Abstract
Hyperalgesia in animal injury models is linked to activation of descending raphespinal modulatory circuits originating in the rostral ventromedial medulla (RVM). A neurokinin-1 (NK-1) receptor antagonist microinjected into the RVM before or after inflammation produced by complete Freund’s adjuvant (CFA) resulted in an attenuation of thermal hyperalgesia. A transient (acute) or a continuous infusion of Substance P (SP) microinjected into the RVM of non-inflamed animals led to similar pain hypersensitivity. Intrathecal pretreatment or post-treatment of a 5-HT3 receptor antagonist (Y-25130 or ondansetron) blocked the SP-induced hyperalgesia. The SP-induced hyperalgesia was both GABAA and NMDA receptor-dependent after pre- and post-treatment with selective antagonists at the spinal level. A microinjection of SP into the RVM also led to increased NMDA NR1 receptor subunit phosphorylation in spinal cord tissue. The GABAA receptor-mediated hyperalgesia involved a shift in the anionic gradient in dorsal horn nociceptive neurons and an increase in phosphorylated NKCC1 protein (isoform of the Na-K-Cl cotransporter). Following a low dose of SP infused into the RVM, intrathecal muscimol (GABAA agonist) increased SP-induced thermal hyperalgesia, phosphorylated NKCC1 protein expression, and NMDA NR1 subunit phosphorylation in the spinal cord. The thermal hyperalgesia was blocked by intrathecal gabazine, the GABAA receptor antagonist, and MK-801, the NMDA receptor channel blocker. These findings indicate that NK-1 receptors in the RVM are involved in SP-induced thermal hyperalgesia, this hyperalgesia is 5-HT3-receptor dependent at the spinal level, and involves the functional interaction of spinal GABAA and NMDA receptors.
Keywords: substance P, inflammation, brainstem, serotonin, NMDA receptor, GABAA receptor
Brainstem descending pathways constitute a major mechanism in pain modulation. Hyperalgesia in animal injury models is linked to activation of descending raphespinal modulatory circuits originating in the rostral ventromedial medulla (RVM) (Ren and Dubner, 2002; Porreca et al., 2002; Vanegas and Schaible, 2004). These circuits include facilitatory and inhibitory mechanisms. Little is known about the chemical mediators that contribute to initiation and maintenance of descending facilitation and their role in inflammation-induced hyperalgesia.
We have now confirmed our earlier preliminary report (LaGraize et al., 2005) that substance P (SP) and its neurokinin-1 (NK-1) tachykinin receptor in the RVM participate in mechanisms of descending facilitation and behavioral hyperalgesia. Our results are consistent with recent studies showing that SP microinjected into the RVM induces hyperalgesia in non-inflamed animals and NK-1R antagonists in the RVM attenuate inflammation-induced hyperalgesia (Budai et al., 2007; Pacharinsak et al., 2008; Hamity et al., 2010). The hyperalgesic effects of SP and its NK-1 receptor mimic those produced by peripheral inflammation. SP inputs to the nucleus raphe magnus (NRM) in the RVM originate in other brainstem regions (Beitz, 1982). There is moderate localization of the NK-1R in the RVM (Maeno et al., 1993; Nakaya et al., 1994; Saffroy et al., 2003) and spinomedullary NK-1R expressing neurons that project to the dorsolateral funiculus modulate descending circuitry (Khasabov et al., 2005).
The major objective of our study was to determine how descending circuitry was functionally linked to spinal mechanisms leading to behavioral hyperalgesia. The influence of direct primary nociceptive afferent input, in addition to RVM descending input, on inflammation-induced spinal mechanisms of hyperalgesia, led us to mainly focus on the descending effects of RVM NK-1R activation of spinal circuitry in non-inflamed animals. Several potential mechanisms can account for the effects of RVM NK-1R activation on spinal circuitry. There is dense serotonergic innervation in the spinal dorsal horn (Ruda et al., 1982; Ruda, 1988), which is primarily derived from the RVM (LaMotte, 1988; Fields et al., 1991; Wei et al., 1999; Mason, 2001; Millan, 2002). NMDA receptor activation in the RVM also contributes to descending facilitation (Miki et al., 2002; Urban and Gebhart, 1998). The cellular mechanisms mainly responsible for descending inhibition also include excitatory amino acid receptors (Guan et al., 2002, 2003, 2004; Miki et al., 2002). Glutamatergic stimulation of RVM neurons results in partial mediation of descending inhibition by spinal GABAA receptors (McGowan and Hammond, 1993). However, depolarizing shifts in the anionic reversal potential of spinal GABA-responsive neurons occurring after nerve injury, contribute to spinal hyperexcitability (Coull et al., 2003) and may play a role in descending facilitation. Additionally, inflammation-induced hyperalgesia involves spinal hyperexcitability that is NMDA-receptor (NMDAR) dependent (Guo et al., 2002, 2004). Thus, activation of RVM-spinal neurons may include both facilitatory and inhibitory mechanisms involving the release of 5-HT, GABA and glutamate, and activation of their receptors at the spinal level. Our findings show that the net effect of SP and its receptor in producing descending facilitation and hyperalgesia involves all of these mechanisms at the spinal level.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague–Dawley rats (80–300 g; Harlan, Indianapolis, IN, USA) were used for the behavioral, pharmacological and western blot experiments. Male Sprague–Dawley (80–100 g) rats were used for the electrophysiology experiments. All animals were housed and maintained on a 12:12 light:dark cycle with free access to food and water throughout the study. The animals were maintained and cared for in accordance to the guidelines outlined by the International Association for the Study of Pain (Zimmerman, 1983). The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Maryland Dental School. All behavioral testing was conducted during the light cycle. Additionally, behavioral testing was performed “blind” with respect to the injected substances.
Chronic cannula implants
Cannulae were implanted into the RVM five to nine days prior to behavioral testing. All animals were deeply anesthetized by an i.p. injection of Nembutal (50 mg/kg; Abbott Laboratories, Chicago, IL, USA). The animals were then positioned in a stereotaxic frame with blunt-tipped ear bars. Following a midline incision, a burr hole was drilled and a guide cannula was lowered into the RVM (AP: 11.0; L: 0.0; D: 9.5 mm). The guide cannula was then secured with cranioplastic cement and the incision was sutured. To prevent clogging of the guide cannula, a dummy cannula was inserted until the microinjection was administered.
Microinjections in cannulated animals
Animals (250–300 g) were lightly restrained, the dummy cannula was removed and an internal cannula extending 0.5 mm below the guide cannula was inserted. SP 0.005 µg, 0.05 µg, or 0.5 µg, L-733,060 (NK-1 receptor antagonist), 1 µg, 3 µg, or 5 µg, L-732,138 (NK-1 receptor antagonist), 0.236 pg, 2.36 pg, or 23.6 pg, L-733,061(inactive enantiomer of L-733,060), 5 µg, or vehicle was microinjected using a 1-µl syringe which was attached to the internal cannula by PE20 tubing. A volume of 500 nl was injected over a 90 s period and the internal cannula remained untouched for an additional 60 s to allow for absorption into the brain region and to minimize the spread of the solution along the track of the cannula.
Implantation of osmotic pumps
Cannulae attached to Alzet osmotic pumps (Model 1003D; Durect Corporation, Cupertino, CA, USA) were implanted into the RVM of male rats (80–100 g for electrophysiology experiments and 200–225 g for behavioral/western blot experiments). Animals were deeply anesthetized using isoflurane anesthesia. The animals were then positioned in the stereotaxic frame and the cannula attached to an osmotic pump filled with either saline or SP 0.2 ng/h or 10 ng/h was lowered into the RVM (AP: 10.0; L: 0.0; D: 8.8 mm). The cannula was then secured as indicated above.
Intrathecal injection
The intrathecal (i.t.) injection was performed by lumbar puncture under isoflurane anesthesia (2–3% induction; 1% maintenance) using methodology adapted from Hylden and Wilcox (1980). Briefly, animals were secured in the stereotaxic frame and a 30 gauge needle was inserted into the intervertebral space between the spinous and transverse processes between L4 and L5. Once the needle was in position, a 10 µl injection of 50 µg Y-25130 (5-HT3 receptor antagonist), 10 µg ondansetron (5-HT3 receptor antagonist), 31.5 µg MK-801 (NMDA receptor antagonist), 55 ng or 74 ng gabazine (GABAA receptor antagonist), 0.1 µg muscimol (GABAA receptor agonist), or saline was administered. Some animals received two intrathecal injections (10 µl each). After recovery, animals were examined and if motor impairment was observed, animals were excluded from the experiment. Following intrathecal injection, animals were tested for response to a thermal stimulus at various time points between 30 min and 48 h.
Hindpaw inflammation
For behavioral experiments, unilateral hindpaw inflammation was induced by injecting 0.05 ml complete Freund’s adjuvant (CFA; 0.025 mg Mycobacterium tuberculosis; 1:1 oil/saline) s.c. into the plantar surface of the left hindpaw (ipsilateral paw). For Western blot experiments, bilateral hindpaw inflammation was induced in a similar fashion. CFA-injected animals show no disruption of grooming behavior, locomotor activity, weight gain, and exploratory behavior.
Measurement of thermal paw withdrawal latency
Behavioral testing was performed using identical methods for both inflamed and non-inflamed groups. The paw withdrawal latency, a measure of behavioral hyperalgesia, was determined following previously described methods (Hargreaves et al., 1988). Rats were placed on an elevated glass surface under an inverted transparent plastic cage and allowed to acclimate for 10–20 min. A radiant thermal stimulus was applied to the plantar surface of each hindpaw from underneath the glass floor. The paw withdrawal latency was determined to the nearest 0.1 s using a stopwatch. The voltage of the bulb was adjusted to result in an average paw withdrawal latency of 10–12 s in non-inflamed animals. A 20 s cutoff was used to prevent tissue damage. Three trials (with an inter-trial interval of at least 5 min) were determined for each hindpaw and the average of the trials was used as the mean thermal paw withdrawal latency.
Pretreatment schedule of thermal testing in inflamed animals
Following baseline thermal testing, animals received a 500 nl microinjection of an NK-1 receptor antagonist (L-733,060 (3 or 5 µg), L-732,138 (2.36 or 23.6 pg), L-733,061 (5 µg; inactive enantiomer of L-733,060), or vehicle into the RVM. Ten min following the NK-1 receptor antagonist microinjection, inflammation was induced in the hindpaw. Latencies in response to the thermal stimulus applied to each hindpaw were obtained 30 min, 2 h, 5 h, and 24 h following the induction of inflammation.
Post-treatment schedule of thermal testing in inflamed animals
Following baseline thermal testing, inflammation was induced and 24 h later, thermal latencies for each hindpaw were determined. Animals then received a 500 nl microinjection of L-733,060 (1 or 5 µg), L-732,138 (0.236 or 2.36 pg), L-733,061 (5 µg), or vehicle into the RVM. Latencies in response to the thermal stimulus applied to each hindpaw were obtained 30 min, 2 h, 5 h, and 24 h following microinjection. Additional inflamed animals received an intrathecal injection of Y-25130 and were tested 30 min, 2 h, 4 h, 6 h, 24 h and 48 h after intrathecal injection.
Thermal testing in non-inflamed animals following a transient acute injection of SP in the RVM
Following baseline thermal testing, animals received a 500 nl microinjection of SP (0.005, 0.05, or 0.5 µg) or vehicle into the RVM. Latencies in response to a thermal stimulus applied to each hindpaw were obtained 30 min, 2 h, 5 h, and 24 h following microinjection.
Thermal testing in non-inflamed animals following chronic osmotic pump administration of SP in the RVM
Following baseline thermal testing, an osmotic pump that administered 0.2 ng/h or 10 ng/h SP or vehicle was implanted into the RVM. Latencies in response to a thermal stimulus applied to each hindpaw were obtained 24 h following pump implant.
Western blot
The tissues were homogenized in solubilization buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 1 mM Na3VO4, 1 U/mL aprotinin, 20 µg/mL leupetin, 20 µg/mL pepstatin A). The homogenate was centrifuged at 20,200×g for 10 min at 4 °C. The supernatant was removed. The protein concentration was determined using a detergent-compatible protein assay with a bovine serum albumin standard. Each sample contains proteins from one animal. The proteins (50 µg) were separated on 7.5% SDS-PAGE and blotted on a nitrocellulose membrane. The blots were blocked with 5% milk in Tris-buffered saline (TBS) buffer and then incubated with the NK-1R (anti-rabbit; 1:1000, Santa Cruz), NR1-ser896 (anti-rabbit; 1:1000, Millipore), NKCC1 (anti-rabbit; 1:1000, Millipore), or anti-phospho-NKCC1 antibody R5 (anti-rabbit; 1:5000, Flemmer et al., 2002) antibody. The membrane was washed with TBS and incubated with anti-rabbit IgG (1:3000; Cell Signaling). The immunoreactivity was detected using enhanced chemiluminescence (ECL; GE Healthcare). The loading and blotting of the amount of protein was verified by reprobing the membrane with anti-β-actin antiserum (Sigma) and with Coomassie Blue staining.
Spinal cord slice preparation and electrophysiology
The methods for slice preparation of 30–40-day old rat spinal cords were the same as described previously (Yang et al., 2001). Briefly, 24 h after either saline- or SP-filled osmotic pumps were implanted, the animal was anesthetized with Nembutal sodium solution (50 mg/kg, i.p.). The lumbosacral spinal cords with attached dorsal and ventral roots were isolated and placed in a mixed gas (95% O2–5% CO2) equilibrated high sucrose solution (in mM: 50 sucrose, 95 NaCl, 3.6 KCl, 0.5 CaCl2, 1.2 NaH2PO4, 6 MgCl2, 26 NaHCO3, and 12 glucose) at 1–3 °C. After cutting all the roots near the root entry zone, the pia-arachnoid was removed. The spinal cord was then mounted on a microslicer to excise 250–350 µm thick transverse slices, which were then pre-incubated in mixed gas bubbled recording solution (in mM: 126 NaCl, 26 NaHCO3, 10 glucose, 2.5 KCl, 2 CaCl2, 2 MgCl2, and 1.25 NaH2PO4) in a chamber for at least 1 h at 36 °C.
For electrophysiological recordings, the slice was transferred to a recording chamber of 0.8 ml volume and perfused continuously (11–12 ml/min) with recording solution at room temperature. During recording GABA-induced GABAA currents, 0.5 µM tetrodotoxin (TTX) was present in the bath to block action potential generation and thus, network activity; 10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 30 µM d-2-amino-5-phosphonovaleric acid (AP-5) were added to the bath to block glutamatergic transmission. All solutions were bubbled with 95% O2–5% CO2, pH 7.4. Gramacidin perforated-patch recordings with pipette resistance of 4–8 MΩ were performed on neurons from the superficial spinal cord. In brief, the very tip of the pipette was filled with a gramicidin-free intracellular solution containing the following (in mM): 130 caesium gluconate, 20 KCl, 2 MgCl2, 0.5 EGTA, 2 Na2-ATP, 0.5 Na2-GTP, and 10 HEPES. The remainder of the pipette was filled with the same solution containing gramicidin (freshly dissolved in DMSO to prepare a stock solution of 4 mg/mL and then diluted to a final concentration of 40 µg/mL). Gramicidin-perforated whole-cell recordings were obtained from dorsal horn neurons visually identified under Nomarski optics using a water immersion objective. Gigaseal formation was performed, then the configuration was left intact and the current response to a test pulse was monitored. The cell capacitance manifested itself after 20–40 min, with decreasing access resistance as indicated by a progressively faster capacitative transient. After the capacitative transient and series resistance (8–40 MΩ) were stable, the drug perfusion began. Exogenous GABA (1 mM, 30 s) was applied by changing the perfusion solution to evoke a GABA response. All currents were filtered at 2 kHz and digitized at 5 kHz using software of Pulse V8.76 running on an EPC 10 amplifier. The resting membrane potential (VRest) for every neuron was measured. On each neuron, GABA currents were recorded at the holding potentials of −90 mV, VRest, −50 mV, −30 mV, and −10 mV, with a washout of 5 min, after each recording. The reversal potential of GABA-evoked current (EGABA) for each neuron was calculated by SigmaPlot 9.0 based on peak amplitude values at different holding potentials. The cells included in this experiment were restricted to those exhibiting an initial VRest that was more hyperpolarized than −55 mV.
Histology
Following behavioral testing, animals were anesthetized with pentobarbital and perfused with saline and 4% paraformaldehyde. 60-µm coronal sections of RVM tissue stained with Cresyl Violet were examined under magnification to determine cannula placement according to the atlas of Paxinos and Watson (2008).
Statistical analyses
Paw withdrawal latencies for inflamed and non-inflamed animals in the various microinjection/osmotic pump groups prior to or following the induction of inflammation/intrathecal injection was performed using a mixed model ANOVA. Following overall parametric analyses, differences were further analyzed using Fisher’s LSD post hoc comparisons. Electrophysiological recordings were analyzed using an unpaired t-test. Alpha level was set at 0.05 for all statistical tests.
Drugs
SP (m.w., 1347.63), L-733,061 (m.w., 439.82), MK-801 (m.w., 337.37), gabazine (m.w., 368.23), muscimol (m.w., 114.1) and ondansetron (m.w., 365.85) were obtained from Sigma (St. Louis, MO, USA) and L-733,060 (m.w., 439.83), L-732,138 (m.w., 472.39) and Y-25130 (m.w., 386.28) were obtained from Tocris (Ellisville, MO, USA). SP was dissolved in phosphate buffered saline (PBS). Y-25130, MK-801, gabazine, muscimol, and ondansetron were dissolved in physiological saline. L-733,060 and L-733,061 were dissolved in dimethyl sulfoxide.
RESULTS
Role of NK-1 receptors in the RVM in the initiation and maintenance of inflammation-induced behavioral hyperalgesia
We first tested the hypothesis that NK-1 receptors are involved in descending facilitation of behavioral hyperalgesia after inflammation. Using paw withdrawal latency as a measure of behavioral hyperalgesia, we examined the response to thermal stimuli in CFA-treated animals that received a microinjection of one of two NK-1 receptor antagonists, L-733,060 or L-732,138, or vehicle microinjection, into the RVM 10 min prior to the induction of inflammation (Fig. 1A, B). The microinjection of L-733,060 or L-732,138 into the RVM led to significant increases in ipsilateral paw withdrawal latencies and the effects of L-732,138 were dose-dependent. The thermal stimulus was applied at different time points between 0.5 and 24 h after the induction of inflammation and the findings were compared to the groups that received microinjection of the inactive isomer, L-733,061, or vehicle. There were no effects of the microinjection of either NK-1 receptor antagonist on the contralateral paw withdrawal latency (data not shown). These findings indicate that RVM pretreatment with NK-1 receptor antagonists attenuates inflammation-induced behavioral hyperalgesia and suggests a role of SP and its NK-1 receptor in the initiation of descending facilitation.
Fig. 1.
Thermal paw withdrawal latencies for CFA-treated animals that received an NK-1 antagonist or vehicle microinjected into the RVM before or after the induction of inflammation. (A) Mean thermal paw withdrawal latencies (+SEM) of the ipsilateral paw for CFA-treated animals that received L-733,060 (3 or 5 µg), L-733,061 (5 µg), or vehicle microinjected into the RVM before the induction of inflammation. * P<0.05, ** P<0.01 versus 5 µg L-733,061 at that time point. vehicle, n=6; 5 µg L-733,061, n=6; 3 µg L-733,060, n=6; 5 µg L-733,060, n=5. B represents baseline. (B) Mean thermal paw withdrawal latencies (+SEM) of the ipsilateral paw for CFA-treated animals that received L-732,138 (2.36 or 23.6 pg) or vehicle microinjected into the RVM before the induction of inflammation. * P<0.05, *** P<0.001 versus vehicle at that time point. # P<0.05 versus 2.36 pg L-732,138 at that time point. vehicle, n=5; 2.36 pg L-732,138, n=6; 23.6 pg L-732,138, n=5. B represents baseline. (C) Mean thermal paw withdrawal latencies (+SEM) of the ipsilateral paw for CFA-treated animals that received L-733,060 (1 or 5 µg), L-733,061 (5 µg), or vehicle microinjected into the RVM after the induction of inflammation. * P<0.05, ** P<0.01, *** P<0.001 versus 5 µg L-733,061 at that time point. vehicle, n=6; 5 µg L-733,061, n=6; 1 µg L-733,060, n=6; 5 µg L-733,060, n=5. B1 represents baseline prior to CFA and B2 represents baseline after CFA but before microinjection. (D) Mean thermal paw withdrawal latencies (+SEM) of the ipsilateral paw for CFA-treated animals that received L-732,138 (0.236 or 2.36 pg) or vehicle microinjected into the RVM after the induction of inflammation. * P<0.05, ** P≤0.01 versus vehicle at that time point. # #P=0.01 versus 0.236 pg L-732,138 at that time point. vehicle, n=5; 2.36 pg L-732,138, n=6; 23.6 pg L-732,138, n=3. B1 represents baseline prior to CFA and B2 represents baseline after CFA but before microinjection.
Post-treatment of the NK-1 receptor antagonists 24 h after the induction of inflammation also led to significant dose-dependent increases in ipsilateral paw withdrawal latencies in response to a thermal stimulus applied at different time points between 0.5 and 24 h after the RVM microinjection as compared to the groups that received microinjection of the inactive isomer, L-733,061, or vehicle (Fig. 1C, D). There were no effects of the microinjection of either NK-1 receptor antagonist on the contralateral paw withdrawal latency (data not shown). These findings indicate that RVM post-treatment with NK-1 receptor antagonists also attenuates inflammation-induced behavioral hyperalgesia and suggests a role of SP and its receptor in the maintenance of the descending facilitation.
The cannula tip placements for inflamed animals that received a microinjection of an NK-1 receptor antagonist in the RVM are shown in Fig. 2A. All of the cannula placements were between −9.80 and −11.60 mm relative to bregma according to the atlas of Paxinos and Watson (2008). The effective placements were in two subregions of the RVM [raphe magnus nucleus (RMg) and gigantocellular reticular nucleus, alpha part (GiA)]. None were in the more lateral subregion of the RVM [lateral paragigantocellular nucleus (LPGi)]. Fig. 2C shows the absence of any effect of misplaced cannulae.
Fig. 2.
Histological representation of cannula tip placements within the RVM (closed circles) and outside of the RVM (open circles) for inflamed animals that received a microinjection of an NK-1 receptor antagonist or a saline vehicle in the RVM (A) and non-inflamed animals that received an acute microinjection of SP or saline or which received an osmotic pump implanted into the RVM that chronically infused SP or vehicle (B). All of the cannula placements were between −9.68 and −11.60 mm relative to bregma according to the atlas of Paxinos and Watson (2008). The effective placements were in two subregions of the RVM [raphe magnus nucleus (RMg) and gigantocellular reticular nucleus, alpha part (GiA)]in non-inflamed and CFA-injected rats and none were in the more lateral subregion of the RVM [lateral paragigantocellular nucleus (LPGi)]. Since there were so few ineffective placements in the non-inflamed rats receiving SP microinjections, we performed experiments in which the placements were made outside the RVM to examine the anatomical specificity of the SP microinjections. The location of these placements are shown in (B) (open circles). Mean thermal paw withdrawal latencies (+SEM) of the ipsilateral paw for CFA-inflamed animals that received a microinjection of an NK-1 receptor antagonist in an effective (Hit) or ineffective (Miss) cannula placement (C) or for non-inflamed animals that received chronic infusion of SP in an effective or ineffective cannula placement (D) are shown. In (C), B1 represents baseline prior to CFA and B2 represents baseline after CFA but before microinjection. In (D), B1 represents baseline prior to osmotic pump implant and B2 represents baseline after osmotic pump implant but before an intrathecal saline injection. * P<0.05, *** P<0.001 versus the miss group at that time point.
Upregulation of NK-1 receptors in the RVM following peripheral inflammation
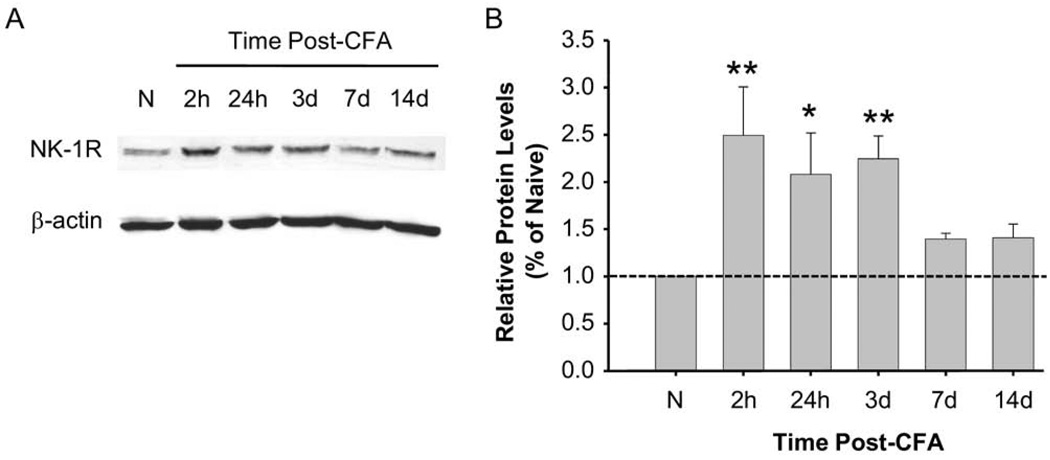
The attenuation of behavioral hyperalgesia following RVM microinjection of NK-1 receptor antagonists strongly suggests SP–NK-1 receptor signaling in the RVM after inflammation. This led us to examine the effect of hindpaw inflammation on NK-1 receptor protein expression in the RVM. Western blots demonstrated a significant time-dependent upregulation of the NK-1 receptor in the RVM from 2 h to 3 d following inflammation (Fig. 3A, B), indicating that NK-1 receptor protein expression is enhanced in the RVM following peripheral inflammation.
Fig. 3.
NK-1 receptor expression in the RVM. (A) The Western blot represents NK-1 receptor protein levels at different time points (2 h to 14 d) following CFA injection. β-actin was used as a loading control to normalize the level of NK-1 receptor protein in each lane. (B) The histogram summarizes the relative NK-1 receptor protein levels in separate Western blot experiments in rat RVM tissue following inflammation as compared to RVM tissue in naive animals (n=3 per time point). * P<0.05, ** P≤0.01. Protein levels relative to the vehicle control (naive and dashed line) were calculated.
Exogenous SP induces descending facilitation
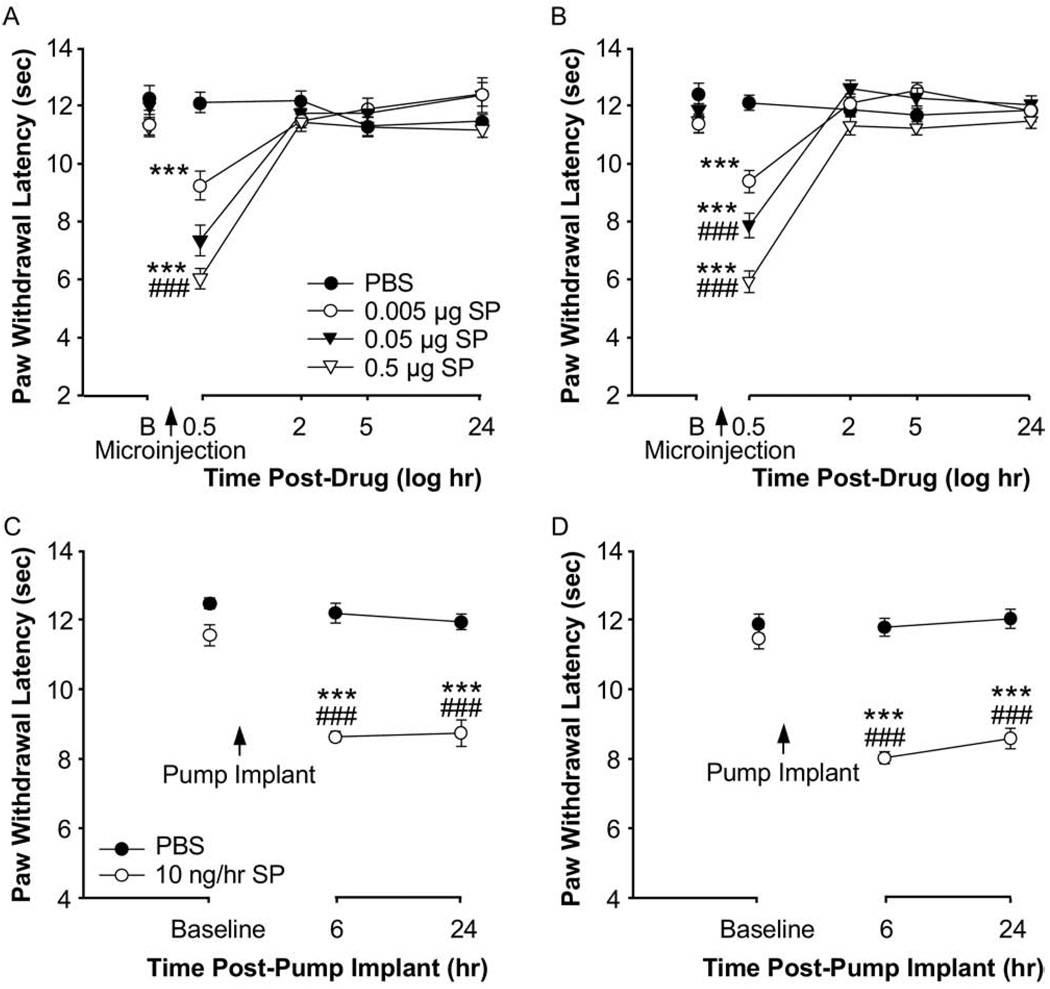
The transient increase in thermal pain hypersensitivity 30 min following SP microinjection into the RVM has been recently reported (Hamity et al., 2010). Using non-inflamed animals, we tested the hypothesis that exogenous SP microinjected into the RVM induces descending facilitation of behavioral hyperalgesia similar to the effects produced by inflammation. The microinjection of SP into the RVM led to transient but significant dose-dependent decreases in paw withdrawal latencies to thermal stimuli for both the right paw (P<0.001) and the left paw (P<0.001) 0.5 h following the microinjection as compared to the group that received vehicle (Fig. 4A, B).
Fig. 4.
Thermal paw withdrawal latencies for groups of adult non-inflamed animals that received SP (0.005, 0.05, or 0.5 µg) or PBS microinjected into the RVM and 30–40-day old non-inflamed animals that received a constant infusion by osmotic pump of SP (10 ng/h) or PBS into the RVM. (A) and (B) Mean thermal paw withdrawal latencies (+SEM) of the left paw (A) and right paw (B) for adult non-inflamed animals that received SP or PBS microinjected into the RVM. *** P<0.001 versus PBS at that time point. ### P<0.001 versus 0.005 µg SP at that time point. PBS, n=6; 0.005 µg SP, n=6; 0.05 µg SP, n=6; 0.5 µg SP, n=6. B represents baseline. (C) and (D) Mean thermal paw withdrawal latencies (+SEM) of the left paw (C) and the right paw (D) for 30–40-day old non-inflamed animals that received an osmotic pump infusion of either SP or PBS into the RVM. * P<0.05, *** P<0.001 versus PBS at that time point. ###P<0.001 versus baseline for that group. PBS, n=5; 10 ng/h, n=5.
We then further determined if the SP-induced hyperalgesia mimicked that produce by inflammation and would persist in the presence of a continuous infusion of 10 ng/h SP into the RVM via an osmotic pump. Six and 24 h following an osmotic pump implant, animals that received a SP infusion into the RVM had significantly lower paw withdrawal latencies for the right paw (P<0.001) and left paw (P<0.001) as compared to animals that received a vehicle infusion or as compared to baseline paw withdrawal latencies (Fig. 4C, D). The magnitude of the change in paw withdrawal latencies produced by the continuous infusion of SP was similar to the change produced by the dose of CFA that was used. Overall, the results indicate that this method of SP-induced hyperalgesia mimics in persistence and magnitude the hyperalgesia produced by CFA-induced inflammation and permits the study of post-treatment manipulations after the induction of the behavioral hyperalgesia.
The cannula tip placements for non-inflamed animals that received an acute microinjection of SP or saline, or which received an osmotic pump implanted into the RVM that chronically infused SP or vehicle, are shown in Fig. 2B. All of the cannula placements were between −9.68 and −11.60 mm. The effective placements were in RMg and GiA. None were in the more lateral subregion of the RVM (LPGi). Since there were so few ineffective placements, we performed experiments in which the placements were made outside the RVM to examine the anatomical specificity of the SP microinjections. The location of these placements are shown Fig. 2B (open circles) and their lack of effects are shown in Fig. 2D.
In subsequent experiments described below, we used the transient (acute) and continuous SP microinjection models to determine the spinal cord mechanisms participating in the initiation and maintenance of behavioral hyperalgesia produced by SP RVM infusion. Similar to the findings shown in Fig. 4, in the experiments described in Figs. 5–8, we found that the effects of RVM SP microinjection on right and left hindpaw withdrawal latencies to thermal stimulation were almost identical and, therefore, these data have been combined.
Fig. 5.
Thermal paw withdrawal latencies for animals that received a 5-HT3 receptor antagonist or vehicle injected intrathecally after CFA- or before or after SP-induced hyperalgesia. (A) Mean thermal paw withdrawal latencies (+SEM) of the ipsilateral paw for saline- or CFA-treated animals that received an intrathecal injection of 50 µg Y-25130 or vehicle injected after the induction of inflammation. * P<0.05, *** P<0.001 versus CFA+vehicle at that time point. ### P<0.001 versus saline+Y-25130 1 h following CFA injection and prior to intrathecal injection. CFA+vehicle, n=4; CFA+Y-25130, n=4; saline+Y-25130, n=4. B represents baseline. (B) Mean thermal paw withdrawal latencies (+SEM) of both paws combined for animals that received 130 nmol Y-25130 or saline injected intrathecally prior to a 0.05 µg SP or vehicle microinjection in the RVM. * P<0.05, *** P<0.001 versus saline+Y-25130 at that time point. ## P<0.01 versus SP+saline at that time point. saline+Y-25130, n=4; SP+saline, n=6; SP+Y-25130, n=4. B represents baseline. (C) Mean thermal paw withdrawal latencies (+SEM) of both paws combined for 30–40-day old animals that received 50 µg Y-25130 or vehicle injected intrathecally following delivery of a chronic infusion of 10 ng/h SP or saline into the RVM. B1 and B2 represent baselines before and after 24 h SP chronic infusion in (C) and (D). * P<0.05, ** P≤0.01 versus SP+saline at that time point. Both SP groups were significantly different from the saline+saline group at all time points following implant of the osmotic pump. Additionally, ## P≤0.01 versus saline+saline at that time point and @ P<0.05, @@ P<0.01 versus SP+Y-25130 group at that time point. saline+saline, n=6; saline+Y-25130, n=4; SP+saline, n=6; SP+Y-25130, n=5. (D) Mean thermal paw withdrawal latencies (+SEM) of both paws combined for 30–40-day old animals that received 10 µg ondansetron or vehicle injected intrathecally following delivery of a chronic infusion of 10 ng/h SP or saline into the RVM. *** P<0.001 versus SP+saline at that time point. # P<0.05 versus saline+saline at that time point. saline+saline, n=6; saline+ondansetron, n=6; SP+saline, n=6; SP+ondansetron, n=6.
Fig. 8.
Thermal paw withdrawal latencies for animals that received an NMDA receptor antagonist or vehicle injected intrathecally prior to or following SP-induced hyperalgesia. (A) Mean thermal paw withdrawal latencies (+SEM) of both paws combined for animals that received 31.5 µg MK-801 or vehicle injected intrathecally prior to a 0.05 µg SP or saline microinjection in the RVM. *** P<0.001 versus saline+MK801 at that time point. ### P<0.001 versus SP+saline at that time point. saline+MK801, n=6; SP+saline, n=5; SP+MK801, n=6. B represents baseline. (B) Mean thermal paw withdrawal latencies (+SEM) of both paws combined for 30–40-day old animals that received 31.5 µg MK-801 or vehicle injected intrathecally following osmotic pump delivery of a chronic 24 h infusion of either 10 ng/h SP or saline microinjection in the RVM. * P<0.05 versus saline+saline at that time point. Additionally, both SP infused groups were significantly different from saline+saline at all time points following implantation of the pump. # P<0.05, ## P<0.01 versus SP+saline at that time point. saline+saline, n=4; saline+MK801, n=5; SP+saline, n=6; SP+MK801, n=6. B1 represents baseline. B2 and B3 represent baseline 6 h and 24 h respectively following osmotic pump implant but before intrathecal injection. (C) Chronic infusion of SP (10 ng/h) into the RVM increases the expression of the phosphorylated NR1 subunit of the NMDA receptor at the Ser896 site (pNR1-Ser896) in spinal cord tissue. The spinal cord tissue was collected 24 h after SP microinjection into the RVM. The Western blot from one experiment shows that there was an increase in relative pNR1-Ser896 protein levels after SP microinjection as compared to the vehicle condition. The average increase in relative expression in three separate experiments was statistically significant (300.3%±7.9%, n=3, P<0.001). β-actin was used as a loading control to normalize the level of pNR1 protein in each lane and protein levels in the vehicle control and experimental conditions were calculated.
Intrathecal administration of a 5-HT3 receptor antagonist blocks inflammation- and SP-induced behavioral hyperalgesia
The major goal of this study was to determine the spinal cord circuitry that contributed to the behavioral hyperalgesia following RVM SP microinjection and mimicked the effects of CFA-induced inflammation. The spinal cord dorsal horn has dense serotonergic innervation (Ruda et al., 1982; Ruda, 1988; Wei et al., 1999), which originates from the RVM (see Millan, 2002 for review). While many 5-HT receptor subtypes are involved in descending inhibition (Hoyer et al., 1994; Millan, 2002), recent work has focused attention on the role of the 5-HT3 receptor subunit in descending facilitation (Suzuki et al., 2002, 2004a,b). Additionally, 5-HT3 receptors are present on excitatory neurons in the dorsal horn (Maxwell et al., 2003; Conte et al., 2005). Using inflamed and non-inflamed animals, we tested the hypothesis that 5-HT3 receptors in the spinal cord modulate CFA-induced or SP-induced behavioral hyperalgesia.
The intrathecal injection of Y-25130, a selective 5-HT3 receptor antagonist, 24 h after the induction of CFA-induced inflammation, led to significantly increased paw withdrawal latencies of the ipsilateral paw (P<0.001) in response to a thermal stimulus administered at different time points between 0.5 and 48 h after the Y-25130 intrathecal injection, but not after administration of saline intrathecally (Fig. 5A). These findings suggest that the behavioral hyperalgesia after CFA-induced inflammation is 5-HT3 receptor-dependent and likely dependent upon descending facilitation originating from the brain stem medulla, the source of 5-HT.
An intrathecal injection of the same dose of Y-25130 administered to non-inflamed animals that received a subsequent 0.05 µg SP microinjection into the RVM attenuated the SP-induced hindpaw hyperalgesia (Fig. 5B; P<0.01) 0.5 h following the SP microinjection as compared to animals that received a saline intrathecal injection. These findings indicate that intrathecal pretreatment with a 5-HT3 receptor antagonist attenuates SP-induced behavioral hyperalgesia. In animals subject to a continuous infusion of SP into the RVM via an osmotic pump, behavioral hyperalgesia was maintained for at least 24 h at which time Y-25130 intrathecal administration significantly attenuated the hindpaw hyperalgesia for 0.5 to 2.0 h (Fig. 5C). Similar to Y-25130, another 5-HT3 receptor antagonist, ondansetron, attenuated the hyperalgesic effects of a continuous infusion of SP into the RVM (Fig. 5D). Taken together, these results suggest a role of spinal cord 5-HT3 receptors in the induction and persistence of descending facilitation and hyperalgesia produced by the microinjection of SP into the RVM.
Intrathecal administration of a GABAA receptor antagonist reverses SP-induced behavioral hyperalgesia
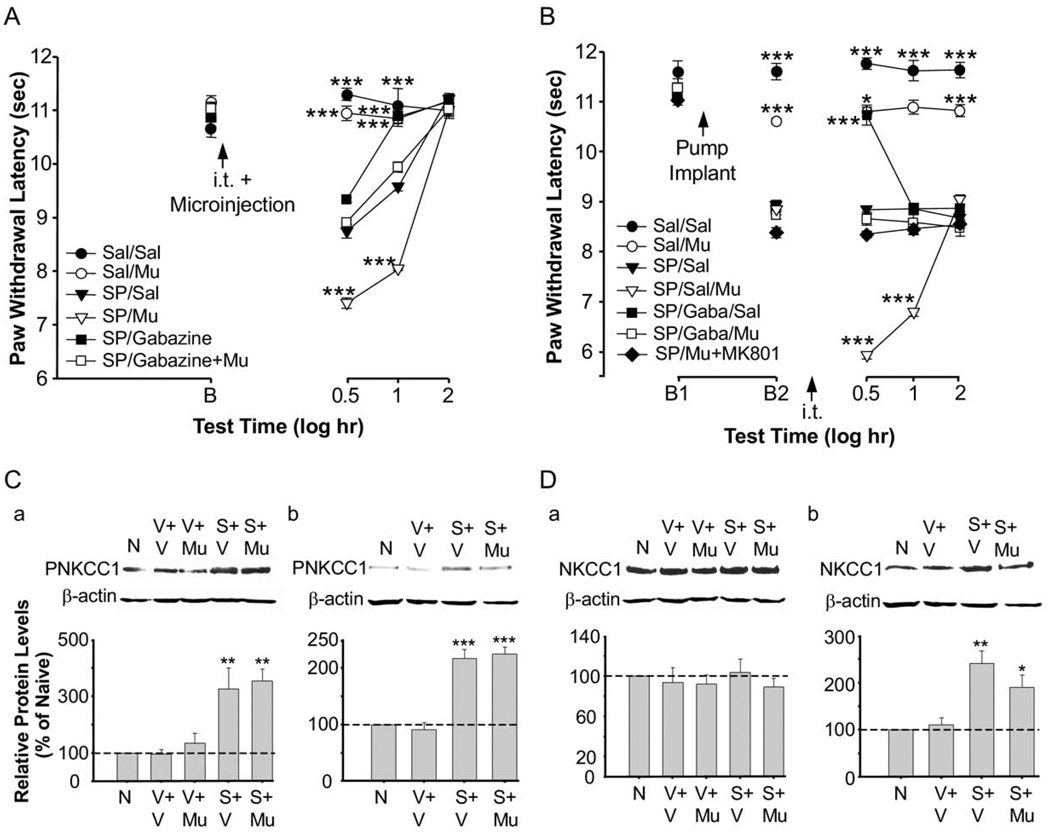
There are many GABA-containing interneurons in the spinal dorsal horn (Todd and McKenzie, 1989). Additionally, there are extensive direct GABAergic projections from the RVM to the spinal cord dorsal horn which suggests a role for GABA in spinal processing of nociception (Antal et al., 1996). A microinjection of L-glutamate into the RVM leads to descending inhibition that is partially blocked by antagonism of GABAA receptors in the spinal cord (McGowan and Hammond, 1993; Kato et al., 2006). It is also now known that following nerve injury there are shifts in the anionic reversal potential of spinal GABA-responsive neurons which can reduce GABA-induced spinal inhibition and possibly result in excitatory effects (Coull et al., 2003). Using non-inflamed animals, we tested the hypothesis that GABAA receptors at the spinal level play a role in descending facilitation induced by RVM SP administration. Post-treatment with an intrathecal injection of gabazine, a GABAA receptor antagonist, in non-inflamed animals that received a 24 h chronic infusion of SP via an osmotic pump, completely reversed the SP-induced behavioral hyperalgesia (Fig. 6A). These findings suggest that the GABAA receptor is involved in the maintenance of behavioral hyperalgesia after RVM SP administration.
Fig. 6.
(A) Thermal paw withdrawal latencies for 30–40-day old animals that received a GABAA receptor antagonist or vehicle injected intrathecally following chronic infusion of SP for 24 h. Mean thermal paw withdrawal latencies (+SEM) of both paws combined for animals that received either a 55 µg or 74 µg dose of gabazine or vehicle injected intrathecally following delivery of a chronic infusion of either 10 ng/h SP or saline microinjection into the RVM. ** P<0.01 versus saline+saline at that time point, *** P<0.001 versus saline+saline at that time point. # P<0.05, ## P<0.01 versus SP+saline at that time point. saline+saline, n=12; saline+gabazine, n=12; SP+saline, n=12; SP+gabazine, n=12. B1 represents baseline, B2 and B3 represent baseline 6 h and 24 h respectively after osmotic pump implant, but before intrathecal injection. (B) GABA-evoked currents from a spinal cord preparation in which 30–40-day old rats had SP- and saline-filled osmotic pumps implanted 24 h previously. Sample traces from saline-injected (vehicle, left) and SP-injected rats (SP, right) from perforated-patch recordings at different holding potentials (Vh). When the holding potential was equal to the resting potential (VRest), GABA induced an outward (inhibitory) current in the saline-injected rat (Vh=−68 mV) and induced an inward (excitatory) response in the cell from the SP-microinjected rat (Vh=−70 mV). (C) The current-voltage plot based on responses from the saline-treated (open circles) and SP-treated (closed circles) animals shown in (B). (C) Mean (+SEM) GABA-induced current reversal potentials (EGABA) from control (n=5) and SP-microinjected (n=5) animals. ** P<0.01. See text for more details.
GABA induces depolarization in dorsal horn neurons following intra-RVM SP microinjection
We tested the hypothesis that after RVM SP microinjection, GABA contributes to behavioral hyperalgesia by inducing depolarization in spinal neurons via a shift in the GABA ionic reversal potential leading to a reduction in spinal inhibitory tone. GABA currents were examined in an in vitro spinal preparation in which SP had previously been continuously infused (10 ng/h) in vivo into the RVM for 24 h. The average VRest was similar between vehicle- and SP-treated groups (71.0+1.1 vs. 68.0+1.4 mV, P=0.13). In slices from SP-treated animals, when the holding potential was set at the VRest, GABA (1 mM, 30 s) induced an inward current (depolarization; Fig. 6B, C). This was the case for four of the five cells (EGABA=61.8+1.9 mV, n=5). In saline-treated rats, when the holding potential was set at the VRest, GABA current was outward (hyperpolarization; Fig. 6B, C) in all five neurons tested. The EGABA of vehicle-treated animals (−70.4+1.2 mV, n=5) was also significantly more negative than that found in SP-treated animals (P<0.01; Fig. 6 Inset). These results suggest that descending facilitation induced by RVM SP administration produces GABAA receptor-evoked depolarization and an increase in excitation of dorsal horn neurons.
Intrathecal administration of a GABAA receptor agonist enhances SP-induced behavioral hyperalgesia
We next tested the hypothesis that treatment with a GABAA receptor agonist enhances hyperalgesia induced by RVM SP administration. Both the transient and continuous infusion models were used in non-inflamed animals. We used a low dose of SP (0.2 ng/h) to avoid a possible ceiling effect of SP in response to administration of a GABA agonist, muscimol. Intrathecal muscimol (0.1 µg) significantly enhanced the behavioral hyperalgesia produced by SP in both the transient and continuous infusion models (Fig. 7A, B). The muscimol-induced enhancement of hyperalgesia was attenuated by intrathecal gabazine (Fig. 7A, B). There was also an increase in threonine phosphorylation of NKCC1 protein (isoform of the Na-K-Cl cotransporter) in the spinal cord as compared to naïve animals and saline controls (P<0.01) using a phosphospecific antibody (anti-phospho-NKCC1 antibody R5; Flemmer et al., 2002) in the transient (30 min after SP microinjection) and 24 h continuous infusion models (Fig. 7C-a, b). In contrast, the expression of NKCC1 protein increased only in the 24 h infusion model (Fig. 7D-a, b). Phosphorylation of threonine in the N-terminus of NKCC1 increases NKCC1 activity (Coull et al., 2003; Kahle et al., 2005). The finding supports our hypothesis that depolarizing shifts in GABA-induced membrane potentials at the spinal level participate in the SP-induced hyperalgesia. The shift in the GABA ionic reversal potential shown earlier suggests that this increase in NKCC1-P was in dorsal horn neurons.
Fig. 7.
Functional interaction of RVM SP-induced GABA depolarization, NKCC1 protein and phosphorylation, and behavioral hyperalgesia. (A) Mean thermal paw withdrawal latencies (+SEM) of both paws combined for animals that received 0.1 µg muscimol, 55 µg gabazine, 55 µg gabazine+0.1 µg muscimol, or vehicle injected intrathecally prior to a 0.005 µg SP or saline microinjection in the RVM. *** P<0.001 versus SP/saline at that time point. saline/saline, n=5; saline/muscimol, n=5; SP/saline, n=6; SP/muscimol, n=5; SP/gabazine, n=5; SP/gabazine+muscimol, n=5. B represents baseline. (B) Mean thermal paw withdrawal latencies (+SEM; both paws combined) for 30–40-day old animals that received a GABAA receptor antagonist, a GABAA receptor agonist, both, or vehicle injected intrathecally following low dose chronic infusion of SP for 24 h. Doses of drugs—intrathecal: 0.1 µg muscimol, 55 µg gabazine, or saline; RVM: 0.2 ng/h SP or saline via chronic infusion. *** P<0.001 versus SP/saline at that time point. saline/saline, n=12; saline/muscimol, n=5; SP/saline, n=6; SP/saline+muscimol, n=6; SP/gabazine+saline, n=6; SP/gabazine+muscimol, n=6; SP/muscimol+MK801, n=5. B1 represents baseline and B2 represents baseline 24 h after osmotic pump implant, but before intrathecal injection. (C) Western blot analysis for NKCC1 protein phosphorylation after acute microinjection of 0.005 µg SP or vehicle (C-a) or chronic infusion of 0.2 ng/h SP or vehicle (C-b). Intrathecal injections consisted of vehicle or 0.1 µg muscimol. NKCC1 phosphorylation was increased as compared to naive animals following an intrathecal injection of muscimol combined with an acute microinjection of SP (P<0.01) or chronic infusion of SP (P<0.001). (D) NKCC1 protein was increased as compared to naive animals following an intrathecal injection of vehicle (P<0.01) or muscimol (P<0.05) in animals with a chronic infusion of SP into the RVM (D-b). This increase in NKCC1 protein was not seen in animals that received an acute microinjection of SP into the RVM (D-a.) Spinal cord tissue was collected 30 min following microinjections (C-a, D-a) or intrathecal injections in the chronic infusion groups (C-b, D-b). β-actin was used as a loading control to normalize the levels of protein in each lane. The histogram shows the mean levels of protein normalized with β-actin (n=3 per group) and expressed as a percentage of the control. The dashed line indicates the control level. N, naive; V, vehicle; Mu, muscimol; S, SP.
Intrathecal administration of an NMDA receptor antagonist attenuates SP-induced behavioral hyperalgesia
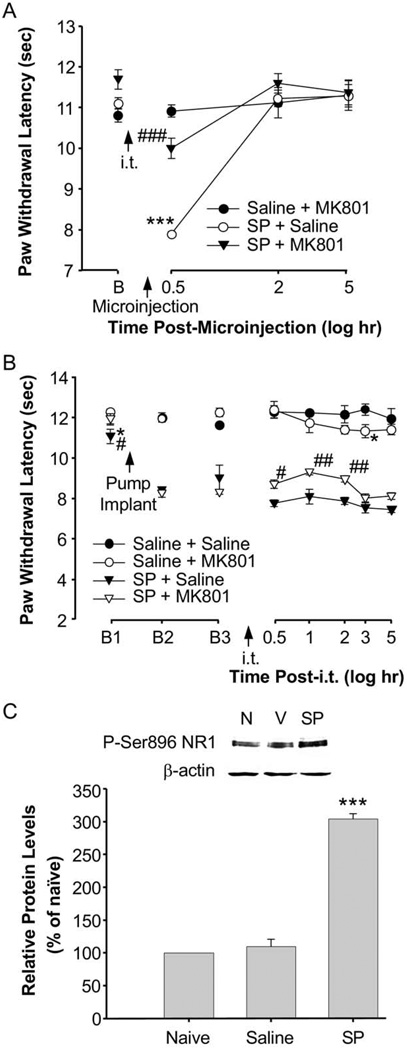
Central sensitization or activity-dependent plasticity in the dorsal horn of the spinal cord occurs following activation of ionotropic and G-protein coupled glutamate receptors (Woolf and Thompson, 1991; Dubner and Ruda, 1992; Ren et al., 1992; Neugebauer et al., 2000; Woolf and Salter, 2000). Previously, we have shown that following inflammation, behavioral hyperalgesia is correlated with increases in the phosphorylation of subunits of the NMDA receptor at the spinal level (Guo et al., 2002, 2004, 2005). In the present study, we tested the hypothesis that descending facilitation and behavioral hyperalgesia following RVM SP microinjection are NMDA receptor-dependent in non-inflamed rats. A prior (10 min) intrathecal injection of MK-801, an NMDA receptor channel blocker, in non-inflamed animals that subsequently received a SP microinjection into the RVM, attenuated the SP-induced hyperalgesia (P<0.001) following the microinjection as compared to animals receiving a saline intrathecal injection (Fig. 8A). We then determined if SP-induced behavioral hyperalgesia maintained by continuous infusion of SP into the RVM was also NMDA receptor-dependent. Twenty-four hours after a SP infusion into the RVM, the intrathecal administration of the NMDA receptor channel blocker, MK-801, at the same dose as that given as a pretreatment, attenuated the maintenance of SP-induced behavioral hyperalgesia (P<0.01) (Fig. 8B). These findings indicate that both the initiation and the maintenance of SP-induced behavioral hyperalgesia are NMDA-receptor dependent at the spinal level. In support of these findings, Western blot analyses (Fig. 8C) showed that the microinjection of SP into the RVM resulted in increased expression of the NR1 subunit of the NMDA receptor at the serine 896 phosphorylation site in the dorsal horn at the 24 h time point (P<0.001).
The functional interaction of spinal GABAA and NMDA receptor activation after RVM SP administration
The same low dose of RVM SP and intrathecal muscimol was used to examine the effect of GABAA receptor activation on NMDA receptor function in the SP continuous infusion model. We first determined that the NMDA receptor channel blocker, M-801, attenuated the SP-induced hyperalgesia enhanced by the GABA agonist, muscimol, (Fig. 7B). Western blot analyses showed that animals receiving a SP microinjection into the RVM and intrathecal muscimol exhibited a significant increase of NMDA NR1 subunit receptor phosphorylation as compared to all control groups (Fig. 9). The increased expression of NR1 phosphorylation was blocked by the GABAA antagonist, gabazine, injected intrathecally 2 min prior to muscimol. The increase in NR1 subunit phosphorylation is likely due to the combined effects of exogenous muscimol (GABA-like) administration and endogenous GABA released in the spinal cord following RVM SP. It appears that descending facilitatory mechanisms and hyperalgesia after RVM SP involve the synaptic depolarization associated with GABAA receptor activation and its functional interaction with the NMDA receptor.
Fig. 9.
NMDA NR1 subunit phosphorylation increases following chronic low dose SP administration in the RVM and muscimol injected intrathecally. Western blot analysis shows a significant increase in NR1 subunit receptor phosphorylation in the spinal cord following intrathecal muscimol in animals with a low dose (0.2 ng/h for 24 h) chronic infusion of SP in the RVM as compared to control groups (P<0.001). The effect of muscimol was blocked by intrathecal administration of gabazine. Intrathecal drug doses were the same as in Fig. 7. Spinal cord tissue was collected 30 min following intrathecal injections. The Western blots represent protein levels using the phosphorylation-specific antibody for NR1 Ser896. β-actin was used as a loading control to normalize the levels of protein in each lane. The histogram shows the mean levels of NR1 Ser896 protein normalized with β-actin (n=3–4 per group) and expressed as a percentage of the control. The dashed line indicates the control level. N, naive; V, vehicle; S, SP; Mu, muscimol; Ga, gabazine. *** P<0.001 versus SP/vehicle.
DISCUSSION
The present experiments provide evidence that RVM NK-1 receptors contribute to behavioral hyperalgesia after inflammation and that RVM SP microinjection induces and maintains hyperalgesia involving descending facilitatory mechanisms linked to 5HT-3, NMDA and GABA receptor spinal circuitry. We found that: (1) antagonism of NK-1 receptors in the RVM attenuated CFA-induced hyperalgesia, (2) there was increased RVM NK-1 expression at 2 h to 3 d following the onset of inflammation, (3) the effect of SP microinjected into the RVM in non -inflamed animals mimicked inflammatory hyperalgesia, (4) pretreatment and post-treatment antagonism of 5-HT3 receptors in the spinal cord blocked SP-induced hyperalgesia, (5) the transient and continuous infusion of SP in the RVM resulted in hyperalgesia that was attenuated by intrathecal treatment with a GABAA selective receptor antagonist and enhanced by treatment with muscimol, a GABAA receptor agonist, (6) SP microinjection into the RVM shifted the GABA-induced anionic reversal potential in spinal neurons and increased NKCC1 phosphorylation, leading to membrane depolarization, inward excitatory currents, and enhanced hyperalgesia, (7) the RVM SP-induced hyperalgesia was blocked after both pre- and post-treatment with the NMDA channel blocker, MK-801, at the spinal level, and (8) RVM SP infusion combined with intrathecal muscimol enhanced spinal NMDA receptor subunit phosphorylation and increased thermal hyperalgesia that was blocked by MK-801, suggesting the functional interaction of spinal GABAA and NMDA receptors.
The finding that NK-1-receptors in the RVM contribute to CFA-induced hyperalgesia confirms our previous preliminary data (LaGraize et al., 2005). Other studies have now demonstrated the attenuation of inflammatory hyperalgesia by RVM microinjection of NK-1 receptor antagonists, after intraplantar injection of capsaicin or CFA (Budai et al., 2007; Pacharinsak et al., 2008; Hamity et al., 2010). We now show that both pretreatment and post-treatment with an NK-1 receptor antagonist result in an attenuation of the inflammatory hyperalgesia, indicating that SP and its receptor participate in the persistence as well as the initiation of the hyperalgesia. Western blot experiments support these findings in showing that there was increased NK-1 receptor protein expression from 2 h to 3 days after inflammation. We also show that SP administration into the RVM evokes behavioral hyperalgesia mimicking inflammation-induced hyperalgesia supporting previous findings (LaGraize et al., 2006; Hamity et al., 2010). Neurons whose activation by SP leads to descending facilitation may correspond to “on” cells described in the RVM after CFA treatment (Miki et al., 2002) because SP activates only this type of RVM neuron (Budai et al., 2007).
The present experiments demonstrated that spinal antagonism of 5-HT3 receptors affected CFA- and RVM SP-induced hyperalgesia. Previous studies have reported that descending facilitation originating in the RVM is mediated by 5-HT3 receptors in the spinal cord (Green et al., 2000; Suzuki et al., 2002; Zeitz et al., 2002). In addition, depletion of endogenous RVM 5-HT attenuates hyperalgesia suggesting that descending serotonergic mechanisms contribute to the descending facilitation (Wei et al., 2010). In longer term models of inflammation due to osteoarthritis, facilitatory effects on low-threshold mechanical responses also appear to be mediated via 5-HT3 receptor activation and may contribute to secondary hyperalgesia (Rahman et al., 2009). The presence of an excitatory spino-bulbospinal loop has been proposed that involves spinal NK-1 containing neurons, ascending projections to the brainstem and descending projections that mediate facilitation via 5-HT3 spinal receptors (Suzuki et al., 2002). We have previously shown that BDNF and its TrkB receptor play a role in 5-HT receptor-mediated descending facilitatory mechanisms after CFA-induced inflammation (Guo et al., 2006; unpublished findings). An involvement of RVM “on” cells containing mu-opioid receptors has been suggested as another 5-HT3 receptor-mediated descending mechanism (Budai et al., 2007; Bee and Dickenson, 2007). Our present studies demonstrate that descending projections also involve RVM NK-1 receptor activation by released SP, which may originate from the PAG, the nucleus reticularis paragigantocellularis, the nucleus cuneiformis, the trigeminal subdivision of the lateral reticular nucleus, or the superior central raphe nucleus (Beitz, 1982).
The colocalization of NK-1 receptors and 5-HT in RVM neurons in inflamed and non-inflamed animals appears to be minimal. NK-1 receptor immunoreactive neurons have previously been found in all brainstem raphe nuclei, however, in low numbers compared with serotonergic neurons (Commons and Valentino, 2002; Leger et al., 2002). Consistent with this result is the finding that tryptophan hydroxylase-immunoreactive profiles in the RVM did not co-localizewith the NK-1 receptor (Zhang and Hammond, 2009). It appears that RVM 5-HT neurons are mainly activated indirectly by SP release from non-5-HT RVM neurons. This conclusion is supported by electrophysiological findings in RVM that exogenous SP did not produce inward currents in serotonergic neurons whereas many non-serotonergic neurons exhibited such currents (Zhang and Hammond, 2009).
Our behavioral results after intrathecal administration of 5-HT3 receptor antagonists indicate its involvement in descending facilitation. 5-HT3 receptors are located on axon terminals in the spinal cord and immunocytochemically labeled 5-HT3 receptors are decreased by 80% after rhizotomy (Maxwell et al., 2003; Kia et al., 1995; Zeitz et al., 2002). Presynaptic 5-HT3 receptors appear to mediate the activation of GABA-containing neurons (Chameau and van Hooft, 2006; Fukushima et al., 2009) although few dorsal horn immunoreactive 5-HT3 receptors colocalize with glutamate decarboxylase (Maxwell et al., 2003). In contrast, findings indicating that many 5-HT3A receptor-containing axons in the spinal cord activate excitatory neurons (Conte et al., 2005) are consistent with our findings of a 5-HT3 excitatory receptor function in the spinal cord. It also has been reported that inhibitory GABAergic and glycinergic RVM-spinal pathways are not influenced by 5-HT3 antagonists (Kato et al., 2006).
GABA-responsive neurons provide another mechanism by which spinal circuitry contributes to descending facilitation of behavioral hyperalgesia. We found that a GABAA receptor antagonist, gabazine, administered after SP RVM treatment, attenuated behavioral hyperalgesia. This is in contrast to the intrathecal effects of GABAA antagonists alone which cause mechanical hyperalgesia in naive animals (Anseloni and Gold, 2008). We tested the effects of RVM SP on postsynaptic GABA currents in single cell perforated patch clamp recordings from dorsal horn neurons. Our findings indicated a depolarizing shift in the chloride equilibrium potential after SP administration and an increase in GABA depolarizing currents, both suggesting a reduction in the inhibitory effects of GABA. Consistent with this hypothesis, we found that intrathecal muscimol, a GABAA receptor agonist, enhanced SP-induced hyperalgesia as compared to the effects of RVM SP followed by intrathecal saline. Furthermore, the transient (30 min) and continuous infusion (24 h) of RVM SP increased the phosphorylation of NKCC1, the Na-K-Cl - co-transporter. Although an increase in NKCC1 protein expression in the spinal cord occurred after SP continuous infusion at 24 h and has been found previously in a model of arthritis (Morales-Aza et al., 2004), these protein changes were absent in the transient (30 min) SP infusion model and cannot totally explain the presence of GABA depolarizing currents. The increase in NKCC1 phosphorylation found in both SP infusion models likely leads to higher chloride intracellular levels, changes in the chloride equilibrium potential (Kahle et al., 2005; Price et al., 2005) and a loss of inhibitory tone. These results are consistent with the previous spinal effects of peripheral nerve injury indicating a reduction in inhibitory GABA tone and a switch in GABA function from inhibitory to excitatory (Coull et al., 2003, 2005). Our findings are the first report of the involvement of GABA-A receptors and NKCC1 phosphorylation at the spinal level in mechanisms of descending facilitation.
NMDA receptors play an important role in the cellular cascade leading to spinal hyperexcitability and resultant behavioral hyperalgesia after injury (Dubner and Ren, 2004; Woolf and Thompson, 1991; Ren et al., 1992; Woolf and Salter, 2000). We previously have shown that CFA-induced hyperalgesia is NMDA receptor-dependent and correlated with phosphorylation of NMDA receptor subunits in the spinal dorsal horn (Guo et al., 2002, 2004). Our present findings are the first reports linking descending facilitatory mechanisms to these spinal cord events. Behavioral hyperalgesia initiated via activation of NK-1 receptors at the RVM level is attenuated by the intrathecal administration of NMDA receptor antagonists, indicating it is partially NMDA receptor-dependent at the spinal level. Our finding that SP microinjection into the RVM also leads to enhanced phosphorylation of the spinal NMDA NR1 receptor subunit, further supports a role of the NMDA receptor in spinally-mediated behavioral hyperalgesia. Our findings of NMDA and 5-HT3 receptor-dependent descending facilitation at the spinal level in the initiation and maintenance of behavioral hyperalgesia after inflammation or SP infusion are in contrast to the role of these descending systems only in the later maintenance of hyperalgesia seen in models of nerve injury (Burgess et al., 2002).
It appears that NMDA receptor-containing neurons may be a major integrative sensory pathway contributing to the initiation and persistence of behavioral hyperalgesia. We have shown here that the GABAA receptor agonist, muscimol, administered intrathecally after low dose SP RVM treatment, enhanced NMDA receptor subunit phosphorylation. Together with the finding that an NMDA receptor channel blocker attenuated the SP-induced hyperalgesia enhanced by muscimol, these results suggest that the reduction in inhibitory GABA tone produced by RVM SP enhances hyperalgesia via its interaction with the NMDA receptor. These findings suggest that one integrative sensory pathway participating in descending facilitatory mechanisms and hyperalgesia involves the release of spinal 5-HT and GABA and their functional coupling to the NMDA receptor. The downstream interaction of spinal 5-HT3, GABA and NMDA receptors links them to the initiation and maintenance of descending facilitation leading to behavioral hyperalgesia.
Acknowledgments
This work was supported by NIH NS060735, NS059028, DE018573, and T32DE007309. The authors would like to thank Dr. Forbush at Yale University for providing us the anti-phospho-NKCC1 antibody R5.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CFA
complete Freund’s adjuvant
- EGABA
GABA-evoked current reversal potential
- GiA
gigantocellular reticular nucleus, alpha part
- i.t.
intrathecal
- LPGi
lateral paragigantocellular nucleus
- m.w.
molecular weight
- NK-1
neurokinin-1
- NMDAR
NMDA-receptor
- NRM
nucleus raphe magnus
- PBS
phosphate buffered saline
- pNR1-Ser896
phosphorylated NR1 subunit of the NMDA receptor at the Ser896 site
- RMg
raphe magnus nucleus
- RVM
rostral ventromedial medulla
- SEM
standard error of the mean
- SP
substance P
- TrkB
tyrosine kinase B
- Vh
holding potential
- VRest
resting membrane potential
REFERENCES
- Anseloni VC, Gold MS. Inflammation-induced shift in the valence of spinal GABA-A receptor-mediated modulation of nociception in the adult rat. J Pain. 2008;9:732–738. doi: 10.1016/j.jpain.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal M, Petko M, Polgar E, Heizmann CW, Storm-Mathisen J. Direct evidence of an extensive GABAergic innervations of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neuroscience. 1996;73:509–518. doi: 10.1016/0306-4522(96)00063-2. [DOI] [PubMed] [Google Scholar]
- Bee LA, Dickenson AH. Rostral ventromedial medulla control of spinal sensory processing in normal and pathophysiological states. Neuroscience. 2007;147:786–793. doi: 10.1016/j.neuroscience.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The nuclei of origin of brain stem enkephalin and substance P projection to the rodent nucleus raphe magnus. Neuroscience. 1982;7:2753–2768. doi: 10.1016/0306-4522(82)90098-7. [DOI] [PubMed] [Google Scholar]
- Budai D, Khasabov SG, Mantyh PW, Simone DA. NK-1 receptors modulate the excitability of ON cells in the rostral ventromedial medulla. J Neurophysiol. 2007;97:1388–1395. doi: 10.1152/jn.00450.2006. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P, van Hooft JA. Serotonin 5-HT(3) receptors in the central nervous system. Cell Tissue Res. 2006;326:573–581. doi: 10.1007/s00441-006-0255-8. [DOI] [PubMed] [Google Scholar]
- Commons KG, Valentino RJ. Cellular basis for the effects of substance P in the periaqueductal gray and dorsal raphe nucleus. J Comp Neurol. 2002;447:82–97. doi: 10.1002/cne.10228. [DOI] [PubMed] [Google Scholar]
- Conte D, Legg ED, McCourt AC, Silajdzic E, Nagy GG, Maxwell DJ. Transmitter content, origins and connections of axons in the spinal cord that possess the serotonin (5-hydroxytryptamine) 3 receptor. Neuroscience. 2005;134:165–173. doi: 10.1016/j.neuroscience.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ren K. Brainstem mechanisms of persistent pain following injury. J Orofac Pain. 2004;18:299–305. [PubMed] [Google Scholar]
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl cotransporter NKCC1 detected with a phosphor-specific antibody. J Biol Chem. 2002;27:37551–37558. doi: 10.1074/jbc.M206294200. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Ohtsubo T, Tsuda M, Yanagawa Y, Hori Y. Facilitatory actions of serotonin type 3 receptors on GABAergic inhibitory synaptic transmission in the spinal superficial dorsal horn. J Neurophysiol. 2009;102:1459–1471. doi: 10.1152/jn.91160.2008. [DOI] [PubMed] [Google Scholar]
- Green G, Scarth J, Dickenson A. An excitatory role for 5-HT in spinal inflammatory nociceptive transmission; state-dependent actions via dorsal horn 5-HT3 receptors in the anaesthetized rat. Pain. 2000;89:81–88. doi: 10.1016/S0304-3959(00)00346-8. [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo W, Robbins MT, Dubner R, Ren K. Changes in AMPA receptor phosphorylation in the rostral ventromedial medulla after inflammatory hyperalgesia in rats. Neurosci Lett. 2004;366:201–205. doi: 10.1016/j.neulet.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo W, Zou SP, Dubner R, Ren K. Inflammation-induced upregulation of AMPA receptor subunit expression in brain stem pain modulatory circuitry. Pain. 2003;104:401–413. doi: 10.1016/s0304-3959(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Guan Y, Terayama R, Dubner R, Ren K. Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. J Pharmacol Exp Ther. 2002;300:513–520. doi: 10.1124/jpet.300.2.513. [DOI] [PubMed] [Google Scholar]
- Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wei F, Zou S-P, Sugiyo S, Ikeda T, Robbins MT, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zou S-P, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci. 2002;22:6208–6217. doi: 10.1523/JNEUROSCI.22-14-06208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zou S-P, Ikeda T, Dubner R, Ren K. Rapid and lasting increase in serine phosphorylation of rat spinal cord NMDAR1 and GluR1 subunits after peripheral inflammation. Thalamus Relat Syst. 2005;3:9–18. [Google Scholar]
- Hamity MV, White SR, Hammond DL. Effects of neurokinin-1 receptor agonism and antagonism in the rostral ventromedial medulla of rats with acute or persistent inflammatory nociception. Neuroscience. 2010;165:902–913. doi: 10.1016/j.neuroscience.2009.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EF, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hylden JLK, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Rinehart J, de Los Heros P, Louvi A, Meade P, Vazquez N, Hebert SC, Gamba G, Gimenez I, Lifton RP. WNK3 modulates transport of Cl- in and out of cells: implications for control of cell volume and neuronal excitability. Proc Natl Acad Sci U S A. 2005;102:16783–16788. doi: 10.1073/pnas.0508307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G, Yasaka T, Katafuchi T, Furue H, Mizuno M, Iwamoto Y, Yoshimura M. Direct GABAergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo] patch-clamp analysis in rats. J Neurosci. 2006;26:1787–1794. doi: 10.1523/JNEUROSCI.4856-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabov SG, Ghilardi JR, Mantyh PW, Simone DA. Spinal neurons that express NK-1 receptors modulate descending controls that project through the dorsolateral funiculus. J Neurophysiol. 2005;93:998–1006. doi: 10.1152/jn.01160.2003. [DOI] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, McKernan RM, Laporte AM, Lombard MC, Bourgoin S, Hamon M, Verge D. Localization of 5-HT3 receptors in the rat spinal cord: immunohistochemistry and in situ hybridization. Neuroreport. 1995;6:257–261. doi: 10.1097/00001756-199501000-00008. [DOI] [PubMed] [Google Scholar]
- LaGraize SC, Guo W, Ren K, Dubner R. Antagonism of neurokinin-1 receptors in the rostral ventromedial medulla attenuate inflammation-induced thermal hyperalgesia in rats. Program No. 31394. Vol. 12. Washington, DC: Soc Neurosci.; 2005. [Google Scholar]
- LaGraize SC, Guo W, Ren K, Dubner R. Substance P in the rostral ventromedial medulla induces thermal hyperalgesia and enhances inflammation-induced hyperalgesia in rats. Program No. 248. Vol. 18. Atlanta: Soc Neurosci.; 2006. [Google Scholar]
- LaMotte CC. Lamina X of primate spinal cord: distribution of five neuropeptides and serotonin. Neuroscience. 1988;25:639–658. doi: 10.1016/0306-4522(88)90265-5. [DOI] [PubMed] [Google Scholar]
- Leger L, Gay N, Cespuglio R. Neurokinin NK1- and NK3-immunoreactive neurons in serotonergic cell groups in the rat brain. Neurosci Lett. 2002;323:146–150. doi: 10.1016/s0304-3940(01)02543-5. [DOI] [PubMed] [Google Scholar]
- Maeno H, Kiyama H, Tohyama M. Distribution of the substance P receptor (NK-1 receptor) in the central nervous system. Brain Res Mol Brain Res. 1993;18:43–58. doi: 10.1016/0169-328x(93)90172-l. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Kerr R, Rashid S, Anderson E. Characterisation of axon terminals in the rat dorsal horn that are immunoreactive for serotonin 5-HT3A receptor subunits. Exp Brain Res. 2003;149:114–124. doi: 10.1007/s00221-002-1339-7. [DOI] [PubMed] [Google Scholar]
- McGowan MK, Hammond DL. Antinociception produced by microinjection of L-glutamate into the ventromedial medulla of the rat: mediation by spinal GABAA] receptors. Brain Res. 1993;620:86–96. doi: 10.1016/0006-8993(93)90274-q. [DOI] [PubMed] [Google Scholar]
- Miki K, Zhou Q, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brainstem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–760. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Morales-Aza BM, Chillingworth NL, Payne JA, Donaldson LF. Inflammation alters cation chloride cotransporter expression in sensory neurons. Neurobiol Dis. 2004;17:62–69. doi: 10.1016/j.nbd.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Chen PS, Willis WD. Groups II and III metabotropic glutamate receptors differentially modulate brief and prolonged nociception in primate STT cells. J Neurophysiol. 2000;84:2998–3009. doi: 10.1152/jn.2000.84.6.2998. [DOI] [PubMed] [Google Scholar]
- Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. NK-1 receptors in the rostral ventromedial medulla contribute to hyperalgesia produced by intraplantar injection of capsaicin. Pain. 2008;139:34–46. doi: 10.1016/j.pain.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Paxinos P, Watson C. The rat brain in stereotaxic coordinates. 6th ed. New York, NY: Academic Press; 2008. [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem. 2005;5:547–555. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman W, Bauer CS, Bannister K, Vonsy JL, Dolphin AC, Dickenson AH. Descending serotonergic facilitation and the antinociceptive effects of pregabalin in a rat model of osteoarthritic pain. Mol Pain. 2009;5:45. doi: 10.1186/1744-8069-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Ren K, Hylden JLK, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Ruda MA. Spinal dorsal horn circuitry involved in the brain stem control of nociception. Prog Brain Res. 1988;77:129–140. doi: 10.1016/s0079-6123(08)62780-6. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Coffield J, Steinbusch HW. Immunocytochemical analysis of serotonergic axons in laminae I and II of the lumbar spinal cord of the cat. J Neurosci. 1982;2:1660–1671. doi: 10.1523/JNEUROSCI.02-11-01660.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffroy M, Torrens Y, Glowinski J, Beaujouan JC. Autoradio-graphic distribution of tachykinin NK2 binding sites in the rat brain: comparison with NK1 and NK3 binding sites. Neuroscience. 2003;116 doi: 10.1016/s0306-4522(02)00748-0. 773. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rahman W, Hunt SP, Dickenson AH. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurons following peripheral nerve injury. Brain Res. 2004a;1019:68–76. doi: 10.1016/j.brainres.2004.05.108. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004b;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Todd AJ, McKenzie J. GABA-immunoreactive neurons in the dorsal horn of the rat spinal cord. Neuroscience. 1989;31:799–806. doi: 10.1016/0306-4522(89)90442-9. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. The glutamate synapse: a target in the pharmacological management of hyperalgesic pain states. Prog Brain Res. 1998;116:407–420. doi: 10.1016/s0079-6123(08)60452-5. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Schaible H-G. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wei F, Dubner R, Ren K. Laminar-selective noradrenergic and serotoninergic modulation includes spinoparabrachial cells after inflammation. Neuroreport. 1999;10:1757–1761. doi: 10.1097/00001756-199906030-00024. [DOI] [PubMed] [Google Scholar]
- Wei F, Dubner R, Zou S, Ren K, Bai G, Wei D, Guo W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci. 2010;30:8624–8636. doi: 10.1523/JNEUROSCI.5389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Yang K, Li Y-Q, Kumamoto E, Furue H, Yoshimura M. Voltage-clamp recordings of postsynaptic currents in substantia gelatinosa in vitro] and its applications to assess synaptic transmission. Brain Res Protoc. 2001;7:235–240. doi: 10.1016/s1385-299x(01)00069-1. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hammond DL. Substance P enhances excitatory synaptic transmission on spinally projecting neurons in the rostral ventromedial medulla after inflammation injury. J Neurophysiol. 2009;102:1139–1151. doi: 10.1152/jn.91337.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]