Abstract
Immediate early genes (IEGs) typically are the first genetic responders to a variety of cellular activations. The IEG that encodes activity-regulated cytoskeleton-associated protein (arc/arg3.1) has attracted much interest because its mRNA is transported to and translated near activated synapses. Moreover, arc has been implicated in both long-term potentiation (LTP) and long-term depression (LTD). However, little is known about the time course of altered arc expression during LTP and LTD. Here we characterized arc mRNA levels in area CA1 of the adult rat hippocampus in vivo after LTP-and LTD-inducing stimulations that were identical except for the temporal patterning of the stimulation pulses. We observed a persistent increase in arc mRNA level during LTP. In contrast, during LTD, arc mRNA level first was decreased and then transiently increased relative to control level. These findings demonstrate that arc mRNA is regulated differently during LTP and LTD, and they provide evidence for stimulation-induced down-regulation of mRNA availability during LTD. Findings of abbreviated LTD when transcription was inhibited indicate that the prolonged maintenance of the type of NMDA receptor-dependent LTD studied here requires de novo transcription. Furthermore, lack of evidence for a LTD-associated change in the mRNA level of the IEG zif268 demonstrates that the decrease in arc mRNA during LTD is not a general genetic response. Thus, the regulation of arc expression not only differs between LTP and LTD but also diverges from that of other IEGs implicated in activity-dependent synaptic plasticity.
Keywords: zif268, immediate early gene, synaptic plasticity, NMDA receptor, RNA stability
Introduction
Alteration in the expression of immediate early genes (IEGs) is the first genetic response to many kinds of cellular activations (Dragunow, 1996; Lanahan and Worley, 1998; Miyashita et al., 2008). Many IEGs regulated by neural activity encode transcription factors; however, some encode effector proteins. One effector protein-encoding gene that has received much attention in the context of activity-dependent synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD), is the gene that encodes activity-regulated cytoskeleton-associated protein (arc, also known as arg3.1) (Bramham et al., 2010; Bramham et al., 2008; Tzingounis and Nicoll, 2006). Arc mRNA levels were found to be increased and shown to be transported to activated synapses after LTP-inducing stimulation in hippocampus (Lyford et al., 1995; Steward et al., 1998; Steward and Worley, 2001a; Steward and Worley, 2001b); genetic deletion of arc was shown to be associated with impaired LTP and LTD in hippocampal slice preparations (Plath et al., 2006); and acute knock-down of arc was shown to disrupt LTP in area CA1 as well as the dentate gyrus of the hippocampus (Guzowski et al., 2000; Messaoudi et al., 2007). Furthermore, consistent with the idea of a link between activity-dependent synaptic plasticity and the establishment of long-term memories (Bliss and Collingridge, 1993; Braunewell and Manahan-Vaughan, 2001; Malenka and Bear, 2004), mice in which arc was either knocked out or acutely knocked down were found to exhibit consolidation deficits (Guzowski et al., 2000; Plath et al., 2006). However, an increase in arc expression after LTP-inducing stimulation has not been observed consistently (French et al., 2001; Miyashita et al., 2009), and overexpression of arc was found to be associated with a reduction in AMPA receptor-mediated currents and occlusion of N-methyl-D-aspartate (NMDA) receptor-dependent LTD (Rial Verde et al., 2006).
Despite much interest in arc as an early responder to plasticity-inducing synaptic input and a critical player in the establishment of long-term synaptic modification, relatively little is known about the time course of altered arc expression after LTP- or LTD-inducing stimulation. Miyashita et al. (2009) observed an increase in arc expression within minutes after LTP induction in area CA1; others reported an increase in arc mRNA levels 30 min to 4 hours after LTP induction in the dentate gyrus (Lyford et al., 1995; Steward et al., 1998). These findings suggest that arc induction in response to LTP-inducing stimulation is very rapid and quite prolonged. Such comparisons across hippocampal subregions, however, may not be warranted, especially in light of differential rates of arc induction in areas CA3 versus CA1 in response to behavioral manipulations (Gusev et al., 2005; Miyashita et al., 2009). The profile of arc expression during LTP in a given brain region, specifically one known to undergo experience-dependent plasticity, therefore remains to be determined.
There currently is no information about changes in arc expression during LTD. Based on findings of disrupted NMDA receptor-dependent LTD in the presence of transcription inhibitors (Kauderer and Kandel, 2000), together with the aforementioned observations that arc overexpression leads to LTD occlusion (Rial Verde et al., 2006), one might predict that LTD-inducing stimulation triggers an increase in arc expression. On the other hand, results showing no effect of transcription inhibitors on NMDA receptor-dependent LTD (Manahan-Vaughan et al., 2000) suggest that arc expression is not increased during LTD or that any increase is non-consequential for the establishment of this form of LTD.
In this study we aimed to characterize arc expression during NMDA receptor-dependent LTP and LTD in area CA1 of the adult rat hippocampus in vivo. To avoid that any differences in expression profiles between LTP and LTD that could be attributed to procedural differences, such as amount or duration of stimulation, we kept all of the stimulation parameters identical but varied only the temporal pattern of the plasticity-inducing stimulation between LTP- and LTD-experiments. Our results show that arc mRNA levels are regulated very differently during the two forms of synaptic plasticity. Whereas LTP is associated with a persistent increase in arc expression, LTD is associated a rapid decrease followed by a transient increase in arc mRNA level. These findings provide evidence for stimulation-induced down-regulation of transcriptional product during LTD.
Materials and Methods
In Vivo Electrophysiology
Electrophysiological methods were used as previously described (Thiels et al., 2002; Thiels et al., 1992). All procedures were in compliance with and approved by the Institutional Animal Care and Use Committee, University of Pittsburgh. Briefly, male Sprague Dawley rats (Hilltop, Scottdale, PA; 250–350 g) were anesthetized first with an intraperitoneal (i.p.) injection of 8% chloral hydrate in 150 mM NaCl (0.4 g/kg) and then maintained under constant anesthesia throughout the remainder of the recording session with intravenous (i.v.) injection of the same anesthetic (0.15 g/kg/h). Rats were placed in a stereotaxic apparatus, and an incision was made on the scalp. After the skin was retracted, one small hole was drilled into the skull on the left side (relative to bregma: AP, −1.7 mm; ML, −1.1 mm) and one on the right side (relative to bregma: AP, −3.6 mm; ML, +2.3 mm). After removal of dura mater, a pair of bipolar metal electrodes, insulated except for the 100 μm to 150 μm at the tip, was lowered into area CA3 of the left dorsal hippocampus (final DV placements relative to the surface of the brain: about −3.5 mm) and a glass electrode, filled with 2 M saline (impedance of 0.9–1.4 MΩ), was lowered to either str. pyramidale or, in separate groups of animals, str. radiatum of area CA1 of the right dorsal hippocampus (final DV placements relative to the surface of the brain: about −1.8 mm and −2.1 mm, respectively). An input-output (I/O) function that relates the intensity of commissural stimulation (20–200 μA; 100-μs duration) to the amplitude of the evoked CA1 pyramidal cell population spike was determined at the beginning of each experiment. An additional I/O function that relates the intensity of commissural stimulation to the initial slope of the evoked CA1 population EPSP was determined for experiments involving recordings in str. radiatum. A stimulation intensity that produced a response with an amplitude that was 30% to 40% of the maximum amplitude of the evoked population spike for str. pyramidale recordings or an initial slope that was 30% to 40% of the maximum slope of the evoked population EPSP for str. radiatum recordings before delivery of high-frequency or paired-pulse stimulation (HFS and PPS, respectively) was used for test pulses (a series of 10 pulses at 0.1 Hz, delivered at 5-min intervals before and after HFS or PPS). Baseline response level was determined by delivery of successive series of test pulses for a total of 15 min to 20 min before HFS or PPS, i.e., until stable baseline responding had been established. LTP was induced by applying HFS (4 trains of 100 pulses at 100 Hz delivered at 130-sec intervals), and LTD was induced by applying PPS (200 pairs of pulses with a 25-ms interval delivered at 0.5 Hz) to the commissural fibers using a stimulation intensity that was 60% to 75% of the maximum amplitude of the population spike, as determined with the first I/O function. Thus, the total number of stimulation pulses (400 pulses), total duration of patterned stimulation (400 sec), and stimulation intensity were essentially identical between the two protocols. After termination of HFS or PPS, additional series of test pulses were delivered for a total of either 10 min, 30 min, 60 min, or 120 min using the same stimulation intensity as was used for test pulses before HFS or PPS delivery. Recorded data were amplified, filtered (0.1 Hz – 10 kHz), digitized, and stored on computer disk for later analysis. In experiments requiring local drug infusion, a glass pipette (tip inner diameter = 35–50 μm) connected to a positive-pressure syringe pump (Harvard Apparatus, Holliston, MA) was lowered into area CA1 within 200 μm to 300 μm of the tip of the recording electrode. D-aminophosphonovaleric acid (D-APV; 0.5 mM in the drug pipette; dissolved in 150 mM NaCl; Tocris, Ellisville, MO) was infused continuously (6–8 nl/min) from 60 min before HFS or PPS until the end of recording. Actinomycin D (ActD; 0.8 mM in the drug pipette; dissolved in 0.5% methanol/95% 150 mM NaCl; Calbiochem, La Jolla, CA) was infused over the course of 15 min to 20 min (50–60 nl/min) beginning 90 min before HFS or PPS. At the end of electrophysiological recording, rats were decapitated, the brains removed rapidly, and the right hippocampus excised in the presence of ice-cold artificial cerebrospinal fluid. Within one min of brain removal, a 1 mm3-piece of tissue from dorsal area CA1 (recording site) and an equal-sized piece of tissue from ventral area CA1 (within-subject control) of the right hippocampus were dissected out and transferred to separate, color-coded 1.5-ml tubes maintained on dry ice for instantaneous freezing of the tissue samples. The samples were stored at −80°C until molecular analysis.
Quantitative PCR (qPCR)
The experimenter conducting the PCR analyses was blind to the color code until completion of the experiments. From each tissue sample, total RNA was isolated using Trizol® reagent (Invitrogen, Carlsbad, CA) according to manufacturer instructions. Isolated RNA was treated with DNAse I (Invitrogen) prior to reverse transcription in order to remove genomic DNA. The integrity of the RNA was confirmed by agarose gel electrophoresis, and the yield was determined by measurement of the absorbance at 260–280 nm. Using the Superscript III kit (Invitrogen) 0.5 μg total RNA was reverse-transcribed with poly-dT primers, nucleotides, and buffers in a total reaction volume of 20 μl according to manufacturer instructions. In each well, 2 μl of the appropriate cDNA was added to the qPCR reaction mixture, which consisted of 12.5 μl SYBR-green master mix (SA Biosciences, Frederick, MD), 6 μl of primer mixture containing 400 nM of each forward and reverse primers (final concentration of each ~100 nM), and 4.5 μl nuclease-free water, to bring the total volume in each well to 25 μl. A minus reverse transcriptase negative control was included in all cases. Reactions were performed in triplicates using a Biorad IQ real-time thermocycler (Hercules, CA) with an initial Taq polymerase activation at 95°C for 10 min, followed by 50 cycles each of which involved 30 sec at 95°C, 30 sec at 60°C, and 30 sec at 72°C. The efficiency (E) of each primer set was determined using a serial dilution of standard cDNA, which was generated by reverse transcription of 2 μg of total RNA extracted from whole hippocampus using polydT primers in a reaction volume of 20 μl. The threshold cycle (Ct) for each sample was chosen to lie within the early exponential rise phase, and any wells with multiple peaks during the melting-point analysis were excluded from analysis. Data were normalized to glyceraldehyde 3-phosphate dehydrogenase (gapdh) run in the same qPCR experiment. Gene expression in dorsal area CA1 (experimental tissue), relative to expression in ventral area CA1 (within-subject control tissue), was determined by the efficiency-corrected delta cycle threshold (ΔCt) method using the following formula: relative quantity (RQ)arc,zif268 = Earc,zif268Ct(ventral)−Ct(dorsal)/EgapdhCt(ventral)−Ct(dorsal).
All primers were selected to have the same melting temperature of 60 °C to enable running them within the same plate. In addition, all primers were designed to be on two exons to allow us to distinguish between mature versus newly synthesized mRNA by the melting curves. In all cases a single peak was observed. Arc, zif268, and gapdh PCR products were cloned using the TA cloning kit (Invitrogen) according to instructions of the manufacturer. The sequence-specific amplification was confirmed by sequencing and subsequent NCBI blast. The primers and their product sizes were as follows:
Arc (fwd): 5′-AGTCTTGGGCAGCATAGCTC-3′, Arc (rev): 5′-GCCGAAGTCTGCTTTTCTTC-3′ (115 bp); Zif268 (fwd): 5′-CAGCGCTTTCAATCCTCAA-3′, Zif268 (rev): 5′-TGGGATAACTTGTCTCCACCA-3′ (119 bp); Gapdh (fwd): 5′-GAAGGGCTCATGACCACAGT-3′, Gapdh (rev): 5′-GGATGCAGGGATGATGTTCT-3′ (117 bp).
Calibration curves for estimating the PCR amplification efficiency of each of the three targets are shown in Supplementary Figure 1.
Statistical analyses
To validate significant levels of synaptic potentiation or depression after HFS or PPS, respectively, two-tailed paired Student’s t-tests were carried out on data collected during the last 5 min before HFS or PPS and during the last 5 min of electrophysiological recording. Differences in level of synaptic change between groups were compared with two-tailed Student’s t-tests for independent groups. To determine whether delivery of baseline stimulation, HFS, or PPS is associated with a significant change in arc or zif268 mRNA level, we first transformed the RQ values calculated as described above on a log2 scale to achieve normal distribution of the data (Bland and Altman, 1996; Kubista et al., 2007) and then tested the resulting distributions against the null-hypothesis of equal mRNA level in dorsal and ventral samples (i.e., a population mean of 0.0) using two-tailed one-sample Student’s t-tests. An α-level of ≤ 0.05 was applied for all comparisons to determine statistical significance.
Results
LTP in area CA1 in vivo is accompanied by a rapid and persistent increase in arc mRNA level
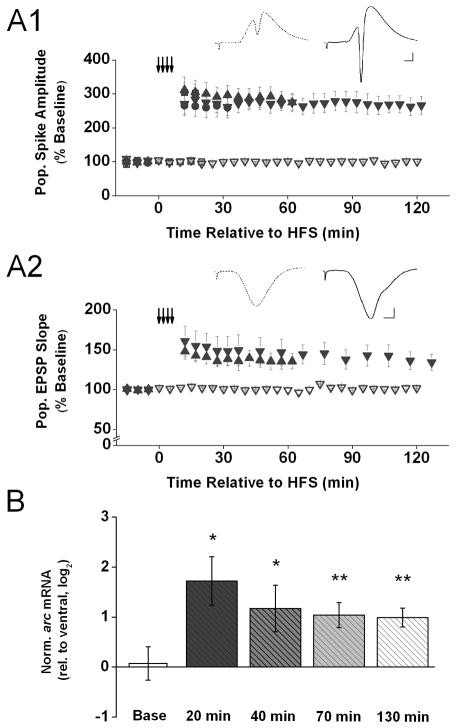
Previous studies on changes in arc expression after induction of LTP in area CA1 in vivo used in situ hybridization-based techniques and have led to conflicting findings, possibly due to procedural differences (French et al., 2001; Miyashita et al., 2009). Both groups of investigators induced LTP in anesthetized rats with two trains of HFS presented 30 sec apart. French and colleagues harvested tissue 30 min after LTP induction and examined IEG induction using autoradiographic in situ hybridization techniques with end-labeled oligodeoxynucleotide probes, whereas Miyashita and colleagues harvested tissue 5 min after LTP induction and used fluorescent in situ hybridization with digoxigenin-labeled intron-enriched riboprobes. French and colleagues did not find evidence for LTP-associated arc induction, whereas Miyashita and colleagues observed a robust increase in arc expression in a large proportion of the cases. Here, we investigated arc expression by applying qPCR on small samples of CA1 tissue that included the recording site and were harvested at different times after LTP-inducing stimulation. To induce LTP, we delivered 4 trains of HFS 130 sec apart to the dorsal CA3 commissural projections to contralateral area CA1; to map out the time course of arc expression, we collected tissue samples either 20 min, 40 min, 70 min, or 130 min after the start of HFS and conducted qPCR on those samples. Our stimulation protocol produced a persistent potentiation of the amplitude of the CA1 population spike evoked by test pulses delivered before and after HFS (Fig 1. A1). The potentiation of the evoked population spike lasted for at least 2 hr (20-min group: t(6) = 6.36; 40-min group: t(6) = 4.64; 70-min group: t(5) = 6.34; and 130-min group: t(4) = 6.15; all p’s < 0.01). Similarly, our HFS protocol produced a persistent potentiation of the initial slope of the CA1 population EPSP, and this potentiation lasted at least 2 hr (Fig 1. A2) (70-min group: t(3) = 4.66; and 130-min group: t(4) = 5.16; both p’s < 0.05). Baseline stimulation only (series of 10 test pulses delivered every 5 min), on the other hand, was not associated with a systematic change in either the amplitude of the evoked population spike (Fig. 1. A1) or the initial slope of the evoked population EPSP (Fig. 1. A2) across the 2.5-hr recording period (population spike: t(2) = 1.15; population EPSP: t(2) = 1.29, both p’s > 0.1). To determine whether arc level is altered after HFS, we compared mRNA levels in dorsal area CA1 near the recording site (experimental tissue) to that in ventral area CA1 (within-subject control). Because the CA3 commissural fibers stimulated in our experiments do not innervate ventral area CA1 (Ishizuka et al., 1990), ventral CA1 constitutes a useful within-subject control. Figure 1B depicts that before delivery of HFS, i.e., under baseline condition, arc mRNA level in dorsal area CA1 was comparable to that in ventral area CA1 (t(8) < 1). In contrast, after 4 trains of HFS, arc mRNA level was significantly higher in experimental relative to control tissue at each of the time points investigated (20 min: t(6) = 3.55, p < 0.02; 40 min: t(6) = 2.52, p < 0.05; 70 min: t(9) = 3.52, p < 0.01; and 130 min: t(9) = 5.03, p < 0.01). Our results confirm the findings by Miyashita et al. (2009) that arc mRNA levels increase rapidly after LTP-inducing stimulation of area CA1 in vivo. Furthermore, our results extend these earlier observations by showing that arc mRNA levels are increased for at least 2 hr after LTP induction in area CA1.
Figure 1. Rapid and persistent increase of arc mRNA level after induction of LTP in area CA1 in vivo.
A1) Means ± s.e.m.s of the amplitude of the population spike, expressed as a percent of baseline, evoked by commissural stimulation before and after 4 trains of high-frequency stimulation (HFS; small down-ward arrows) recorded from animals decapitated either 20 min (filled squares; n = 7), 40 min (filled circles; n = 7), 70 min (filled upward triangles; n = 6), or 130 min (filled down-ward triangles; n = 5) after the onset of HFS, or after either 20 min (open squares; n = 2) or 120 min (open down-ward triangles; n = 3) of only test pulse stimulation after the initial baseline stimulation period. Inserts above show representative waveforms (average of 10 recordings) recorded from an animal 5 min before (stippled line) and 127 min after HFS (solid line). Scale: 2 mV, 2 msec. A2) Similar data as shown above for the initial slope of the population EPSP evoked by commissural stimulation before and after HFS. (n = 3 for baseline, n = 4 for 70 min, n = 5 for 130 min). Scale for the insert is identical. B) Means ± s.e.m.s of arc mRNA level in dorsal area CA1 (experimental), relative to ventral area CA1 (control), detected in tissue samples harvested after either baseline stimulation (Base) or either 20 min, 40 min, 70 min, or 130 min after HFS. Arc expression levels were normalized by gapdh expression in the respective tissue samples and, to approximate a normal distribution of the data, log2 -transformed. Fold differences (FD) can be calculated from log2(RQ) by the formula FD= 2log2(RQ). (n = 8 for baseline, n = 7 for 20 min, n = 7 for 40 min, n = 10 for 70 min, n = 10 for 130 min). Determined Ct values for each of these groups are shown in Table 1 in the Supplementary Information. Two-tailed t-tests indicate a significant increase in arc mRNA above control level at all time points after HFS (* p < 0.05, ** p < 0.01).
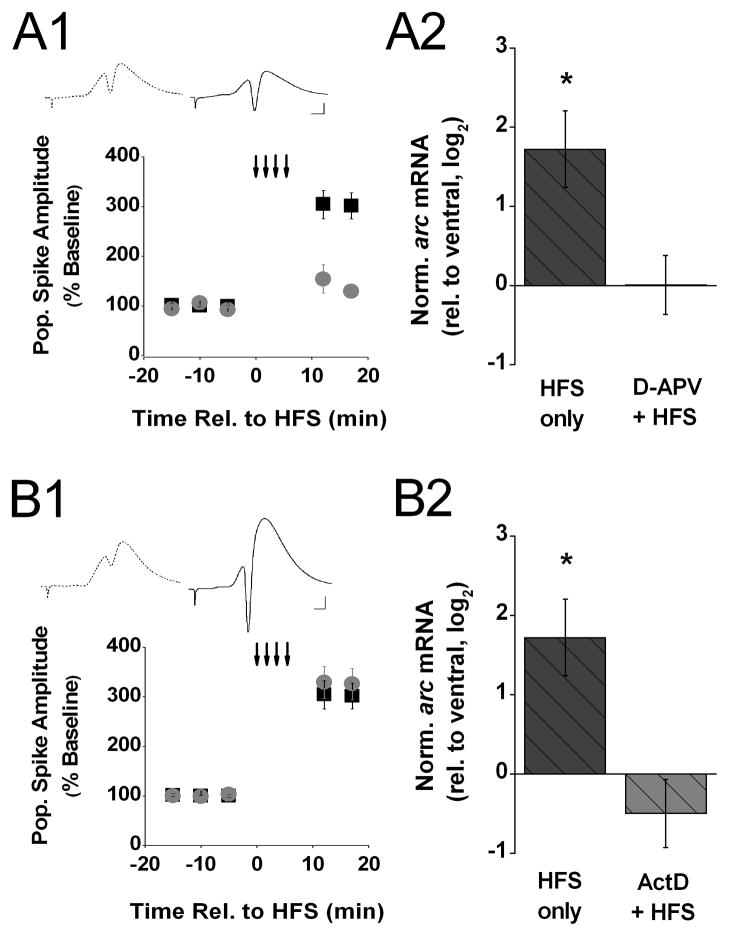
To rule out the possibility that the observed change in arc mRNA level was a non-specific consequence of patterned stimulation, we took advantage of the fact that HFS-induced LTP in area CA1 is dependent on NMDA receptor activation (Thiels et al., 1992). Thus, we infused the NMDA receptor antagonist D-APV (0.5 mM in the drug pipette) into area CA1 near the recording site throughout electrophysiological recording and assessed the effect on arc expression in CA1 tissue samples collected 20 min after HFS. Figure 2. A1 shows that in the presence of D-APV, HFS failed to produce a significant potentiation of the amplitude of the evoked CA1 population spike (t(6) = 2.21, p > 0.07.). Figure 2. A2 shows that the increase in arc mRNA level detected 20 min after HFS in the absence of drug was abolished completely in the presence of D-APV (t(6) < 1). These results confirm that the increase in arc mRNA level after HFS we observed above (Figure 1B) depends on prior induction of LTP. Furthermore, the results suggest that the LTP-associated increase in arc mRNA level is mediated via an NMDA receptor-dependent mechanism.
Figure 2. Increase of arc mRNA level after HFS fails to occur when either NMDA receptors are blocked or new RNA synthesis is inhibited.
A1) Means ± s.e.m.s of the amplitude of the population spike, expressed as a percent of baseline, evoked by commissural stimulation before and after 4 trains of high-frequency stimulation (HFS; small down-ward arrows) recorded from animals decapitated 20 min after HFS delivered in either the presence of D-APV (gray circles, n=6) or the absence of drug (black squares, n=7). The data from the latter group were shown in Fig. 1. A1 and are included here for purposes of comparison. Insert above shows representative waveforms (average of 10 recordings) recorded 5 min before (stippled line) and 17 min after the onset of HFS (solid line) from an animal that received HFS in the presence of D-APV. Scale: 2 mV, 2 ms. A2) Means ± s.e.m.s of arc mRNA level in dorsal area CA1 (experimental), relative to ventral area CA1 (control), detected in tissue samples harvested 20 min after HFS delivered in either the presence of D-APV (D-APV + HFS, gray bar) or the absence of drug (HFS only, black bar). The data from the latter group were shown in Fig. 1. B and are included here for purposes of comparison. Arc expression levels were normalized by gapdh expression in the respective tissue samples and, to approximate a normal distribution of the data, log2 -transformed. Fold differences (FD) can be calculated from log2(RQ) by the formula FD= 2log2(RQ). Determined Ct values for the two groups are shown in Table 1 in the Supplementary Information. Two-tailed t-tests indicate that the significant increase in arc mRNA level 20 min after HFS in the absence of drug was abolished in the presence of D-APV. B1) Means ± s.e.m.s of the amplitude of the population spike, expressed as a percent of baseline, evoked by commissural stimulation before and after 4 trains of HFS (small down-ward arrows) recorded from animals decapitated 20 min after HFS delivered in either the presence of ActD (gray circles, n=9) or the absence of drug (black squares, n=7). The data from the latter group were shown in Fig. 1. A1 and are included here for purposes of comparison. Insert above shows representative waveforms (average of 10 recordings) recorded 5 min before (stippled line) and 17 min after HFS (solid line) from an animal that received HFS in the presence of ActD. Scale: 2 mV, 2 ms. B2) Means ± s.e.m.s of arc mRNA level in dorsal area CA1 (experimental), relative to ventral area CA1 (control), detected in tissue samples harvested 20 min after HFS delivered in either the presence of ActD (ActD + HFS, gray bar) or the absence of drug (HFS only, black bar). The data from the latter group were shown in Fig. 1. B and are included here for purposes of comparison. Arc expression levels were normalized by gapdh expression in the respective tissue samples and, to approximate a normal distribution of the data, log2 -transformed. Fold differences (FD) can be calculated from log2(RQ) by the formula FD= 2log2(RQ). Determined Ct values for the two groups are shown in Table 1 in the Supplementary Information. Two-tailed t-tests indicate that the significant increase in arc mRNA level 20 min after HFS in the absence of drug was abolished in the presence of ActD.
Our observations of an HFS-associated increase in arc mRNA level do not distinguish between the possibility that LTP is associated with an increase in de novo arc transcription versus an increase in arc mRNA stability. To address this issue, we assessed the effect of the general transcription inhibitor ActinomycinD (ActD) on the increase in arc mRNA level observed immediately after HFS. Specifically, we infused ActD (0.8 mM in the drug pipette) into area CA1 near the recording site over the course of about 15 min beginning 90 min before HFS. Dorsal and ventral area CA1 pieces were collected 20 min after HFS. Figure 2. B1 shows that the presence of ActD did not affect the ability of 4 trains of HFS to cause a significant increase in the amplitude of the evoked population spike (t(8) = 6.71, p < 0.01). No effect of the transcriptional inhibitor on the increase in the evoked response immediately after HFS was expected because only late but not early phases of LTP depend on transcription (Abraham and Williams, 2003; Nguyen et al., 1994). Figure 2. B2 shows that the marked increase in arc mRNA level 20 min after HFS observed in the absence of drug failed to emerge in the presence of ActD (t(8) = 1.15, p > 0.1). These results indicate that the observed LTP-associated elevation in arc mRNA level stems from a stimulation-induced increase in arc mRNA synthesis, rather than a decrease in arc mRNA degradation. Taken together, our findings demonstrate that the induction of NMDA receptor-dependent LTP in area CA1 in vivo is associated with a robust, rapid increase in arc transcription and that arc mRNA levels remain elevated above control levels for at least 2 hr after LTP induction.
LTD in area CA1 in vivo is accompanied by a rapid decrease followed by a transient increase in arc mRNA level
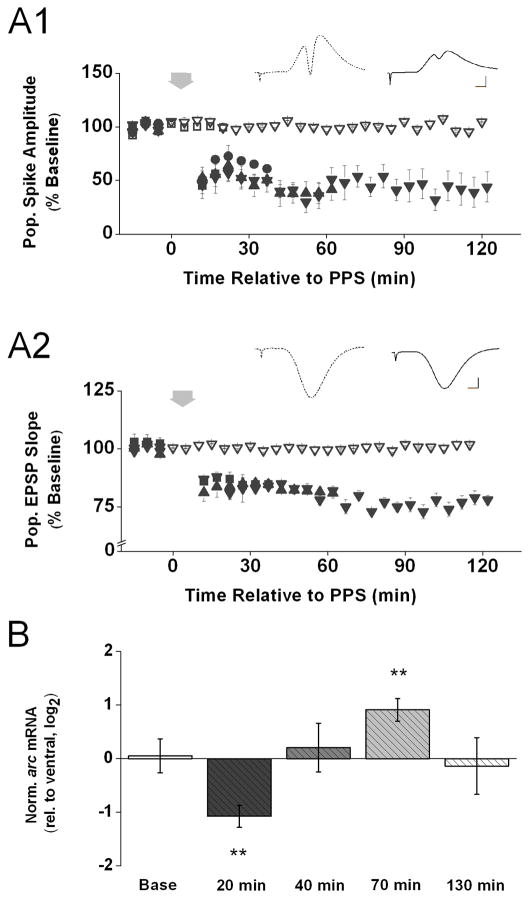
To determine the time course of any changes in arc mRNA level in association with LTD area CA1 in vivo, we followed essentially the same approach as described above. We induced LTD in area CA1 by delivering one train of PPS, which consisted of 200 pairs of pulses with a 25-ms interval presented at 0.5 Hz, to the dorsal CA3 commissural fibers, and we harvested CA1 tissue samples for qPCR analysis either 20 min, 40 min, 70 min, or 130 min after the onset of the PPS. Similar to our previous observations (Thiels et al., 1994), PPS produced a persistent depression of the amplitude of the CA1 population spike (Fig. 3. A1) as well as the initial slope of the CA1 population EPSP (Fig. 3. A2) evoked by test pulses delivered before and after PPS, and these effects lasted for at least 2 hr after PPS (population spike: 20 min: t(6) = 5.15, p < 0.01; 40 min: t(1) = 16.31, p < 0.05; 70 min: t(3) = 5.71, p < 0.02; and 130 min: t(4) = 7.63, p < 0.01; population EPSP: 40 min: t(4) = 10.44; 70 min: t(4) = 6.93; 130 min: t(4) = 12.60, all p’s < 0.01). As in the above experiments, baseline stimulation was not associated with a systematic change in either the amplitude of the evoked population spike (Fig. 3. A1) or the initial slope of the evoked population EPSP (Fig. 3. A2) across the 2.5-hr recording period (population spike: t(2) < 1; population EPSP: t(2) = 1.13, p > 0.1). The results from the qPCR analysis are shown in Figure 3B. Before delivery of PPS, arc mRNA level in dorsal area CA1 did not differ from that in ventral area CA1 (t(6) < 1). However, immediately after PPS, arc mRNA level was significantly decreased relative to the level detected in control tissue (20 min: t(6) = 5.22, p < 0.01). This significant decrease was short-lasting, because 40 min after PPS, a systematic difference in arc mRNA level between experimental and control tissue samples was no longer detectable (t(6) < 1). However, as time since PPS passed, arc mRNA levels began to increase such that 70 min after PPS, mRNA level in experimental CA1 samples was significantly higher relative to that in control samples (t(8) = 4.34, p < 0.01). This increase in arc mRNA level 1 hr after LTD induction, however, also did not persist; mRNA level in experimental CA1 samples no longer differed significantly from control level 130 min after PPS (t(8) < 1). Different from what one might have expected based on previous work showing that genetic reduction of arc interferes with LTD maintenance (Plath et al., 2006) whereas overexpression of arc occludes LTD (Rial Verde et al., 2006), the present results indicate that arc mRNA level undergoes a rapid decrease after LTD-inducing stimulation. Furthermore, our results suggest a remarkable bidirectional regulation of arc mRNA level after LTD induction in area CA1 in vivo, with levels decreasing below baseline during the early phase of LTD, increasing above baseline about 1 hr after LTD induction, and returning to baseline by 2 hr after LTD induction. This profile is distinctly different from the one we observed after induction of LTP.
Figure 3. Rapid decrease followed by transient increase of arc mRNA level after induction of LTD in area CA1 in vivo.
A1) Means ± s.e.m.s of the amplitude of the population spike, expressed as a percent of baseline, evoked by commissural stimulation before and after paired-pulse stimulation (PPS; wide down-ward arrow) recorded from animals decapitated either 20 min (filled squares; n = 7), 40 min (filled circles; n = 2), 70 min (filled upward triangles; n = 5), or 130 min (filled down-ward triangles; n = 5) after the onset of PPS, or after either 20 min (open squares; n = 3) or 120 min (open down-ward triangles; n = 2) of only test pulse stimulation after the initial baseline stimulation period. Inserts above show representative waveforms (average of 10 recordings) recorded from an animal 5 min before (stippled line) and 127 min after PPS (solid line). Scale: 2 mV, 2 msec. A2) Similar data as shown above for the initial slope of the population EPSP evoked by commissural stimulation before and after PPS. (n = 3 for baseline, n = 5 for 40 min, n = 5 for 70 min, n = 5 for 130 min). Scale for the insert is identical. B) Means ± s.e.m.s of arc mRNA level in dorsal area CA1 (experimental), relative to ventral area CA1 (control), detected in tissue samples harvested after either baseline stimulation (Base) or either 20 min, 40 min, 70 min, or 130 min after PPS. Arc expression levels were normalized by gapdh expression in the respective tissue samples and, to approximate a normal distribution of the data, log2 –transformed. Fold differences (FD) can be calculated from log2(RQ) by the formula FD= 2log2(RQ). (n = 8 for baseline, n = 7 for 20 min, n = 7 for 40 min, n = 10 for 70 min, n = 10 for 130 min). Determined Ct values for each of these groups are shown in Table 1 in the Supplementary Information. Two-tailed t-tests indicate a significant decrease in arc mRNA level 20 min after PPS followed by a significant increase in arc mRNA level 70 min after PPS (** p < 0.01).
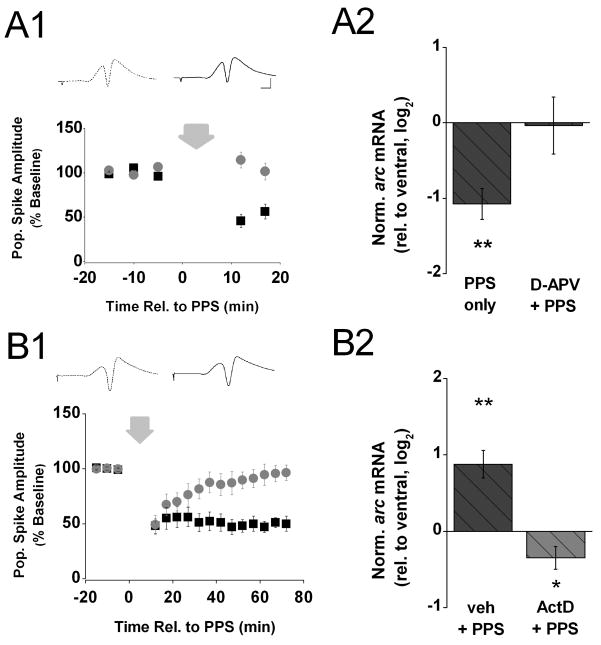
We previously showed that induction of LTD by PPS in area CA1 in vivo is dependent on NMDA receptor activation (Thiels et al., 1994; Thiels et al., 2000). Therefore, to rule out that the observed decrease in arc mRNA level was some non-specific effect of patterned stimulation, we infused D-APV (0.5 mM in the drug pipette) into area CA1 near the recording site throughout the electrophysiological recording session and assessed the effect on arc mRNA level in CA1 tissue samples collected 20 min after PPS. Figure 4. A1 shows that in the presence of D-APV, PPS failed to produce a depression of the amplitude of the evoked CA1 population spike (t(5) < 1). Figure 4. A2 shows that the decrease in arc mRNA level detected 20 min after PPS in the absence of drug failed to emerge in the presence of D-APV (t(5) < 1). These results confirm that the decrease in arc mRNA level after PPS observed above (Figure 3B) depends on prior induction of LTD and is not a non-specific consequence of prior synaptic activation.
Figure 4. Decrease of arc mRNA level after PPS fails to occur when NMDA receptors are blocked.
A1) Means ± s.e.m.s of the amplitude of the population spike, expressed as a percent of baseline, evoked by commissural stimulation before and after PPS (wide down-ward arrow) recorded from animals decapitated 20 min after PPS delivered in either the presence of D-APV (gray circles, n=9) or the absence of drug (black squares, n=7). The data from the latter group were shown in Fig. 3. A1 and are included here for purposes of comparison. Inserts above show representative waveforms (average of 10 recordings) recorded 5 min before (stippled line) and 17 min after PPS (solid line) from an animal that received PPS in the presence of D-APV. Scale: 2 mV, 2 ms. A2) Means ± s.e.m.s of arc mRNA level in dorsal area CA1 (experimental), relative to ventral area CA1 (control), detected in tissue samples harvested 20 min after PPS delivered in either the presence of D-APV (D-APV + PPS, gray bar) or the absence of drug (PPS only, black bar). The data from the latter group were shown in Fig. 3. B and are included here for purposes of comparison. Arc expression levels were normalized by gapdh expression in the respective tissue samples and, to approximate a normal distribution of the data, log2 -transformed. Fold differences (FD) can be calculated from log2(RQ) by the formula FD= 2log2(RQ). Determined Ct values for the two groups are shown in Table 1 in the Supplementary Information. Two-tailed t-tests indicate that the significant decrease of arc mRNA level 20 min after PPS in the absence of drug is abolished in the presence of D-APV. B1) Means ± s.e.m.s of the amplitude of the population spike, expressed as a percent of baseline, evoked by commissural stimulation before and after PPS (wide down-ward arrow) recorded from animals decapitated 70 min after PPS delivered in the presence of either ActD (gray circles, n=6) or vehicle solution (black squares, n=6). Insert above shows representative waveforms (average of 10 recordings) recorded 5 min before (stippled line) and 70 min after PPS (solid line) from an animal that received PPS in the presence of ActD. Scale: 2 mV, 2 ms. B2) Means ± s.e.m.s of arc mRNA level in dorsal area CA1 (experimental), relative to ventral area CA1 (control), detected in tissue samples harvested 70 min after PPS delivered in the presence of either ActD (ActD + PPS, gray bar) or vehicle solution (veh + PPS, black bar). Arc expression levels were normalized by gapdh expression in the respective tissue samples and, to approximate a normal distribution of the data, log2 -transformed. Fold differences (FD) can be calculated from log2(RQ) by the formula FD= 2log2(RQ). Determined Ct values for the two groups are shown in Table 1 in the Supplementary Information. A two-tailed t-test indicates a significant increase in arc mRNA level 70 min after PPS in the presence of vehicle solution (** p < 0.01). This effect was abolished completely in the presence of ActD; in fact, a two-tailed t-test indicates a small but significant decrease in arc mRNA level 70 min after PPS in the presence of ActD (* p < 0.05).
The increase in arc mRNA 70 min after PPS raises the question whether the observed change stems from de novo transcription or a dramatic reduction in arc mRNA degradation. To address this issue, we assessed the effect of ActD on the increase in arc mRNA level observed 70 min after PPS. Specifically, we infused ActD (0.8 mM in the drug pipette) into area CA1 near the recording site over the course of about 15 min beginning 90 min before PPS. Dorsal and ventral area CA1 pieces were collected 70 min after HFS. Figure 4. B1 shows that pre-infusion of ActD did not affect the early phase of LTD, as the amplitude of the evoked population spike immediately after PPS was not distinguishable from the depressed level observed in the vehicle group (for the first 4 test-pulse series after PPS, vehicle vs. ActD, t(10) > 1). However, the initial depression of the evoked response did not last in the group that received ActD but returned to baseline level by the end of the recording period (t(6) < 1). In contrast, the response depression lasted at least until the end of the recording period in vehicle-treated animals (t(6) = 8.40, p < 0.01) (Figure 4. B1). These findings demonstrate that the prolonged maintenance of PPS-induced LTD in area CA1 requires mRNA synthesis. Figure 4. B2 shows that the robust increase in arc mRNA level in dorsal hippocampus 70 min after PPS observed after infusion of vehicle solution (t(6) = 4.83, p < 0.01) was abolished completely after infusion of ActD; in fact, arc mRNA level detected in dorsal CA1 tissue 70 min after PPS in the presence of ActD was slightly but significantly below control level (t(6) = 2.3, p < 0.05). These results indicate that the LTD-associated increase in arc mRNA level 70 min after PPS stems from a stimulation-induced increase in arc mRNA synthesis, rather than a decrease in arc mRNA degradation.
Taken together, these findings demonstrate that the induction of NMDA receptor-dependent LTD in area CA1 in vivo is associated with an immediate, pronounced decrease and a protracted, transient increase in arc mRNA level. The transient increase in arc mRNA level is the result of an increase in arc transcription. Importantly, this increase in arc transcription is necessary for the prolonged maintenance of NMDA receptor-dependent PPS-induced LTD in area CA1 in vivo. Relating these findings to those after LTP induction, our results show that arc mRNA levels are altered during both LTP and LTD in area CA1 in vivo. The pattern of arc mRNA regulation, however, differs markedly between these two forms of synaptic plasticity.
The patterns of change in arc and zif268 mRNA level differ from one another during LTD but not during LTP in area CA1 in vivo
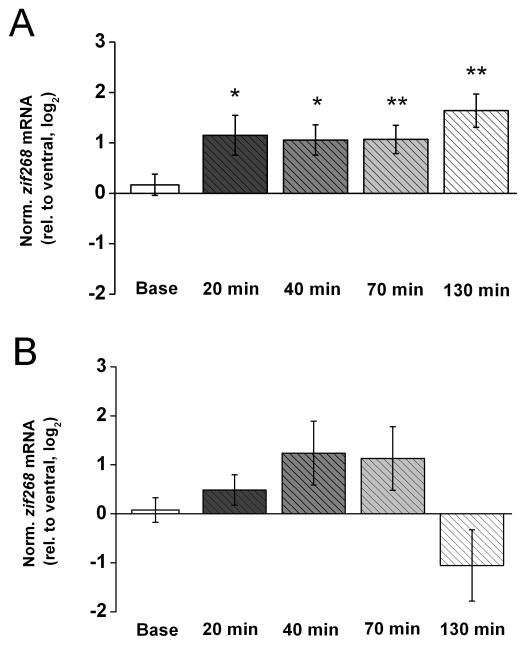
Similar to arc, the IEG zif268 was reported to be regulated in a synaptic activation-dependent manner (Jones et al., 2001; Lindecke et al., 2006). In fact, zif268 was shown to be required for the maintenance of LTP (Davis et al., 2003; Renaudineau et al., 2009). Using the same tissue samples as we used for the analyses of arc mRNA level after induction of LTP, we found that zif268 mRNA level also is increased significantly immediately after LTP induction (Fig. 5A). As was the case with arc, this increase in zif268 mRNA level persisted for at least 2 hr (Base: t(7) < 1; 20 min: t(6) = 2.91, p < 0.05; 40 min: t(6) = 3.53, p < 0.02; 70 min: t(9) = 3.79, p < 0.01; and 130 min: t(9) = 3.86, p < 0.01). In light of an overall similarity in the expression patterns of arc and zif268 during LTP, it became of interest to determine whether these two IEGs also are regulated similarly during LTD. Accordingly, we probed the same LTD- tissue samples as used for the arc analyses for zif268 mRNA. Figure 5B shows that zif268 mRNA level in dorsal area CA1 was comparable to that detected in ventral area CA1 before PPS. Although zif268 mRNA level tended to increase, relative to control level, over the course of the first hour after PPS and then decrease, relative to control level, over the course of the second hour after PPS, none of these trends was statistically significant (Base: t(7) < 1; 20 min: t(6) = 1.56; 40 min: t(6) = 1.92; 70 min: t(7) = 1.74; and 130 min: t(7) = 1.45, all p’s > 0.1). These results indicate that, in contrast to arc mRNA, zif268 mRNA level does not deviate significantly from control level at any time during the first 2 hr after LTD induction. Thus, taken together, these results show that arc and zif268 are regulated differently from one another during LTD, whereas they appear to be regulated in overall similar fashion during LTP in area CA1 in vivo.
Figure 5. Zif268 mRNA levels also increase during LTP but do not change from control levels during LTD in area CA1 in vivo.
A) Means ± s.e.m.s of zif268 mRNA level in dorsal area CA1 (experimental), relative to ventral area CA1 (control), detected in the same tissue samples used in Fig. 1. B. (n = 8 for baseline (Base), n = 7 for 20 min, n = 7 for 40 min, n = 10 for 70 min, n = 10 for 130 min). Zif268 expression levels were normalized by gapdh expression in the respective tissue samples and, to approximate a normal distribution of the data, log2 -transformed. Fold differences (FD) can be calculated from log2(RQ) by the formula FD= 2log2(RQ). Two-tailed t-tests indicate a significant increase in zif268 mRNA above control level at all time points after HFS (* p < 0.05, ** p < 0.01). B) Means ± s.e.m.s of zif268 mRNA level in dorsal area CA1 (experimental), relative to ventral area CA1 (control), detected in tissue samples used in Fig. 3. B. (n = 8 for baseline (Base), n = 7 for 20 min, n = 7 for 40 min, n = 10 for 70 min, n = 10 for 130 min). Zif268 expression levels were normalized and transformed as described above. Determined Ct values for each of these groups are shown in Table 2 in the Supplementary Information. Two-tailed t-tests indicate that the variations in zif268 mRNA level after PPS do not differ significantly from control level for any of the time points.
Discussion
Previous work indicates that arc plays a critical role in LTP, LTD, and homeostatic plasticity (Bramham et al., 2008; Tzingounis and Nicoll, 2006). In this study we characterized the time course of arc expression during LTP and LTD in area CA1 of the adult rat hippocampus in vivo. Based on previous reports of changes in arc mRNA level after LTP induction (Link et al., 1995; Lyford et al., 1995; Messaoudi et al., 2007; Miyashita et al., 2009; Steward et al., 1998; Waltereit et al., 2001) and findings that arc overexpression occludes whereas arc deletion interferes with LTD induction (Plath et al., 2006; Rial Verde et al., 2006), we expected that arc mRNA expression would be increased after LTP-as well as LTD-inducing stimulation. To avoid that any differences in expression profiles between LTP and LTD could be attributed to procedural differences, such as amount of stimulation, we used the same number of stimulation pulses, stimulation intensity, and total duration of stimulation for the induction of LTP and LTD, but varied only the temporal pattern of the stimulation pulses between LTP and LTD experiments. Our results show that the regulation of arc expression differs markedly between LTP and LTD in area CA1 in vivo despite the similar requirement of this gene’s product for both forms of plasticity. Whereas there is a persistent up-regulation of arc during LTP, arc exhibits a biphasic regulation pattern during LTD.
The rapid induction of arc by a wide variety of cellular activations, and the transport to and local translation of arc mRNA near activated synapses (Lyford et al., 1995; Park et al., 2008; Steward et al., 1998; Steward and Worley, 2001a; Steward and Worley, 2001b; Wallace et al., 1998; Waung et al., 2008) have attracted much interest in the fields of molecular, synaptic, and behavioral neuroscience. Arc is a single copy gene that is highly conserved among vertebrates (Bramham et al., 2008; Miyashita et al., 2008), which suggests that arc serves a critical function. Arc is expressed in principal neurons in the brain (Vazdarjanova et al., 2006), and rapid increases in arc mRNA level have been observed after spatial exploration (Chawla et al., 2005; Guzowski et al., 1999; Miyashita et al., 2009; Ramirez-Amaya et al., 2005; Vazdarjanova et al., 2006), implicit and explicit memory tasks (Guzowski et al., 2000; McIntyre et al., 2005; Montag-Sallaz and Montag, 2003; Plath et al., 2006; Soule et al., 2008), LTP-inducing stimulation (Guzowski et al., 2000; Link et al., 1995; Lyford et al., 1995; Waltereit et al., 2001), seizures (Link et al., 1995; Lyford et al., 1995), and stress (Ons et al., 2004). Our findings of a reduction in arc mRNA level after LTD-inducing stimulation demonstrate that not all types of cellular activation lead to arc induction and, hence, that arc mRNA is not a general marker of cellular activity but regulated in differential fashion.
The basis of the decrease in arc mRNA level after LTD induction remains to be determined. One possibility is that the transcription of arc is reduced immediately after PPS. We showed previously that PPS-induced LTD in area CA1 in vivo is associated with an increase in the activity of the serine/threonine protein phosphatases PP1 and PP2A (Thiels et al., 1998). Both PP1 and PP2A can affect the phosphorylation state of transcription factors, such as CREB (Genoux et al., 2002; Hagiwara et al., 1992), including after PPS in area CA1 (Mauna et al., submitted), and they thereby reduce the DNA binding ability of these transcriptional regulators. The promoter sequence of arc contains binding consensus sequences for several transcription factors, including CREB, MEF2, SRF, and Elk-1 (Flavell et al., 2006; Kawashima et al., 2009; Pintchovski et al., 2009; Waltereit et al., 2001). In light of findings that CREB phosphorylation is decreased below basal level during LTD (Thiels et al., 2002; Mauna et al., submitted), it is tempting to speculate that the initial decrease in arc mRNA level observed here is related to the LTD-associated decrease in CREB phosphorylation and, hence, that our results reflect a reduction in transcription. This scenario is consistent with previous work attributing to CREB a possible role in the regulation of arc expression (Kawashima et al., 2009; Waltereit et al., 2001). A prominent regulatory role by CREB does not preclude a contribution by other transcription factors; however, we consider a significant role by either SRF/Elk-1 or MEF2 in the LTD-associated decrease of arc mRNA level less likely because (1) we previously found Elk-1 phosphorylation to be increased immediately after the induction of LTD in area CA1 in vivo (Thiels et al., 2002), and (2) calcineurin, a protein phosphatase that is activated during LTD (Mulkey et al., 1994), was shown to promote, rather than attenuate, MEF2-mediated arc expression (Flavell et al., 2006).
An alternative explanation for the decrease in arc mRNA level immediately after LTD-inducing stimulation is that arc mRNA undergoes rapid degradation in response to the synaptic input pattern. Recently, arc mRNA was shown to be degraded by the nonsense-mediated mRNA decay (NMD) pathway (Giorgi et al., 2007). The NMD pathway targets newly transcribed mRNAs to degradation and thereby limits the total translation time and amount of these mRNAs (Ishigaki et al., 2001; Maquat, 2004). A key RNA-binding protein required for NMD is eIF4AIII (Palacios et al., 2004), and arc was shown to be co-localized with eIF4AIII (Giorgi et al., 2007). When NMD was inhibited in neurons by knockdown of eIF4AIII, the level of both arc mRNA and Arc protein were found to be elevated (Giorgi et al., 2007). Thus, it is possible that PPS triggers increased targeting of arc mRNA by the NMD pathway and consequently initiates degradation of arc mRNA that has rapidly been translated immediately after LTD-inducing stimulation. Future studies focused on targeting of arc mRNA by the NMD pathway and on determining pre-arc mRNA levels may help resolve the question of whether the observed decrease in arc mRNA level stems from a decrease in de novo transcription or an increase in rapid mRNA degradation.
The increase in arc mRNA level about 1 hr after LTD induction revealed to be the result of de novo transcription of arc. Interference with de novo transcription, in turn, prevented the prolonged maintenance of LTD. This effect of transcription inhibition on LTD in area CA1 in vivo is in agreement with a previous report of transcription-dependent NMDA receptor-mediated LTD in area CA1 in vitro (Kauderer and Kandel, 2000; but see Manahan-Vaughan et al., 2000). It remains to be determined whether the increase in arc transcription is critical for LTD maintenance. This intriguing possibility is suggested by findings of impaired LTD in area CA1 of arc knockout mice (Plath et al., 2006).
The increase in arc mRNA level during LTD did not emerge until approximately 1 hr after PPS. It is possible that upregulation of arc transcription is triggered by the initial synaptic input, but that its manifestation at the mRNA level is masked by the mRNA degradation pathway that rapidly degrades translated arc mRNA (Giorgi et al., 2007). Alternatively, the late increase in arc mRNA level may be caused by a latent transcriptional mechanism that operates in parallel with the mechanisms that underlie the initial decrease in arc mRNA level. Future studies assessing pre-arc mRNA levels at various time points after LTD induction may inform whether a prolonged upregulation of arc transcription is masked by rapid mRNA degradation or whether de novo transcription is increased only for a short period of time approximately 1 hr after PPS. Regardless of which specific mechanisms underlie the respective changes in arc mRNA level during LTD, our findings of a distinct bidirectional pattern of change highlight the dynamic nature of arc mRNA regulation during LTD. Furthermore, the findings illustrate that the time point at which mRNA levels are assessed affects greatly the direction of change that is observed. Accordingly, models of arc regulation and its role in synaptic plasticity based on singular temporal measurements may be incomplete.
Similar to observations by others using a range of cellular activation paradigms (Guzowski et al., 2000; Guzowski et al., 1999; Link et al., 1995; Lyford et al., 1995; Steward and Worley, 2001a; Steward and Worley, 2001b), we found that our LTP-inducing stimulation protocol resulted in a persistent increase in arc mRNA level that lasted at least 2 hr. Our findings that the initial increase in arc mRNA level is abolished when transcription was blocked indicates that the change in mRNA level was due to an increase in arc transcription. The prolonged increase in arc mRNA level might be considered surprising given that arc mRNA has a half-life of ~47 min (Vazdarjanova et al., 2006). One possible mechanism underlying the prolonged increase in mRNA level may be extended activation of arc transcription. In addition to CREB, which is phosphorylated rapidly after LTP-inducing stimulation (Impey et al., 1998; Kasahara et al., 2001; Racaniello et al., 2009; Schulz et al., 1999), other transcriptional regulators, including MEF2, SRF/Elk-1, Zeste-like response element binding proteins, or Zif268, may mediate arc transcription and may do so at later time points (Flavell et al., 2006; Kawashima et al., 2009; Li et al., 2005; Pintchovski et al., 2009). Thus, the prolonged elevation in arc mRNA may result from enhanced transcription via a number of different transcription factors operating in sequence. Alternatively, the prolonged increase in arc mRNA may stem from an enhancement of arc mRNA stability after the initial increase in arc transcription. In fact, control of mRNA stability has been implicated to play a critical role in neuronal function and synaptic plasticity (Deschenes-Furry et al., 2006; Perrone-Bizzozero and Bolognani, 2002). Thus, it would be interesting to determine in future work whether LTP-inducing stimulation triggers a reduction in arc mRNA degradation, thereby enabling an increase in arc mRNA availability through an epigenetic mechanism.
In conclusion, our observations reveal that LTP and LTD are associated with different profiles of arc mRNA changes that may result from different combinations of alterations in arc transcription and arc mRNA stability within as well as between LTP and LTD. Taken together with our findings of zif268 mRNA levels during these two forms of synaptic plasticity, it appears that the regulatory mechanisms underlying arc expression differ not only between and within LTP and LTD but also from those of other IEGs implicated in synaptic plasticity. We believe our study presents a useful starting point for future dissection of the varied mechanisms that regulate arc expression during bidirectional plasticity at glutamatergic synapses in hippocampal area CA1.
Supplementary Material
A) Calibration curve for arc. Cycle threshold (Ct) values from qPCR analysis are plotted against different levels of dilution of RNA input (undiluted, 1/5, 1/25, and 1/125). To generate the standard cDNA, we reverse-transcribed 2 μg total RNA extracted from whole hippocampus under identical conditions and at the same time at which we reverse-transcribed total RNA extracted from experimental and control CA1 tissue samples, as described in the Methods. Based on the slope of the calibration curve, the amplification efficiency for the arc primer set was estimated to be 1.96. B) Calibration curve for zif368. Similar data as shown in A. Based on the slope of the calibration curve, the amplification efficiency for the zif268 primer set was estimated to be 1.98. C) Calibration curve for gapdh. Similar data as shown in A. Based on the slope of the calibration curve, the amplification efficiency for the gapdh primer set was estimated to be 1.97.
Acknowledgments
National Institute of Neurological Disorders and Stroke (NINDS)
Grant Number: RO1-046423 to E.T.
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01-046423 to E.T. The authors would like to thank Drs. A. Paula Monaghan-Nichols and Emily Drill for their invaluable advice with the qPCR experiments.
References
- Abraham WC, Williams JM. Properties and mechanisms of LTP maintenance. Neuroscientist. 2003;9(6):463–74. doi: 10.1177/1073858403259119. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. The use of transformation when comparing two means. Bmj. 1996;312(7039):1153. doi: 10.1136/bmj.312.7039.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, et al. The Arc of synaptic memory. Exp Brain Res. 2010;200(2):125–40. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28(46):11760–7. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunewell KH, Manahan-Vaughan D. Long-term depression: a cellular basis for learning? Rev Neurosci. 2001;12(2):121–40. doi: 10.1515/revneuro.2001.12.2.121. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15(5):579–86. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142(1–2):17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- Deschenes-Furry J, Perrone-Bizzozero N, Jasmin BJ. The RNA-binding protein HuD: a regulator of neuronal differentiation, maintenance and plasticity. Bioessays. 2006;28(8):822–33. doi: 10.1002/bies.20449. [DOI] [PubMed] [Google Scholar]
- Dragunow M. A role for immediate-early transcription factors in learning and memory. Behav Genet. 1996;26(3):293–9. doi: 10.1007/BF02359385. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311(5763):1008–12. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- French PJ, O’Connor V, Jones MW, Davis S, Errington ML, Voss K, Truchet B, Wotjak C, Stean T, Doyere V, et al. Subfield-specific immediate early gene expression associated with hippocampal long-term potentiation in vivo. Eur J Neurosci. 2001;13(5):968–76. doi: 10.1046/j.0953-816x.2001.01467.x. [DOI] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418(6901):970–5. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130(1):179–91. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Gusev PA, Cui C, Alkon DL, Gubin AN. Topography of Arc/Arg3.1 mRNA expression in the dorsal and ventral hippocampus induced by recent and remote spatial memory recall: dissociation of CA3 and CA1 activation. J Neurosci. 2005;25(41):9384–97. doi: 10.1523/JNEUROSCI.0832-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20(11):3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2(12):1120–4. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992;70(1):105–13. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21(4):869–83. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Ishigaki Y, Li X, Serin G, Maquat LE. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell. 2001;106(5):607–17. doi: 10.1016/s0092-8674(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295(4):580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4(3):289–96. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kasahara J, Fukunaga K, Miyamoto E. Activation of calcium/calmodulin-dependent protein kinase IV in long term potentiation in the rat hippocampal CA1 region. J Biol Chem. 2001;276(26):24044–50. doi: 10.1074/jbc.M100247200. [DOI] [PubMed] [Google Scholar]
- Kauderer BS, Kandel ER. Capture of a protein synthesis-dependent component of long-term depression. Proc Natl Acad Sci U S A. 2000;97(24):13342–7. doi: 10.1073/pnas.97.24.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, Nonaka M, Adachi-Morishima A, Kyo N, Okamura M, Takemoto-Kimura S, Worley PF, Bito H. Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc Natl Acad Sci U S A. 2009;106(1):316–21. doi: 10.1073/pnas.0806518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubista M, Sindelka R, Tichopad A, Bergkvist A, Lindh D, Forootan A. Real-time PCR data analysis. 9–10. G.I.T. Laboratory Journal; 2007. The Prime Technique; pp. 33–35. [Google Scholar]
- Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70(1–2):37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- Li L, Carter J, Gao X, Whitehead J, Tourtellotte WG. The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors. Mol Cell Biol. 2005;25(23):10286–300. doi: 10.1128/MCB.25.23.10286-10300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindecke A, Korte M, Zagrebelsky M, Horejschi V, Elvers M, Widera D, Prullage M, Pfeiffer J, Kaltschmidt B, Kaltschmidt C. Long-term depression activates transcription of immediate early transcription factor genes: involvement of serum response factor/Elk-1. Eur J Neurosci. 2006;24(2):555–63. doi: 10.1111/j.1460-9568.2006.04909.x. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92(12):5734–8. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14(2):433–45. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A, Frey JU. Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J Neurosci. 2000;20(22):8572–6. doi: 10.1523/JNEUROSCI.20-22-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat LE. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat Rev Mol Cell Biol. 2004;5(2):89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci U S A. 2005;102(30):10718–23. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27(39):10445–55. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Haghighi N, Steward O, Guzowski JF. Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. J Neurosci. 2009;29(4):898–906. doi: 10.1523/JNEUROSCI.4588-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Lewandowski G, Guzowski JF. Networks of neurons, networks of genes: an integrated view of memory consolidation. Neurobiol Learn Mem. 2008;89(3):269–84. doi: 10.1016/j.nlm.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag-Sallaz M, Montag D. Learning-induced arg 3.1/arc mRNA expression in the mouse brain. Learn Mem. 2003;10(2):99–107. doi: 10.1101/lm.53403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369(6480):486–8. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265(5175):1104–7. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Ons S, Marti O, Armario A. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. J Neurochem. 2004;89(5):1111–8. doi: 10.1111/j.1471-4159.2004.02396.x. [DOI] [PubMed] [Google Scholar]
- Palacios IM, Gatfield D, St Johnston D, Izaurralde E. An eIF4AIII-containing complex required for mRNA localization and nonsense-mediated mRNA decay. Nature. 2004;427(6976):753–7. doi: 10.1038/nature02351. [DOI] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59(1):70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone-Bizzozero N, Bolognani F. Role of HuD and other RNA-binding proteins in neural development and plasticity. J Neurosci Res. 2002;68(2):121–6. doi: 10.1002/jnr.10175. [DOI] [PubMed] [Google Scholar]
- Pintchovski SA, Peebles CL, Kim HJ, Verdin E, Finkbeiner S. The serum response factor and a putative novel transcription factor regulate expression of the immediate-early gene Arc/Arg3.1 in neurons. J Neurosci. 2009;29(5):1525–37. doi: 10.1523/JNEUROSCI.5575-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52(3):437–44. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Racaniello M, Cardinale A, Mollinari C, D’Antuono M, De Chiara G, Tancredi V, Merlo D. Phosphorylation Changes of CaMKII, ERK1/2, PKB/Akt Kinases and CREB Activation During Early Long-Term Potentiation at Schaffer Collateral-CA1 Mouse Hippocampal Synapses. Neurochem Res. 2009 doi: 10.1007/s11064-009-0047-0. Epub Sept 3 2009. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25(7):1761–8. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudineau S, Poucet B, Laroche S, Davis S, Save E. Impaired long-term stability of CA1 place cell representation in mice lacking the transcription factor zif268/egr1. Proc Natl Acad Sci U S A. 2009;106(28):11771–5. doi: 10.1073/pnas.0900484106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52(3):461–74. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Siemer H, Krug M, Hollt V. Direct evidence for biphasic cAMP responsive element-binding protein phosphorylation during long-term potentiation in the rat dentate gyrus in vivo. J Neurosci. 1999;19(13):5683–92. doi: 10.1523/JNEUROSCI.19-13-05683.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule J, Penke Z, Kanhema T, Alme MN, Laroche S, Bramham CR. Object-place recognition learning triggers rapid induction of plasticity-related immediate early genes and synaptic proteins in the rat dentate gyrus. Neural Plast. 2008;2008:269097. doi: 10.1155/2008/269097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21(4):741–51. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci U S A. 2001a;98(13):7062–8. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001b;30(1):227–40. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Thiels E, Barrionuevo G, Berger TW. Excitatory stimulation during postsynaptic inhibition induces long-term depression in hippocampus in vivo. J Neurophysiol. 1994;72(6):3009–16. doi: 10.1152/jn.1994.72.6.3009. [DOI] [PubMed] [Google Scholar]
- Thiels E, Kanterewicz BI, Knapp LT, Barrionuevo G, Klann E. Protein phosphatase-mediated regulation of protein kinase C during long-term depression in the adult hippocampus in vivo. J Neurosci. 2000;20(19):7199–207. doi: 10.1523/JNEUROSCI.20-19-07199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E. Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1. J Neurosci. 2002;22(6):2054–62. doi: 10.1523/JNEUROSCI.22-06-02054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Norman ED, Barrionuevo G, Klann E. Transient and persistent increases in protein phosphatase activity during long-term depression in the adult hippocampus in vivo. Neuroscience. 1998;86(4):1023–9. doi: 10.1016/s0306-4522(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Thiels E, Weisz DJ, Berger TW. In vivo modulation of N-methyl-D-aspartate receptor-dependent long-term potentiation by the glycine modulatory site. Neuroscience. 1992;46(3):501–9. doi: 10.1016/0306-4522(92)90139-s. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Nicoll RA. Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron. 2006;52(3):403–7. doi: 10.1016/j.neuron.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498(3):317–29. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Wallace CS, Lyford GL, Worley PF, Steward O. Differential intracellular sorting of immediate early gene mRNAs depends on signals in the mRNA sequence. J Neurosci. 1998;18(1):26–35. doi: 10.1523/JNEUROSCI.18-01-00026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci. 2001;21(15):5484–93. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59(1):84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Calibration curve for arc. Cycle threshold (Ct) values from qPCR analysis are plotted against different levels of dilution of RNA input (undiluted, 1/5, 1/25, and 1/125). To generate the standard cDNA, we reverse-transcribed 2 μg total RNA extracted from whole hippocampus under identical conditions and at the same time at which we reverse-transcribed total RNA extracted from experimental and control CA1 tissue samples, as described in the Methods. Based on the slope of the calibration curve, the amplification efficiency for the arc primer set was estimated to be 1.96. B) Calibration curve for zif368. Similar data as shown in A. Based on the slope of the calibration curve, the amplification efficiency for the zif268 primer set was estimated to be 1.98. C) Calibration curve for gapdh. Similar data as shown in A. Based on the slope of the calibration curve, the amplification efficiency for the gapdh primer set was estimated to be 1.97.