Abstract
BACKGROUND:
Peripheral arterial disease (PAD) is a major risk factor for adverse cardiovascular events. There has been a definite push for wider use of the ankle-brachial index (ABI) as a simple screening tool for PAD. Perhaps this has occurred to the detriment of a thorough physical examination.
OBJECTIVE:
To assess the accuracy of the physical examination to detect clinically significant PAD compared with the ABI.
METHODS:
PADfile, the PAD module of CARDIOfile (the Kingston Heart Clinic’s cardiology database [Kingston, Ontario]), was searched for all patients who underwent peripheral arterial testing. Of 1619 patients, 1236 had all of the necessary data entered. Patients’ lower limbs were divided into two groups: those with a normal ABI between 0.91 and 1.30, and those with an abnormal ABI of 0.90 or lower. Peripheral pulses were graded as either absent or present. Absent was graded as 0/3, present but reduced (1/3), normal (2/3) or bounding (3/3). Femoral bruits were graded as either present (1) or absent (0). Using the ABI as the gold standard, the sensitivity, specificity, negative predictive value (NPV), positive predictive value and overall accuracy were calculated for the dorsalis pedis pulse, the posterior tibial pulse, both pedal pulses, the presence or absence of a femoral bruit and, finally, for a combination of both pedal pulses and the presence or absence of a femoral bruit.
RESULTS:
In 1236 patients who underwent PAD testing and who underwent a complete peripheral vascular physical examination (all dorsalis pedis and posterior tibial pulses palpated and auscultation for a femoral bruit), the sensitivity, specificity, NPV, positive predictive value and accuracy for PAD were 58.2%, 98.3%, 94.9%, 81.1% and 93.8%, respectively.
CONCLUSIONS:
The clinical examination of the peripheral arterial foot pulses and the auscultation for a femoral bruit had a high degree of accuracy (93.8%) for the detection or exclusion of PAD compared with the ABI using the cut-off of 0.90 or lower. If both peripheral foot pulses are present in both lower limbs and there are no femoral bruits, the specificity and NPV of 98.3% and 94.9%, respectively, make the measurement of the ABI seem redundant. The emphasis in PAD detection should be redirected toward encouraging a thorough physical examination.
Keywords: Ankle-brachial index, Peripheral arterial disease, Physical examination
Abstract
HISTORIQUE :
La maladie artérielle périphérique (MAP) est un important facteur de risque d’événements cardiovasculaires. On a observé des pressions évidentes pour utiliser davantage l’indice tibio-brachial (ITB) comme simple outil de dépistage de la MAP. Cette impulsion s’est peut-être faite au détriment d’un examen physique approfondi.
OBJECTIF :
Évaluer la précision de l’examen physique par rapport à l’ITB pour déceler une MAP significative sur le plan clinique.
MÉTHODOLOGIE :
Les chercheurs ont effectué des recherches dans PADfile, le module des MAP de CARDIOfile (base de données cardiologiques de la Kingston Heart Clinic de Kingston, en Ontario) afin de déceler tous les patients qui avaient subi un dépistage de maladie artérielle périphérique. Des 1 619 patients relevés, 1 236 possédaient toutes les données nécessaires. Les patients étaient divisés en deux groupes : ceux à l’ITB normal entre 0,91 et 1,30, et ceux à l’ITB anormal de 0,90 ou moins. Les pouls périphériques étaient classés comme absents ou présents. Les pouls étaient classés comme absents (0/3), présents mais réduits (1/3), normaux (2/3) ou bondissants (3/3). Les souffles fémoraux étaient classés comme présents (1) ou absents (0). Au moyen de l’ITB comme norme de référence, les chercheurs ont calculé la sensibilité, la spécificité, la valeur prédictive négative (VPN), la valeur prédictive positive et la précision globale du pouls dorsal du pied, du pouls tibial postérieur, des deux pouls pédieux, de la présence ou de l’absence de souffle fémoral et, enfin, d’une association des pouls pédieux et de présence ou d’absence de souffle fémoral.
RÉSULTATS :
Chez les 1 236 patients qui ont subi un dépistage des MAP et un examen physique vasculaire périphérique complet (palpation de tous les pouls pédieux et tibiaux postérieurs et auscultation du souffle fémoral), la sensibilité, la spécificité, la VPN, la valeur prédictive positive et la précision de la MAP s’élevaient à 58,2 %, 98,3 %, 94,9 %, 81,1 % et 93,8 %, respectivement.
CONCLUSIONS :
L’examen clinique des pouls pédieux des artères périphériques et l’auscultation du souffle fémoral avaient un fort taux de précision (93,8 %) pour dépister ou exclure la MAP par rapport à l’ITB au moyen de la valeur seuil de 0,90 ou moins. Si les deux pouls pédieux périphériques sont présents dans les deux membres inférieurs et qu’on ne repère pas de souffle fémoral, la spécificité de la VPN correspondant à 98,3 % et à 94,9 %, respectivement, rendent la mesure de l’ITB redondante. Il faudrait recommencer à favoriser l’examen physique approfondi pour dépister une MAP.
Lower extremity peripheral arterial disease (PAD) affects approximately 12% of older patients in the general population (1–3). The diagnosis of PAD is considered to be a major risk factor for future cardiovascular (CV) events and mortality (1,2,4–14). Previous investigations have also shown that the risk of mortality in patients with asymptomatic PAD is similar to those with severe or symptomatic PAD (10,15). Similarly, the health-related quality of life of patients with PAD is similar in patients with other forms of CV disease (CVD) (16), and PAD has even been associated with higher rates of depression (17).
A clinical tool for both the diagnosis of PAD and the assessment of global CV risk stratification is the ankle-brachial index (ABI). The ABI is calculated as the ratio of the highest brachial systolic pressure to the highest systolic pressure in either the dorsalis pedis (DP) or the posterior tibial (PT) artery, and is most often measured using a handheld Doppler ultrasound device. The current ABI reference standard of 0.90 or lower has a sensitivity of 90% and a specificity of 98% for the detection of a hemodynamically significant stenosis of 50% or greater proximally in the lower limb (18,19). In our laboratory, an ABI of 0.96 to 1.30 is considered to be normal. An ABI of between 0.91 and 0.95 is a ‘grey zone’ and in this group, we closely examine the segmental pressures, continuous-wave Dopplers, toe-brachial index and pulse volume recordings to help decide on normality. An ABI of between 0.81 and 0.90 is considered to be mild PAD. An ABI of 0.50 to 0.80 is considered to be moderate PAD and an ABI of lower than 0.50 is considered to be severe PAD. An abnormally high ABI (greater than 1.30) occurs in patients with incompressible arteries, due to calcification of the vascular media. This is most often seen in elderly and diabetic patients. A high ABI (greater than 1.30) is also associated with increased CV risk (20).
Despite the advent of advanced diagnostic techniques, physicians are further required to determine which patients might benefit the most from further testing. Previous studies have investigated the usefulness of the peripheral vascular physical examination to identify patients with an abnormal ABI (0.90 or lower). However, differences in reference standards, patient populations and methodology make drawing conclusions regarding the utility of the physical examination for the detection of PAD difficult. Furthermore, current data are lacking regarding the accuracy of the physical examination in a community-based outpatient setting.
Our objective was to determine the accuracy of the peripheral vascular examination to detect the presence or absence of PAD as defined by an ABI of 0.90 or lower among patients referred to the Kingston Heart Clinic (Kingston, Ontario) for peripheral physiological arterial testing for suspected PAD or for ABI screening in high-risk groups.
METHODS
Patient population
The Kingston Heart Clinic is a community-based outpatient cardiology practice. PADfile, the PAD module of CARDIOfile (the clinic’s cardiology database), was searched for all patients who underwent peripheral arterial testing. All tests were performed between December 2005 and February 2010. Patients were referred either for suspected PAD or because they were at high risk for PAD (ie, patients older than 70 years of age, diabetic patients 50 to 69 years of age, smokers 50 to 69 years of age or patients with intermediate Framingham risk scores of 10% to 19%). Of the 1619 patients who underwent peripheral arterial testing, 228 were excluded due to an abnormally high ABI (greater than 1.30) in either the left leg, the right leg or both legs. The clinic’s own data indicated, quite clearly, that these patients did not have obstructive PAD. In fact, the high ABI group had a very similar profile to the normal ABI group including the physical examination (Table 1). As expected, the high ABI group had a higher prevalence of diabetes and was, on average, older than the normal ABI group. What was unexpected was that the high ABI group had a much lower prevalence of smoking (12.7% versus 24.2%; P<0.005) (Table 1). Furthermore, the intention of the present study was to compare the accuracy of the physical examination to detect obstructive PAD (ABI 0.90 or lower) with the accuracy of detecting no PAD (ABI 0.91 to 1.30). A further 156 patients were excluded because at least one physical examination field was missing in CARDIOfile. This was due, in large part, to the femoral bruit field being empty (the right in 154 patients, the left in 147 and either in 155). Some foot pulse data were also absent (the right DP in 56 patients, the right PT in 57 and both in 56; and the left DP in 56, the left PT in 57 and both in 56). Absent foot pulses alone only reduced the number of patients by one. The indications for PAD testing are shown in Table 2.
TABLE 1.
Characteristics of patients with an abnormally high ankle-brachial index (ABI; greater than 1.30) in either the right leg, the left leg or both legs, compared with patients with normal ABIs in both legs
| High ABI (n=142) | Normal ABI (n=920) | P | |
|---|---|---|---|
| Male/female ratio | 128/14 | 625/295 | <0.0001 |
| Age, years, mean ± SD | 67.6±8.9 | 65.5±10.3 | <0.05 |
| ABI right leg, mean ± SD | 1.35±0.11 | 1.13±0.09 | <0.0001 |
| ABI left leg, mean ± SD | 1.34±0.14 | 1.13±0.09 | <0.0001 |
| Abnormal right TBI* | 23/119 (16.2) | 166/754 (18.0) | NS |
| Abnormal right TBI† | 13/129 (9.1) | 102/818 (11.1) | NS |
| Abnormal left TBI* | 22/120 (15.5) | 177/743 (19.2) | NS |
| Abnormal left TBI† | 18/124 (12.7) | 110/810 (12.0) | NS |
| Femoral bruits | 9/133 (6.3) | 82/838 (8.9) | NS |
| Normal examination‡ | 92/50 (64.8) | 563/357 (61.2) | NS |
| Hypertensive | 86/56 (60.6) | 615/305 (66.8) | NS |
| Dyslipidemic | 111/31 (78.2) | 662/258 (71.9) | NS |
| Smokers | 18/124 (12.7) | 223/697 (24.2) | <0.005 |
| Diabetic | 52/90 (36.6) | 259/661 (28.1) | <0.05 |
Data presented as yes/no (% yes) unless otherwise indicated. Fisher’s exact test was used for differences between proportions. An unpaired t test was used for differences between means.
Strict toe-brachial index (TBI) cut-off of lower than 0.72;
Conservative cut-off for a TBI of lower than 0.66;
Normal examination means that all four distal foot pulses are present and there are no femoral bruits bilaterally. NS Nonsignificant
TABLE 2.
Indications for peripheral arterial disease testing (n=1236)
| Indication according to vascular screening | n |
|---|---|
| Age ≥ 70 years* | 519 |
| Smokers 50 to 69 years of age* | 218 |
| Diabetes 50 to 69 years of age* | 153 |
| Intermediate Framingham risk (10% to 19%) <70 years* | 148 |
| Total | 1038 |
| Indication for ankle-brachial index measurement | |
| No symptoms (190 fit into a vascular screening group) | 275 |
| Leg discomfort | 246 |
| Symptomatic peripheral arterial disease | 171 |
| Abnormal foot pulses | 102 |
| Cold feet | 89 |
| Erectile dysfunction | 66 |
| Peripheral ulceration | 36 |
| Skin colour changes | 18 |
| Peripheral bruits | 14 |
| Postrevascularization | 5 |
| Not stated | 8 |
| Vascular screening indication only* | 206 |
| Total | 1236 |
At least one of the four vascular screening indications were present
Physiological PAD testing
PAD testing was performed using a Nicolet VasoGuard (CareFusion Corporation, USA) physiological testing system using the four-cuff method for segmental pressures and pulse volume recordings. Proximal vessels were isonated using a 4 MHz transducer. Distal vessels were isonated using an 8 MHz transducer for the continuous-wave Dopplers. The ABI and toe-brachial index were recorded in all patients. Patients’ lower limbs were divided into two groups: those with an ABI of greater than 0.90 and 1.30 or lower, and those with an ABI of 0.90 or lower. The examination of the peripheral pulses, and the auscultation for ileofemoral and femoral bruits were performed by one registered nurse (CT) who was specifically trained in the vascular examination. Peripheral pulses were graded as either absent or present. Absent was graded as 0/3, present but reduced was graded as 1/3, normal was graded as 2/3 and a bounding pulse was graded as 3/3. Femoral bruits were graded as either present or absent based on auscultation. The Edinburgh questionnaire (21) was not specifically used; however, claudication was believed to be present when the patient experienced leg discomfort with exercise that was relieved by rest within 1 min to 5 min.
Data interpretation
Using the ABI as the gold standard, the sensitivity, specificity, positive predictive value, negative predictive value (NPV), accuracy, positive likelihood ratio and negative likelihood ratio were calculated in the usual manner for the DP pulse, the PT pulse, both DP and PT pulses, the presence or absence of a femoral bruit, for a combination of the DP pulse, PT pulse and the presence or absence of a femoral bruit, and for claudication. The ABI data are presented for the total number of legs tested (n=2472). An unpaired t test was used to detect differences between means. Fisher’s exact test was used to detect differences between proportions. The level of significance was adjusted using the Bonferroni correction method for multiple comparisons.
RESULTS
The mean (± SD) age of the entire population (n=1236) was 66.6±10.5 years. There were 821 men (66.4%) and 415 women (33.6%). A total of 751 patients (60.8%) had a history of CVD (previous myocardial infarction, stroke/transient ischemic attack or revascularization). A total of 900 patients (72.8%) were treated with a statin for dyslipidemia, 862 (69.7%) were being treated for hypertension, 375 (30.3%) had diabetes and 336 (27.2%) were current smokers. A total of 1158 patients (93.7%) had at least one major CV risk factor. Concomitant drug therapy is shown in Table 3.
TABLE 3.
Concomitant drug therapy
| Drug | n | % |
|---|---|---|
| Acetylsalicylic acid total | 886 | 71.7 |
| Acetylsalicylic acid alone | 749 | 60.6 |
| Acetylsalicylic acid/clopidogrel | 95 | 7.7 |
| Clopidogrel alone | 40 | 3.2 |
| Acetylsalicylic acid/dipyridamole | 7 | 0.6 |
| Acetylsalicylic acid/warfarin | 30 | 2.4 |
| Acetylsalicylic acid/warfarin/clopidogrel | 5 | 0.4 |
| Warfarin alone | 57 | 4.6 |
| Total antiplatelet or antithrombotic | 983 | 79.5 |
| Statin | 805 | 65.1 |
| ACEI alone | 533 | 43.1 |
| Angiotensin receptor blocker alone | 145 | 11.7 |
| ACEI/angiotensin receptor blocker combined | 8 | 0.6 |
| Total ACEI/angiotensin receptor blocker | 686 | 55.5 |
ACEI Angiotensin-converting enzyme inhibitor
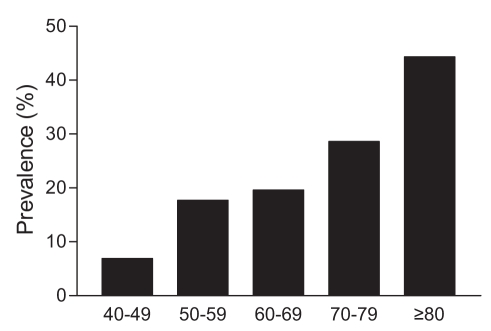
In 928 patients (75.1%), both ABIs were normal (greater than 0.90 and 1.30 or lower). A total of 348 patients (28.1%) had at least one abnormal ABI (0.90 or lower), and in 166 of these patients (13.4%), both ABIs were abnormal. The prevalence of PAD in the population, defined as an ABI of 0.90 or lower, was 28.1%. Prevalence according to deciles of age is shown in Figure 1.
Figure 1).
Prevealence of peripheral arterial disease according to deciles of age (years)
The sensitivity, specificity, positive predictive value, NPV and accuracy of the vascular physical examination to detect an ABI of 0.90 or lower are shown in Table 4. All components of the vascular physical examination had a relatively low sensitivity and high specificity for detecting an ABI of 0.90 or lower. A combination of all components of the physical examination had a higher accuracy to detect an abnormal ABI (0.90 or lower), compared with any other component of the physical examination. In a leg with a completely normal vascular examination (all DP and PT pulses present and the absence of a femoral bruit), the specificity, NPV and accuracy were 98.3%, 94.9% and 93.8%, respectively. The more complete the physical examination, the higher the accuracy when compared with the ABI (Table 4).
TABLE 4.
Results for the detection of an ankle-brachial index of 0.90 or lower
| Peripheral arterial disease parameter | n | Sensitivity | Specificity | PPV | NPV | Accuracy | + LR | −LR |
|---|---|---|---|---|---|---|---|---|
| Claudication | 2472 | 78.6 | 67.6 | 34.7 | 92.0 | 68.9 | 2.3 | 0.38 |
| DP pulse only | 2472 | 63.9 | 80.6 | 43.2 | 90.7 | 77.5 | 3.3 | 0.45 |
| PT pulse only | 2472 | 70.0 | 83.4 | 49.3 | 92.3 | 80.9 | 4.2 | 0.36 |
| Femoral bruit only | 2472 | 36.1 | 92.0 | 51.1 | 86.2 | 81.6 | 4.5 | 0.69 |
| Both pedal pulses* | 1888 | 73.0 | 91.5 | 65.5 | 93.9 | 88.2 | 8.6 | 0.30 |
| Both DP and PT pulses and femoral bruit† | 1524 | 58.2 | 98.3 | 81.1 | 94.9 | 93.8 | 34.2 | 0.43 |
When two * or three † variables must all be either normal or abnormal, there is diminishing return and, therefore, the numbers decrease. + LR Positive likelihood ratio; −LR Negative likelihood ratio; DP Dorsalis pedis; NPV Negative predictive value; PPV Positive predictive value; PT Posterior tibial
A comparison of patients with a completely normal peripheral arterial examination (all four pedal pulses present and no femoral bruits; n=575) and patients with an abnormal peripheral examination (absent DP and PT pulses and the presence of a femoral bruit on either the right leg, the left leg or both legs; n=90) is shown in Table 5. As seen, these patients were older, more likely to be smokers, more likely to be hypertensive and more likely to be diabetic. There was no significant difference in male to female distribution and no significant difference in the incidence of dyslipidemia.
TABLE 5.
Characteristics of patients with absent dorsalis pedis pulse, absent posterior tibial pulse and a femoral bruit in the right leg, the left leg or both legs (abnormal examination) compared with patients with none of these findings on both legs
| Abnormal examination (n=90) | Normal examination (n=575) | P | |
|---|---|---|---|
| Male/female ratio | 57/33 | 402/173 | 0.2212* |
| Age, years | 69.8±11.2 | 64.0±9.8 | <0.0001** |
| Ankle-brachial index right leg | 0.78±0.21 | 1.15±0.08 | <0.0001** |
| Ankle-brachial index left leg | 0.72±0.23 | 1.14±0.08 | <0.0001** |
| Hypertensive, n (%) | 76 (84.4) | 368 (64.0) | <0.0001* |
| Dyslipidemic, n (%) | 74 (82.2) | 417 (72.5) | 0.0536* |
| Smokers, n (%) | 39 (43.3) | 137 (23.8) | <0.0005* |
| Diabetic, n (%) | 38 (42.2) | 152 (26.4) | <0.005* |
Data presented as mean ± SD unless otherwise indicated.
Fisher’s exact test for differences between proportions;
Unpaired t test for differences between means. P<0.00625 (ie, 0.05/8) was considered to be significant using the Bonferroni correction for multiple comparisons
DISCUSSION
There is little doubt that PAD is a major cause of morbidity and diminished quality of life, and a major risk factor for adverse CV events including mortality. The ABI is a well-validated tool for categorizing disease severity and assessing CV risk.
Previous studies have assessed the use of the vascular physical examination for detecting PAD; however, some studies (22–27) included only symptomatic patients, some (24,28–30) included only asymptomatic patients, and other studies (28,31) exclusively studied patients with diabetes mellitus. Disparities in the reference standard for disease detection (ie, ABI cut-off) also make consensus on the usefulness of the vascular physical examination difficult (32). Current data concerning the accuracy of the physical examination to detect an ABI of 0.90 or lower in a heterogeneous population are lacking.
Our study included a large number of men and women, both with and without diabetes, and both symptomatic and asymptomatic. Only 171 patients (13.8%) were referred for PAD testing due to symptoms associated with PAD, whereas 890 patients (72%) referred had factors associated with increased vascular risk.
Claudication alone had a relatively poor accuracy to detect an abnormal ABI, and was a poor predictor of an abnormal ABI. Only 34.7% of patients with claudication had an ABI of 0.90 or lower. These data are not surprising and, in accordance with previous studies (29,32), reflect the fact that claudication is dependent on the functional demand of the circulation and lower limb pain may be masked by adequate collateral circulation.
The most striking finding from our data was the very high specificity, NPV and accuracy for all pulses present in the absence of a femoral bruit in predicting a normal ABI. The high specificity (98.3%) indicates that patients with an abnormal vascular physical examination should be directed toward ABI measurement. The rather high positive likelihood ratio (odds of an abnormal ABI in a patient with an abnormal versus a normal vascular physical examination) of 34.2 also indicated that patients lacking both DP and PT pulses in the presence of a femoral bruit would likely benefit from having their ABI measured. Previous studies (24,25,27–29) have examined combinations of pulse palpation for detecting an abnormal ABI; however, we are unaware of previous studies investigating a combination of both an abnormal pulse and a femoral bruit. Previous studies (32) investigating combinations of pulse palpation alone have found more modest positive likelihood ratios – similar to those observed in our study.
Conversely, the high NPV indicates that 94.9% of patients with a normal vascular physical examination have a normal ABI. The overall accuracy of an abnormal vascular physical examination was 93.8%. Therefore, our data suggest that a complete vascular physical examination can exclude patients from redundant ABI testing, and ABI measurement should be focused toward patients with an abnormal vascular physical examination.
Limitations
Although the Kingston Heart Clinic is a community-based outpatient cardiac facility, it is a major outpatient cardiac referral centre for CVD in southeastern Ontario. As such, there is a higher prevalence of peripheral vascular disease in those patients referred. Although there is a higher prevalence of PAD, we do not believe this should detract from the most important finding, which is the importance of a complete peripheral arterial examination that includes all four pedal pulses and the auscultation for a femoral bruit before embarking on the measurement of the ABI.
Another issue is the experience of the person (CT) who performed the majority of the clinical examinations and, therefore, the general application of our data to the practicing physician. Surely, this is the wrong message to be sending. The message should be that with the same application and dedication to the peripheral arterial physical examination, anyone can reliably expect to achieve similar results. The registered nurse (CT) had no expertise in the peripheral arterial examination and the first 85 cases were performed under the supervision of a physician (MFM). We believe that anyone can be taught this examination and eliminate unnecessary ABI measurements. We also believe that most physicians have the necessary expertise and all that is needed is the application.
There could be some concern regarding the exclusion of patients with a high ABI (greater than 1.30). First, we would simply say that our intention was to compare a normal ABI (0.91 to 1.30) with an ABI that clearly indicates obstructive PAD (ie, an ABI of 0.90 or lower). Second, there is overwhelming evidence that an ABI of 0.90 or lower has a high sensitivity and specificity for a stenosis of greater than 50% somewhere in the leg proximally, usually the aorto-iliac or superficial femoral systems on the affected side. Third, there is no evidence from our data that a high ABI is associated with obstructive PAD disease proximally. This is supported by showing that a completely normal toe-brachial index (TBI) (0.72 or greater with a strict definition) that is not affected by peripheral arterial calcification was seen in approximately 85% of our high ABI patients. Using the more conservative definition of a normal TBI (0.66 or greater), this percentage of normal TBIs in the high ABI group increases to almost 93%. Furthermore, these percentages are not significantly different from the normal ABI group, and the high ABI group had a similar profile to the normal ABI group including the physical examination (Table 1). It is well known that patients with a high ABI have a higher CV risk, but we believe this is most likely explained by the higher prevalence of diabetes and the higher mean age in this group (Table 1). Therefore, we believe that including the high ABI in the normal ABI group would be methodologically wrong and, even if we had, it would not have affected the overall results of the physical examination.
CONCLUSION
Vascular physical examination of the lower limbs provides valuable information when investigating lower limb PAD. The clinical examination of the peripheral arterial foot pulses and the auscultation for a femoral bruit has a high degree of accuracy (93.8%) for the detection or exclusion of PAD when compared with the ABI using the cut-off of 0.90 or lower. If both peripheral foot pulses are present in both lower limbs and there are no femoral bruits, the specificity and NPV of 98.3% and 94.9%, respectively, make the measurement of ABI seem redundant. The emphasis in PAD detection should be directed toward encouraging a thorough physical examination.
REFERENCES
- 1.Criqui MH, Denenberg JO, Langer RD, Fronek A. The epidemiology of peripheral arterial disease: Importance of identifying the population at risk. Vasc Med. 1997;2:221. doi: 10.1177/1358863X9700200310. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FGR, Housely E, Cawood EHH, MacIntyre CCA, Ruckley CV, Prescott RJ. Edinburgh Artery Study: Prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–92. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 4.Doobay AV, Anand SS. Sensitivity and specificity of the ankle-brachial index to predict future cardiovascular outcomes: A systematic review. Arterioscler Thromb Vasc Biol. 2005;25:1463–9. doi: 10.1161/01.ATV.0000168911.78624.b7. [DOI] [PubMed] [Google Scholar]
- 5.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The strong heart study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 6.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–28. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 7.Vogt MT, McKenna M, Anderson SJ, Wolfson SK, Kuller LH. The relationship between ankle-arm index and mortality in older men and women. J Am Geriatr Soc. 1993;41:523. doi: 10.1111/j.1532-5415.1993.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MMG, Feinglass J, Slavensky R, Pearce WH. The ankle-brachial index as a predictor of survival in patients with peripheral vascular disease. J Gen Intern Med. 1994;9:445–9. doi: 10.1007/BF02599061. [DOI] [PubMed] [Google Scholar]
- 9.Perlstein TS, Creager MA. The ankle-brachial index as a biomarker of cardiovascular risk: It’s not just about the legs. Circulation. 2009;120:2033–5. doi: 10.1161/CIRCULATIONAHA.109.907238. [DOI] [PubMed] [Google Scholar]
- 10.Diehm C, Allenberg JR, Pittrow D, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–61. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 11.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: Morbidity and mortality implications. Circulation. 2006;114:688–99. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 13.Ankle Brachial Index Collaboration Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heald CL, Fowkes FGR, Murray GD, Price JF. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis. 2006;189:61–9. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Leng GC, Lee AJ, Fowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–81. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 16.Regensteiner JG, Hiatt WR, Coll JR, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13:15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 17.McDermott MMG, Greenland P, Guralnik JM, et al. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med. 2003;18:461–7. doi: 10.1046/j.1525-1497.2003.20527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouriel K, McDonnell AE, Metz CE, Zarins CK. Critical evaluation of stress testing in the diagnosis of peripheral vascular disease. Surgery. 1982;91:686. [PubMed] [Google Scholar]
- 19.Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg. 1969;56:676–9. doi: 10.1002/bjs.1800560910. [DOI] [PubMed] [Google Scholar]
- 20.Allison MA, Hiatt WR, Hirsch AT, Coll JR, Criqui MH. A high ankle-brachial index is associated with increased cardiovascular disease morbidity and lower quality of life. J Am Coll Cardiol. 2008;51:1292–8. doi: 10.1016/j.jacc.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 21.Leng GC, Fowkes FGR. The Edinburgh Claudication Questionnaire: An improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101–9. doi: 10.1016/0895-4356(92)90150-l. [DOI] [PubMed] [Google Scholar]
- 22.Carter SA. Arterial auscultation in peripheral vascular disease. JAMA. 1981;246:1682–6. [PubMed] [Google Scholar]
- 23.Christensen JH, Freundlich M, Jacobsen BA, Falstiejensen N. Clinical relevance of pedal pulse palpation in patients suspected of peripheral arterial insufficiency. J Intern Med. 1989;226:95–9. doi: 10.1111/j.1365-2796.1989.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 24.Hiatt WR, Marshall JA, Baxter J, et al. Diagnostic methods for peripheral arterial disease in the san luis valley diabetes study. J Clin Epidemiol. 1990;43:597–606. doi: 10.1016/0895-4356(90)90164-k. [DOI] [PubMed] [Google Scholar]
- 25.Kazmers A, Koski MF, Groehn H, et al. Assessment of noninvasive lower extremity arterial testing versus pulse exam. Am Surgeon. 1996;62:315–9. [PubMed] [Google Scholar]
- 26.Nicholson ML, Byrne RL, Steele GA, Callum KG. Predictive value of bruits and doppler pressure measurements in detecting lower limb arterial stenosis. European J Vasc Surg. 1993;7:59–62. doi: 10.1016/s0950-821x(05)80545-6. [DOI] [PubMed] [Google Scholar]
- 27.Stoffers HEJH, Kester ADM, Kaiser V, Rinkens PELM, Knottnerus JA. Diagnostic value of signs and symptoms associated with peripheral arterial occlusive disease seen in general practice: A multivariable approach. Med Decis Making. 1997;17:61–70. doi: 10.1177/0272989X9701700107. [DOI] [PubMed] [Google Scholar]
- 28.Boyko EJ, Ahroni JH, Davignon D, Stensel V, Prigeon RL, Smith DG. Diagnostic utility of the history and physical examination for peripheral vascular disease among patients with diabetes mellitus. J Clin Epidemiol. 1997;50:659–68. doi: 10.1016/s0895-4356(97)00005-x. [DOI] [PubMed] [Google Scholar]
- 29.Criqui MH, Fronek A, Klauber MR, Barrett-Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: Results from noninvasive testing in a defined population. Circulation. 1985;71:516–22. doi: 10.1161/01.cir.71.3.516. [DOI] [PubMed] [Google Scholar]
- 30.Farkouh ME, Oddone EZ, Simel DL. Improving the clinical examination for a low ankle-brachial index. Int J Angiol. 2002;11:41–5. [Google Scholar]
- 31.Tan MH, Gwee HM, Yeo PP, Cheah JS, Lim P. Accuracy of clinical evaluation in diagnosing arterial occlusive disease of the lower extremity. Singapore Med J. 1982;23:194–7. [PubMed] [Google Scholar]
- 32.Khan NA, Rahim SA, Anand SS, Simel DL, Panju A. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536–46. doi: 10.1001/jama.295.5.536. [DOI] [PubMed] [Google Scholar]