Abstract
Background
Poor sleep quality, dysregulation of hormones and increased inflammatory cytokines are all associated with risk for postpartum major depression (PPMD). We evaluated change over time in sleep quality and hormones during the first 17-weeks postpartum, as well as a single cytokine measure, and their association with PPMD recurrence.
Methods
Participants were pregnant women (N = 56), with past histories of MDD/PPMD but not depressed in their current pregnancy. The Pittsburgh Sleep Quality Index (PSQI) and blood samples were collected 8 times during the first 17 weeks postpartum. Estradiol, prolactin and cortisol, and a single measure of IL-6 were assayed. Recurrence was determined by two consecutive 21-item Hamilton Rating Scale for Depression (HRSD) scores ≥ 15 and clinician interview.
Results
In analyses of time to PPMD recurrence, poor sleep quality, but none of the hormones, was associated with PPMD recurrence (p < .05) after controlling for medication assignment. With every one point increase in PSQI scores across time, a woman's risk for recurrence increased by approximately 25% There was no significant association between PSQI scores and IL-6 concentrations in early postpartum (χ2= 0.98, p = .32).
Conclusions
Poor sleep quality across the first 17 weeks post delivery increases the risk for recurrent PPMD among women with a history of MDD. Changes in the hormonal milieu were not associated with recurrence. Further exploration of the degree to which poor sleep contributes to hormonal and cytokine dysregulation and how they are involved in the pathophysiology of PPMD is warranted.
1. Introduction
Postpartum onset major depression (PPMD) is a serious public health concern (Wisner et al., 2006). Approximately 14.5% of women will experience an incident episode, and 25% will experience a recurrent episode (Wisner et al., 2004). Women who experience PPMD are more likely to have impaired maternal-infant relationships (Gavin et al., 2005;Moehler et al., 2006), difficulties adhering to recommended preventative health services for the infant (Logsdon et al., 2006), and diminished maternal role gratification (Logsdon, Wisner, and Pinto-Foltz, 2006). Depression and its consequences can persist from months to years after childbirth, with lingering limitations in physical and psychological functioning after recovery from depressive episodes (Kendler et al., 1993).
Self-reported sleep disturbances are not only a common feature of depression but they are a diagnostic criterion (American Psychiatric Association, 2000). Complaints of poor sleep are reported in up to 90% of people with diagnosed depression (Tsuno et al., 2005). Both epidemiologic and clinical studies have shown that disturbed sleep is a prodromal symptom of both new and recurrent depressive episodes (Breslau et al., 1996;Ford et al., 1989;Perlis et al., 1997;Perlman et al., 2006). The extension of this relationship to depression occurring in the postpartum period has been evaluated with self-reported depressive symptoms (Goyal et al., 2007;Wolfson et al., 2003) rather than clinical diagnosis. Recently, we have shown a striking relationship between poor sleep quality in late pregnancy and clinically diagnosed recurrence of PPMD after 4 weeks postpartum (Okun et al., 2009).
Alterations in the hormonal and cytokine milieu may contribute to risk of postpartum depression (Bloch et al., 2003;Maes et al., 2000). Hormones such as estradiol, prolactin and cortisol peak during the last few weeks of pregnancy, followed by a drastic drop in levels following delivery and into the early postpartum period (Abou-Saleh et al., 1998;Ancelin et al., 2007). The rate of change in the hormone levels that occurs from pre-pregnancy to post-delivery is considered a key determinant in the increase in depressive symptoms and risk of PPMD (Ancelin, Scali, and Ritchie, 2007;Bloch, Daly, and Rubinow, 2003;Soares et al., 2008). Similarly, the `cytokine hypothesis of depression' states that both the etiology and pathophysiology of depression are linked to dysregulation of inflammatory cytokines (Maes, 1994). Puerperal women may be particularly vulnerable because inflammatory cytokines increase significantly during the last trimester of pregnancy in preparation for delivery (Romero et al., 2006). Women who report increased depressive symptoms in the postpartum have corresponding higher levels of proinflammatory cytokines (Maes, Lin, Ombelet, Stevens, Kenis, De Jongh, Cox, and Bosmans, 2000).
Disturbed sleep may be an antecedent to hormonal or cytokine changes. However, the mechanisms underlying these relationships have not been systematically evaluated (Cover et al., 1994;Irwin et al., 1992;Motivala et al., 2005). In the current study, we assessed whether poor sleep during the postpartum contributes to PPMD recurrence and if this relationship is affected by changes in pregnancy-related hormones. We hypothesized that women with poor sleep quality while controlling for decreased estradiol, prolactin, or cortisol levels across the postpartum would be more likely to recur within 17 weeks postpartum than women with better sleep quality. We were also interested in whether sleep quality and IL-6 concentrations during early postpartum would be associated with recurrence. Thus, we assessed if poor sleep quality and higher IL-6 concentrations in early postpartum would be observed among women who recurred within 17 weeks postpartum.
Methods and Materials
Sample
Pregnant women (N = 56) with past histories of an episode of PPMD/MDD, enrolled in a randomized clinical trial (RCT) designed to test the efficacy of prophylactic antidepressant medication use, were included in the analyses. A detailed description of the participants and the procedures have been published elsewhere (Wisner et al., 2001;Wisner, Perel, Peindl, Hanusa, Piontek, and Findling, 2004). Briefly, all participants reported at least one episode of major depression either in or outside the postpartum period within 5 years of enrollment but none were depressed at gestational week 36 of the current pregnancy. Women were randomly assigned to either an active medication or placebo immediately following delivery. Upon delivery participants were randomly assigned to either nortriptyline (N = 20), sertraline (N =10) or placebo (N = 26).
Procedures
Approval was obtained from the Case Western Reserve University institutional review board, which was the setting in which the original research was conducted. Written informed consent was obtained from all subjects. Details of the procedures are published elsewhere (Okun, Hanusa, Hall, and Wisner, 2009;Wisner, Perel, Peindl, Hanusa, Findling, and Rapport, 2001;Wisner, Perel, Peindl, Hanusa, Piontek, and Findling, 2004). Briefly, participants were seen at 2, 3, 4, 6, 8, 11, 14, and 17 weeks postpartum for assessment of depressive symptoms and sleep quality. Blood samples were obtained during these visits.
Measures and Instruments
The Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989), a 19-item questionnaire, was used to measure habitual sleep quality over the previous month. It is composed of seven subscales assessing habitual duration of sleep, nocturnal sleep disturbances, sleep latency, sleep quality, daytime dysfunction, sleep medication usage and sleep efficiency. Each subscale has a possible score 0-3, with an overall global score 0–21. The PSQI and its psychometric properties have been validated in various psychiatric populations (Buysse, Reynolds, Monk, Berman, and Kupfer, 1989), but not in pregnant women, particularly with children at home. In reports of various non-pregnant cohorts, the sensitivity and specificity are favorable when using a cutoff of ≤ 5 (Buysse, Reynolds, Monk, Berman, and Kupfer, 1989).
The 21-item Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960) is a commonly used measure of depressive symptomatology in clinical psychiatric research in both pregnant and postpartum populations (Wisner, Perel, Peindl, Hanusa, Findling, and Rapport, 2001). Scores are continuous, with a range from 0–52.
Definition of Recurrence
Women were administered the HRSD at each visit during the first 17 weeks of the postpartum period to assist in identifying a recurrent episode (Wisner, Perel, Peindl, Hanusa, Findling, and Rapport, 2001). If a woman endorsed symptoms of depression (HRSD ≥ 15), the participant was evaluated again within the following 7-day period. A second HRSD ≥ 15 prompted a clinical evaluation by two board certified psychiatrists that the clinical presentation met DSM-IV (American Psychiatric Association, 2000) criteria for major depressive disorder. Clinical interviews included the Structured Clinical Interview for Depression to diagnosis depression (First MB et al., 1996). If both evaluators concurred, the participant was identified as having a depression recurrence.
Hormone Assays
Blood samples were collected with chilled Vacutainer EDTA tubes and centrifuged immediately. Plasma was separated and stored at −80°C until assayed. Duplicates that deviated more than 5% were rejected and analyses were repeated.
Estradiol
Plasma estradiol levels were determined using a double antibody assay (Diagnostic Products Corporation (DPC), Los Angeles). Samples of 200μl were measured in duplicate. The limit of quantitative detectability was 2.0 pg/ml. Interassay %Coefficient of Variability (C.V.) was 2.8% – 5.8%.
Prolactin
Plasma prolactin levels were determined by a 100T commercial assay kit (Nichols Institute Diagnostics, San Juan Capistrano, CA). Samples of 50μl were assayed in duplicate. The limit of quantitative detectability was 0.25 ng/ml. Interassay %Coefficient of Variability (C.V.) ranged between 5.3% – 8.8%.
Cortisol
Cortisol was measured using the Coat-A-Count assay (Diagnostic Products Corporation (DPC), Los Angeles). Samples of 25μl were assayed in duplicate, Limit of Quantitative Detectability 0.2 μg/dl, Interassay %Coefficient of variability (C.V.) 4.7 – 7.4.
Cytokine Assays
IL-6 was assayed in one batch using a high sensitivity quantitative sandwich enzyme immunoassay kit (R & D Systems, Minneapolis, MN). The assay standard range is 0.156 – 10pg/ml. IL-6 levels were extrapolated from the standard curve with linear regression from a log-linear curve. All samples were run in duplicate and coefficient variation between samples was < 10%. Since the assay of IL-6 was conducted post hoc and not part of the initial study design, availability of viable samples was greatly reduced. Thus, only a single sample of IL-6 was available and reported here.
Statistical Considerations
Descriptive statistics were used to characterize the sample. Analyses were conducted using SAS 9.2. Repeated measures mixed linear models were estimated to test (1) the association between time and change in both hormone levels and sleep quality; and (2) the association between change in hormone levels and change in sleep quality adjusted for time, age, medication, and HRSD scores. All models included a random intercept and assumed an unstructured covariance matrix. In order to meet the normality assumptions of these models, each hormone was transformed by taking the natural log. Cox proportional hazards models were estimated to test the association between recurrence and both sleep quality and hormone level adjusting for medication. When the models included cortisol, estradiol, or prolactin, the covariates were time-dependent. In the model including IL-6, the covariates were not time-dependent. Each model was estimated with and without an interaction term between PSQI and hormone level. Models were considered significant at a p-value < .05.
Results
Table 1 shows the demographic characteristics of the sample. Women were approximately 31 years of age and mostly Caucasian (93%). Participants reflected a wide distribution of socioeconomic status (SES) and the majority (95%) were married or living with a partner. Among this sample, 12 (21.4%) had a recurrent PPMD episode within 17 weeks postpartum.
Table 1.
Sample characteristics*.
| Characteristics | N=56 |
|---|---|
| Age (years) | 31.1 ± 4.1 |
| White race | 52 (92.9) |
| Socio-economic class | |
| Lower | 13 (23.2) |
| Lower middle | 11 (19.6) |
| Middle | 9 (16.1) |
| Upper middle | 14 (25.0) |
| Upper | 9 (16.1) |
| Married/cohabiting | 53 (94.6) |
| Parity | 2.0 ± 0.8 |
| Study medication | |
| Placebo | 26 (46.4) |
| Nortriptyline | 20 (35.7) |
| Sertraline | 10 (17.9) |
Data presented as mean ± SD or n (%N)
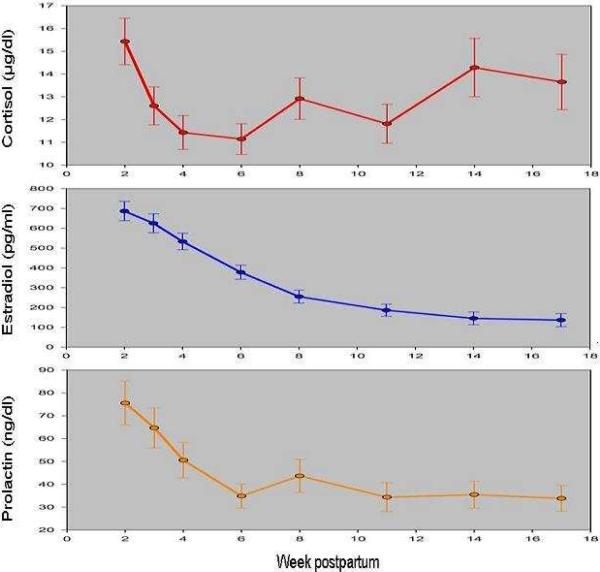
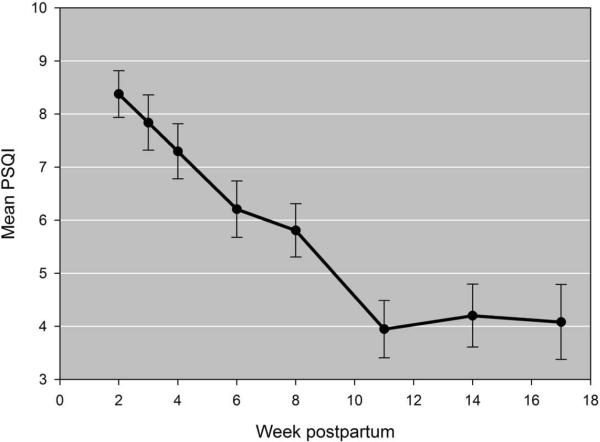
PSQI scores changed significantly over time controlling for each hormone, age, medication assignment and HRSD scores (p-value < .001). Likewise, each hormone changed significantly over time controlling for PSQI scores, age, medication assignment, and HRSD scores (p-values < .001). Tukey-adjusted pairwise comparisons revealed that PSQI scores significantly declined from week 2 to week 11 postpartum (p-values < .05) (Figure 1). In summary, sleep quality slowly improves, but remains poor (using a cut-off score of >5 to indicate poor sleep) for the first 8–10 weeks postpartum after which scores begin to stabilize. Estradiol concentrations show a steady and significant decline each week (2–17) across postpartum. Cortisol concentrations, on the other hand, show an immediate drop week 2 post-delivery with means gravitating towards levels observed at delivery by week 17. Lastly, prolactin concentrations decline from week to post-delivery to about week 4 then level off (Figure 2).
Figure 1.
Mean Pittsburgh Sleep Quality Index (PSQI) score across weeks 2 – 17 postpartum. Scores are significantly higher in the first 8 weeks compared to weeks 11–17 postpartum.
Figure 2.
Mean hormone concentrations of cortisol (μg/ml), estradiol (pg/ml) and prolactin (ng/ml) are plotted for weeks 2 –17 postpartum. All hormones significantly decreased over time, with the exception of cortisol which subsequently increased to near post-delivery values between weeks 11 and 17 postpartum.
Regressing time to recurrence on both PSQI scores and hormone levels using Cox proportional hazard models revealed that only PSQI scores were significantly related to recurrence. None of the covariates were significantly associated with recurrence. We found that for every one point increase in the PSQI score across time, a woman's risk for recurrence was increased by 23.9% when controlling for medication and change in estradiol levels (χ2 = 3.96, p = .05). A nearly identical relationship was observed for prolactin. With every one point increase in PSQI scores across time, a woman's risk for recurrence was increased by 24.2% when controlling for medication and change in prolactin levels (χ2 = 4.35, p = .04). Lastly, with every one point increase in PSQI scores across time, a woman's risk for recurrence increased by 26.8% when controlling for medication and change in cortisol levels (χ2 = 5.66, p = .02). There were no significant interactions between change in PSQI scores and change in estradiol (χ2 = .47, p = .49), cortisol (χ2 = 1.13, p = .29), or prolactin (χ2 = 2.58, p = .11).
Based on current literature we were interested in whether poor sleep quality and higher IL-6 concentrations in early postpartum would be observed among women who recurred within 17 weeks postpartum. Cross-sectional IL-6 data were available from 33 women. We assessed the relationship between week-matched (weeks 2, 3, or 4) PSQI scores and IL-6 concentrations and their relationship to risk of recurrence. Neither PSQI score (χ2 = 1.54, p = .21) nor IL-6 concentration (χ2 = 0.07, p = .79) were associated with recurrence controlling for medication assignment; however, the PSQI by IL-6 interaction approached significance (χ2 = 3.44, p = .06).
Discussion
We found that poor sleep quality during the first 17 weeks postpartum, but not levels of estradiol, prolactin or cortisol, was significantly associated with increased risk for a recurrent PPMD episode. Although neither sleep quality nor IL-6 concentrations in early postpartum contributed to increased risk, an interaction between the two may contribute to risk. Both sleep quality and changes in biomarkers have been independently implicated in recurrent depression (Bloch, Daly, and Rubinow, 2003; Breslau, Roth, Rosenthal, and Andreski, 1996; Jolley et al., 2007; Maes, Lin, Ombelet, Stevens, Kenis, De Jongh, Cox, and Bosmans, 2000; Perlis, Giles, Buysse, Tu, and Kupfer, 1997). However, the existing evidence of an interaction between the two and risk of recurrent depression is minimal. The current findings provide additional support for the hypothesis proposed by other investigators of heterogeneity and differential vulnerabilities with regards to risk for recurrence. For example, women who recur shortly after delivery are considered biologically vulnerable to the dramatic drop in reproductive hormones (Bloch et al., 2006). On the other hand, women who develop PPMD several weeks post delivery are considered psychosocially vulnerable (Okun, Hanusa, Hall, and Wisner, 2009). Moreover, the interpretations of these data suggest that an individual's trajectory on any of these measures may substantially deviate from the group pattern.
The finding that poor sleep quality is a significant risk factor for recurrent PPMD is consistent with several reports (Breslau, Roth, Rosenthal, and Andreski, 1996; Ford and Kamerow, 1989; Okun, Hanusa, Hall, and Wisner, 2009; Perlis, Giles, Buysse, Tu, and Kupfer, 1997). More importantly, these findings extend the existing literature such that they provide evidence that enduring sleep disturbances are likely crucial elements in determining risk for recurrent depression.
We could not test the hypothesis that rate of change in the hormonal milieu occurring from pre- to post-delivery is a key determinant in the increase in depressive symptoms and risk of PPMD (Ancelin, Scali, and Ritchie, 2007; Bloch, Daly, and Rubinow, 2003; Soares and Zitek, 2008). This was due to the design of the parent study and when the hormone assays were conducted. It is possible that the dramatic drop observed in pregnancy-related hormones by other investigators hit a plateau by our first assessment. There is support for this hypothesis as shown by the therapeutic effects observed following administration of these hormones to women with PPMD (Epperson et al., 1999). However, the empirical support for an effect of rate of change post-delivery, particularly in estradiol or cortisol, contributing significantly to clinical disease is scant Prolactin, on the other hand, has been associated with symptomatology but this relationship is moderated by breastfeeding status (Abou-Saleh, Ghubash, Karim, Krymski, and Bhai, 1998; Bloch, Daly, and Rubinow, 2003; Harris et al., 1989; Nott et al., 1976). Future evaluations of this relationship are merited in order to prospectively assess hormones during the prenatal as well as the postnatal period.
We did not find support for a relationship between poor sleep quality and concentrations of IL-6 and recurrent PPMD. The week at which the assessment was made could partially explain the results. Previous reports have evaluated cytokines within a few days post-delivery when they are noted to be higher in women with a history of PPMD (Maes, Lin, Ombelet, Stevens, Kenis, De Jongh, Cox, and Bosmans, 2000; Maes et al., 2001). Although there is no clinically established cutoff for IL-6 and increased risk of PPMD, we found clinically relevant differences when two clinically suggestive cutoffs were applied. When IL-6 > 1.5 pg/ml was used we found that 29% above this cutoff recurred versus 0% with concentrations below; and if > 2 pg/ml was used then 29% recurred vs 12.5%.
Our null finding may be a result of the parent study's design rather than the absence of a relationship. The parent studies were prophylactic drug trials in which women were randomized to an antidepressant medication or placebo following delivery in an effort to prevent a recurrent episode during a highly vulnerable period (Wisner, Perel, Peindl, Hanusa, Findling, and Rapport, 2001; Wisner, Perel, Peindl, Hanusa, Piontek, and Findling, 2004). Circulating levels of estradiol (Taylor et al., 2004), cortisol (Horstmann et al., 2009) and proinflammatory cytokines (Basterzi, 2005 14482 /id} are noted to decrease following SSRI treatment, whereas prolactin has been noted to increase following SSRI treatment (Torre et al., 2007). Thus, use of medication may mask a more complicated relationship between sleep, biomarkers and recurrence; one that we were unable to discern with current statistical control measures.
Although our findings are significant, we recognize several limitations. First, this sample of women is not generalizable to all pregnant/postpartum women. These women were considered vulnerable and high risk. Another limitation is that sleep quality was assessed via self-report. Future findings may be bolstered with objective sleep data. In spite of this, the PSQI has been shown to have good validity in depression and pregnancy (Buysse, Reynolds, Monk, Berman, and Kupfer, 1989;Carpenter et al., 1998;Smyth, 2000). Moreover, the availability of longitudinal sleep quality data (8 scores in total across 17 weeks) and the small variance speaks to the reliability of the PSQI. The evaluation of estradiol, prolactin and cortisol in this study was dependent on available data, and the lack of data between delivery and two weeks postpartum likley prevented our ability to detect a relationship between the hormonal decrease and depression risk (Abou-Saleh, Ghubash, Karim, Krymski, and Bhai, 1998;Bloch, Daly, and Rubinow, 2003;Harris, Johns, Fung, Thomas, Walker, Read, and Riad-Fahmy, 1989;Nott, Franklin, Armitage, and Gelder, 1976;O'Hara et al., 1991).. Future studies will examine additional pregnancy-related hormones including progesterone and CRH. With regards to the IL-6 data, we acknowledge the limitation of having a small sample size and a single measure of inflammation. However, it provided an opportunity to explore the sleep and cytokine relationship as a risk factor for PPMD.
In summary, diminished sleep quality during the first several weeks postpartum is associated with an increased risk recurrent PPMD. Clinically this information is useful since sleep is a modifiable behavior. With the recognition and appreciation that sleep is a significant correlate of PPMD recurrence, clinicians and patients alike can incorporate behavioral interventions to improve sleep quality during the perinatal period and subsequently yield positive benefits with regards to both the increased inflammatory response noted to occur following delivery (Maes, Ombelet, De Jongh, Kenis, and Bosmans, 2001) and depression risk. .
Acknowledgements
The authors would like to thank Dr. Barbara Hanusa for her initial contributions to the statistical analyses and to Andrea Confer for data management.
This work was supported by “NARSAD, The World's Leading Charity Dedicated to Mental Health Research” 1998 Independent Investigator Award. This research was completed during the author's work at Women's Behavioral Health CARE. This author is currently affiliated with VA Pittsburgh Healthcare System, Center for Health Equity Research and Promotion as well as, Department of Psychiatry, University of Pittsburgh Medical Center.
Initial funding for this study and Dr. Wisner's time was supported by National Institute of Mental Health grants MH R01 60335 and MH R01 53735. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Dr. Wisner's time was supported by National Institute of Mental Health grants MH R01 60335 and MH R01 53735. Dr. Wisner is a member of the speakers' bureau for GlaxoSmithKline and has received grant support from Pfizer, funding from Wyeth to study desmethylvenlafaxine for postpartum depression is pending. No pharmaceutical companies were involved in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. Drs. Okun, Perel, and Wisniewski and Mrs. Luther and Prather report no competing interests. Dr. Okun's time was supported by NR 08103.
Disclosure Statement This is not an industry supported study. Dr. Wisner received a donation from Novo-Gyne of transdermal placebo patches for an NIMH funded study of estradiol patch for postpartum depression treatment. Dr. Wisner also serves on an Advisory Board for Eli Lilly Corporation. The remaining authors have indicated no biomedical financial interests or other conflicts of interest.
References
- 1.Abou-Saleh MT, Ghubash R, Karim L, Krymski M, Bhai I. Hormonal aspects of postpartum depression. Psychoneuroendocrinology. 1998;23(5):465–475. doi: 10.1016/s0306-4530(98)00022-5. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 3.Ancelin ML, Scali J, Ritchie K. Hormonal therapy and depression: are we overlooking an important therapeutic alternative? J. Psychosom. Res. 2007;62(4):473–485. doi: 10.1016/j.jpsychores.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr. Psychiatry. 2003;44(3):234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 5.Bloch M, Rotenberg N, Koren D, Klein E. Risk factors for early postpartum depressive symptoms. Gen. Hosp. Psychiatry. 2006;28(1):3–8. doi: 10.1016/j.genhosppsych.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol. Psychiatry. 1996;39(6):411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 7.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J. Psychosom. Res. 1998;45(1 Spec No):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 9.Cover H, Irwin M. Immunity and depression: insomnia, retardation, and reduction of natural killer cell activity. J. Behav. Med. 1994;17(2):217–223. doi: 10.1007/BF01858106. [DOI] [PubMed] [Google Scholar]
- 10.Epperson CN, Wisner KL, Yamamoto B. Gonadal steroids in the treatment of mood disorders. Psychosom. Med. 1999;61(5):676–697. doi: 10.1097/00006842-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 11.First MB, Gibbon M, Spitzer RL, Williams JBW. Biometrics Research. New York: 1996. User's Guide for the Structured Interview for DSM-IV Axis I Disorders - Research Version (SCID-1, version 2.0, February 1996 final version) [Google Scholar]
- 12.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 13.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet. Gynecol. 2005;106(5 Pt 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 14.Goyal D, Gay CL, Lee KA. Patterns of sleep disruption and depressive symptoms in new mothers. J. Perinat. Neonatal Nurs. 2007;21(2):123–129. doi: 10.1097/01.JPN.0000270629.58746.96. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris B, Johns S, Fung H, Thomas R, Walker R, Read G, Riad-Fahmy D. The hormonal environment of post-natal depression. Br. J. Psychiatry. 1989;154:660–667. doi: 10.1192/bjp.154.5.660. [DOI] [PubMed] [Google Scholar]
- 17.Horstmann S, Binder EB. Pharmacogenomics of antidepressant drugs. Pharmacol. Ther. 2009;124(1):57–73. doi: 10.1016/j.pharmthera.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Irwin M, Smith TL, Gillin JC. Electroencephalographic sleep and natural killer activity in depressed patients and control subjects. Psychosom. Med. 1992;54:10–21. doi: 10.1097/00006842-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Jolley SN, Elmore S, Barnard KE, Carr DB. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol. Res. Nurs. 2007;8(3):210–222. doi: 10.1177/1099800406294598. [DOI] [PubMed] [Google Scholar]
- 20.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of 1-year prevalence of major depression in women. Arch. Gen. Psychiatry. 1993;50(11):843–852. doi: 10.1001/archpsyc.1993.01820230009001. [DOI] [PubMed] [Google Scholar]
- 21.Logsdon MC, Wisner KL, Pinto-Foltz MD. The impact of postpartum depression on mothering. J. Obstet. Gynecol. Neonatal Nurs. 2006;35(5):652–658. doi: 10.1111/j.1552-6909.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- 22.Maes M. Cytokines in major depression. Biol. Psychiatry. 1994;36(7):498–499. doi: 10.1016/0006-3223(94)90652-1. [DOI] [PubMed] [Google Scholar]
- 23.Maes M, Lin AH, Ombelet W, Stevens K, Kenis G, De Jongh R, Cox J, Bosmans E. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology. 2000;25(2):121–137. doi: 10.1016/s0306-4530(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 24.Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J. Affect. Disord. 2001;63(1–3):85–92. doi: 10.1016/s0165-0327(00)00156-7. [DOI] [PubMed] [Google Scholar]
- 25.Moehler E, Brunner R, Wiebel A, Reck C, Resch F. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch. Womens Ment. Health. 2006;9(5):273–278. doi: 10.1007/s00737-006-0149-5. [DOI] [PubMed] [Google Scholar]
- 26.Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom. Med. 2005;67(2):187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- 27.Nott PN, Franklin M, Armitage C, Gelder MG. Hormonal changes and mood in the puerperium. Br. J. Psychiatry. 1976;128:379–383. doi: 10.1192/bjp.128.4.379. [DOI] [PubMed] [Google Scholar]
- 28.O'Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J. Abnorm. Psychol. 1991;100(1):63–73. doi: 10.1037//0021-843x.100.1.63. [DOI] [PubMed] [Google Scholar]
- 29.Okun ML, Hanusa BH, Hall M, Wisner KL. Sleep complaints in late pregnancy and the recurrence of postpartum depression. Behav. Sleep Med. 2009;7(2):106–117. doi: 10.1080/15402000902762394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J. Affect. Disord. 1997;42(2–3):209–212. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 31.Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar. Disord. 2006;8(3):271–274. doi: 10.1111/j.1399-5618.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin. Fetal Neonatal Med. 2006;11(5):317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth C. The Pittsburgh Sleep Quality Index (PSQI) Director. 2000;8(1):28–29. [PubMed] [Google Scholar]
- 34.Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J. Psychiatry Neurosci. 2008;33(4):331–343. [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor GT, Farr S, Klinga K, Weiss J. Chronic fluoxetine suppresses circulating estrogen and the enhanced spatial learning of estrogen-treated ovariectomized rats. Psychoneuroendocrinology. 2004;29(10):1241–1249. doi: 10.1016/j.psyneuen.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Torre DL, Falorni A. Pharmacological causes of hyperprolactinemia. Ther. Clin. Risk Manag. 2007;3(5):929–951. [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuno N, Besset A, Ritchie K. Sleep and depression. J. Clin. Psychiatry. 2005;66(10):1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- 38.Wisner KL, Chambers C, Sit DK. Postpartum depression: a major public health problem. JAMA. 2006;296(21):2616–2618. doi: 10.1001/jama.296.21.2616. [DOI] [PubMed] [Google Scholar]
- 39.Wisner KL, Perel JM, Peindl KS, Hanusa BH, Findling RL, Rapport D. Prevention of recurrent postpartum depression: a randomized clinical trial. J. Clin. Psychiatry. 2001;62(2):82–86. doi: 10.4088/jcp.v62n0202. [DOI] [PubMed] [Google Scholar]
- 40.Wisner KL, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RL. Prevention of postpartum depression: a pilot randomized clinical trial. Am. J. Psychiatry. 2004;161(7):1290–1292. doi: 10.1176/appi.ajp.161.7.1290. [DOI] [PubMed] [Google Scholar]
- 41.Wolfson AR, Crowley SJ, Anwer U, Bassett JL. Changes in sleep patterns and depressive symptoms in first-time mothers: last trimester to 1-year postpartum. Behav. Sleep Med. 2003;1(1):54–67. doi: 10.1207/S15402010BSM0101_6. [DOI] [PubMed] [Google Scholar]