal eye fields (FEF; 21, 22) and the superior colliculus (SC; 23) is necessary for saccadic preparation and inhibition. In contrast, neurons in the medial frontal cortex generate performance monitoring signals associated with errors, reward and conflict(24, 25), which may contribute to specific behavioral adjustments based on trial history(26).
The countermanding task was included in a testing battery, resulting from a recent NIH-initiative, for evaluating changes in cognition in clinical trials(27). However, few studies have examined countermanding performance in schizophrenia. Both longer(28, 29) and equal(30) SSRTs have been reported in schizophrenia from the manual countermanding task. Discrepant findings are potentially due to task-specific factors that affect estimation of SSRT(31). The present study is the first investigation of stopping behavior and RT adjustments based on trial history using the saccadic countermanding task in schizophrenia. There are several advantages to using the oculomotor version of this task. A substantial body of work has investigated neurophysiological mechanisms instantiating the inhibition and monitoring of saccades in nonhuman primates performing this task(32). In addition, a formal mathematical model was developed that accounts for behavior(20), and it has recently been elaborated to account for activity in single neurons during saccade countermanding(33). Accordingly, this paradigm allows us to make clear assumptions about what is being inhibited and monitored, to estimate when inhibition is occurring, and to understand how inhibition and monitoring of saccades is being supported in the brain. In this way, this task permits specific hypotheses to be drawn about the nature of putative deficits in response inhibition and monitoring. The use of saccadic versus manual tasks in schizophrenia has an additional advantage. Slowing in manual RT has been consistently reported in schizophrenia(34), but the latency of reflexive saccades is generally normal(35, 36). Thus, it is argued that saccadic tasks of cognitive control in schizophrenia minimize confounding effects of impairments in the basic response system (37).
Our aim in the current study was to examine inhibition and monitoring of saccades in healthy individuals and patients with schizophrenia. We also examined working memory deficits(1) in relation to potential countermanding deficits. Thus, we sought to expand our understanding of the specific nature of cognitive control deficits in schizophrenia.
Methods and Materials
Participants
Individuals who met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for schizophrenia (SZ) were recruited from outpatient psychiatric facilities in Nashville, TN. Diagnoses were confirmed using structured clinical interviews (SCID-IV; 38). All patients were taking atypical antipsychotic medications, with the exception of one patient taking Depakote. Healthy, unmedicated control subjects (HC) without a personal and family history of DSM-IV Axis I disorders were recruited from the same community by advertisements.
Clinical symptoms were assessed with the Brief Psychiatric Rating Scale (BPRS)(39), the Scale for the Assessment of Positive Symptoms (SAPS)(40), and the Scale for the Assessment of Negative Symptoms (SANS)(41). Social and occupational functioning was assessed by the Social Functioning Scale (SFS(42). The Adult North American Reading Test (ANART)(43, 44) or Wechsler Abbreviated Scale of Intelligence (WASI) (45) was used to assess IQ. Although mean IQ and education were lower in SZ compared with HC, their mean IQ was in the normal range, and the average SZ had achieved a high school education. Moreover, IQ has not been found to be related to response inhibition(10, 18) or to the RT cost of task-switching(10). Handedness was assessed using the Modified Edinburgh Handedness Inventory(46).
All participants were screened to exclude substance use, neurological disorders, history of head injury, inability to fixate, and excessive sleepiness. All subjects had normal or corrected-to-normal vision. Two SZ were excluded based on countermanding task performance, as outlined in the Statistical Methods section. Analyses were conducted on the remaining 17 SZ and 16 HC. SZ and HC were matched for age, sex, and handedness; demographic data are presented in Table 1. All subjects gave written informed consent approved by the Vanderbilt Institutional Review Board and were paid.
Table 1.
Demographic characteristics of the patient and control groups.
| Patients Mean (s.d.) | Controls Mean (s.d.) | t | p | |
|---|---|---|---|---|
| Age | 36.0 (7.7) | 34.9 (7.9) | 0.4 | 0.70 |
| Sex | 6F/11M | 7F/9M | Phi=0.2 | 0.73 |
| Edinburgh handedness | 51.5 (54.9) | 56.7 (67.7) | 0.4 | 0.70 |
| Years of Education | 13.4 (1.9) | 16.2 (2.1) | 4.0 | 0.0003 |
| IQ | 102.6 (10.8) | 110.5 (4.6) | 2.7 | 0.01 |
| SAPS | 13.8 (19.1) | |||
| SANS | 20.8 (16.7) | |||
| BPRS | 11.8 (7.1) | |||
| SFS Total Score | 132.3 (24.4) | 156.8 (14.6) | 3.4 | 0.002 |
| SFS Employment Score | 5.2 (3.8) | 9.7 (0.7) | 4.6 | <0.0001 |
The Phi value is the result of a Fishers exact test.
Apparatus and Stimuli
Eye position was monitored using the EyeLink II eyetracker (SR Research, Canada) at a sampling rate of 250 Hz with average gaze position error <0.5°, noise limited to <0.01° RMS. Saccades were detected on-line using a velocity criterion (35°/sec). Subjects were seated 57cm from the computer monitor with their head in a chinrest. The fixation and targets subtended 1° and were light gray (34 cd/m2) on a darker gray (18 cd/m2) background.
Design and Procedure
Countermanding Task
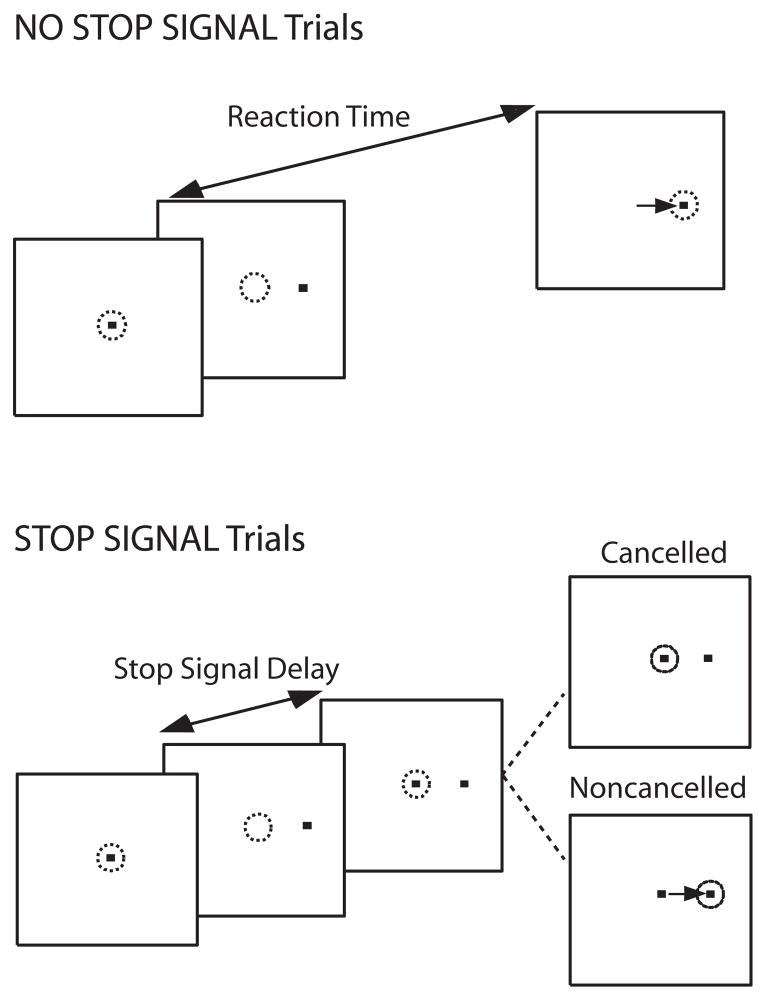
Subjects performed a saccadic countermanding task (Figure 1). Seventy percent of the trials were no-stop signal trials. These trials required subjects to fixate on a central spot until it disappeared (after a random delay between 500–1000 ms) and a peripheral target appeared at one of two randomly selected locations (left or right) equidistant (8.5°) from the central fixation spot. Subjects were instructed to look directly at the target as quickly as possible. The remaining 30% of trials were stop signal trials. These trials were initially identical to the no-stop signal trials, but the fixation spot was re-illuminated after a variable delay (stop signal delay; SSD) following target presentation, cuing subjects to inhibit a saccade to the target. Stop signal trials were labeled cancelled or noncancelled based on whether subjects inhibited or failed to inhibit the saccade, respectively. Response inhibition becomes more difficult with increasing SSDs. SSDs were dynamically adjusted using a 1-up/1-down tracking procedure, thereby ensuring successful inhibition on 50% of the stop signal trials(47). The initial SSD was set at 225 ms and increased or decreased by 47 ms when the subject succeeded or failed to inhibit, respectively. The testing session consisted of a practice block of 60 trials, and 4 experimental blocks of 120 trials each.
Figure 1.
Saccade countermanding task. Dotted circles indicates gaze position, and the arrow indicates the direction of the saccade. All trials began with the presentation of a central fixation spot. After a variable fixation length, the fixation spot disappeared, and, simultaneously, a target appeared at an eccentric location to the right or left of central fixation. On 30% of trials (stop signal trials), the fixation spot was reilluminated at some delay, referred to as stop signal delay (SSD), following target onset. Fixation reillumination was the cue for the subject to withhold a saccade to the target. Trials in which the subject was successful in maintaining fixation were referred to as cancelled trials, and trials in which the subject made a saccade to the eccentric target were referred to as noncancelled trials. For the remaining 70% of trials (no-stop signal trials), fixation was not reilluminated and the subject was instructed to make a saccade to the peripheral target.
Behavioral performance was evaluated through measurements of saccadic RT on no-stop signal and noncancelled trials, and mean SSD. At each SSD, we quantified the proportion of trials in which a participant successfully inhibited a saccade. The proportion of cancelled trials at each delay is referred to as the inhibition function.
Performance in the stop signal task can be accounted for by a mathematical model that assumes a race between independent processes that generate (GO) and inhibit (STOP) the movement(20). The response is executed if the GO process finishes first and inhibited if the STOP process finishes first. The latency of the GO process can be measured directly from the observable RTs, but the latency of the STOP process is estimated. The independent race model provides an estimate of the time needed to respond to the stop signal and cancel the movement, referred to as the stop signal reaction time (SSRT). See Supplement 1 for description of SSRT calculation. The slope of the inhibition function is thought to reflect variability in the STOP and GO RT and the ability to trigger an inhibitory response. The slope can be corrected for variability in GO RT by applying a Z-transformation to the SSDs (48), which expresses them in terms of the latency relative to finishing times of GO and STOP processes standardized with respect to variability in GO RT using the equation:
To index response monitoring, RT was examined as a function of trial history. Mean RT was computed separately for no-stop signal trials preceding and following no-stop signal trials, correctly cancelled stop signal trials, and noncancelled stop signal trials (i.e. stop-task errors). RTs on no-stop signal trials preceding and following two consecutive stop signal trials were included in this analysis only if the response on the two stop signal trials was the same. Post-cancelled slowing was calculated as the difference between mean RT for no-stop signal trials preceding and following a cancelled trial. Likewise, post-error slowing was calculated as the difference between mean RT for no-stop signal trials preceding and following a noncancelled trial.
Verbal and Spatial Working Memory (WM) Tasks
Verbal working memory (VWM) was measured using the Letter Number Sequencing task(49) in which subjects were verbally presented a series of letters and numbers and asked to report the numbers in numerical order, followed by the letters in alphabetical order. VWM scores were unavailable for one patient and one control.
Spatial working memory (SWM) was assessed using a delayed-response task. Subjects fixated centrally. Then a target (black circle subtending 2°) was presented for 300ms at one of eight locations 12° from the central fixation spot, followed by a delay of 8s. During the delay, numbers were presented centrally, in descending order in steps of four. Subjects were instructed to note any subtraction errors in order to prevent verbal rehearsal and maintain central fixation. After the delay, subjects were asked to indicate location of the target, then indicate if they noticed a subtraction error using the keypad. There were 48 trials. SWM scores were unavailable for two patients and one control.
Statistical Methods
All tests were two-tailed except otherwise specified. Subjects were excluded from analyses if the adaptive tracking procedure in the stop signal task was ineffective, defined by a proportion of cancelled responses lying outside a 95% binomial confidence interval around p=0.5.
Results
Table 2 shows stop signal performance and RT adjustments for SZ and HC.
Table 2.
Performance characteristics of the patient and control groups.
| HC Mean (s.d.) | SZ Mean (s.d.) | t-statistic | p | |
|---|---|---|---|---|
| Probability of Inhibition (%) | 50.7 (4.2) | 48.0 (4.6) | 1.7 | 0.09 |
| No-stop signal RT (ms) | 273 (55) | 283 (59) | 0.5 | 0.60 |
| Noncancelled RT(ms) | 222 (40) | 232 (44) | 0.7 | 0.50 |
| SSRT (ms) | 124 (24) | 147 (31) | 2.5 | 0.02 |
| Post-error slowing (ms) | 40 (22) | 48 (38) | 0.7 | 0.50 |
| Post-cancelled slowing (ms) | 24 (22) | 51 (42) | 2.3 | 0.03 |
Probability of inhibition
The dynamic tracking procedure was successful, and the mean proportion of noncancelled trials was 49%. The two groups did not differ in the proportion of noncancelled trials. For each subject, the estimated slope of the inhibition function plotted against ZRFT was calculated (Figure 2). There was no group difference in the slope of the Z-transformed inhibition function (t(31) = 1.3, p = 0.20), providing evidence for equal variability in the inhibitory process for both groups.
Figure 2.
Individual normalized inhibition functions for healthy controls (HC; dotted lines) and schizophrenia patients (SZ; solid lines). Probability of inhibition is plotted as a function of a Z score that measures time relative to the finish time of the GO and STOP processes in standard deviation units using the formula: ZRFT = (mean no-stop signal RT − SSD − SSRT)/standard deviation of no-stop signal RT. Separate cumulative Weibull functions are fit to the normalized inhibition functions for patients and controls.
No-stop signal and noncancelled RT
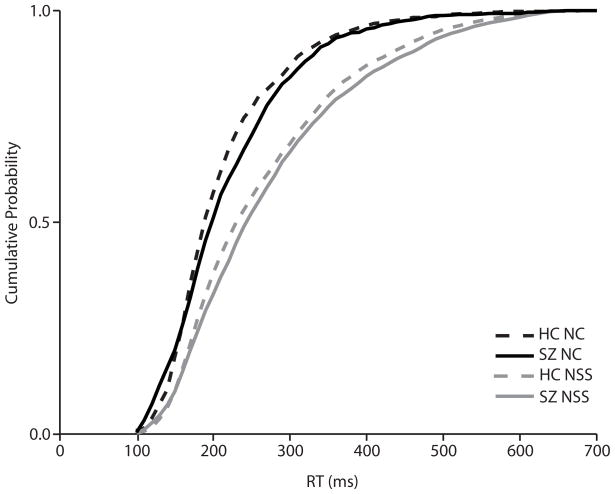
The effect of trial type (no-stop signal or noncancelled) on RT was assessed with a repeated-measures ANOVA with group as a between-subjects variable and trial type as a within-subjects variable. There was a significant effect of trial type (F(1,31) = 106.7, p < 0.0001), with no-stop signal trials being slower than noncancelled trials. There was no main effect of group (F(1,31) = 0.38, p = 0.54) or group-by-trial type interaction effect (F(1,31) = 0.0003, p = 0.99). Cumulative RT distributions are presented in Figure 3.
Figure 3.
Cumulative distributions of saccade latencies in no-stop signal (grey) and noncancelled (black) trials for healthy controls (HC; dotted) and schizophrenia (SZ; solid) groups.
SSRT
SSRT was significantly longer in SZ (t(31)=2.5, p=0.02), who required more time to inhibit a saccade than HC.
RT adjustments across three trials in sequence
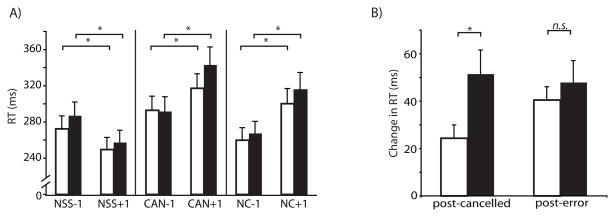
RT adjustment effects are presented in Figure 4. To assess effects of trial history on current no-stop signal trial, a repeated-measures ANOVA was conducted on no-stop signal RTs with diagnostic group as a between-subject variable and critical trial (no-stop signal, cancelled, noncancelled) and history (before or after critical trial) entered as within-subject variables. There was a significant effect of history (F(1, 31) = 68.2, p < 0.0001), and critical trial (F(2,62) = 61.5, p < 0.0001). Notably, there was a significant history-by-critical trial interaction (F(2,62) = 57.3, p < 0.0001).
Figure 4.
Mean no-stop signal RT as a function of trial history. A. Mean no-stop signal RT (with standard error) for trials following (+1) and preceding (−1) no-stop signal (NSS), cancelled (CAN) and noncancelled (NC) trials for healthy controls (empty bars) and schizophrenia patients (filled bars). B. Mean post-cancelled and post-error slowing.
Planned contrasts revealed that RTs for no-stop signal trials were slower when they followed cancelled (F(1,62) = 54.4, p < 0.0001) and noncancelled (F(1,62) = 74.0, p < 0.0001) trials than when they preceded them. This suggests that presenting a stop signal increases saccadic RT on the subsequent trial, whether or not the saccade was cancelled. When three no-stop trials were presented in a row, participants got faster throughout (F(1,62)=25.8, p<0.0001). Additionally, planned contrasts revealed significant differences between RTs of the trials preceding each of the critical trial types. Trials preceding cancelled trials were slower than those preceding both noncancelled (F(1, 62)=30.7, p<0.0001) and no-stop signal trials (F(1,62)=5.9, p=0.02). This suggests when subjects are responding slower, they are more likely to be able to cancel a saccade on the subsequent trial. Trials preceding no-stop signal trials were slower than those preceding noncancelled trials (F(1,62)=9.7, p=0.003). Likewise, this suggests that faster saccadic RT may result in subsequent failure to cancel a saccade.
There was no main effect of group (F(1,31)=0.32, p=0.58), but there was a significant group-by-history effect (F(1, 31) = 4.23, p=0.05). Planned contrasts revealed slower performance in SZ compared to HC and this difference was more pronounced after the critical trial (F(1,31)=26.0, p < 0.0001) than before the critical trial (F(1,31)=4.8, p=0.04). There was a trend towards a group-by-history-by-critical trial effect (F(2, 62) = 2.7, p = 0.07). Independent t-tests were conducted to assess group differences in post-cancelled and post-error slowing and speeding following no-stop signal trials. SZ slowed down significantly more following cancelled trials than HC (t(31)=2.3, p=0.03). There were no group differences in post-error slowing (t(31)=0.7, p=0.50) or speeding following no-stop signal trials (t(31)=1.7. p=0.10).
Symptom and social functioning
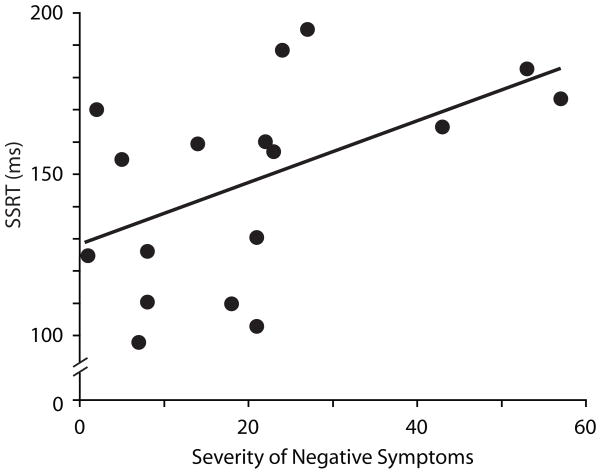
Spearman rank-correlation coefficients were used to evaluate the association between the severity of psychiatric symptoms and behavioral measures in SZ. SANS score was positively correlated with SSRT (rs = 0.61, p = 0.009); those with increased negative symptoms needed more time to inhibit saccades (Figure 5). SANS score did not correlate with any of the other behavioral measures, and SAPS and BPRS scores were not correlated with any of the behavioral measures.
Figure 5.
Relationship between SSRT and severity of negative symptoms, indexed by SANS score, in schizophrenia patients. Greater SANS scores represent more severe negative symptomology.
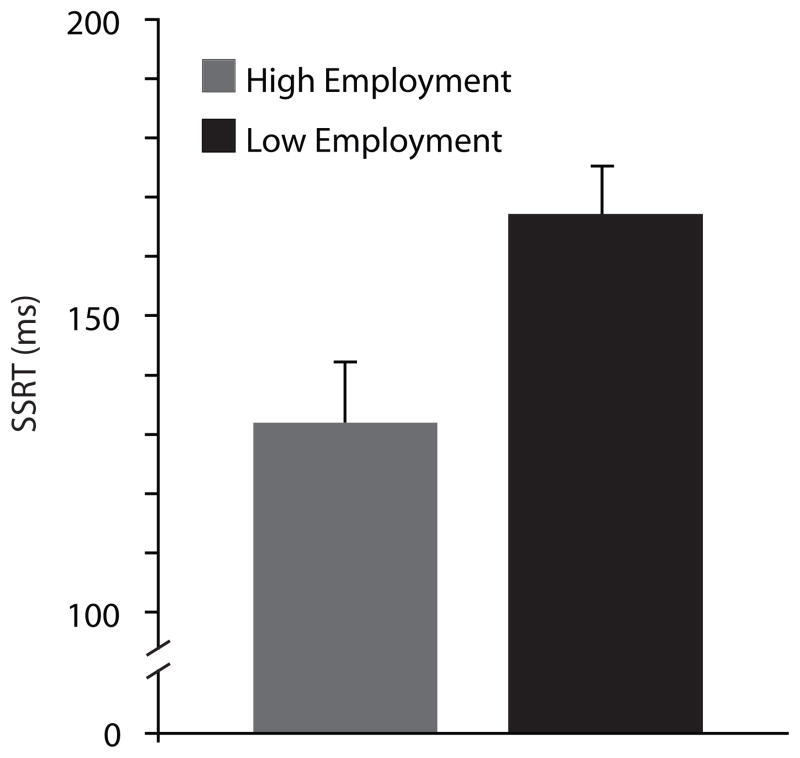
Since scores on the Employment subscale of the SFS were bimodally distributed in SZ, a median split was performed on the scores, and independent t-tests were conducted to compare behavioral measures in those scoring high and low on occupational functioning. SSRT was significantly longer in the low compared to high employment group, (t(14) = 2.8, p = 0.02) (Figure 6). That is, better occupational functioning was associated with less time needed to inhibit a planned movement in SZ. There was no significant difference in post-error or post-cancelled slowing between employment groups, and no significant relationship between SFS total score and countermanding task performance was observed.
Figure 6.
SSRT (plus standard error) for schizophrenia patients scoring high (grey) and low (black) on SFS employment subscale, defined by median split within patient group.
Interestingly, both SZ participants who were excluded from analyses based on performance indices would have fallen into the low employment group and their SANS scores were above the group mean.
Working Memory
Since we had an a priori hypothesis of poorer WM performance in SZ(1), one-tailed independent t-tests were conducted to compare WM performance between groups. SZ had significantly fewer correct sequences (M = 13.6, s.d. = 3.5) on the VWM task (t(28)=1.9, p=0.03, 1-tailed) than HC (M = 15.7, s.d. = 2.4). Since variances were unequal, a Welch’s t-test was used to compare accuracy on the SWM task. SZ (M=89.0%, s.d. = 10.9%) were less accurate than HC (M=96.8%, s.d.=4.9%) (t(19.4)=2.5, p=0.01,1-tailed).
Pearson product-moment correlation coefficients were used to evaluate the association between WM performance and SSRT, post-error slowing, and post-cancelled slowing in HC and SZ. One-tailed tests of significance were conducted to examine the strength of the correlation between WM and SSRT, given their purported relationship(50). In patients, the relationship between SSRT and VWM performance was significant (rs=−0.45, p=0.05, 1-tailed). That is, better VWM was associated with less time needed to cancel a planned saccade. There were no other significant correlations between WM performance and countermanding measures.
Discussion
Schizophrenia was associated with increased latency to inhibit a planned saccade. Longer SSRT was found in patients despite having equal sensitivity to the stop signal and similar latencies to initiate a saccade. Further, SSRT was related to increased negative symptoms and poorer occupational functioning, indicating the clinical relevance of these findings. In addition, patients made appropriate RT adjustments following errors, but slowed down significantly more than controls following correctly inhibited saccades.
Importantly, the performance of both patients and controls satisfied two criteria for the race model to hold. First, the probability of successfully inhibiting decreased with longer SSDs. After normalizing each individual’s SSD with respect to their mean and variance of no-stop signal RT, the slopes of the two groups’ inhibition functions were not statistically different, suggesting equal variability in SSRT and probability that the inhibitory process was triggered. Second RTs were shorter for noncancelled than no-stop signal trials, indicating that only the fastest GO processes were fast enough to escape inhibition. There was no group difference in the latency to initiate a saccade, consistent with prior findings (34).
For the most part, our data conform to the existing cognitive control literature in schizophrenia. Increased SSRT in patients indicates that they needed more time to inhibit a saccade, consistent with mounting evidence for impaired response inhibition in schizophrenia (See Introduction). We also found that patients had WM deficits, and poorer WM was related to longer SSRT. Further studies are necessary to investigate the degree to which increased SSRT might be due to inappropriate use of WM to trigger the stop process.
In our analysis of RT adjustments based on trial history, we found that both groups were faster on no-stop signal trials when they were preceded by no-stop signal trials versus cancelled and noncancelled trials. Post-cancelled slowing has been observed in both humans and nonhuman primates performing this task (26, 51–53, but see 54). Likewise, post-error slowing is commonly observed in speeded response tasks(55), including the manual (52, 54) and saccadic (56, 57; but see 26 for exception and 60 for methodological explanation for inconsistent post-error findings) countermanding tasks. There was no group difference in post-error slowing, consistent with prior reports(17, 58). These data suggest that the ability to evaluate performance and make appropriate short-term changes to repsonse strategy is spared in schizophrenia. Further, we found no group difference in speeding following no-stop signal trials. However, patients slowed down significantly more than controls following inhibited saccades. This finding is in line with recent evidence of prolonged effects of prior antisaccades on saccadic latency in schizophrenia(59–61), which are interpreted as abnormal perseveration in the saccadic response system (59). The degree to which increased post-cancelled slowing in patients represents a purposeful adjustment of response strategy is unclear. Although our findings partially replicate existing response inhibition and response monitoring data in schizophrenia using other cognitive tasks, the advantage of using the saccadic countermanding paradigm is the leverage it gives us on understanding the neural mechanisms of these abnormalities. In the following sections, we relate our findings in SZ to the extensive neurophysiology literature on this task.
Potential neural mechanisms underlying abnormal saccade countermanding in schizophrenia
Neurophysiological research in nonhuman primates has identified neural mechanisms by which saccades are inhibited in the countermanding task in the FEF and SC where GO and STOP processes have been mapped on to saccade- and fixation-related neurons, respectively. On no-stop signal and noncancelled trials, activity in saccade-related neurons reaches a threshold and the saccade is executed(62, 63). On correctly cancelled trials, activity in saccade-related neurons begins to decay following the stop signal but before SSRT, while activity in fixation neurons begins to grow(22, 23). Thus, activity in gaze-shifting and gaze-holding neurons in FEF and SC appear to play a crucial role in the control of saccades. See Supplement 1 for discussion of countermanding performance in schizophrenia in the context of computational models of interacting neurons in FEF and SC.
Although not explored in nonhuman primates performing the saccadic countermanding task, other brain regions are thought to play a role in inhibition of eye movements. Data from single unit recordings in nonhuman primates (64, 65) and human functional imaging studies (fMRI; 66) point to the role of subthalamic nucleus (STN) in response inhibition. Additionally, deep brain stimulation of STN in patients with Parkinson’s disease improved inhibitory control and resulted in shorter manual SSRT(67). A role of the right inferior frontal gyrus in countermanding movements has also been described (68). Although there have not been any neuroimaging studies of the countermanding task in schizophrenia, fMRI studies that have examined neural activity during the antisaccade task suggest abnormalities in fronto-striatal-thalamo-cortical circuits(69, 70).
Potential neural mechanisms underlying abnormal RT adjustments following cancelled saccades
Neural correlates of response monitoring and performance adjustments have also been investigated on a single-cell level in the saccadic countermanding task, with a focus on the role of medial frontal structures. Activity in a subpopulation of SEF neurons following correctly inhibited saccades is thought to reflect conflict between incompatible gaze-shifting and gaze-holding signals in FEF. SEF can bias saccadic latency via connections to cortical and subcortical oculomotor regions(32) and appears to be the basis of slowing following cancelled saccades (71).
Based on these findings, a few potential hypotheses emerge regarding the mechanism of enhanced slowing following cancelled saccades in schizophrenia. Because the inhibitory process might take longer to complete in schizophrenia, as indexed by longer SSRT, the saccade could be cancelled at a longer delay following the rise of movement-related activity in FEF and SC, leading to more co-activation and subsequent conflict between gaze-holding and gaze-shifting neurons on correctly cancelled saccades. Alternatively, gaze-holding and gaze-shifting related neurons might be co-activated longer in patients with schizophrenia, resulting in a longer period of conflict. SEF would signal longer conflict between mutually incompatible responses, resulting in prolonged RTs on subsequent trials. Finally, neurons in the SEF of patients with schizophrenia could be more sensitive to conflict between mutually incompatible responses or exert more powerful biasing effects on structures directly implicated in saccade initiation. Although functional SEF abnormalities have been noted during smooth pursuit(72) and volitional saccade tasks(73, 74) in schizophrenia, findings of abnormal SEF activity are not consistent(69, 70, 75, 76).
Limitations
A limitation of the present study is the unclear effect of neuroleptics in saccade inhibition and monitoring. However, previous studies suggest that atypical neuroleptics improve, but do not normalize, antisaccade performance(77). If deficits in countermanding and antisaccade tasks reflect inhibition impairments, longer SSRT in SZ is unlikely to be a result of neuroleptics. Further, administration of haloperidol had no significant effect on post-error slowing in healthy individuals(78, 79). Finally, in our study, chlorpromazine equivalent dose was not related to any countermanding measures (See Supplement 1).
Conclusions and implications
We found that patients with schizophrenia needed more time to inhibit a planned saccade, which was related to negative symptom severity and occupational functioning. Further, patients exhibited more pronounced RT effects after saccade inhibition. These findings are consistent with functional abnormalities in FEF and SEF. Further, these results indicate the potential of this task to measure improvements in cognitive functioning with psychopharmacological treatment and have implications for inclusion in cognitive remediation batteries, which have shown success in improving psychosocial functioning in patients with schizophrenia (80, 81).
Supplementary Material
Acknowledgments
We would like to thank Natasha Matthews and Amanda Cumming for their assistance with clinical interviews and subject recruitment. This work was supported in part by F31-MH085405-01 (KNT), NARSAD (SP), MH073028 (SP), P30-HD015052 (SP, JDS, KNT), F32-EY016679 (LB), FA9550-07-1-0192 (LB, JDS, GDL), P30-EY08126 (LB, JDC), MH055806 (JDS), the E. Bronson Ingram Chair in Neuroscience (JDS) and MH073878 (GDL).
Footnotes
Financial disclosure
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 2.Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- 3.Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- 4.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanes DP, Schall JD. Countermanding saccades in macaque. Vis Neurosci. 1995;12:929–937. doi: 10.1017/s0952523800009482. [DOI] [PubMed] [Google Scholar]
- 7.Rabbitt P. Introduction: methodologies and models in the study of executive function. In: Rabbitt P, editor. Methodology of Frontal and Executive Function. East Sussex: Psychology Press; 1997. pp. 1–38. [Google Scholar]
- 8.Donohoe G, Reilly R, Clarke S, Meredith S, Green B, Morris D, et al. Do antisaccade deficits in schizophrenia provide evidence of a specific inhibitory function? J Int Neuropsychol Soc. 2006;12:901–906. doi: 10.1017/S135561770606108X. [DOI] [PubMed] [Google Scholar]
- 9.Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biol Psychiatry. 2000;48:210–221. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- 10.Friedman NP, Miyake A, Corley RP, Young SE, Defries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychol Sci. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- 11.Friedman NP, Miyake A. The relations among inhibition and interference control functions: a latent-variable analysis. J Exp Psychol Gen. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- 12.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 13.Rabbitt P. Introduction: Methodologies and models in the study of executive function. In: Rabbitt P, editor. Methodology of frontal and executive function. Hove, UK: Psychology Press; 1997. pp. 1–38. [Google Scholar]
- 14.Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 15.Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 2002;111:22–41. [PubMed] [Google Scholar]
- 16.Malenka RC, Angel RW, Hampton B, Berger PA. Impaired central error-correcting behavior in schizophrenia. Arch Gen Psychiatry. 1982;39:101–107. doi: 10.1001/archpsyc.1982.04290010073013. [DOI] [PubMed] [Google Scholar]
- 17.Polli FE, Barton JJ, Vangel M, Goff DC, Iguchi L, Manoach DS. Schizophrenia patients show intact immediate error-related performance adjustments on an antisaccade task. Schizophr Res. 2006;82:191–201. doi: 10.1016/j.schres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Logan GD. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego: Academic Press; 1994. pp. 189–239. [Google Scholar]
- 19.Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev. 2009;33:647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 21.Brown JW, Hanes DP, Schall JD, Stuphorn V. Relation of frontal eye field activity to saccade initiation during a countermanding task. Exp Brain Res. 2008;190:135–151. doi: 10.1007/s00221-008-1455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanes DP, Patterson WF, 2nd, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- 23.Paré M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci. 2003;23:6480–6489. doi: 10.1523/JNEUROSCI.23-16-06480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 25.Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- 26.Emeric EE, Brown JW, Boucher L, Carpenter RH, Hanes DP, Harris R, et al. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Res. 2007;47:35–49. doi: 10.1016/j.visres.2006.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barch DM, Braver TS, Carter CS, Poldrack RA, Robbins TW. CNTRICS final task selection: executive control. Schizophr Bull. 2009;35:115–135. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enticott PG, Ogloff JR, Bradshaw JL. Response inhibition and impulsivity in schizophrenia. Psychiatry Res. 2008;157:251–254. doi: 10.1016/j.psychres.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Huddy VC, Aron AR, Harrison M, Barnes TR, Robbins TW, Joyce EM. Impaired conscious and preserved unconscious inhibitory processing in recent onset schizophrenia. Psychol Med. 2009;39:907–916. doi: 10.1017/S0033291708004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badcock JC, Michie PT, Johnson L, Combrinck J. Acts of control in schizophrenia: dissociating the components of inhibition. Psychol Med. 2002;32:287–297. doi: 10.1017/s0033291701005128. [DOI] [PubMed] [Google Scholar]
- 31.Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- 32.Schall JD, Boucher L. Executive control of gaze by the frontal lobes. Cogn Affect Behav Neurosci. 2007;7:396–412. doi: 10.3758/cabn.7.4.396. [DOI] [PubMed] [Google Scholar]
- 33.Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol Rev. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- 34.Nuechterlein KH. Reaction time and attention in schizophrenia: a critical evaluation of the data and theories. Schizophr Bull. 1977;3:373–428. doi: 10.1093/schbul/3.3.373. [DOI] [PubMed] [Google Scholar]
- 35.Holzman PS, Proctor LR, Hughes DW. Eye-tracking patterns in schizophrenia. Science. 1973;181:179–181. doi: 10.1126/science.181.4095.179. [DOI] [PubMed] [Google Scholar]
- 36.Gale HJ, Holzman PS. A new look at reaction time in schizophrenia. Schizophr Res. 2000;46:149–165. doi: 10.1016/s0920-9964(00)00006-2. [DOI] [PubMed] [Google Scholar]
- 37.Reuter B, Kathmann N. Using saccade tasks as a tool to analyze executive dysfunctions in schizophrenia. Acta Psychol (Amst) 2004;115:255–269. doi: 10.1016/j.actpsy.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 38.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders. New York: Biometrics Research Department; 1995. [Google Scholar]
- 39.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Report. 1962;10:799–812. [Google Scholar]
- 40.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- 41.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- 42.Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- 43.Blair JR, Spreen O. Predicting Premorbid IQ: A revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- 44.O’Carroll RO, Walker M, Dunan J, Murray C, Blackwood D, Ebmeier K, et al. Selecting controls for schizophrenia research studies: The use of the national adult reading test. Schizophrenia Research. 1992;8:137–141. doi: 10.1016/0920-9964(92)90030-9. [DOI] [PubMed] [Google Scholar]
- 45.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 46.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropyschologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 47.Osman A, Kornblum S, Meyer DE. Does motor programming necessitate response execution? J Exp Psychol Hum Percept Perform. 1990;16:183–198. doi: 10.1037//0096-1523.16.1.183. [DOI] [PubMed] [Google Scholar]
- 48.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 49.Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 50.Goldman-Rakic PS. Circuitry of the pre- frontal cortex and the regulation of behavior by representational memory. In: Plum F, Mountcastle V, editors. Handbook of physiology: Sec 1 The nervous system. Bethesda, MD: American Physiological Society; 1987. pp. 373–347. [Google Scholar]
- 51.Cabel DW, Armstrong IT, Reingold E, Munoz DP. Control of saccade initiation in a countermanding task using visual and auditory stop signals. Exp Brain Res. 2000;133:431–441. doi: 10.1007/s002210000440. [DOI] [PubMed] [Google Scholar]
- 52.Rieger M, Gauggel S. Inhibitory after-effects in the stop signal paradigm. Br J Psychology. 1999;90:509–518. [Google Scholar]
- 53.Kornylo K, Dill N, Saenz M, Krauzlis RJ. Cancelling of pursuit and saccadic eye movements in humans and monkeys. J Neurophysiol. 2003;89:2984–2999. doi: 10.1152/jn.00859.2002. [DOI] [PubMed] [Google Scholar]
- 54.Verbruggen F, Logan GD, Liefooghe B, Vandierendonck A. Short-term aftereffects of response inhibition: repetition priming or between-trial control adjustments? J Exp Psychol Hum Percept Perform. 2008;34:413–426. doi: 10.1037/0096-1523.34.2.413. [DOI] [PubMed] [Google Scholar]
- 55.Rabbitt PM. Errors and error-correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- 56.Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J Cogn Neurosci. 2008;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nelson MJ, Boucher L, Logan GD, Palmeri TJ, Schall JD. Nonstationary response time in stopping and stepping saccade tasks. Attention, Perception & Psychophysics. doi: 10.3758/APP.72.7.1913. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathalon DH, Bennett A, Askari N, Gray EM, Rosenbloom MJ, Ford JM. Response-monitoring dysfunction in aging and Alzheimer’s disease: an event-related potential study. Neurobiol Aging. 2003;24:675–685. doi: 10.1016/s0197-4580(02)00154-9. [DOI] [PubMed] [Google Scholar]
- 59.Barton JJ, Cherkasova MV, Lindgren KA, Goff DC, Manoach DS. What is perseverated in schizophrenia? Evidence of abnormal response plasticity in the saccadic system. J Abnorm Psychol. 2005;114:75–84. doi: 10.1037/0021-843X.114.1.75. [DOI] [PubMed] [Google Scholar]
- 60.Franke C, Reuter B, Breddin A, Kathmann N. Response switching in schizophrenia patients and healthy subjects: effects of the inter-response interval. Exp Brain Res. 2009 doi: 10.1007/s00221-009-1871-9. [DOI] [PubMed] [Google Scholar]
- 61.Franke C, Reuter B, Schulz L, Kathmann N. Schizophrenia patients show impaired response switching in saccade tasks. Biol Psychol. 2007;76:91–99. doi: 10.1016/j.biopsycho.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- 63.Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol. 1995;73:2313–2333. doi: 10.1152/jn.1995.73.6.2313. [DOI] [PubMed] [Google Scholar]
- 64.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 65.Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J Cogn Neurosci. 2006;18:626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- 68.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Raemaekers M, Jansma JM, Cahn W, Van der Geest JN, van der Linden JA, Kahn RS, et al. Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Arch Gen Psychiatry. 2002;59:313–320. doi: 10.1001/archpsyc.59.4.313. [DOI] [PubMed] [Google Scholar]
- 70.Tu PC, Yang TH, Kuo WJ, Hsieh JC, Su TP. Neural correlates of antisaccade deficits in schizophrenia, an fMRI study. J Psychiatr Res. 2006;40:606–612. doi: 10.1016/j.jpsychires.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 71.Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- 72.Hong LE, Tagamets M, Avila M, Wonodi I, Holcomb H, Thaker GK. Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:726–732. doi: 10.1016/j.biopsych.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 73.Camchong J, Dyckman KA, Austin BP, Clementz BA, McDowell JE. Common neural circuitry supporting volitional saccades and its disruption in schizophrenia patients and relatives. Biol Psychiatry. 2008;64:1042–1050. doi: 10.1016/j.biopsych.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Camchong J, Dyckman KA, Chapman CE, Yanasak NE, McDowell JE. Basal ganglia-thalamocortical circuitry disruptions in schizophrenia during delayed response tasks. Biol Psychiatry. 2006;60:235–241. doi: 10.1016/j.biopsych.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 75.Keedy SK, Ebens CL, Keshavan MS, Sweeney JA. Functional magnetic resonance imaging studies of eye movements in first episode schizophrenia: smooth pursuit, visually guided saccades and the oculomotor delayed response task. Psychiatry Res. 2006;146:199–211. doi: 10.1016/j.pscychresns.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 76.McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, et al. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biol Psychiatry. 2002;51:216–223. doi: 10.1016/s0006-3223(01)01204-5. [DOI] [PubMed] [Google Scholar]
- 77.Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Longitudinal studies of antisaccades in antipsychotic-naive first-episode schizophrenia. Psychol Med. 2006;36:485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- 78.de Bruijn ER, Sabbe BG, Hulstijn W, Ruigt GS, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Res. 2006;1105:122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 79.Zirnheld PJ, Carroll CA, Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. J Cogn Neurosci. 2004;16:1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]
- 80.McGurk SR, Mueser KT, Feldman K, Wolfe R, Pascaris A. Cognitive training for supported employment: 2–3 year outcomes of a randomized controlled trial. Am J Psychiatry. 2007;164:437–441. doi: 10.1176/ajp.2007.164.3.437. [DOI] [PubMed] [Google Scholar]
- 81.McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.