Abstract
Objectives
Muscle pain from different activities was tested with the muscle pain expected to vary in ways that may clarify mechanisms of activity-induced exacerbation of myofascial pain.
Methods
Participants (N = 20; 45% women; 23 years old (SD = 2.09)) consented to participate in a six session protocol. Bilateral muscle pain ratings and pressure pain thresholds (PPTs) were collected before and for 4 days after lengthening (i.e., eccentric) muscle contractions were completed with the non-dominant elbow flexors to induce delayed-onset muscle pain. The muscle pain ratings were collected with the arms in several conditions (e.g., resting, moving, contracting in a static position) and PPTs were collected with the arms.
Results
In the ipsilateral arm, muscle pain ratings at rest and during activity significantly increased while PPTs significantly decreased after the eccentrics (η 2s = .17 – .54). The greatest increases in pain occurred during arm extension without applied load, in which there was more stretching but less force than isometrics. In the contralateral arm, neither muscle pain nor PPTs changed from baseline.
Discussion
These results resemble previous electrophysiology studies showing differential sensitization across stimuli and support that increased depth of information about aggravating activities from clinical patients is needed.
Keywords: delayed-onset muscle soreness, stretch injury
Introduction
Pain can occur during activity in healthy adults.1–7 Activity can also exacerbate the preexisting pain of clinical patients. For example, patients with osteoarthritis,8,9 low back pain,10,11 chronic regional myalgia,12 fibromyalgia syndrome,13–17 migraine,18 neuropathic pain,19,20 neuromuscular disease,21 and post-surgical pain22 have reported acute exacerbations of pain with activity. Thus, activity should be investigated as a potential source of spikes in patients’ pain.
Along with ratings of clinical pain, activity has been reported to stimulate nociceptors in basic electrophysiology studies. A classic investigation by Mense and Meyer23 found that Group III and IV muscle nociceptors were activated by noxious pressure, noxious stretch, and noxious contraction force. Further, after muscle inflammation, nociceptors started to respond to innocuous levels of these stimuli. Also, a more recent investigation by Ro and colleagues24 reported that the number of c-fos containing cells in trigeminal brainstem nuclei increased with masseter muscle inflammation and further increased with jaw movement. Thus, the nociceptors innervating muscle sensitize after inflammation and this sensitization is manifested as an enhanced response to joint movement and muscle stimulation.
Pain and nociceptor activation vary with both the intensity1,23,7 and type of activity25,26,23,27 so the activity’s characteristics need to be considered. For example, recalled knee pain from weight bearing activities was more strongly associated with radiographic severity of arthritis than recalled knee pain from non-weight bearing activities.28 Therefore, examination of different activities may advance our understanding of the mechanisms of activity-induced exacerbation of pain.
Activity-induced exacerbations of pain can be investigated by comparing pain from activities that differ in controlled ways. For example, Sullivan and colleagues29 have developed an innovative canister lifting task for patients with low back pain, in which the canister weight and canister distance from patients are varied. An alternative and novel approach is to induce delayed-onset muscle pain in healthy participants with controlled exercise and then investigate how controlled activities affect the pain. Such an approach is clinically-relevant because induced delayed-onset muscle pain interferes with normal daily activities outside of the laboratory and generates self-care behaviors such as stretching and massaging the muscles.30,31
The purpose of this investigation was to test the hypothesis that delayed-onset muscle pain would vary across activities even when the activities differ in simple ways. The delayed-onset muscle pain was induced with lengthening (i.e., eccentric) contractions of the non-dominant elbow flexors and the activities assessed were normal movements and contractions of the elbow. The identification of activities that most increase pain in temporarily damaged muscles may lead to insights into the mechanisms of activity-induced exacerbations of pain in patients with myofascial pain.
Materials and Methods
Participants
Participants (N = 20; 45% women) with an average age of 23 years (SD = 2.09) consented to participate in a six session protocol that was approved by the University of Missouri’s Health Science Institutional Review Board. The restrictions for participation were the following: (a) had not engaged in upper body strength training on a regular basis (i.e., two times per week) for consecutive weeks within the previous six months, (b) were not currently experiencing arm pain, (c) had no history of upper arm injury within the previous six months, and (d) no chronic pain conditions. In addition, participants were screened by questionnaire for potential risk factors to the exercise protocol (e.g., excessive swelling, loss of range or motion, exertional rhabdomyolysis). Furthermore, participants were restricted from the following behaviors: smoking 3 hours prior to a session, consuming any food or drink except water 8 hours prior to a session, and taking analgesics throughout the study period.
Measures
Muscle pain ratings
In order to evaluate the multidimensional nature of pain,32 ratings of muscle pain intensity and muscle pain unpleasantness in both arms were assessed before and after lengthening (i.e., eccentric) muscle contractions with 0–100 numeric scales. More specifically, ratings were collected while the participants’ arms were (1) stationary at approximately 90° of elbow flexion, (2) moving through active range of motion without applied load to full elbow flexion, (3) moving through active range of motion without applied load to full elbow extension, and (4) during five repetition maximal strength tests (5 RM) at 90° of elbow flexion. The anchors of the pain intensity scales were “no pain” and “most intense pain sensation imaginable.” The anchors of the pain unpleasantness scales were “no unpleasantness” and “most unpleasant imaginable.” Numeric pain scales have been found to be reliable and valid.33
Pressure Pain Threshold
Pressure pain threshold (PPT) was defined as the point at which a pressure stimulus first became painful. The pressure stimulus was applied at 25% of the distance from the cubital fossa to the greater tuberosity of the humerus while both arms were stationary at approximately 90° of elbow flexion. Using a hand-held 10 kg dolorimeter with a 1 cm rubber tip (Pain Diagnostics Inc.), pressure was increased at a rate of about 1 kg/s until the participant first reported feeling pain. The average of two repeated measurements was analyzed for each arm.
Procedures
After a familiarization session, participants visited the laboratory for five consecutive days. Muscle pain ratings were collected for both arms during rest, flexion, and extension and pressure pain thresholds were assessed for both arms at rest. Lastly, participants were positioned in a muscle testing apparatus (Biodex System 3; Biodex Medical Systems, Shirley, NY) so that muscle pain could be measured during maximal isometric (i.e., static) contractions by both arms. For the isometric contraction test, the participants completed a 5 repetition maximal (5 RM) test with 2 minutes of rest in between each repetition at 90° of elbow flexion.
Following the isometric tests, eccentric contractions of the participants’ non-dominant elbow flexors were completed with the muscle testing apparatus to induce delayed-onset muscle pain. (The non-dominant arm was defined as the contralateral arm to the arm with which the participants wrote.) More specifically, the participants performed 3 sets of 12 maximal eccentric repetitions with a rest period of 60 s in between each set. Eccentric contractions were completed at a velocity of 90°/s through the participants’ active range of motion.
It is important to clarify how the state of the elbow flexor muscles varied across the conditions within the study. The elbow flexors were agonists during flexion and isometrics with less force produced and more shortening during the unloaded flexion than the isometrics. The elbow flexors were also agonists during eccentrics with more force produced and more lengthening than during the unloaded flexion and isometrics. In contrast, the elbow flexors were antagonists during unloaded extension with less force produced and similar lengthening to eccentrics.34
After the eccentric exercise, participants were given a rest period of about 1 hour. During this time, they were instructed to continue adherence to the pre-session restrictions, but they were allowed to leave the laboratory if they desired. After the rest period, muscle pain and pressure pain thresholds were assessed again in the same manner as before the eccentric exercise. Then the session was terminated and participants were reminded of the schedule and restrictions for the subsequent sessions, which included avoiding any self-care behaviors for muscle pain (e.g., ice or heat application, stretching, massage, etc.).
Participants returned to the laboratory at one, two, three, and four days after the eccentric contractions in order for us to evaluate changes in muscle pain across time. These sessions were held either in the morning or afternoon hours in congruence with the previous session so that all the sessions of a single participant were either in the morning or afternoon. During each laboratory session, the muscle pain measures were repeated. Muscle pain ratings and pressure pain thresholds were completed as previously described.
Data Analyses
In order to test our hypotheses for changes in muscle pain ratings, we conducted repeated measures analyses of variance (ANOVAs) with three factors: ARM (ipsilateral – eccentric contractions - and contralateral), CONDITION (resting, flexing, extending, and maximally contracting in static position), and TIME (pre-exercise, 1-hr post-exercise, 1 day, 2 days, 3 days, 4 days) with pain intensity or pain unpleasantness as the dependent variable. Significant 3-way interactions were followed up with CONDITION by TIME repeated measures ANOVAs within each arm, which were followed up with CONDITION repeated measures ANOVAs within each time point.
In order to test our hypotheses for changes in pressure pain thresholds, we conducted repeated measures ANOVAs with two factors: ARM (ipsilateral – eccentric contractions - and contralateral) and TIME (pre-exercise, 1-hr post-exercise, 1 day, 2 days, 3 days, 4 days). Significant 2-way interactions were followed up with TIME repeated measures ANOVAs within each arm and pairwise comparisons.
All analyses were conducted using SPSS software (SPSS, Inc., Chicago, IL) with Greenhouse-Geisser correction of degrees of freedom to adjust for violations of sphericity. Statistical significance was defined as p < .05 and eta squared (η 2) was calculated to determine the meaningfulness of the results. Eta squared values of .01, .06, and .14 corresponded to small, medium, and large effect sizes, respectively.35
Results
Muscle Pain Ratings
Pain intensity and unpleasantness were affected by a large interaction among the factors of arm, arm condition, and measurement time point (ARM by CONDITION by TIME: F 15, 255 = 8.35, p < .001, η 2 = .33 and F 15,240 = 7.44, p < .001, η 2 = .32). Follow-up analyses within the ipsilateral arm, revealed that the ratings changed differently across time depending upon the arm condition. However, in general, both pain intensity and pain unpleasantness increased significantly by large amounts to a peak at 2 days post-exercise with the largest increases occurring during arm extension, next largest during flexion and isometrics, and smallest at rest. (See Table 1.)
Table 1.
Follow-up CONDITION × TIME mixed repeated measures ANOVA results within each arm. Shaded results were statistically significant (p < .05).
| Source | Arm | Pain Intensity | Pain Unpleasantness | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | η2 | df | F | p | η2 | ||
| CONDITION × TIME |
ipsilateral | 15,255 | 8.72 | <.001 | .34 | 15,255 | 8.67 | <.001 | .34 |
| contralateral | 15,270 | 1.15 | .339 | .06 | 15,255 | 1.95 | .141 | .10 | |
| CONDITION | ipsilateral | 3, 51 | 15.00 | <.001 | .47 | 3, 51 | 14.09 | <.001 | .45 |
| contralateral | 3,54 | 11.06 | .003 | .38 | 3,51 | 6.74 | .018 | .28 | |
| TIME | ipsilateral | 5,51 | 10.40 | .002 | .38 | 5,51 | 12.62 | <.001 | .43 |
| contralateral | 5,90 | 1.56 | .214 | .08 | 5,85 | 1.81 | .164 | .10 | |
Additional analyses comparing arm condition within each time point showed that before exercise, both pain intensity and unpleasantness were higher during the isometric maximal contraction than at rest and during flexion and extension. However, this pattern changed after the eccentric contractions. One hour after the exercise, there were non-significant and small differences among the arm condition for pain intensity or unpleasantness. One to four days after the exercise, both pain intensity and unpleasantness were the highest during extension, followed by flexion and isometrics, and were lowest at rest. (See Table 2 and Figures 1 and 2.)
Table 2.
Follow-up CONDITION repeated measures ANOVA results within each time point for the ipsilateral arm. Shaded results were statistically significant (p < .05).
| Source | Pain Intensity | Pain Unpleasantness | ||||||
|---|---|---|---|---|---|---|---|---|
| df | F | p | η2 | df | F | p | η2 | |
| Baseline | 3,54 | 8.01 | .010 | .31 | 3,54 | 9.08 | .006 | .26 |
| 1 Hour | 3,57 | 2.41 | .111 | .11 | 3,57 | 0.86 | .433 | .04 |
| 1 Day | 3,57 | 13.75 | <.001 | .42 | 3,57 | 12.85 | <.001 | .40 |
| 2 Days | 3,57 | 14.64 | <.001 | .44 | 3,57 | 14.55 | <.001 | .43 |
| 3 Days | 3,57 | 19.54 | <.001 | .51 | 3,57 | 17.04 | <.001 | .47 |
| 4 Days | 3,57 | 13.66 | <.001 | .43 | 3,54 | 12.56 | <.001 | .41 |
Figure 1.
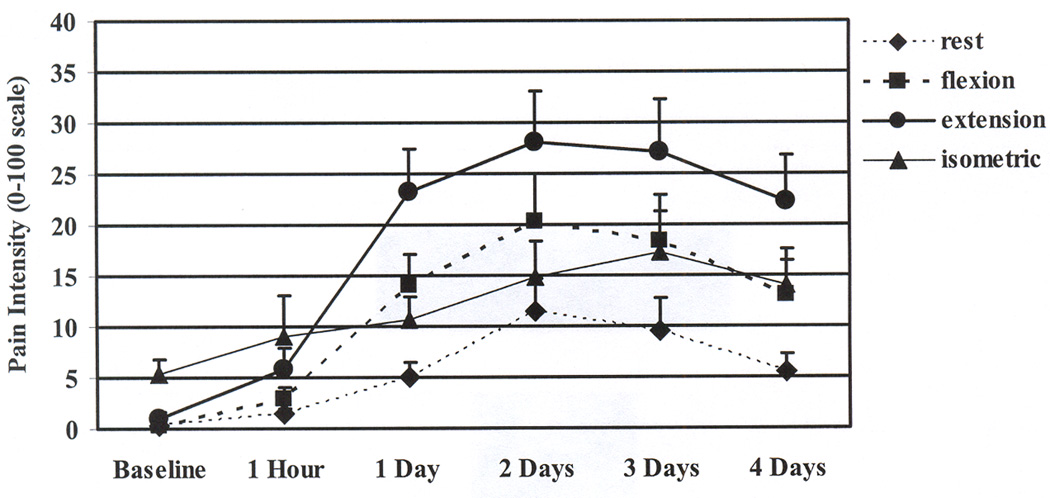
Means and standard errors for the ratings of muscle pain intensity in the ipsilateral arm before and across 4 days after the eccentric contractions. Ratings at all time points increased from baseline when the ipsilateral arm was resting, flexing, and extending (p < .05). Ratings did not change significantly from baseline when the ipsilateral arm was maximally contracting in a isometric position.
Figure 2.
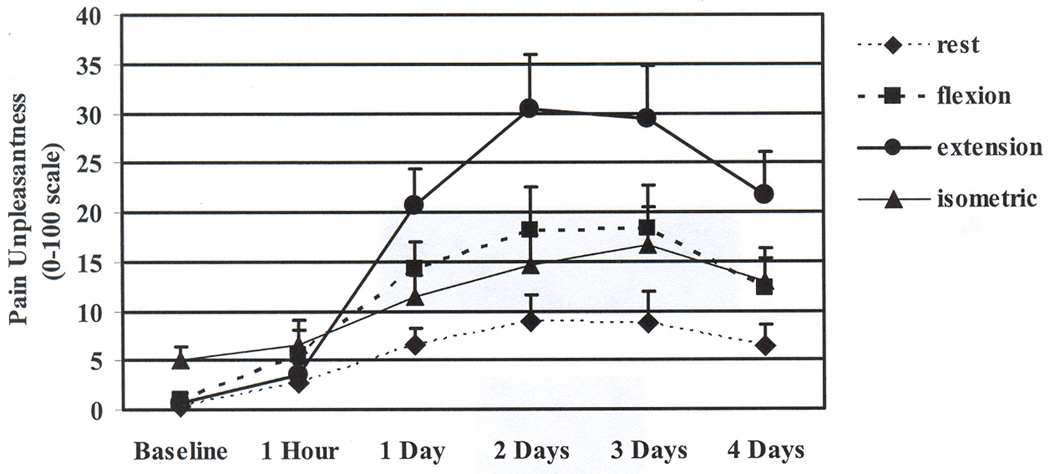
Means and standard errors for the ratings of muscle pain unpleasantness in the ipsilateral arm before and across 4 days after the eccentric contractions. Ratings at all time points increased from baseline when the ipsilateral arm was extending (p < .05). Ratings at 1 to 4 days increased from baseline when the ipsilateral arm was resting, flexing, and maximally contracting in a static position (p < .05).
A different pattern of results was observed for the contralateral arm. The muscle pain ratings for the contralateral arm were unaffected by the ipsilateral arm’s eccentric contractions so that the pain in the contralateral arm remained highest during maximal contractions as was found in the ipsilateral arm before the eccentric contractions. (See Table 3.)
Table 3.
Means and standard errors for the ratings of muscle pain intensity and unpleasantness in the contralateral arm before and across 4 days after the eccentric contractions.
| Baseline | 1 Hour | 1 Day | 2 Days | 3 Days | 4 Days | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | |
| Pain Intensity |
||||||||||||
| Rest | 0.26 | 0.19 | 0.30 | 0.18 | 0.52 | 0.44 | 0.48 | 0.43 | 0.26 | 0.22 | 0.30 | 0.23 |
| Flexion | 0.11 | 0.06 | 1.17 | 0.54 | 1.00 | 0.68 | 1.30 | 0.89 | 0.30 | 0.23 | 0.39 | 0.27 |
| Extension | 0.85 | 0.52 | 0.91 | 0.42 | 0.87 | 0.48 | 1.04 | 0.52 | 0.43 | 0.25 | 0.35 | 0.23 |
| Isometric* | 5.30 | 1.42 | 6.33 | 1.74 | 6.52 | 1.82 | 5.25 | 1.57 | 5.02 | 1.49 | 4.96 | 1.70 |
| Pain Unpleasantness |
||||||||||||
| Rest | 0.39 | 0.27 | 0.35 | 0.23 | 0.43 | 0.43 | 0.26 | 0.22 | 0.22 | 0.22 | 0.35 | 0.25 |
| Flexion | 0.22 | 0.15 | 0.87 | 0.50 | 0.73 | 0.50 | 0.61 | 0.34 | 0.35 | 0.24 | 0.52 | 0.37 |
| Extension | 0.70 | 0.34 | 0.83 | 0.48 | 1.00 | 0.52 | 0.91 | 0.40 | 0.39 | 0.25 | 0.39 | 0.27 |
| Isometric* | 6.09 | 1.92 | 5.55 | 1.83 | 6.35 | 2.14 | 3.98 | 1.35 | 5.05 | 1.71 | 3.87 | 1.50 |
Pain ratings when the contralateral arm was maximally contracting in a static position were higher than when resting, flexing, and extending (p < .05).
Pressure Pain Thresholds
Pressure pain thresholds after the eccentric contractions changed across time differently in the ipsilateral and contralateral arms (ARM by TIME: F 5,90 = 10.74, p < .001, η 2 = .37), but the pressure pain thresholds were generally lowest at 1 and 2 days post-exercise (TIME effect F 5,90 = 4.33, p = .007, η 2 = .19) and lower in the ipsilateral arm than the contralateral arm (ARM effect F 1,18 = 7.44, p = .014, η 2 = .29). More specifically, within the ipsilateral arm, pressure pain thresholds decreased significantly by a large amount and were lowest at 1 day, 2 days, and 3 days after exercise (F 5,90 = 8.99, p < .001, η 2 = .33). Within the contralateral arm, non-significant and small changes from baseline were detected. (F 5,90 = 2.53, p = .068, η 2 = .12). (See Figure 3.)
Figure 3.
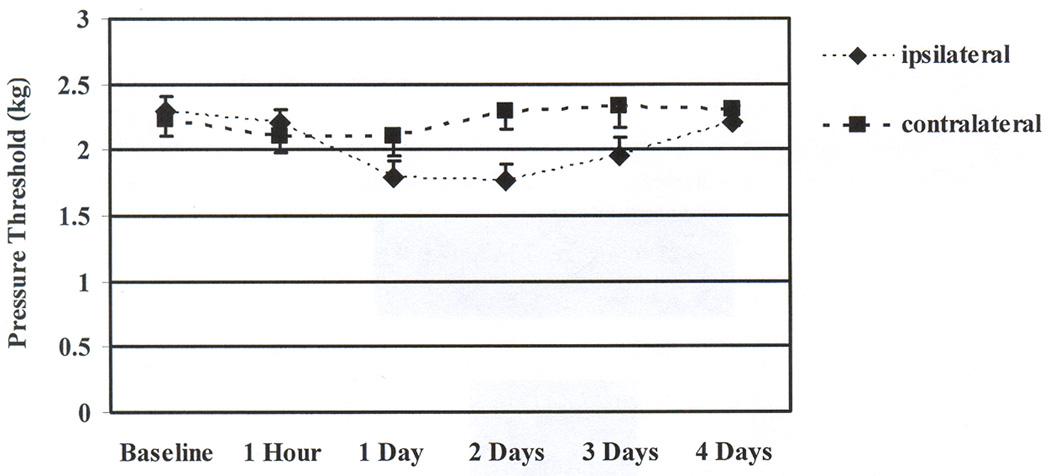
Means and standard errors for the pressure pain thresholds before and across 4 days after the eccentric contractions. Pressure pain thresholds at 1 to 3 days were significantly decreased from baseline for the ipsilateral arm. Pressure pain thresholds did not change significantly from baseline for the contralateral arm.
Discussion
This investigation detected that the eccentric muscle contractions successfully induced spontaneous pain (i.e., pain at rest) and allodynia to movement and pressure. Few investigations of eccentric contractions have reported muscle pain at rest. Of those that have assessed muscle pain at rest, one study detected increased resting pain36 while two other studies did not.37,38 Thus, the literature is currently mixed and more research is needed because pain at rest is clinically relevant due to its occurrence with clinical pain in humans and peripheral and central sensitization in animals.39–41
The movement and pressure allodynia that we detected are consistent with numerous studies of post-exercise muscle pain, but the novel pain measurement methodology in this study enabled comparison of specific types of movements and/or contractions. The muscle pain ratings differed depending upon the activity with lengthening producing the greatest pain in the damaged muscles. These findings resemble Mense and Meyer’s23 observation that inflammation-induced sensitization of Group III and IV fibers differed across stimuli (e.g., stretch, contraction, etc.). Thus, the findings support the potential of Mense and Meyer’s findings with induced muscle inflammation in cats to translate to temporary endogenous muscle damage in humans.
As stated previously, the elbow flexors were agonists and shortening during unloaded flexion and isometrics and antagonists and lengthening during unloaded extension. Because the movement allodynia was highest during extension when the damaged muscles are antagonists and lengthening, it appears that stretch sensitive peripheral afferents were particularly sensitized by the eccentric contractions. , low threshold stretch receptors may be behaving as nociceptors and/or high threshold stretch sensitive nociceptors may have lowered activation thresholds. In fact, inflammation can make low threshold mechanoreceptors act like nociceptors 42–46 and can lower the stimulation thresholds of nociceptors,39,41 which is important because inflammation does occur with muscle damage from eccentric muscle contractions.47,48 It may be possible to use animal models of eccentric contractions, such as the one developed by Taguchi and colleagues,49,50 to compare the activation level of stretch sensitive peripheral afferents to different types of activities before and after the eccentric contractions.
Our findings confirm that assessments of activity-related pain are affected by relatively simple differences among the activities. Detailed information about the characteristics of aggravating activities from clinical pain patients beyond “least, usual, worst, and current” pain ratings may enable important biomechanical modifications to how activities are performed and improved prescriptions for therapeutic exercise. For example, patients with myofascial pain may benefit from incorporation of assistive devices (e.g., reaching tools) and/or strengthening exercises that minimize muscle lengthening. Reducing activity-related pain may reduce activity avoidance and deconditioning because numerous studies have found that pain impairs adherence to therapeutic exercise in patients with chronic pain.51–59
Unique strengths of this study’s methodology were measuring bilateral muscle pain responses across 4 days post-exercise and our assessment of muscle pain ratings when both arms were performing different activities. Limitations of the investigation were the generally low levels of induced muscle pain and the absence of additional sensory tests such as temporal summation to heat or pressure. Future studies could easily address these limitations and further advance our understanding of the mechanisms and treatments for activity-related pain.
Acknowledgements
Support was provided by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1KO1 AR050146-01A1) and the University of Missouri (Research Council) to Dr. Erin A Dannecker. The authors would like to thank all the participants who volunteered for this investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cook DB, O'Connor PJ, Ray CA. Muscle pain perception and sympathetic nerve activity to exercise during opioid modulation. Am J Physiol Regul Integr Comp Physiol. 2000;279:1565–1573. doi: 10.1152/ajpregu.2000.279.5.R1565. [DOI] [PubMed] [Google Scholar]
- 2.Green S, Langberg H, Skovgaard D, et al. Interstitial and arterial-venous [K+] in human calf muscle during dynamic exercise: effect of ischaemia and relation to muscle pain. J Physiol. 2000;529(Pt 3):849–861. doi: 10.1111/j.1469-7793.2000.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollander DB, Kilpatrick MW, Ramadan ZG, et al. Load rather than contraction type influences rate of perceived exertion and pain. J Strength Cond Res. 2008;22:1184–1193. doi: 10.1519/JSC.0b013e31816a8bc2. [DOI] [PubMed] [Google Scholar]
- 4.Ljunggren G, Ceci R, Karlsson J. Prolonged exercise at a constant load on a bicycle ergometer: ratings of perceived exertion and leg aches and pain as well as measurements of blood lactate accumulation and heart rate. Int J Sports Med. 1987;8:109–116. doi: 10.1055/s-2008-1025651. [DOI] [PubMed] [Google Scholar]
- 5.Maixner W, Gracely RH, Zuniga JR, et al. Cardiovascular and sensory responses to forearm ischemia and dynamic hand exercise. Am J Physiol Regul Integr Comp Physiol. 1990;259:1156–1163. doi: 10.1152/ajpregu.1990.259.6.R1156. [DOI] [PubMed] [Google Scholar]
- 6.Motl RW, Gliottoni RC, Scott JA. Self-efficacy correlates with leg muscle pain during maximal and submaximal cycling exercise. J Pain. 2007;8:583–587. doi: 10.1016/j.jpain.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Noble BJ, Borg GA, Jacobs I, et al. A category-ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc. 1983;15:523–528. [PubMed] [Google Scholar]
- 8.Badley EM, Papageorgiou AC. Visual analogue scales as a measure of pain in a study of overall pain and pain in individual joints at rest and on movement. J Rheumatol. 1989;16:102–105. [PubMed] [Google Scholar]
- 9.Focht BC, Ewing V, Gauvin L, et al. The unique and transient impact of acute exercise on pain perception in older, overweight, or obese adults with knee osteoarthritis. Ann Behav Med. 2002;24:201–210. doi: 10.1207/S15324796ABM2403_05. [DOI] [PubMed] [Google Scholar]
- 10.Robinson ME, Dannecker EA, George SZ, et al. Sex differences in the associations among psychological factors and pain report: a novel psychophysical study of patients with chronic low back pain. J Pain. 2005;6:463–470. doi: 10.1016/j.jpain.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Van Dillen LR, Sahrmann SA, Norton BJ, et al. The effect of modifying patient-preferred spinal movement and alignment during symptom testing in patients with low back pain: a preliminary report. Arch Phys Med Rehabil. 2003;84:313–322. doi: 10.1053/apmr.2003.50010. [DOI] [PubMed] [Google Scholar]
- 12.Roe C, Knardahl S, Vollestad NK. Muscle activation during isometric contractions in workers with unilateral shoulder myalgia. J Musculoskelet Pain. 2000;8:57–73. [Google Scholar]
- 13.Cook DB, Nagelkirk PR, Poluri A, et al. The influence of aerobic fitness and fibromyalgia on cardiorespiratory and perceptual responses to exercise in patients with chronic fatigue syndrome. Arthritis Rheum. 2006;54:3351–3362. doi: 10.1002/art.22124. [DOI] [PubMed] [Google Scholar]
- 14.Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain. 2007;11:39–47. doi: 10.1016/j.ejpain.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Mengshoel AM, Vollestad NK, Forre O. Pain and fatigue induced by exercise in fibromyalgia patients and sedentary healthy subjects. Clin Exp Rheumatol. 1995;13:477–482. [PubMed] [Google Scholar]
- 16.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Valkeinen H, Hakkinen A, Hannonen P, et al. Acute heavy-resistance exercise-induced pain and neuromuscular fatigue in elderly women with fibomyalgia and in healthy controls. Arthritis Rheum. 2006;54:1334–1339. doi: 10.1002/art.21751. [DOI] [PubMed] [Google Scholar]
- 18.Martins IP, Gouveia RG, Parreira E. Kinesiophobia in migraine. J Pain. 2006;7:445–451. doi: 10.1016/j.jpain.2006.01.449. [DOI] [PubMed] [Google Scholar]
- 19.Gustin SM, Wrigley PJ, Gandevia SC, et al. Movement imagery increases pain in people with neuropathic pain following complete thoracic spinal cord injury. Pain. 2008;137:237–244. doi: 10.1016/j.pain.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Hensing GK, Sverker AM, Leijon GS. Experienced dilemmas of everyday life in chronic neuropathic pain patients--results from a critical incident study. Scand J Caring Sci. 2007;21:147–154. doi: 10.1111/j.1471-6712.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 21.Phillips M, Flemming N, Tsintzas K. An exploratory study of physical activity and perceived barriers to exercise in ambulant people with neuromuscular disease compared with unaffected controls. Clin Rehabil. 2009;23:746–755. doi: 10.1177/0269215509334838. [DOI] [PubMed] [Google Scholar]
- 22.Morris ME, Henderson IW. Analgesic activity of floctafenine after cholecystectomy. Clin Pharmacol Ther. 1978;23:383–389. doi: 10.1002/cpt1978234383. [DOI] [PubMed] [Google Scholar]
- 23.Mense S, Meyer H. Bradykinin-induced modulation of the response behaviour of different types of feline group III and IV muscle receptors. J Physiol. 1988;398:49–63. doi: 10.1113/jphysiol.1988.sp017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ro JY, Harriott A, Crouse U, et al. Innocuous jaw movements increase c-fos expression in trigeminal sensory nuclei produced by masseter muscle inflammation. Pain. 2003;104:539–548. doi: 10.1016/S0304-3959(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 25.Barr S, Bellamy N, Buchanan WW, et al. A comparative study of signal versus aggregate methods of outcome measurement based on the WOMAC Osteoarthritis Index. Western Ontario and McMaster Universities Osteoarthritis Index. J Rheumatol. 1994;21:2106–2112. [PubMed] [Google Scholar]
- 26.Bellamy N, Buchanan WW, Goldsmith CH, et al. Signal measurement strategies: are they feasible and do they offer any advantage in outcome measurement in osteoarthritis? Arthritis Rheum. 1990;33:739–745. doi: 10.1002/art.1780330518. [DOI] [PubMed] [Google Scholar]
- 27.Papageorgiou AC, Badley EM. The quality of pain in arthritis: the words patients use to describe overall pain and pain in individual joints at rest and on movement. J Rheumatol. 1989;16:106–112. [PubMed] [Google Scholar]
- 28.Duncan R, Peat G, Thomas E, et al. Symptoms and radiographic osteoarthritis: not as discordant as they are made out to be? Ann Rheum Dis. 2007;66:86–91. doi: 10.1136/ard.2006.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan MJ, Thibault P, Andrikonyte J, et al. Psychological influences on repetition-induced summation of activity-related pain in patients with chronic low back pain. Pain. 2009;141:70–78. doi: 10.1016/j.pain.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Dannecker EA, Gagnon CM, Jump RL, et al. Self-care behaviors for muscle pain. J Pain. 2004;5:521–527. doi: 10.1016/j.jpain.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Dannecker EA, Gormley VL, Robinson ME. Sex differences in muscle pain: self-care behaviors and effects on daily activities. J Pain. 2008;9:200–209. doi: 10.1016/j.jpain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price DD, Harkins SW, Baker C. Sensory-affective relationships among different types of clinical and experimental pain. Pain. 1987;28:297–307. doi: 10.1016/0304-3959(87)90065-0. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of pain assessment. New York: Guilford Press; 2001. pp. 135–151. [Google Scholar]
- 34.Neumann DA. Kinesiology of the musculoskeletal system: foundations for rehabilitation. 2nd ed. St. Louis, MO: Mosby Elsevier; 2010. [Google Scholar]
- 35.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 36.Frey Law LA, Evans S, Knudtson J, et al. Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J Pain. 2008;9:714–721. doi: 10.1016/j.jpain.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Nie H, Kawczynski A, Madeleine P, et al. Delayed onset muscle soreness in neck/shoulder muscles. Eur J Pain. 2005;9:653–660. doi: 10.1016/j.ejpain.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Slater H, Arendt-Nielsen L, Wright A, et al. Experimental deep tissue pain in wrist extensors-a model of lateral epicondylalgia. Eur J Pain. 2003;7:277–288. doi: 10.1016/S1090-3801(02)00141-6. [DOI] [PubMed] [Google Scholar]
- 39.Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaible HG, Schmelz M, Tegeder I. Pathophysiology and treatment of pain in joint disease. Adv Drug Deliv Rev. 2006;58:323–342. doi: 10.1016/j.addr.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Dougherty PM, Sluka KA, Sorkin LS, et al. Neural changes in acute arthritis in monkeys. I. Parallel enhancement of responses of spinothalamic tract neurons to mechanical stimulation and excitatory amino acids. Brain Res. 1992;17:1–13. doi: 10.1016/0165-0173(92)90002-4. [DOI] [PubMed] [Google Scholar]
- 43.Neumann S, Doubell TP, Leslie T, et al. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 44.Sluka KA, Doughtery PM, Sorkin LS, et al. Neural changes in acute arthritis in monkeys. III. Changes in substance P, calcitonin gene-related peptide and glutamate in the dorsal horn of the spinal cord. Brain Res Rev. 1992;17:29–38. doi: 10.1016/0165-0173(92)90004-6. [DOI] [PubMed] [Google Scholar]
- 45.Xu GY, Zhao ZQ. Change in excitability and phenotype of substance P and its receptor in cat Abeta sensory neurons following peripheral inflammation. Brain Res. 2001;923:112–119. doi: 10.1016/s0006-8993(01)03203-6. [DOI] [PubMed] [Google Scholar]
- 46.Zheng JH, Song XJ. Abeta-afferents activate neurokinin-1 receptor in dorsal horn neurons after nerve injury. Neuroreport. 2005;16:715–719. doi: 10.1097/00001756-200505120-00012. [DOI] [PubMed] [Google Scholar]
- 47.MacIntyre DL, Sorichter S, Mair J, et al. Markers of inflammation and myofibrillar proteins following eccentric exercise in humans. Eur J Appl Physiol. 2001;84:180–186. doi: 10.1007/s004210170002. [DOI] [PubMed] [Google Scholar]
- 48.Smith L. Acute inflammation: The underlying mechanism in delayed onset muscle soreness? Med Sci Sports Exerc. 1991;23:542–551. [PubMed] [Google Scholar]
- 49.Taguchi T, Matsuda T, Tamura R, et al. Muscular mechanical hyperalgesia revealed by behavioural pain test and c-Fos expression in the spinal dorsal horn after eccentric contraction in rats. J Physiol. 2005;564:259–268. doi: 10.1113/jphysiol.2004.079483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taguchi T, Sato H, Mizumura K. Augmented mechanical response of muscle thin-fiber sensory receptors recorded from rat muscle-nerve preparations in vitro after eccentric contraction. J Neurophysiol. 2005;94:2822–2831. doi: 10.1152/jn.00470.2005. [DOI] [PubMed] [Google Scholar]
- 51.Dobkin PL, Abrahamowicz M, Fitzcharles MA, et al. Maintenance of exercise in women with fibromyalgia. Arthritis Rheum. 2005;53:724–731. doi: 10.1002/art.21470. [DOI] [PubMed] [Google Scholar]
- 52.Kraus H, Nagler W, Melleby A. Evaluation of an exercise program. Am Fam Physician. 1983;28:153–158. [PubMed] [Google Scholar]
- 53.Litcher-Kelly L, Stone AA, Broderick JE, et al. Associations among pain intensity, sensory characteristics, affective qualities, and activity limitations in patients with chronic pain: a momentary, within-person perspective. J Pain. 2004;5:433–439. doi: 10.1016/j.jpain.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Mailloux J, Finno M, Rainville J. Long-term exercise adherence in the elderly with chronic low back pain. Am J Phys Med Rehabil. 2006;85:120–126. doi: 10.1097/01.phm.0000197580.64079.3d. [DOI] [PubMed] [Google Scholar]
- 55.Medina-Mirapeix F, Escolar-Reina P, Gascon-Canovas JJ, et al. Predictive factors of adherence to frequency and duration components in home exercise programs for neck and low back pain: an observational study. BMC Musculoskelet Disord. 2009;10:155. doi: 10.1186/1471-2474-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minor MA, Brown JD. Exercise maintenance of persons with arthritis after participation in a class experience. Health Ed Q. 1993;20:83–95. doi: 10.1177/109019819302000108. [DOI] [PubMed] [Google Scholar]
- 57.Mori DL, Sogg S, Guarino P, et al. Predictors of exercise compliance in individuals with Gulf War veterans illnesses: Department of Veterans Affairs Cooperative Study 470. Mil Med. 2006;171:917–923. doi: 10.7205/milmed.171.9.917. [DOI] [PubMed] [Google Scholar]
- 58.Sluijs EM, Kok GJ, van der Zee J. Correlates of exercise compliance in physical therapy. Phys Ther. 1993;73:771–782. doi: 10.1093/ptj/73.11.771. [DOI] [PubMed] [Google Scholar]
- 59.van Santen M, Bolwijn P, Landewe R, et al. High or low intensity aerobic fitness training in fibromyalgia: does it matter? J Rheumatol. 2002;29:582–587. [PubMed] [Google Scholar]


