Abstract
The principal aim of this study was to synthesize and characterize pH-sensitive biodegradable triblock copolymers containing a hydrophobic polyacetal segment for controlled drug delivery. Poly(ethylene glycol)-poly(ethyl glyoxylate)-poly(ethylene glycol) (PEG-PEtG-PEG) triblock copolymers with PEG molecular weights 500 (PEtG-PEG500) and 750 (PEtG-PEG750) were synthesized by PEtG end-capping with methoxy PEG via a carbamate linkage. Synthesized amphiphilic PEG-PEtG-PEG was characterized by 1H-NMR spectroscopy. Molecular weights of PEtG-PEG500 and PEtG-PEG750 were determined to be 2,823 and 3,387, respectively, by gel permeation chromatography. The polymers with a biodegradable polyacetal block underwent pH-dependent degradation via an acid-catalyzed hydrolysis. Paclitaxel (PTX)-loaded polymeric micelles were prepared by a dialysis method and the amount of PTX incorporated into the polymeric micelle formulations was 45,000 times greater than the water solubility of PTX at room temperature. The polymeric micelles prepared from the amphiphilic PEG-PEtG-PEG triblock copolymers have released the loaded PTX in a pH-dependent manner. The novel PEtG-based amphiphilic block copolymers can find applications for targeted and controlled drug delivery to the acidic environments found in tumors and intracellular compartments.
Keywords: biodegradable, controlled release, pH-sensitive, polyacetal, polymeric micelles
1. Introduction
Bioresponsive drug delivery systems have recently attracted much attention because of their capability to release incorporated bioactive compounds in a responsive manner in accordance with the signals stemming from disease-associated microenvironment changes. Such systems have frequently been engineered to respond to pH changes (Ganta et al., 2008), temperature changes (Bikram et al., 2008), biological molecules (Miyata et al., 2002) and proteolytic enzymes (Law and Tung, 2009). Among them, especially, pH-sensitive drug delivery systems have been intensively studied because of their potential advantage of controlled drug release in mild acidic environments encountered in solid tumors, inflammatory tissues and intracellular endosomal/lysosomal compartments (Lee et al., 2008).
For pH-responsive targeted drug delivery, various systems have been developed to include liposomes, drug-polymer conjugates, dendrimers and polymeric micelles. pH-sensitive liposomes have been prepared with PEG-di-orthoester-lipid conjugate (Guo and Szoka, 2001), fusogenic peptide (Yamada et al., 2005) or pH-sensitive dioleoylphosphatidyl-ethanolamine (DOPE) (Karanth and Murthy, 2007). To elicit pH-sensitivity in mildly acidic physiological conditions, various synthetic approaches have been adopted to include acid-labile linkages between polymer and a drug in main backbones or side chains. Block copolymers conjugated with adriamycin via acid-labile hydrazone bonds self-assemble into polymeric micelles which release conjugated drug in an acidic intracellular compartment upon cleavage of hydrazone bonds (Bae et al., 2007). The hydrazone linkage has also been used for tumor-targeted delivery of doxorubicin in multi-arm block copolymer micelles (Prabaharan et al., 2009).
Acetal linkages have also been exploited for the development of pH-sensitive drug carriers because they undergo a rapid hydrolysis at an acidic pH. Particularly it is shown that the degradation of acetals mainly results in alcohols and aldehydes which are usually biocompatible and do not cause local acidosis which is frequently associated with polyester biodegradation (Khaja et al., 2007). Acetal-based acid-labile drug carriers have been developed as drug-polymer conjugates (Gillies et al., 2004), polymersomes (Chen et al., 2010) and thermoreversible gel with pH-dependent degradation (Garripelli et al., 2010). Furthermore, polyacetals comprising of repeated acetal bonds have been utilized for the preparation of doxorubicin-polyacetal conjugates (Tomlinson et al., 2003) and a particulate delivery system for proteins (Khaja et al., 2007; Paramonov et al., 2008).
Polyacetals have also been synthesized from alkyl glyoxylate by anionic or cationic polymerization. Alkyl glyoxylates have been considered as a useful reagent in organic chemistry due to the reactivity at α-keto-ester functionality. Among alkyl glyoxylates, methyl glyoxylate (MG) has been first polymerized in industry for a potential application as a detergent builder or a complexing agent (Brachais et al., 1997). The synthesized polyacetal, poly(methyl glyoxylate) (PMG), has also been examined for biomedical applications such as drug delivery and tissue engineering because it underwent hydrolytic degradation in an aqueous environment (Brachais et al., 1998b, 1999). Especially for a drug delivery application, PMG has been formulated into a hydrophobic matrix incorporating metoprolol (Brachais et al., 1998a), and its saponified amphiphilic copolymer has enhanced the bioavailability of progesterone in a sublingual dosage form (Vaugelade et al., 2001). Even though the expected main degradation product from PMG is glyoxylic acid which is the primary precursor of oxalic acid and an intermediate in the conversion of glycolic acid to glycine in the body, a critical limitation of PMG for biomedical applications is to release methanol upon polymer degradation. To overcome the limitation, poly(ethyl glyoxylate) (PEtG) has been recently synthesized by anionic polymerization of ethyl glyoxylate (EtG) (Burel et al., 2003). The ultimate degradation products of PEtG, ethanol and glyoxylic acid, may justify the great potential of PEtG for pharmaceutical applications (Belloncle et al., 2008). Biodegradability and presumed biocompatibility of PEtG upon acid-catalyzed hydrolysis make PEtG as a suitable material for the development of a pH-sensitive drug delivery system.
In this study, amphiphilic triblock copolymers were synthesized from a hydrophobic polyacetal, PEtG. To provide an amphiphilic property as well as biocompatibility, hydrophilic methoxy poly(ethylene glycol) (mPEG) was attached to the both ends of the PEtG block. Poorly water-soluble anticancer drug, paclitaxel (PTX), was selected as a model drug for the preparation of polymeric micelles. To investigate pH-dependent polymer degradation and PTX release, in vitro drug release studies were performed in release media with varied pH.
2. Materials and methods
2.1. Materials
EtG was purchased from Fluka Chemical Company (Milwaukee, WI, USA) as a 50% w/v solution in toluene. Hexamethylene diisocyanate (HMDI), dibutyltin dilaurate (DBTDL),triethylamine (TEA) and mPEG of molecular weights 500 and 750 (mPEG500 and mPEG750, respectively) were obtained from Sigma (St. Louis, MO, USA). Paclitaxel (PTX) was supplied by LC laboratories®(Woburn, MA, USA). All other chemicals were of reagent grade and used without further purification.
2.2. Synthesis of PEG-PEtG-PEG triblock copolymers
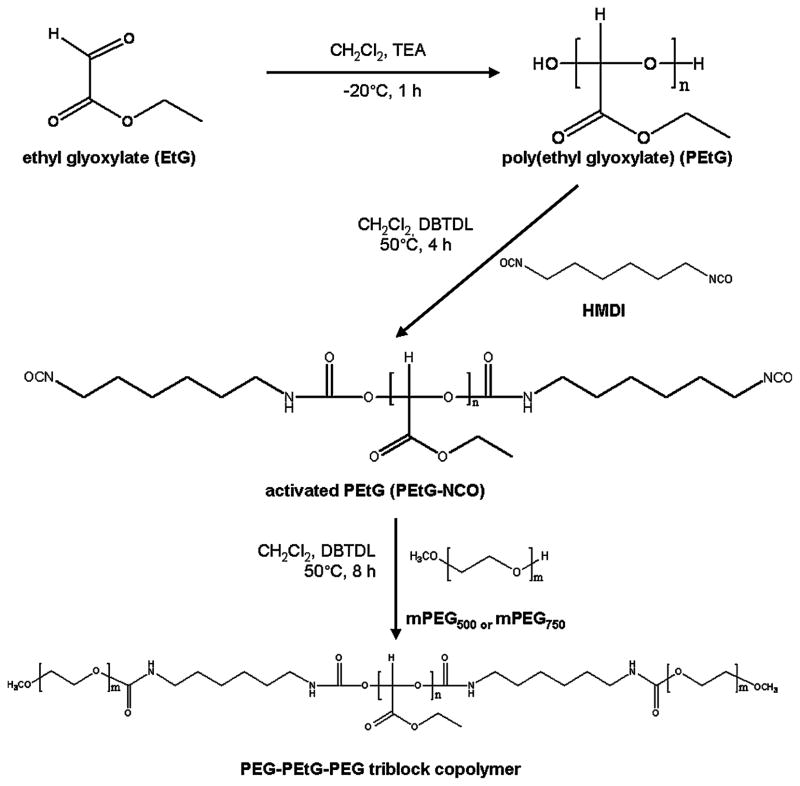
The EtG solution in toluene (50%, w/w) was purified by distillation at 50°C under vacuum (48 mmHg) over P2O5. The clear yellow liquid monomer was added to CH2Cl2 and polymerized for 1 h at −20°C under nitrogen atmosphere by an anionic polymerization using TEA as an initiator. The synthesized PEtG was mixed with an excess amount of HMDI (more than 5 molar equivalents to PEtG) and then DBTDL was added as a catalyst. The mixture was stirred for 30 min at room temperature and reacted for another 6 h at 50°C under reflux. The activated PEtG, PEtG-NCO, was isolated by the precipitation of the mixture in an excess amount of petroleum ether. The precipitate was washed twice with petroleum ether and finally dissolved in CH2Cl2 for the next end-capping reaction with mPEG. An excess amount of dried mPEG in CH2Cl2 (more than 2 molar equivalents to PEtG) was slowly added to the activated PEtG solution using a dropping funnel. The end-capping reaction proceeded for another 8 h at 50°C under reflux in the presence of DBTDL as a catalyst. At the end of reaction, the reactant was precipitated and washed twice with petroleum ether. The crude polymer was dissolved in deionized water and precipitated by heating the polymer solution at 70°C to remove remaining unreacted mPEG. The final polymer precipitate was freeze-dried to give a soft brown waxy solid. PEtG-based block copolymers with different molecular weights of mPEG, PEtG-PEG500 and PEtG-PEG750, were finally obtained.
2.3. Characterization of PEG-PEtG-PEG triblock copolymers
The chemical structures of the PEG-PEtG-PEG triblock copolymers were analyzed by 1H NMR. The polymer was dissolved in CDCl3 and NMR spectra were recorded on a 400 MHz NMR spectrometer (Bruker Ultrashield 400 PLUS, Germany). The molecular weights of the synthesized polymers were determined by gel permeation chromatography (GPC). GPC was run on a Waters Breeze system equipped with a binary pump (Waters 1525), a refractive index detector (Waters 2414), and a Styragel HR4E column Styragel HR4E column (300 × 7.8 mm I.D., 5 μm particle size). THF as a mobile phase was eluted at a flow rate of 1.0 mL/min at 25°C. Polystyrene standards in the molecular weight range of 600–20,000 daltons were also run to obtain a calibration curve. The molecular weights of the polymers were calculated from the retention times of the polymers using the calibration curve. The critical micelle concentrations (CMCs) of the polymers were determined by a dye solubilization method using 1,6-diphenyl-1,3,5-hexatriene (DPH) (Ahn et al., 2005). Polymer solutions in the concentration range of 1.0 × 10−4 to 1.0 wt% were prepared by serial dilutions. Then, 25 μL of 0.4 mM DPH solution in methanol was added to 2.5 mL of each polymer solution and the polymer solution was incubated for 24 h in a dark place. The difference in the absorbance of DPH at 377 and 391nm was plotted against polymer concentrations. A cross-point of the two extrapolated lines was defined as the CMC of the polymer.
2.4. pH-dependent degradation of polymers
The solutions of PEG-PEtG-PEG triblock copolymers were prepared at a concentration of 5 wt% in deionized water. Then, 200 μL of the polymer solution was placed in a 1.5mL microcentrifuge tube and 800 μL of phosphate buffer with different pH value (pH 5.0, 6.5 and 7.4) was added. After incubation at 37°C for a predetermined time, a polymer solution was frozen and lyophilized for a GPC measurement. The dried polymer was dissolved in 1mL dichloromethane and centrifuged at 10,000 g for 10 min to remove the precipitated buffer components. The supernatant was collected and vacuum dried to prepare GPC sample. GPC was run to determine the distribution of polymer molecular weight as described in Section 2.3.
2.5. Preparation of polymeric micelles
PTX (15 mg) and a PEG-PEtG-PEG triblock copolymer (50 mg) were completely dissolved in 500 μL of acetone and the organic solvent was removed in vacuum. Then, 1 mL of phosphate buffered saline (PBS, pH 7.4) was added to the dried mixture and the solution was mixed overnight at room temperature under magnetic stirring at a speed of 200 rpm The clear solution was dialyzed against 2 L of PBS using a cellulose dialysis membrane with a molecular cutoff value of 1,000. The dialysis media were refreshed every 12 h for 2 days. The dialysate was filtered through a 0.45 μm syringe filter and freeze-dried for storage.
2.6. Characterization of polymeric micelles
The mean particle size and polydispersity index (PI) of polymeric micelles were measured by a dynamic light scattering method using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). The zeta potential of the polymeric micelles was also determined by the measurement of electrophoretic mobility on the instrument. All measurements were performed at room temperature and PBS was used for the dilution of samples if necessary. The formation of polymeric micelles from PEG-PEtG-PEG triblock copolymers was confirmed by transmission electron microscopy (TEM). Briefly, 1 μL of solution sample was place on a TEM grid and the TEM grid treated with the sample was negatively stained for 30 sec with 2% (w/v) phosphotungstic acid. Excess staining solution was carefully removed by touching the grid edge with filter paper. After air-drying the sample overnight, TEM images of negatively stained specimens were obtained using a Tecnai G2 F30 (FEI Co., Eindhoven, Netherlands) operated at 300 kV.
The loading efficiency of PTX in the polymeric micelle was determined by direct measurements PTX concentration after dissolving the dried PTX-containing micelles in acetonitrile. The amount of PTX was determined by a HPLC method. The HPLC system consisted of a binary pump (Waters 1525), a UV detector (Waters 2487) and an autosampler (Waters 717). Analytical column was Waters C18 Symmetry column (150 × 3.9 mm I.D., 5 μm particle size) and the eluent was a mixture of acetonitrile and water (55:45, v/v). The flow rate was set at 1.0 mL/min and the injection volume was 20 μL. PTX was detected at an absorption wavelength of 227 nm.
2.7. pH-dependent PTX release from polymeric micelles
In vitro PTX release from polymeric micelles was studied at pHs 5.0, 6.5 and 7.4. An amount of polymeric micelles equivalent to 100 μg of PTX was taken and suspended with 1 mL of PBS. The PTX-incorporated polymeric micelle solution was placed into a cellulose dialysis bag with a molecular cutoff value of 1,000. The dialysis bag was placed in 40 mL of release media containing 0.8 M sodium salicylate. Release media were stirred magnetically at a speed of 100 rpm at 37°C. At each designated time point, 1 mL of sample was collected and the same volume of fresh release media was added to maintain the total volume. Samples were collected over 24 h. The amounts of released PTX were determined by the HPLC method described in Section 2.6.
2.8. Statistical analysis
Statistical analyses of data were performed using Student’s t-test and ANOVA. The differences were considered significant for p value of <0.05.
3. Results
3.1. Characterization of PEG-PEtG-PEG triblock copolymers
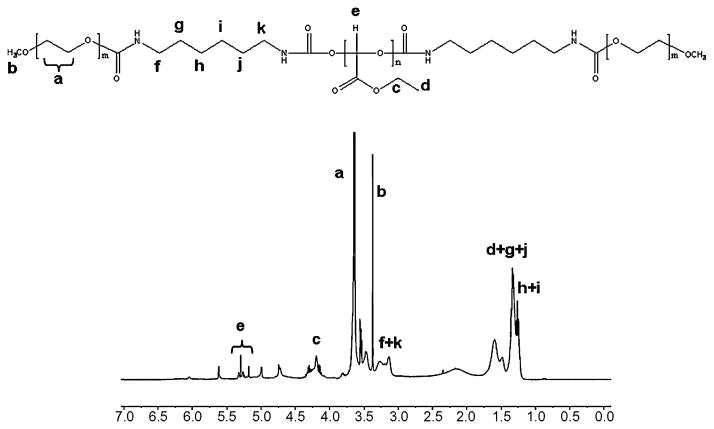
PEG-PEtG-PEG triblock copolymers, PEtG-PEG500 and PEtG-PEG750, were synthesized by anionic polymerization and the following simple mPEG end-capping using as shown in Figure 1. An acid-labile polyacetal, PEtG, was synthesized by an anionic polymerization of freshly distilled EtG. The hydroxyl extremities of PEtG were reacted with isocyanate groups of HMDI to give stable carbamate bonds and reaction sites available for end-capping with mPEG. Remaining isocyanate groups in the activated PEtG underwent an end-capping reaction with mPEG of molecular weights 500 and 750 to achieve biodegradable BAB type triblock copolymers. The polymer synthesis was confirmed by the analysis of chemical structures using NMR spectroscopy. The NMR spectrum of PEtG-PEG500 in Figure 2 showed characteristic peaks of mPEG at 3.6 ppm (−CH2CH2O-) and 3.3 ppm (CH3O-) while presenting methylene (−CH2-) and methyl (CH3-) protons of ethyl ester group in PEtG at 4.2 and 1.3 ppm, respectively. The formation of acetal bond in PEtG was confirmed by the methine (-CH) protons at 5.2 – 5.4 ppm.
Figure 1.
Reaction scheme for the synthesis of PEG-PEtG-PEG block copolymers.
Figure 2.
1H NMR spectrum of PEtG-PEG500 in CDCl3
GPC was used to determine number-average molecular weights (Mn) and polydispersities of PEG-PEtG-PEG triblock copolymers. As summarized in Table 1, the molecular weights of PEtG-PEG500 and PEtG-PEG750 were determined to be 2,823 and 3,387, respectively. It is postulated that the molecular weight difference between PEtG-PEG500 and PEtG-PEG750 seems to be originated from the difference in mPEG molecular weight. The difference in theoretical molecular weights between PEtG-PEG500 and PEtG-PEG750 was calculated to be 500 with an assumption that complete end-capping of PEtG with mPEG at both ends. The difference in the polymer molecular weights was determined to be 560 by GPC measurements, which was in accordance with the theoretical difference in polymer molecular weights. The molecular weight of hydrophobic PEtG block was calculated to be 1,800 by the subtraction of the total PEG molecular weight from the copolymer molecular weight.
Table 1.
Characterization results of synthesized PEG-PEtG-PEG block copolymers.
Number average molecular weight determined by GPC
Polydispersity based on GPC measurements
CMCs of PEtG-PEG500 and PEtG-PEG750 were determined by a hydrophobic dye solubilization method using DPH. The hydrophobic dye used in this study has a tendency to partition into the hydrophobic core of the micelles resulting in a sudden increase in dye solubility and UV absorbance at the CMCs. The CMCs of the triblock copolymers were determined by the extrapolation of the absorbance versus logarithmic polymer concentration. The CMCs of PEtG-PEG500 and PEtG-PEG750 were found to be 0.0050 (± 0.0004) wt% and 0.0049 (± 0.0003) wt%, respectively (Table 2). There was no noticeable CMC difference between PEtG-PEG500 and PEtG-PEG750.
Table 2.
Particle sizes, polydispersity indices, zeta potentials of prepared polymeric micelles with or without PTX incorporation. PTX-loading efficiencies into the polymeric micelles were also determined (n=3)
| Formulation | Mean diameter (nm) a | Polydispersity index b | Zeta potential (mV) | Loading efficiency (%) |
|---|---|---|---|---|
| PEtG-PEG500 | 11.6 ± 0.2 | 0.137 ± 0.004 | − 8.00 ± 0.70 | - |
| PEtG-PEG500 + PTX | 46.0 ± 0.9 | 0.214 ± 0.011 | − 8.21 ± 0.70 | 93.4 ± 1.6 |
| PEtG-PEG750 | 14.3 ± 0.2 | 0.112 ± 0.008 | − 7.90 ± 0.68 | - |
| PEtG-PEG750 + PTX | 47.4 ± 1.2 | 0.235 ± 0.016 | − 8.10 ± 1.44 | 95.0 ± 0.5 |
Hydrodynamic diameter determined by dynamic light scattering
Relative width of the radius distribution determined by dynamic light scattering
3.2. pH-dependent polymer degradation
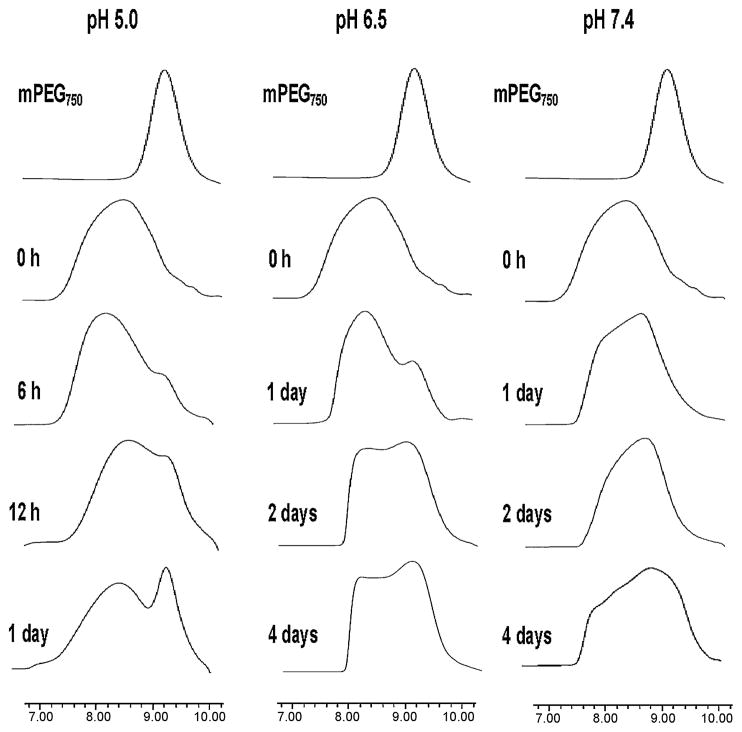
The pH-dependent degradation of PEtG-PEG500 and PEtG-PEG750 was studied at different pHs. The degradation of PEG-PEtG-PEG triblock copolymers was determined by the changes in molecular weight distribution upon GPC measurements. GPC chromatograms only for PEtG-PEG750 degradation are presented in Figure 3 because PEtG-PEG500 showed a similar result. PEtG-based amphiphilic block copolymer degraded more rapidly in an acidic pH than in neutral pH. At pH 5.0, the peak of mPEG750 started to appear after 6 h incubation at 37°C and the peak was more pronounced after 1 day. Degradation of the polyacetal block in an acidic pH might release mPEG750. The degradation of PEtG-PEG750 at pH 6.5 was much slower than that at pH 5.0. The peak of mPEG750 appeared after 1 day incubation and the intensity of the peak even after 4 day was not even comparable to that from a sample incubated for 1 day at pH 5.0. However, the distinct mPEG750 peak was not clearly observed even after 4 days incubation at pH 7.4. It is interesting to note that polymer degradation at neutral pH made the original polymer band shift gradually towards a longer retention time without a clear demonstration of the release of free mPEG750 on GPC chromatograms. It was assumed that the slower polymer degradation at neutral pH released a small amount of mPEG750 with small PEtG fragments, which could not be visualized on the GPC chromatogram. These polymer degradation results clearly support that the synthesized polyacetal-based block copolymers underwent an acid-catalyzed hydrolysis.
Figure 3.
pH-dependent degradation of PEtG-PEG750 determined by GPC measurements. Phosphate buffers of pHs 5.0, 6.5 and 7.4 were used for polymer degradation. The PEG-PEtG-PEG block copolymers released free mPEG upon polymer degradation.
3.3. Characterization of polymeric micelles
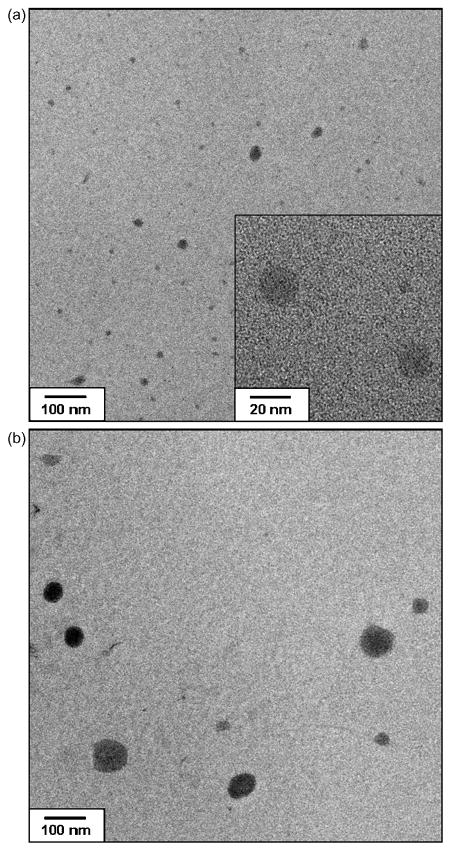
PTX-loaded polymeric micelles were prepared by a dialysis method and lyophilized for storage. The prepared PTX-loaded polymeric micelles could be easily reconstituted prior to experiments. The physicochemical properties of the polymeric micelles were examined in terms of particle size, PI, zeta potential and drug loading efficiency (Table 2). The mean diameters of PTX-free polymeric micelles prepared from PEtG-PEG500 and PEtG-PEG750 were 11.6 nm and 14.3 nm, respectively. These small particle sizes can be attributed to the low molecular weight of the triblock copolymers. When PTX was incorporated into the polymeric micelles, the mean diameters of the micelles increased to 46.0 nm and 47.4 nm for PEtG-PEG500 and PEtG-PEG750, respectively. As shown in Figure 4, the particle size increase of PEtG-PEG polymeric micelles by PTX incorporation was confirmed by TEM. The particle size increase observed with TEM images is in accordance with the results of particle size analysis. The PIs of polymeric micelles also increased to be greater than 0.2 with PTX incorporation into the micelles. Even though the PIs of PTX-loaded polymeric micelles was greater than 0.2, the distribution of particle size was still unimodal. The polymeric micelle-based PTX formulation was readily injectable via a 25 G syringe. The determined zeta potential of the PTX-loaded polymeric micelles was slightly negative, − 8 mV, which was similar to that of PTX-free micelles. These results consistent with the reported results that the incorporation of PTX into nanocarriers such as micelles and nanoparticles did not alter the zeta potential of the carriers (Liu et al., 2005; Wang et al., 2005; Liang et al., 2006; Lee et al., 2007).
Figure 4.
TEM images of (a) PTX-free and (b) PTX-loaded polymeric micelles prepared from PEtG-PEG500
The concentrations of PTX in polymeric micelle solutions were determined to be 14.0 and 14.3 mg/mL for PEtG-PEG500 and PEtG-PEG750 polymeric micelles, respectively. PTX loading into the polymeric micelles was calculated by the following equation.
PTX loading efficiencies into PEtG-PEG500 and PEtG-PEG750 polymeric micelles were calculated to be 93.4% and 95.0%, respectively. PTX contents in polymeric micelles (the amount of PTX in polymeric micelles /total weight of PTX-loading micelles × 100%) were also close to 30% (28.0% for PEtG-PEG500 and 28.5% for PEtG-PEG750). Taken together, the achieved PTX concentrations using the polymeric micelle formulations were 45,000 times greater than the water solubility of PTX (0.3 μg/mL) at room temperature.
3.4. pH-dependent PTX release from polymeric micelles
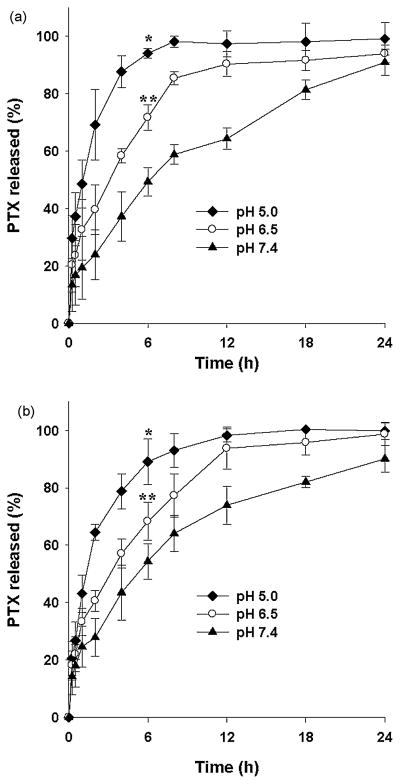
In order to evaluate the controlled drug delivery capability of PEtG-based copolymer micelles, in vitro PTX release from the polymeric micelles was tested over 24 h at different pHs. As shown in Figure 5, the PTX release from the polymeric micelles was significantly dependent on the pH of the release medium. In the case of PEtG-PEG500 polymeric micelles, about 50% of incorporated PTX was released within 1 h at pH 5.0 while only 20% of PTX was released at pH 7.4. Cumulative percentages of released PTX were 49.3%, 71.7%, and 94.1% at pH 7.4, 6.5 and 5.0, respectively, after 6 h drug release. However, it is shown that more than 90% of incorporated PTX was released within 24 h at all pHs chosen for the drug release study. With respective to in vitro PTX release, PEtG-PEG750 polymeric micelles were slightly different from PEtG-PEG500 polymeric micelles. PEtG-PEG750 polymeric micelles released 54.4%, 68.3%, and 89.1% of PTX within 6 h at pH 7.4, 6.5 and 5.0, respectively. In vitro PTX release at different pHs has demonstrated that the polymeric micelles prepared from PEtG-based amphiphilic block copolymers can release PTX via pH-dependent polymer degradation.
Figure 5.
PTX release from (a) PEtG-PEG500 polymeric micelles and (b) PEtG-PEG750 polymeric micelles at pHs 5.0, 6.5 and 7.4. Phosphate buffer solutions containing 0.8 M sodium salicylate were used as release media. *Significant difference from the cumulative percent PTX release at pH 6.5 after 6 h drug release (p<0.05). **Significant difference from the cumulative percent PTX release at pH 7.4 after 6 h drug release (p<0.05). The results are expressed as the mean ± SD (n=3).
4. Discussion
As for desirable targeted drug delivery, drug delivery systems should be able to unload cargo drug molecules in response to pathophysiological changes occurred in the microenvironment of target tissues such as pH changes and/or the expression of biomolecules and enzymes. In the present study, we have considered acidic pH in intracellular compartments, tumor and inflammatory tissues as an important endogenous signal for the design of a bioresponsive drug vehicle. Indeed, the extracellular pH of solid tumors is acidic (pH 6.5–7.2) compared to normal tissues or blood (pH 7.4) (Leeper et al., 1994; Ojugo et al., 1999). This slightly acidic microenvironment inside tumors is primarily originated from the increased glycolysis in cancer, which may be related to the invasive properties of cancer cells that subsequently lead to the destruction of extracellular matrices and normal tissues (Yamagata et al., 1998). In addition, the pH of intracellular compartments during the endocytic pathway varies from 5.5–6.0 in endosomes to 4.5–5.0 in lysosomes which are significantly lower than cytosolic pH (Mellman et al., 1986). Thus, acid-labile polyacetals have been considered as promising materials for the development of pH-responsive drug delivery systems targeting tumors and intracellular drug trafficking.
For a biodegradable pH-responsive polymeric drug delivery system, acetal-based PEtG was synthesized and modified further into an amphiphilic block copolymer. Anionically polymerized PEtG requires a correct end-capping reaction, otherwise it depolymerizes because of the low ceiling temperature. It has been suggested that the end-capping of polyglyoxylates via etherification or esterification can not prevent the spontaneous depolymerization in diluted media at room temperature. After the end-capping a polyglyoxylate with isocyanates such as 2-methyl-2-propenoyl isocyanate, phenyl isocyanate and HMDI, the stability of the final polymer could be improved and thus depolymerization could be inhibited (Brachais et al., 1997). In this study, we used HMDI for both PEtG end-capping and mPEG conjugation. An excess molar amount of HMDI over PEtG, more than 5 molar equivalents to PEtG, was used for the synthesis of PEtG-NCO to prevent the chain extension mediated by the reaction between PEtG and HMDI (−PEtG-HMDI-PEtG-). mPEG modification with HMDI has been widely accepted for polymer coupling and other conjugation reactions (Jeong et al., 1999; Peterson et al., 2002). With mPEG coupling to PEtG, the final PEG-PEtG-PEG copolymers became water-soluble and amphiphilic,indicating that the polymers would have a surfactant-like property. Indeed, both PEtG-PEG500 and PEtG-PEG750 showed CMCs. However, the obtained CMC values were close enough to imply that a slight increase in the molecular weight of hydrophilic PEG block (theoretically 500 in total) did not cause any noticeable difference in the hydration of the hydrophobic dye via micelle formation.
GPC chromatograms of PEG-PEtG-PEG block copolymers degraded at different pHs indicated that PEtG-based block copolymers underwent pH-sensitive polymer degradation. Proposed main mechanism for PEtG degradation would be the combination of polymer chain scission and hydrolysis of side ester groups. The expected degradation products of PEtG are ethyl glyoxylate hydrate (EtGH), ethanol and glyoxylic acid hydrate (GAH) which can be readily hydrated in water. EtGH is known to be rapidly converted into GAH and ethanol in water. However, it has been reported that the hydrolysis of side ester groups in PEtG is rather slow at neutral pH and starts after at least 7 days of incubation in water (Belloncle et al., 2008). The hydrolysis of the side ester groups can result in free carboxylic acids which lower the pH and in turn auto-accelerate the hydrolysis of polymers via cleavages of acetal bonds. Therefore, the use of a buffer can slow down polymer degradation even after 7 days at neutral pH. Our observation with GPC measurements and size analysis confirms the effect of a buffer on the degradation of PEG-PEtG-PEG block copolymers. The polymer degradation in a pH 7.4 phosphate buffer did not cause any noticeable changes in the distribution of polymer molecular weight. Moreover, the particle sizes of polymeric micelles were not changed even after 7 days incubation regardless of PTX loading (data not shown). With the hydrolysis of the side ester groups in PEtG, the sizes of polymeric micelles should be increased by the swelling of the micelles because of repulsive ionic interactions between negatively charged carboxylic acids in the micelle core. The observations based on GPC measurements and micelle sizes consistently postulate that the polymers are stable at least for 7 days in an aqueous medium and the polymer chain cleavage mediated by acid-catalyzed hydrolysis of acetal bonds primarily contributes for the pH-dependent degradation of the polymers.
PTX-loading into the polymeric micelles by dialysis has increased the size of micelles, which can be attributed to the incorporation of PTX molecules into the core of polymeric micelles via hydrophobic interaction. It is known that hydrophobic drugs act as space fillers in the hydrophobic micelle core which increase the size of micelles (Huh et al., 2008). The zeta potential of PTX-loaded micelles was similar to that of PTX-free micelles with a value close to - 8 mV. There was no noticeable difference in zeta potentials between PTX-free and PTX-loaded micelles, indirectly indicating that the polymers were not degraded during micelle preparation. The hydrolysis of the side ester groups, which would be the primary mechanism for PEtG degradation at neutral pH, is expected to expose carboxyl anions and make the zeta potential of polymeric micelles more negative.
PTX release from the PEG-PEtG-PEG polymeric micelles was dependent on the pH of the release medium. Sodium salicylate was included in the release media to achieve a concentration of 0.8 M to warrant sink conditions during in vitro PTX release study. The addition of sodium salicyate has been widely accepted for in vitro PTX release study because it increases PTX solubility without affecting physical properties of polymeric micelles (Huh et al., 2008). Moreover, it has been reported that the presence of sodium salicylate in release media did not affect the release rate of PTX (Cho et al., 2004, Huh et al., 2005). In this study, released PTX samples were collected only for 24 h because more than 90% of incorporated PTX was released within 24 h. This result is comparable to the previous work with hydrotropic polymer micelles (Huh et al., 2008). It is also worthwhile to mention that a type of pH-sensitive polymeric micelle released most of the incorporated drug within 24 h (Alani et al., 2010). Additionally, PTX degradation can be observed in an aqueous medium within 24 h incubation in water (Kim et al.,2008). There was no marked difference between PTX release profiles obtained with PEtG-PEG500 and PEtG-PEG750 micelles. The slight molecular weight difference between the block copolymers resulted from the size difference in hydrophilic PEG block did not seem to influence the overall physicochemical properties of resulting polymeric micelles including particle size, PI, zeta potential, drug loading and drug release as well. However, the PEtG-based block copolymers greatly enhanced the solubility of PTX in water via micelle formation and allowed pH-dependent drug release which can be an important benefit in the design of targeted drug delivery. Moreover, the suitable particle size and outer PEG shell of the PTX-loaded PEG-PEtG-PEG micelles (below 50 nm but greater than 5 nm) may avoid the clearance of the particles via the RES uptake in the liver and rapid renal filtration. The prolonged blood circulation of the PTX-loaded polymeric micelles would allow preferential accumulation of the nano-sized micellar drug carriers in tumor sites by the enhanced permeability and retention (EPR) effect (Greish, 2007).
In conclusion, pH-sensitive biodegradable block copolymers comprising of hydrophobic PEtG and hydrophilic PEG was successfully synthesized and characterized. The synthesized polymers underwent acid-catalyzed degradation and their polymeric micelles were able to release incorporated hydrophobic cancer drug in a pH-dependent manner. The synthesized amphiphilic block copolymers would be suitable for controlled drug delivery directed to the slightly acidic microenvironments found in inflamed tissues, tumors or endosomal/lysosomal compartments of cells.
Acknowledgments
This work was supported in part by NIH/NCRR-P20RR021929 and Korea Research Foundation Grant (KRF-2007-357-E0041) from the Korean Government (MOEHRD) (J-K Kim).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn JS, Suh JM, Lee M, Jeong B. Slow eroding biodegradable multiblock poloxamer copolymers. Polym Int. 2005;54:842–847. [Google Scholar]
- Alani AW, Bae Y, Rao DA, Kwon GS. Polymeric micelles for the pH-dependent controlled, continuous low dose release of paclitaxel. Biomaterials. 2010;31:1765–1772. doi: 10.1016/j.biomaterials.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y, Nishiyama N, Kataoka K. In vivo antitumor activity of the folate-conjugated pH-sensitive polymeric micelle selectively releasing adriamycin in the intracellular acidic compartments. Bioconjug Chem. 2007;18:1131–1139. doi: 10.1021/bc060401p. [DOI] [PubMed] [Google Scholar]
- Belloncle B, Burel F, Oulyadi H, Bunel C. Study of the in vitro degradation of poly(ethyl glyoxylate) Polym Deg Stab. 2008;93:1151–1157. [Google Scholar]
- Bikram M, West JL. Thermo-responsive systems for controlled drug delivery. Expert Opin Drug Deliv. 2008;5:1077–1091. doi: 10.1517/17425247.5.10.1077. [DOI] [PubMed] [Google Scholar]
- Brachais CH, Huguet J, Bunel C. Synthesis, characterization and stabilization of poly(methyl glyoxylate) Polymer. 1997;38:4959–4964. [Google Scholar]
- Brachais CH, Duclos R, Vaugelade C, Huguet J, Capelle-Hue ML, Bunel C. Poly(methylglyoxylate), a biodegradable polymeric material for new drug delivery systems. Int J Pharm. 1998a;169:23–31. [Google Scholar]
- Brachais CH, Huguet J, Bunel C, Brachais L. In vitro degradation of poly(methyl glyoxylate) in water. Polymer. 1998b;39:883–890. [Google Scholar]
- Brachais CH, Huguet J, Bunel C, Brachais L. Identification of small molecules formed from polymethyl glyoxylate degradation in vitro. Polym Deg Stab. 1999;64:243–249. [Google Scholar]
- Burel F, Rossignol L, Pontvianne P, Hartman J, Couesnon N, Bunel C. Synthesis and characterization of poly(ethyl glyoxylate) - a new potentially biodegradable polymer. e-Polymers. 2003;31:1–12. [Google Scholar]
- Chen W, Meng F, Cheng R, Zhong Z. pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: a comparative study with micelles. J Control Release. 2010;142:40–46. doi: 10.1016/j.jconrel.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Cho YW, Lee J, Lee SC, Huh KM, Park K. Hydrotropic agents for study of in vitro paclitaxel release from polymeric micelles. J Control Release. 2004;97(2):249–257. doi: 10.1016/j.jconrel.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Garripelli VK, Kim JK, Namgung R, Kim WJ, Repka MA, Jo S. A novel thermosensitive polymer with pH-dependent degradation for drug delivery. Acta Biomater. 2010;6:477–485. doi: 10.1016/j.actbio.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target. 2007;15:457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- Gillies ER, Goodwin AP, Fréchet JM. Acetals as pH-sensitive linkages for drug delivery. Bioconjug Chem. 2004;15(6):1254–1263. doi: 10.1021/bc049853x. [DOI] [PubMed] [Google Scholar]
- Guo X, Szoka FC., Jr Steric stabilization of fusogenic liposomes by a low-pH sensitive PEG-diortho ester-lipid conjugate. Bioconjug Chem. 2001;12:291–300. doi: 10.1021/bc000110v. [DOI] [PubMed] [Google Scholar]
- Huh KM, Lee SC, Cho YW, Lee J, Jeong JH, Park K. Hydrotropic polymer micelle system for delivery of paclitaxel. J Control Release. 2005;101(1–3):59–68. doi: 10.1016/j.jconrel.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Huh KM, Min HS, Lee SC, Lee HJ, Kim S, Park K. A new hydrotropic block copolymer micelle system for aqueous solubilization of paclitaxel. J Control Release. 2008;126:122–129. doi: 10.1016/j.jconrel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong B, Bae YH, Kim SW. Thermoreversible gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions. Macromolecules. 1999;32:7064–7069. doi: 10.1002/(sici)1097-4636(200005)50:2<171::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Karanth H, Murthy RS. pH-sensitive liposomes--principle and application in cancer therapy. J Pharm Pharmacol. 2007;59:469–483. doi: 10.1211/jpp.59.4.0001. [DOI] [PubMed] [Google Scholar]
- Khaja SD, Lee S, Murthy N. Acid-degradable protein delivery vehicles based on metathesis chemistry. Biomacromolecules. 2007;8:1391–1395. doi: 10.1021/bm061234z. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim JY, Huh KM, Acharya G, Park K. Hydrotropic polymer micelles containing acrylic acid moieties for oral delivery of paclitaxel. J Control Release. 2008;132:222–229. doi: 10.1016/j.jconrel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law B, Tung CH. Proteolysis: a biological process adapted in drug delivery, therapy, and imaging. Bioconjug Chem. 2009;20:1683–1695. doi: 10.1021/bc800500a. [DOI] [PubMed] [Google Scholar]
- Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Control Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Lim SJ, Kim CK. Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials. 2007;28(12):2137–2146. doi: 10.1016/j.biomaterials.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Leeper DB, Engin K, Thistlethwaite AJ, Hitchon HD, Dover JD, Li DJ, Tupchong L. Human tumor extracellular pH as a function of blood glucose concentration. Int J Radiat Oncol Biol Phys. 1994;28:935–943. doi: 10.1016/0360-3016(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Liang HF, Chen SC, Chen MC, Lee PW, Chen CT, Sung HW. Paclitaxel-loaded poly(gamma-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system against cultured HepG2 cells. Bioconjug Chem. 2006;17(2):291–299. doi: 10.1021/bc0502107. [DOI] [PubMed] [Google Scholar]
- Liu SQ, Tong YW, Yang YY. Thermally sensitive micelles self-assembled from poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly(D,L-lactide-co-glycolide) for controlled delivery of paclitaxel. Mol Biosyst. 2005;1(2):158–165. doi: 10.1039/b501756b. [DOI] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Miyata T, Uragami T, Nakamae K. Biomolecule-sensitive hydrogels. Adv Drug Deliv Rev. 2002;54:79–98. doi: 10.1016/s0169-409x(01)00241-1. [DOI] [PubMed] [Google Scholar]
- Ojugo AS, McSheehy PM, McIntyre DJ, McCoy C, Stubbs M, Leach MO, Judson IR, Griffiths JR. Measurement of the extraceullar pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous 19F and 31P probes. NMR Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Paramonov SE, Bachelder EM, Beaudette TT, Standley SM, Lee CC, Dashe J, Fréchet JM. Fully acid-degradable biocompatible polyacetal microparticles for drug delivery. Bioconjug Chem. 2008;19:911–919. doi: 10.1021/bc7004472. [DOI] [PubMed] [Google Scholar]
- Petersen H, Fechner PM, Fischer D, Kissel T. Synthesis, characterization, and biocompatibility of polyethyleneimine-graft-poly(ethylene glycol) block copolymers. Macromolecules. 2002;35:6867–6874. [Google Scholar]
- Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S. Amphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery. Biomaterials. 2009;30:5757–5766. doi: 10.1016/j.biomaterials.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Tomlinson R, Heller J, Brocchini S, Duncan R. Polyacetal-doxorubicin conjugates designed for pH-dependent degradation. Bioconjug Chem. 2003;14:1096–1106. doi: 10.1021/bc030028a. [DOI] [PubMed] [Google Scholar]
- Vaugelade C, Rohmer AC, Burel F, Belleney J, Duclos R, Bunel C. Progesterone freeze-dried systems in sublingual dosage form. Int J Pharm. 2001;229:67–73. doi: 10.1016/s0378-5173(01)00817-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Mongayt D, Torchilin VP. Polymeric micelles for delivery of poorly soluble drugs: preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly(ethylene glycol)-lipid conjugate and positively charged lipids. J Drug Target. 2005;13(1):73–80. doi: 10.1080/10611860400011935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Shinohara Y, Kakudo T, Chaki S, Futaki S, Kamiya H, Harashima H. Mitochondrial delivery of mastoparan with transferrin liposomes equipped with a pH-sensitive fusogenic peptide for selective cancer therapy. Int J Pharm. 2005;303:1–7. doi: 10.1016/j.ijpharm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Hasuda K, Stamato T, Tannock IF. The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br J Cancer. 1998;77:1726–1731. doi: 10.1038/bjc.1998.289. [DOI] [PMC free article] [PubMed] [Google Scholar]