Abstract
BACKGROUND:
Dyslipidemia results from consumption of a diet rich in saturated fatty acids and is usually associated with cardiovascular disease. A diet rich in unsaturated fatty acids is usually associated with improved cardiovascular condition.
OBJECTIVE:
To investigate whether a high-fat diet rich in unsaturated fatty acids (U-HFD) – in which fatty acid represents approximately 45% of the total calories – impairs the cardiovascular system.
METHODS:
Male, 30-day-old Wistar rats were fed a standard (control) diet or a U-HFD containing 83% unsaturated fatty acid for 19 weeks. The in vivo electrocardiogram, the spectral analysis of heart rate variability, and the vascular reactivity responses to phenylephrine, acetylcholine, noradrenaline and prazosin in aortic ring preparations were analyzed to assess the cardiovascular parameters.
RESULTS:
After 19 weeks, the U-HFD rats had increased total body fat, baseline glucose levels and feed efficiency compared with control rats. However, the final body weight, systolic blood pressure, area under the curve for glucose, calorie intake and heart weight/final body weight ratio were similar between the groups. In addition, both groups demonstrated no alteration in the electrocardiogram or cardiac sympathetic parameters. There was no difference in the responses to acetylcholine or the maximal contractile response of the thoracic aorta to phenylephrine between groups, but the concentration necessary to produce 50% of maximal response showed a decrease in the sensitivity to phenylephrine in U-HFD rats. The cumulative concentration-effect curve for noradrenaline in the presence of prazosin was shifted similarly in both groups.
CONCLUSIONS:
The present work shows that U-HFD did not impair the cardiovascular parameters analyzed.
Keywords: Cardiovascular, High-fat diet, Obesity, Unsaturated fatty acid
Abstract
HISTORIQUE :
La dyslipidémie découle de la consommation d’un régime riche en acides gras saturés et s’associe généralement à une maladie cardiovasculaire. Par contre, un régime riche en acides gras non saturés est généralement lié à une amélioration de l’état cardiovasculaire.
OBJECTIF :
Explorer si un régime cétogène riche en acides gras non saturés (RC-N), dans lequel les acides gras représentent environ 45 % des calories totales, nuit au système cardiovasculaire.
MÉTHODOLOGIE :
Des rats mâles Wistar de 30 jours ont été nourris au moyen d’un régime standard (groupe témoin) ou d’un RC-N contenant 83 % d’acides gras non saturés pendant 19 semaines. Les chercheurs ont analysé l’électrocardiogramme in vivo, l’analyse spectrale de la variabilité du rythme cardiaque et les réponses de réactivité vasculaire à la phényléphrine, à l’acétylcholine, à la noradrénaline et à la prazosine dans les préparations de l’anneau aortique afin d’évaluer les paramètres cardiovasculaires.
RÉSULTATS :
Au bout de 19 semaines, les rats RC-N présentaient une masse grasse totale, une glycémie de départ et une capacité de transformation des aliments plus élevées que les rats témoins. Cependant, le poids corporel final, la tension artérielle systolique, la surface sous la courbe de glycémie, l’apport calorique et le ratio entre le poids du cœur et le poids corporel final étaient similaires entre les deux groupes. De plus, les deux groupes n’ont démontré aucune altération de l’électrocardiogramme ou des paramètres sympathiques cardiaques. La réponse à l’acétylcholine ou la réponse contractile maximale de l’aorte thoracique à la phényléphrine ne différait pas entre les groupes, mais la concentration nécessaire pour produire 50 % de la réponse maximale était moins sensible à la phényléphrine chez les rats RC-N. La déviation de la courbe concentration-effet cumulative de noradrénaline en présence de prazosine était similaire entre les deux groupes.
CONCLUSIONS :
Les présents travaux révèlent qu’un RC-N ne nuisait pas aux paramètres cardiovasculaires analysés.
Diet and lifestyle modifications have long been advocated to decrease the risk of cardiovascular disease (CVD) (1), which is one of the main causes of death worldwide (2). Dietary fatty acids of varying chain length and degree of saturation differentially alter plasma lipoprotein profiles and the subsequent risk of developing CVD. A lipoprotein profile imbalance is associated with obesity, among others (3). Obesity, which is characterized by the accumulation of excessive body fat, is considered to be a global epidemic and represents an important public health problem (4,5).
Although the etiology of obesity is complex, distinct risk factors have been implicated in its development, especially hypercaloric intake (6). Recent investigations (7–10) have demonstrated that obesity decreases life expectancy and is related to numerous medical complications such as type 2 diabetes mellitus, dyslipidemia and CVDs. To understand the physiopathology of the abnormalities secondary to obesity, various animal models have been proposed – using either genetic or dietetic approaches (11,12). Although it is clear that genetic factors contribute to the propensity of an individual to become obese, the overconsumption of a high-calorie diet may promote a positive energy balance and lead to the development of overweight and obesity states (13).
Previous studies have indicated that human obesity promotes electrocardiographic (ECG) changes (14,15) such as prolongation of the QT interval (16,17). This abnormality is considered to be a precursor of malignant arrhythmias and sudden death (18,19). Although QT interval lengthening has been found to be associated with obesity (20), the mechanism responsible for such an association remains elusive. Corbi et al (21) found a significant relationship between heart rate-corrected QT intervals, and plasma adrenaline and noradrenaline concentrations, and suggested autonomic nervous system dysfunction as a possible mechanism of the prolonged heart rate-corrected QT intervals in patients with visceral obesity. Moreover, no study has analyzed the ECG and the QT interval in diet models of obesity.
Despite the unequivocal relationship between obesity and CVD, the effect of obesity on vascular function in experimental studies presents divergent results. Although some authors have found attenuated vasorelaxation responses to acetylcholine (ACh) (22), other investigators have verified that obesity does not impair endothelium-dependent vasodilation (23,24). Furthermore, studies examining the contractile responses to adrenergic agonists in different animal models of obesity have yielded contradictory results. Although Naderali et al (22) did not find differences in contractile responses to noradrenaline, other authors (23,24) observed enhanced vascular contractility in diet-induced obesity. Hypercholesterolemia (25), genetic hyperlipidemias (26) or a single high-fat meal (27) decreases endothelial function, suggesting that a regular high-fat diet might lead to endothelial dysfunction.
Administration of a high-fat diet is an experimental model that reproduces many features of human obesity (28,29). Classically, the high-fat diets used in animal models contain a high fraction of saturated fatty acid (approximately 90%), with a low fraction of monounsaturated and polyunsaturated fatty acid (30). However, few studies have tested, at least to our knowledge, whether a high-fat diet rich in unsaturated fatty acids (U-HFD) – containing 83% unsaturated fatty acid – impairs the cardiovascular system after 19 weeks. Thus, in the present study, we examined the ECG profile, the autonomic function and the vascular reactivity of rats fed a U-HFD to assess such a situation.
METHODS
Animal model and experimental protocol
All experiments and procedures were performed consistent with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (USA) and were approved by the Botucatu Medical School Ethics Committee, Universidade Estadual Paulista (São Paulo, Brazil).
Thirty-day-old, male Wistar rats were divided into two groups. One group was fed a standard diet (controls; n=10), containing 11.2% of kilocalories from fat, 55.5% from carbohydrate and 33.3% from protein, for 19 consecutive weeks. The other group received a U-HFD (n=9), containing 45.2% of kilocalories from fat, 28.6% from carbohydrate and 26.2% from protein, for 19 consecutive weeks. The U-HFD (4.5 kcal/g) was more calorie rich than the standard diet (3.3 kcal/g) because of the higher amount of energy from fat – composed of 17% saturated and 83% unsaturated fatty acids. All rats were housed in individual cages in an environmentally controlled clean-air room at a mean (± SD) temperature of 23±3°C, with a 12 h light/dark cycle (lights on at 06:00) and 60±5% relative humidity. Food consumption was measured daily and body weight was determined once a week. Weekly caloric intake was calculated by the average weekly food consumption calorie value of each diet. Feed efficiency, which is the ability to translate calories consumed into body weight, was also evaluated. Initial body weight, final body weight (FBW), heart weight (HW) and the HW/FBW ratio were analyzed.
Body fat
After the animals had been anesthetized (sodium pentobarbital 50 mg/kg intraperitoneal), decapitated and thoracotomized, the fat pads of adipose tissue were dissected and weighed. Total body fat was measured from the sum of the individual fat pad weights: epididymal fat plus retro-peritoneal fat plus visceral fat. The adiposity index was calculated as the following (31):
Systolic blood pressure
At the end of the experiment, the systolic blood pressure was assessed by the noninvasive tail-cuff method using an electrosphygmomanometer (Narco BioSystems, International Biomedical Inc, USA) (32). The average of two pressure readings was recorded for each measurement.
Insulin-tolerance test
After 19 weeks of low- and high-fat dietary feeding, the rats were fasted for 6 h and subjected to an insulin-tolerance test. To measure glucose, blood samples were drawn from the tip of the tail at baseline and after intraperitoneal administration of regular insulin (Novolin R, Novo Nordisk, Denmark) at a dose of 1.5 IU/kg body weight (33). Subsequently, blood samples were collected at 0 min (basal), 15 min, 30 min, 45 min, 60 min and 90 min. Glucose levels were determined using the Accu-Chek Go Kit glucose analyzer (Roche Diagnostic Brazil Ltda, Brazil). Insulin resistance was determined by the area under the curve for glucose (0 min to 90 min).
ECG
At the start of the 19th week, the rats were anesthetized (ketamine/xylazine 80/20 mg/kg intraperitoneal). Using a surgical procedure, two steel electrodes were implanted subcutaneously near the apex and base of the heart, resulting in a lead placed close to D2. Electrodes were exteriorized through the back of the neck and terminated in a custom-made connector. ECG recordings in the conscious state were made 24 h after the surgical procedure; this was conducted while the unrestricted animals rested quietly in individual cages. Recordings were always started 10 min to 15 min after linking the connector, through flexible cables, to the differential alternating-current amplifier (Model 1700; A-M Systems, USA), and were conducted in all animals for 3 min 10 s (these additional 10 s were recorded to ensure 180 s of artifact-free tachograms; nevertheless, when RR artifacts were removed, it never reached more than 1% of detected intervals). ECG signals were acquired at a sampling rate of 10 kHz and an amplitude resolution of 16 bits (A/D interface Digidata 1322A; Axon Instruments, USA). All recordings were conducted in a constant environment, during the same period of the morning (06:00 to 09:00).
The ECG parameters measured were PR, QT, RR and QRS intervals. Data were analyzed using Clampfit 10.1 (Axon Instruments, USA).
The assessment of cardiac autonomic control was made using heart rate variability (HRV) analysis, and the signal processing in the present study was performed using MATLAB-based algorithms (MathWorks, USA). ECGs were first band-pass filtered (2 Hz to 300 Hz) and, after R-wave peak detection, 180 s tachograms were generated, containing all heart period fluctuations within this time segment. In the time domain, the following indexes were obtained: mean RR interval, SD of the RR intervals, and square root of the mean squared differences of successive RR intervals. For spectral (frequency domain) analysis of HRV, tachograms were resampled to equal intervals by the spline cubic interpolation method at 10 Hz, and the linear trend was removed. Power spectrum was obtained using a fast Fourier transform-based method (Welch’s periodogram: 256 points, 50% overlap and Hamming window). Two frequency bands were determined: low frequency (LF; 0.2 Hz to 0.8 Hz) and high frequency (HF; 0.8 Hz to 2.5 Hz). Power (in ms2) was estimated as the area under the spectrum within these frequency ranges.
Vascular reactivity
The animals were sacrificed by decapitation under sodium pentobarbital (50 mg/kg intraperitoneal) anesthesia. The thoracic aortas were removed, cleaned of connective tissue and prepared for isometric tension recording. The aortas were cut into 2 mm to 3 mm rings that were placed in a vertical chamber filled with saline solution composed of 120 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 18 mM NaHCO3, 1.2 mM CaCl2 and 11 mM glucose (pH 7.4), and oxygenated with carbogen gas mixture (95% O2, 5% CO2) at 37±0.5°C. Each ring was mounted between two hooks in which one hook was attached to a force transducer (Grass model FT-03, Grass Technologies, USA) whose signal was conditioned by a Cyberamp (Axon Instruments Inc, USA); contractile response was displayed and stored in a computer for future analysis using Axoscope software (Axon Instruments Inc). Preparations were stabilized under 1 g resting tension for 2 h. After the equilibrium period, the integrity of the endothelium was tested by the addition of ACh (10 μM) after precontraction with phenylephrine (PE; 10 μM). The endothelium was considered to be intact if the Ach-induced relaxation of the precontracted aorta was greater than 80%. The aortic rings were then washed and equilibrated for another 40 min period before initiating the experimental protocol. Increasing concentrations of PE (1 nM to 10 μM) were added to the tissue bath. When the contractile response to PE reached a plateau, ACh was added in a cumulative manner (1 nM to 10 μM). Also, to assess the contribution of the alpha-1 (α1)-adrenergic receptors in the aortas of both control and U-HFD rats, cumulative concentration-effect curves for noradrenaline were generated for isolated aortas in the absence or presence of the reversible α1-antagonist prazosin (10–8 M).
Statistical analysis
Values were compared between groups using the Student’s t test. The mean weekly body weight and the insulin-tolerance test were analyzed by repeated-measures two-way ANOVA and complemented by Bonferroni’s post hoc test for multiple comparisons. Contraction responses are expressed as the percentage of maximal PE-induced contraction. Relaxation responses are expressed as the percentage relaxation of the precontraction induced by 10 μM PE. Concentration-response curves were analyzed by nonlinear regression using GraphPad Prism version 4.0 (GraphPad Software Inc, USA). Both the maximal contraction or maximal relaxation (Emax) and the concentration necessary to produce 50% of maximal response (EC50) were determined. Data are presented as mean ± SEM.
RESULTS
General characteristics of rats
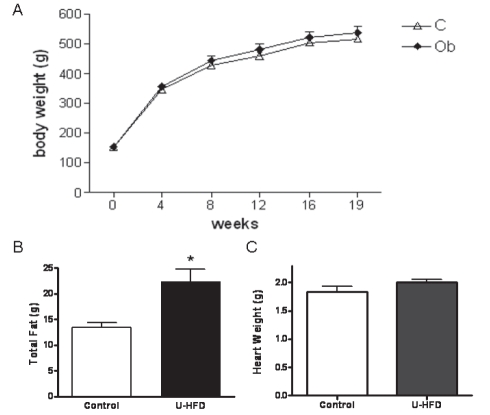
The general characteristics of the rats are shown in Table 1. There was no difference between groups in the evolution of body weight after 19 weeks (Figure 1A).
TABLE 1.
General and nutritional characteristics of study rats
| Variables |
Group |
|
|---|---|---|
| Control diet (n=10) | U-HFD (n=9) | |
| Initial body weight, g | 153±1 | 152±1 |
| Final body weight, g | 516±10 | 537±22 |
| Food consumption, g/day | 28.4±0.3 | 22.6±0.3* |
| Caloric intake, g × kcal/day | 83.9±1.5 | 82.6±3.3 |
| Feed efficiency, % | 3.25±0.04 | 3.49±0.09* |
| Systolic blood pressure, mmHg | 136±4 | 128±4 |
| Glucose, mg/dL | 88±1 | 95±1* |
| AUC | 5082±95 | 5278±92 |
| Epididymal fat, g | 6.06±0.47 | 9.44±1.13* |
| Retroperitoneal fat, g | 5.98±0.46 | 12.60±1.05* |
| Visceral fat, g | 1.49±0.14 | 2.44±0.23* |
| Total body fat, g | 13.5±0.8 | 24.5±2.3* |
| Adiposity index, % | 2.64±0.18 | 4.51±0.29* |
Data presented as mean ± SEM.
P<0.05 versus controls (Student’s t test for independent samples). AUC Area under the curve for glucose (insulin-tolerance test); U-HFD High-fat diet rich in unsaturated fatty acids
Figure 1).
A After 19 weeks, no difference in body weight was observed. B The high-fat diet rich in unsaturated fatty acids (U-HFD) group showed higher total body fat than the control group. C There was no difference in heart weight between the groups. Results are expressed as means with SEMs. Control diet (C; n=10) and U-HFD (Ob; n=9). *P<0.005
After 19 weeks, the FBW, systolic blood pressure, area under the curve for glucose, and calorie intake were similar between groups. Despite the fact that the U-HFD rats demonstrated less food consumption (P<0.05), their feed efficiency was greater than that of control rats (P<0.03). Furthermore, the adiposity index and plasma glucose concentration were significantly increased in the U-HFD rats (Table 1).
Although FBW was not different between groups, the U-HFD rats showed significantly greater total body fat weight (control 13.5±0.8 g versus U-HFD 24.5±2.3 g, P<0.01; Figure 1B). No difference between groups was observed in HW (control 1.84±0.09 g versus U-HFD 1.99±0.06 g, P>0.05; Figure 1C), nor in the HW/FBW ratio (HW/FBW × 1000) (control 3.57±0.18 versus U-HFD 3.75±0.18, P>0.05).
ECG and HRV
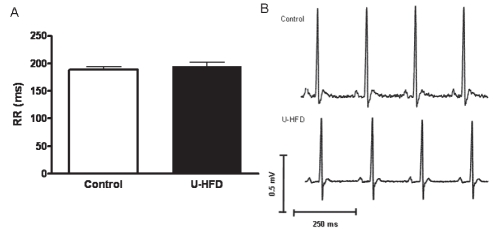
Figure 2 illustrates representative unfiltered tracings of ECG recordings obtained from both control and U-HFD rats. The ECG parameters measured were not statistically different between groups (Table 2).
Figure 2).
Representative electrocardiograms in conscious rats. A Bar graph showing no difference in RR interval between the groups. Results are expressed as means with SEMs. B Representative traces of electrocardiograms from both groups. Control diet (n=10) and high-fat diet rich in unsaturated fatty acids (U-HFD; n=9). P>0.05 for comparison between groups
TABLE 2.
Electrocardiogram parameters
| Interval |
Group |
|
|---|---|---|
| Control diet (n=10) | U-HFD (n=9) | |
| PR | 44.3±1.9 | 44.4±1.9 |
| QRS | 20.3±0.6 | 19.9±0.7 |
| RR | 189±5 | 195±6 |
| QT | 72.4±3.3 | 65.0±2.6 |
| Heart-rate corrected QT | 167±8 | 146±7 |
Data presented as mean ± SEM. There was no statistical difference between groups (Student’s t test for independent samples). U-HFD High-fat diet rich in unsaturated fatty acids
Time-domain HRV analysis revealed no significant difference between groups for the studied indexes, as illustrated in Figure 3 (mean RR interval: control 187.8±6.2 ms versus U-HFD 196.9±6.7 ms; SD of RR intervals: contol 5.2±0.6 ms versus U-HFD 4.9±0.8 ms; square root of the mean squared differences of successive RR intervals: control 3.5±0.7 ms versus U-HFD 3.9±0.7 ms).
Figure 3).
Time-domain heart rate variability indexes for the mean RR interval (RR), the SD of RR intervals (SDNN) and the square root of the mean squared differences of successive RR intervals (RMSSD). Data are presented as means with SEMs. U-HFD High-fat diet rich in unsaturated fatty acids
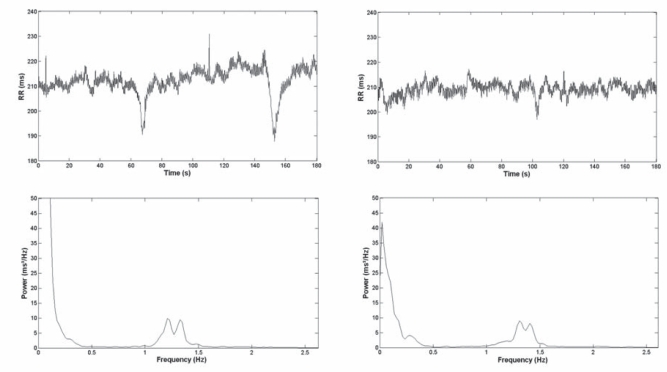
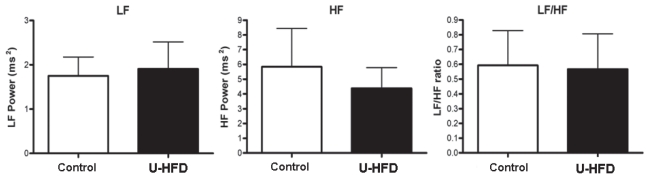
Figure 4 illustrates full-length tachograms and RR power spectra from both a representative control rat and a U-HFD rat. HRV analysis in the frequency domain also failed to reveal significant differences between groups, as illustrated in Figure 5 (LF power: control 1.7±0.4 ms2 versus U-HFD 1.9±0.6 ms2; HF power: control 5.8±2.6 ms2 versus U-HFD 4.4±1.4 ms2; LF/HF ratio: control 0.59±0.2 versus U-HFD 0.57±0.2).
Figure 4).
Full-length tachograms (top) and RR power spectra (bottom) from both a representative control rat (left) and a rat fed a high-fat diet rich in unsaturated fatty acids (right)
Figure 5).
Spectral heart rate variability indexes for low-frequency (LF) RR power, high-frequency (HF) RR power and LF/HF ratio. Data are presented as means with SEMs. U-HFD High-fat diet rich in unsaturated fatty acids
Vascular response
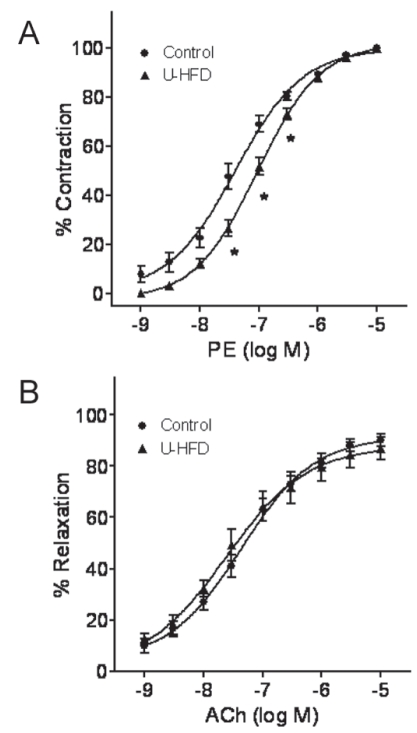
Isometric tension was measured in aortic rings from control and U-HFD rats. The concentration-response curve for PE was shifted slightly to the right in the U-HFD group, although maximal response was not altered (Emax: control 1.2±0.04 versus U-HFD 1.2±0.08 g, P>0.05) (Figure 6A). The U-HFD aorta was less sensitive to the α1-adrenergic agonist as shown by the higher EC50 (EC50: control 53.5±8.1 nM versus U-HFD 87.9±8.1 nM, P<0.05).
Figure 6).
Vascular response to phenylephrine (PE) and acetylcholine (ACh). A Representative curves contractile response to PE. B Concentration-effect curves for ACh in the aorta with intact endothelium. The concentration-response curves of the aortic rings were generated from control rats and rats fed a high-fat diet rich in unsaturated fatty acids (U-HFD). Each point represents mean ± SEM, n=7. *P<0.05 versus the control group
Vasodilation in response to ACh did not differ between control and U-HFD rats (Figure 6B) (Emax: control 90.1±2.4% versus U-HFD 86.9±4.5%, P>0.05). EC50 was 35.8±4.3 nM and 42.2±9.9 nM (P>0.05), respectively.
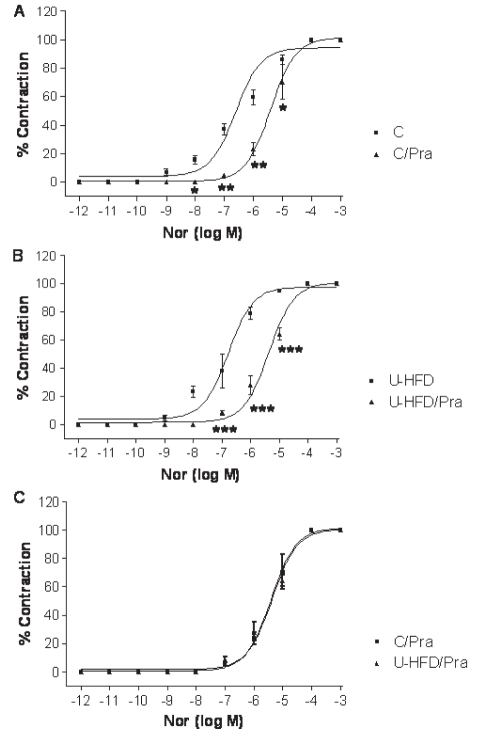
In other experiments, the participation of the alpha-adrenergic pathway was investigated. Thus, the cumulative concentration-effect curves for noradrenaline were generated for isolated aortas in the absence or presence of the reversible α1-antagonist prazosin (10–8 M). In the aortas of both control and U-HFD groups, a significant shift in the presence of prazosin was observed, as shown in Figures 7A and 7B. However, the final response to prazosin was similar for both groups (Figure 7C and Table 3). In addition, no significant differences between groups were found when comparing the EC50 of noradrenaline in the presence of prazosin (P<0.05).
Figure 7).
The vascular response to prazosin was similar in both the high-fat diet rich in unsaturated fatty acids (U-HFD) group and the control (C) group. A Curves of contractile response to noradrenaline (Nor) in the presence or absence of prazosin (Pra) in an aorta from the control group. B Curves of contractile response to Nor in the presence or absence of Pra in aortas from the U-HFD group. C Both groups showed similar pharmacological behaviour to Nor in the presence of the alpha-1-antagonist Pra. Each point represents mean ± SEM, n=6. *P<0.01; **P<0.001; ***P<0.0001 versus C curve
TABLE 3.
The vascular response to prazosin
| Diet | Prazosin use | Maximal response, g | EC50(×10−6M) |
|---|---|---|---|
| Control | Without prazosin | 2.20±0.40 | 0.44±1.4 |
| With prazosin | 2.21±0.70 | 2.06±3.7* | |
| U-HFD | Without prazosin | 1.83±0.65 | 0.16±1.8 |
| With prazosin | 1.84±0.46 | 5.01±1.2** |
Data presented as mean ± SEM.
P<0.003 versus control without prazosin;
P<0.001 versus a high-fat diet rich in unsaturated fatty acids (U-HFD) without prazosin (n=6) (Student’s t test for paired samples). EC50 Concentration necessary to produce 50% of maximal response
DISCUSSION
The results obtained in the present work show that U-HFD did not impair the cardiovascular parameters evaluated, even though a slightly decreased sensitivity to PE was observed in the aortas from the U-HFD animals.
Although no significant difference between groups was observed in the FBW, the U-HFD was of sufficient intensity and duration to increase both the total body fat (81.5%) and the adiposity index of U-HFD animals (Table 1) (34,35). These results are in agreement with several studies (28–30) regarding high-saturated-fat-diet-induced obesity that found no difference in FBW. On the other hand, notwithstanding a significant difference in plasma glucose concentration between the groups, the insulin-tolerance test was not able to demonstrate insulin resistance in the U-HFD rats. In this context, a previous study using an insulin resistance diet model showed that rats that were fed polyunsaturated fatty acid (docosahexaenoic acid) had a short QT interval on the ECG (36). Nevertheless, in our study, no changes were observed in this parameter. Because increased heterogeneity of ventricular repolarization favours the development of malignant ventricular arrhythmias, and prolonged QT interval may reflect this inhomogeneity, the effects of an unsaturated fatty acid diet on the QT interval observed here could explain the antiarrhythmic properties of this dietary habit (for more details, see reference 37).
It is known that cardiac autonomic control plays a fundamental role in the maintenance of cardiac electric stability. HRV analysis represents a powerful tool for the assessment of cardiac autonomic control, both in humans and in animal models. Cardiac vagal impairment, detected by reduced high-frequency power in spectral analysis of HRV, consists of a marker of cardiac electrical instability, and has been shown to constitute an independent prognostic factor for ventricular arrhythmia and sudden cardiac death in cardiac patients, and an independent predictor of cardiovascular events in the general population (38–40). The U-HFD model was not accompanied by a change in autonomic function as evaluated by HRV spectral analysis. To our knowledge, no previous work has assessed HRV in U-HFD rats. However, a previous study (41) showed a strong positive association between RR interval and omega (ω)-3 polyunsaturated fatty acid content in cell membranes, suggesting a lower heart rate in subjects with a high intake of ω-3 polyunsaturated fatty acid. This effect helps to explain the mechanism by which unsaturated fatty acids prevent sudden death and arrhythmias. Also, Christensen et al (42) showed a close positive association between ω-3 polyunsaturated fatty acid and HRV in patients suspected of having ischemic heart disease; it may indicate a protective effect of ω-3 polyunsaturated fatty acids against sudden cardiac death. The same group (42) showed increased HRV in postmyocardial infarction patients who received 5.2 g of ω-3 polyunsaturated fatty acid daily for 12 weeks.
Vascular reactivity
Vascular endothelium plays an essential role in the regulation of vascular tone through the synthesis and liberation of many substances, which can be activated by the stimulation of specific receptors by various agonists (43). Activation of muscarinic receptors in the endothelium by Ach increases the production of nitric oxide, which stimulates soluble guanylate cyclase in vascular smooth muscle cells, leading to increased levels of cyclic guanosine monophosphate and consequent relaxation (44). Our study showed that endothelium-dependent relaxation by ACh did not differ between control and U-HFD animals, suggesting a significant alteration in the nitric oxide-induced vasodilation of U-HFD rat aortas. This result is in agreement with experimental studies that did not observe alteration in ACh-induced vasodilation of aortas from mice (23) or rats (24) treated with a diet rich in fat – mainly saturated fatty acid. These findings suggest that different dietary components may or may not have an effect on vasorelaxation. Humans and animals fed high-fat diets, particularly saturated fat, have shown endothelial abnormalities (27,45). On the other hand, the protective effects of high-carbohydrate (low-saturated fat) diets and diets rich in unsaturated fats are contentious (46,47). Although the high-fat diet used in the present study contained a lower amount of saturated fat and a higher amount of unsaturated fatty acid, which is associated with improved cardiovascular risk (48,49), at high levels they could impair endothelial function (46).
In contrast to our results, some studies (50,51) have shown a decrease in endothelium-dependent vasorelaxation and no alteration in the maximal contractile responses to PE in renal arteries from saturated and unsaturated fatty acid-fed rats, compared with control rats, but there was no marked difference between them (50). These authors used a 34.4% unsaturated fatty acid diet over a 24-week period, while our study used an 83% unsaturated fatty acid diet over a 19-week period. Therefore, the higher percentage of unsaturated fatty acid and the shorter treatment time used in the present study could explain, at least in part, the divergent results observed here when compared with the work of Yu et al (50).
Furthermore, our results showed that endothelium-intact aorta isolated from U-HFD rats exhibited a slight decrease in sensitivity to PE, observed as a change in the EC50 in response to PE. This response could be explained by an alteration in the α1-adrenergic receptors in aortas of U-HFD rats. However, no differences were found in the maximal contraction generated by noradrenaline in aortas from both groups when in the presence of the α1-adrenergic receptor antagonist prazosin. The contractile response of aortic rings from U-HFD rats was very similar to the response obtained in the control group; in both groups, the curve shifted to the right as expected, suggesting that the α1-adrenergic receptor pathway and density were not impaired by the U-HFD. Thus, the results of the present study suggest that systemic vascular smooth muscle contraction in response to α1-adrenoceptor stimulation is conserved after U-HFD.
Furthermore, the results regarding vascular reactivity and control of the autonomic system in the present study were in agreement with the arterial pressure measurement that did not show any differences between the control and the U-HFD groups.
CONCLUSION
The data obtained in the present study indicate that the U-HFD diet increased total adiposity and decreased the vascular sensitivity to PE, but did not impair cardiac electrical and autonomic functions.
Acknowledgments
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo process numbers 07/53267-3 and 2009/03771-2. The authors are grateful to José Georgette, Mário Bruno, Sandra Fábio, Elenize Pereira, Sueli Clara, Vitor Souza, José Aparecido, Rogério Monteiro, Antônio de Lalla, Camila Camacho and Corina Corrêa for their technical assistance and GAP (group to support research) for revising the English text.
Footnotes
NOTE: None of the authors have any conflicts of interest or financial interests to disclose.
REFERENCES
- 1.Curb JD, Wergowske G, Dobbs JC, Abbott RD, Huang B. Serum lipid effects of a high-monounsaturated fat diet based on macadamia nuts. Arch Intern Med. 2000;160:1154–8. doi: 10.1001/archinte.160.8.1154. [DOI] [PubMed] [Google Scholar]
- 2.AHA Heart disease and stroke statistics: 2008 update. < http://www.americanheart.org/presenter.jhtml?identifier1/41928> (Accessed October 2008).
- 3.Matthan NR, Dillard A, Lecker JL, Ip B, Lichtenstein AH. Effects of dietary palmitoleic acid on plasma lipoprotein profile and aortic cholesterol accumulation are similar to those of other unsaturated fatty acids in the F1B golden Syrian hamster. J Nutr. 2009;139:215–21. doi: 10.3945/jn.108.099804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopaschuck GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res. 2007;101:335–47. doi: 10.1161/CIRCRESAHA.107.150417. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien PE, Dixon JB. The extent of the problem of obesity. Am J Surg. 2002;184(6B):4S–8S. doi: 10.1016/s0002-9610(02)01172-8. [DOI] [PubMed] [Google Scholar]
- 6.Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89:2522–5. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- 7.Gordon T, Kannel WB. Obesity and cardiovascular disease: The Framingham Study. Clin Endocrinol Metab. 1976;5:367–75. doi: 10.1016/s0300-595x(76)80026-6. [DOI] [PubMed] [Google Scholar]
- 8.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Study. Circulation. 1983;67:968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 9.Artham SM, Lavie CJ, Patel HM, Ventura HO. Impact of obesity on the risk of heart failure and its prognosis. J Cardiometab Syndr. 2008;3:155–61. doi: 10.1111/j.1559-4572.2008.00001.x. (Rev) [DOI] [PubMed] [Google Scholar]
- 10.Poirier P, Giles TD, Bray GA, et al. American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. (Rev) [DOI] [PubMed] [Google Scholar]
- 11.Carroll JF, Dwyer TM, Grady AW, et al. Hypertension, cardiac hypertrophy, and neurohumoral activity in a new animal model of obesity. Am J Physiol. 1996;271:H373–8. doi: 10.1152/ajpheart.1996.271.1.H373. [DOI] [PubMed] [Google Scholar]
- 12.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–30. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 13.Swinburn B, Egger G. Preventive strategies against weight gain and obesity. Obes Rev. 2002;3:289–301. doi: 10.1046/j.1467-789x.2002.00082.x. [DOI] [PubMed] [Google Scholar]
- 14.Frank S, Colliver JA, Frank A. The electrocardiogram in obesity: Statistical analysis of 1,029 patients. J Am Coll Cardiol. 1986;7:295–9. doi: 10.1016/s0735-1097(86)80494-6. [DOI] [PubMed] [Google Scholar]
- 15.Fraley MA, Birchem JA, Senkottaiyan N, Alpert MA. Obesity and the electrocardiogram. Obes Rev. 2005;6:275–81. doi: 10.1111/j.1467-789X.2005.00199.x. [DOI] [PubMed] [Google Scholar]
- 16.Esposito K, Nicoletti G, Marzano S, et al. Autonomic dysfunction associates with prolongation of QT intervals and blunted night BP in obese women with visceral obesity. J Endocrinol Invest. 2002;25:RC32–5. doi: 10.1007/BF03344061. [DOI] [PubMed] [Google Scholar]
- 17.Papaioannou A, Michaloudis D, Fraidakis O, et al. Effects of weight loss in QT interval in morbidly obese patients. Obes Surg. 2003;13:869–73. doi: 10.1381/096089203322618687. [DOI] [PubMed] [Google Scholar]
- 18.el-Gamal A, Gallagher D, Nawras A, et al. Effects of obesity on QT, RR, and QTc intervals. Am J Cardiol. 1995;75:956–9. doi: 10.1016/s0002-9149(99)80700-0. [DOI] [PubMed] [Google Scholar]
- 19.Vlay SC, Mallis GI, Brown EJ, Cohn PF. Documented sudden cardiac death in prolonged QT syndrome. Arch Intern Med. 1984;144:833–5. [PubMed] [Google Scholar]
- 20.Moss AJ. Measurement of the QT interval and the risk associated with QTc interval prolongation: A review. Am J Cardiol. 1993;72:23B–25B. doi: 10.1016/0002-9149(93)90036-c. [DOI] [PubMed] [Google Scholar]
- 21.Corbi GM, Carbone S, Ziccardi P, et al. FFAs and QT intervals in obese women with visceral adiposity: Effects of sustained weight loss over 1 year. J Clin Endocrinol Metab. 2002;87:2080–3. doi: 10.1210/jcem.87.5.8516. [DOI] [PubMed] [Google Scholar]
- 22.Naderali EK, Pickavance LC, Wilding JPH, Williams G. Diet-induced endothelial dysfunction in the rat is independent of the degree of increase in total body weight. Clin Sci. 2001;100:635–41. doi: 10.1042/cs1000635. [DOI] [PubMed] [Google Scholar]
- 23.Mundy AL, Haas E, Bhattacharya I, et al. Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: Implications for diet-induced obesity. Cardiovasc Res. 2007;73:368–75. doi: 10.1016/j.cardiores.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Smith AD, Brands MW, Wang MH, Dorrance AM. Obesity-induced hypertension develops in young rats independently of the renin-angiotensin-aldosterone system. Exp Biol Med. 2006;231:282–7. doi: 10.1177/153537020623100307. [DOI] [PubMed] [Google Scholar]
- 25.Seiler C, Hess OM, Buechi M, Suter TM, Kayenbuehl HP. Influence of serum cholesterol and other coronary risk factors on vasomotion of angiographically normal coronary arteries. Circulation. 1993;88:2139–48. doi: 10.1161/01.cir.88.5.2139. [DOI] [PubMed] [Google Scholar]
- 26.Mietus-Snyder M, Malloy MJ. Endothelial dysfunction occurs in children with two genetic hyperlipidemias: Improvement with antioxidant vitamin therapy. J Pediatr. 1998;133:35–40. doi: 10.1016/s0022-3476(98)70174-x. [DOI] [PubMed] [Google Scholar]
- 27.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–4. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 28.Lauterio TJ, Bond JP, Ulman EA. Development and characterization of a purified diet to identify obesity-susceptible and resistant rat populations. J Nutr. 1994;124:2172–8. doi: 10.1093/jn/124.11.2172. [DOI] [PubMed] [Google Scholar]
- 29.Woods SC, Seeley RJ, Rushing PA, D’Alessio DA, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–7. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 30.Brufau G, Canela MA, Rafecas M. A high-saturated fat diet enriched with phytosterol and pectin affects the fatty acid profile in guinea pigs. Lipids. 2006;41:159–68. doi: 10.1007/s11745-006-5084-8. [DOI] [PubMed] [Google Scholar]
- 31.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R943–9. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer JM, Pfeffer MA, Frohlich ED. Validity of an indirect tail-cuff method for determining systolic arterial pressure in unanesthetized normotensive and spontaneously hypertensive rats. J Lab Clin Med. 1971;78:957–62. [PubMed] [Google Scholar]
- 33.Carvalho-Filho MA, Ueno M, Hirabara SM, et al. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: A novel mechanism of insulin resistance. Diabetes. 2005;54:959–67. doi: 10.2337/diabetes.54.4.959. [DOI] [PubMed] [Google Scholar]
- 34.Nascimento AF, Sugizaki MM, Leopoldo AS, et al. Misclassification probability as obese or lean in hypercaloric and normocaloric diet. Biol Res. 2009;41:253–9. [PubMed] [Google Scholar]
- 35.Okere IC, Chandler MP, McElfresh TA, et al. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–R44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 36.Rousseau D, Héliès-Toussaint C, Moreau D, Raederstorff D, Grynberg A. Dietary n-3 PUFAs affect the blood pressure rise and cardiac impairments in a hyperinsulinemia rat model in vivo. Am J Physiol Heart Circ Physiol. 2003;285:H1294–302. doi: 10.1152/ajpheart.00651.2002. [DOI] [PubMed] [Google Scholar]
- 37.Lombardi F, Terranova P. Anti-arrhythmic properties of N-3 poly-unsaturated fatty acids (n-3 PUFA) Curr Med Chem. 2007;14:2070–80. doi: 10.2174/092986707781368405. (Rev) [DOI] [PubMed] [Google Scholar]
- 38.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–62. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 39.Routledge HC, Chowdhary S, Townend JN. Heart rate variability – a therapeutic target? J Clin Pharm Ther. 2002;27:85–92. doi: 10.1046/j.1365-2710.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 40.Nolan J, Batin PD, Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure: Results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–6. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 41.Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. Heart rate variability and fatty acid content of blood cell membranes: A dose-response study with n-3 fatty acids. Am J Clin Nutr. 1999;70:331–7. doi: 10.1093/ajcn/70.3.331. [DOI] [PubMed] [Google Scholar]
- 42.Christensen JH, Dyerberg J, Schmidt EB. n-3 fatty acids and the risk of sudden cardiac death assessed by 24-hour heart rate variability. Lipids. 1999;34(Suppl):S197. doi: 10.1007/BF02562287. [DOI] [PubMed] [Google Scholar]
- 43.Arnal JF, Dinh-Xuan AT, Pueyo M, Darblade R, Rami J. Endothelium-derived nitric oxide and vascular physiology and pathology. Cell Mol Life Sci. 1999;55:1078–87. doi: 10.1007/s000180050358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanhoutte PM. Endothelium-dependent hyperpolarizations: The history. Pharmacol Res. 2004;49:503–8. doi: 10.1016/j.phrs.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 45.German JB, Dillard CJ. Saturated fats: What dietary intake? Am J Clin Nutr. 2004;80:550–9. doi: 10.1093/ajcn/80.3.550. [DOI] [PubMed] [Google Scholar]
- 46.Ong PJ, Dean TS, Hayward CS, Della Monica PL, Sanders TA, Collins P. Effect of fat and carbohydrate consumption on endothelial function. Lancet. 1999;354:2134. doi: 10.1016/s0140-6736(99)03374-7. [DOI] [PubMed] [Google Scholar]
- 47.Keogh JB, Grieger JA, Noakes M, Clifton PM. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler Thromb Vasc Biol. 2005;25:1274–9. doi: 10.1161/01.ATV.0000163185.28245.a1. [DOI] [PubMed] [Google Scholar]
- 48.Pelkman CL, Fishell VK, Maddox DH, Pearson TA, Mauger DT, Kris-Etherton PM. Effects of moderate-fat (from monounsaturated fat) and low-fat weight-loss diets on the serum lipid profile in overweight and obese men and women. Am J Clin Nutr. 2004;79:204–12. doi: 10.1093/ajcn/79.2.204. [DOI] [PubMed] [Google Scholar]
- 49.Volek JS, Feinman RD. Carbohydrate restriction improves the features of metabolic syndrome. Metabolic syndrome may be defined by the response to carbohydrate restriction. Nutr Metab. 2005;16:31. doi: 10.1186/1743-7075-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu G, Guang-Yao S, Hui-Juan M, Wen-Jie Z, Yu Z. Effects of long-term high-saturated and unsaturated fatty acid diets on relaxation and contraction of renal arteries in insulin resistant rats Acta Physiologica Sinica. 2007;59:363–8. [PubMed] [Google Scholar]
- 51.Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int J Obes (Lond) 2010;34:165–71. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]