Abstract
For maternal metabolism, pregnancy ends not with delivery, but with weaning. In several recent epidemiological studies, authors have reported an association between duration of breast-feeding and reduced maternal risk of metabolic disease. These findings parallel data from animal models showing favorable changes in metabolism associated with lactation. During gestation, visceral fat accumulates, and insulin resistance and lipid and triglyceride levels increase. These changes appear to reverse more quickly, and more completely, with lactation. In this article, we review animal and human studies regarding the effects of lactation on adiposity, lipid, and glucose homeostasis. We hypothesize that lactation plays an important role in “resetting” maternal metabolism after pregnancy.
Keywords: Lactation, adiposity, glucose homeostasis, hyperlipidemia, diabetes, metabolic syndrome
Evidence continues to accumulate that a woman’s reproductive history affects her long-term risk for metabolic disease. Increasing parity is associated with central adiposity,1 and retained gestational weight gain is associated with adverse outcomes in the next pregnancy2 as well as with long-term obesity risk for the mother.3 Data from several large cohort studies suggest that breast-feeding may reduce a woman’s risk of metabolic complications. Authors have found protective associations between duration of breast-feeding and incidence of type 2 diabetes,4 incidence of myocardial infarction,5 prevalence of the metabolic syndrome,6 and prevalence of hypertension, type 2 diabetes, and hyperlipidemia.7
The physiology of mammalian reproduction provides a biological basis for these associations. During pregnancy, dramatic changes occur in a woman’s metabolism as she accommodates the demands of “metabolizing for two.”8 These changes both support the developing fetus and allow accumulation of energy stores in anticipation of lactation.9 This accumulation is characterized by well-described increases in visceral fat, insulin production, insulin resistance, and circulating lipid levels.10 After birth, we hypothesize that lactation plays a central role in mobilizing these accumulated fat stores and “resetting” maternal metabolism, thereby reducing maternal risk for metabolic disease (Fig. 1). The longer a woman lactates, the more completely she off-loads these accumulated stores. Conversely, when a woman does not lactate, adverse metabolic changes persist for a longer period of time, increasing her disease risk.
Figure 1.
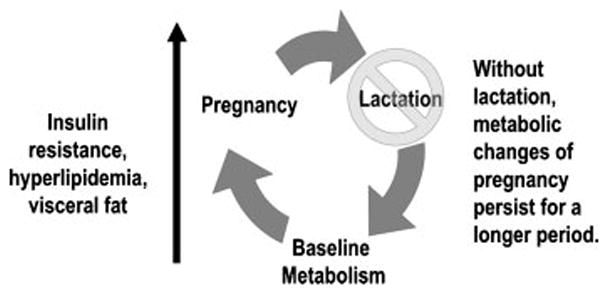
Pregnancy is associated with accumulation of fat stores and deleterious changes in glucose and lipid metabolism. We hypothesize that lactation plays a role in “resetting” maternal metabolism after pregnancy.
Both animal and human studies have examined the relation between lactation and maternal metabolism in the postpartum period and beyond. In this article, we explore the role of lactation in resetting maternal metabolism. Evidence assessed includes controlled experiments in animal models, detailed observational studies of maternal metabolism during lactation, and longitudinal cohort studies examining associations between lactation and incident disease.
HOW DOES LACTATION AFFECT ADIPOSITY?
In well-nourished populations, women frequently retain the weight gained during pregnancy. This retained gain is associated with adverse outcomes in the subsequent pregnancy,2 as well as long-term risk for metabolic disease.3 Evidence from animal and human research suggests that lactation plays a role in mobilizing stored fat after delivery.
Animal Studies
In rodents, adipose tissue is stored during pregnancy and mobilized during lactation. Hamosh et al11 measured regional changes in lipoprotein lipase (LPL) activity as a proxy for fat deposition in the rat. Fat deposition increased during pregnancy, but with lactation, storage of lipids in adipose tissue ceased and uptake into mammary tissue increased as lipids were transferred into milk. At 20 days postpartum, Steingrimdottir et al12 found that lactating animals had smaller adipose cells and lower peripheral LPL activity than nonlactating controls. These regional differences in LPL activity appear to correlate with lasting changes in fat distribution. Moore et al13,14 assessed the effects of a single cycle of pregnancy with or without lactation on fat cell number in rats. After a 21-day postweaning recovery period, animals that had undergone pregnancy without lactation had more parametrial fat cells (10.40 ± 1.87 million versus 5.93 ± 1.22 million cells) than those that had lactated.
Repeated pregnancies without lactation amplify these effects. Zhong et al15 assessed fat mass after three cycles of pregnancies without lactation. Animals were assessed following a 12-week rest period after the third pregnancy. Nonlactated animals had substantially higher percent body fat than lactated or nonmated control animals (16.7% versus 10.2% for lactated and 11.0% for controls, p <0.01). Collectively, these data show that in the rat, lactation plays an important role in mobilizing fat stores that accumulate during pregnancy.
Human Studies
We hypothesize that women who do not lactate may have greater difficulty mobilizing fat accumulated during pregnancy, resulting in greater retained gestational weight gain. This hypothesis is difficult to test in observational studies because multiple factors influence postpartum weight change. To isolate the role of breast-feeding in postpartum weight loss, studies should include data on prepregnancy weight, gestational weight gain, and breast-feeding intention, duration and intensity, as well as postpartum diet, energy expenditure, and intentional weight loss. Intentional dieting is of particular concern because nursing mothers may be reluctant to diet due to concerns about milk production, potentially confounding comparisons of weight loss between breast-feeding and formula-feeding women. Few studies have captured information on all of these factors, contributing to variability in study results.
LACTATION AND WEIGHT CHANGE
Several authors have assessed the relation between duration of lactation and postpartum weight change. Study designs range from small, intensive physiological studies employing calorimetry and water densitometry to large observational cohorts.
Not surprisingly, dietary intake and energy expenditure affect how much weight women lose with lactation. Butte et al16 conducted a detailed study of lactation and weight change among 45 exclusively breast-feeding women in a longitudinal study in Texas. They found a mean mobilization of 156 kcal/d from fat stores during the study period. Women who consumed more calories lost less weight. Goldberg et al17 assessed energy expenditure among 10 women in Cambridge, England. While breast-feeding, subjects consumed ~300 more kcal/d and expended ~200 fewer kcal/d in physical activity than after weaning, supplying the estimated 480 kcal/d needed to support lactation.18 Thus, when ample nutrition is available, women compensate for increased energy demands by increasing intake and decreasing energy expenditure, rather than mobilizing fat stores.
Fat mobilization appears to increase after the first 3 months postpartum, perhaps reflecting changes in the endocrine effects of lactation on maternal appetite as frequency of infant feeds decreases. In the first 2 to 3 months postpartum, several authors19–21 have found that formula-feeding mothers consumed 600 to 800 fewer calories than breast-feeding mothers and lost substantially more weight. From 3 to 6 months post-partum, however, weight loss among breast-feeding women increased substantially. These results suggest that in the early postpartum period, well-nourished women increase energy intake and/or decrease physical activity to meet the energy demands of lactation, whereas beyond 3 months, lactating women are more likely to mobilize fat stores.
Similar results were found by Dewey et al22 in a longitudinal study of 85 California women followed for 24 months. There were no significant differences in weight loss in the first 3 months, but thereafter, women who breast-fed for 12 months or more lost 2 kg more than women who breast-fed for 3 months or less. These differences persisted at 24 months postpartum. Importantly, the study excluded women who were intentionally dieting. In the Stockholm cohort, Ohlin and Rossner,23 similarly reported that greater duration and intensity of breast-feeding were associated with more weight loss from 2.5 to 6 months after delivery; however, overall weight loss from 2.5 to 12 months was similar, regardless of breast-feeding status. In a subsequent analysis,24 the authors found that women who snacked three or more times a day did not lose weight with lactation. Their findings underscore the importance of assessing the effect of dietary habits on weight change during lactation.
Janney et al25 assessed the relation between infant feeding and weight retention among 110 women in Michigan. Using a longitudinal random effects model, they found that women who breast-fed predominantly through 6 months postpartum returned to their prepregnant weight 6 months earlier than those who formula-fed. This association was modified by age and marital status, with slower postpartum weight loss associated with lactation among older women and with postpartum weight gain among lactating unmarried women. Others have found differences in the effects of breast-feeding on weight loss depending on frequency of breast-feeding,26,27 percent body fat,28 and prepregnancy body mass index (BMI).29 These findings underscore the importance of considering subgroup effects when analyzing data on lactation and weight change. Other authors found no association between lactation and postpartum weight change, although studies were limited by small sample size30 and high dropout rates.31
LACTATION AND BODY FAT DISTRIBUTION
Several authors have examined the effect of pregnancy and breast-feeding on body fat distribution. Longitudinal studies of skin-fold thickness during pregnancy and lactation consistently show fat accumulation in the supra-iliac and midthigh regions during pregnancy, with mobilization from these areas postpartum.16,20,32–34 Sohlstrom and Forsum35 confirmed these findings in a longitudinal magnetic resonance imaging study of lactating women. Studies of lipolysis and LPL activity in fat biopsies also show regional deposition of femoral fat during pregnancy and mobilization of these stores during lactation.36 Lassek and Gaulin37 examined the association between regional fat distribution and parity in a cross-sectional study of subjects in the Third National Health and Nutrition Examination Survey. Compared with nulligra-vid and pregnant women, those who were currently breast-feeding and had lactated more than 7 months had smaller suprailiac and thigh skinfolds and smaller hip and thigh circumferences. The authors note that long-chain polyunsaturated fatty acids are concentrated in lower body fat, and they speculate that fat from this region is preferentially mobilized in lactation to support infant brain development.
LONG-TERM ASSOCIATIONS BETWEEN LACTATION AND ADIPOSITY
There is modest evidence that breast-feeding-associated differences in adiposity persist beyond weaning. Gigante et al38 examined the relation between adiposity at 5 years postpartum and duration of breast-feeding for a woman’s last birth and among 372 women in Brazil. Longer duration of breast-feeding was associated with lower percent fat mass. They further reported that women who exclusively breast-fed for 4 months were leaner on all measures than women who had weaned by 4 months, but only differences in percent body fat were statistically significant. Based on these results, the authors concluded that there is a modest relationship between breast-feeding and long-term adiposity, although the association may be confounded by socioeconomic status.
In a study of 4348 women in the Nurses’ Health Study II, Sichieri et al39 found minimal differences in weight trajectory by breast-feeding duration among women who had given birth over a 4-year period. Overall, breast-feeding women gained 1 kg more weight than women who never breast-fed, adjusting for age, physical activity, and baseline BMI. This difference was statistically significant for normal-weight women (BMI <25 kg/m2) who were nulliparous in 1989 (p = 0.02) and for overweight women (BMI ≥ 25 kg/m2) who were primiparous in 1989. Of note, the study did not include data on gestational weight gain, which is a major predictor of retained postpartum weight. With adjustment for weight during pregnancy in a subset of women for whom data were available, normal-weight women who breast-fed lost 1 kg more than those who did not (p <0.05).
In a longitudinal cohort in La Crosse, Wisconsin, Rooney and Schauberger40 found long-term differences in adiposity by breast-feeding history. A decade after the index pregnancy, they reported that women who breast-fed for more than 12 weeks weighed 3.73 kg less than women who had never breast-fed, adjusting for gestational weight gain, weight loss by 6 months postpartum, and aerobic exercise. These findings suggest that lactation may have long-lasting effects on BMI, independent of weight in the early postpartum period. Alternately, differences in prepregnancy BMI or health behaviors associated with more than 12 weeks of breast-feeding may explain the observed association.
Rush et al41 found such confounding in a study of 671 women born around the time of the Dutch famine. Adjusting for socioeconomic confounders, they found that BMI at age 45 was positively associated with parity and negatively associated with number of children breast-fed. However, they also found that the number of children breast-fed was negatively associated with BMI before the first pregnancy. With adjustment for prepregnancy BMI, parity remained strongly associated with BMI at age 45, but there was no relation between breast-feeding and BMI. They concluded that breast-feeding does not, in itself, reduce weight, but rather, women who breast-feed engage in other lifelong behaviors that prevent weight gain.
RANDOMIZED CONTROLLED TRIAL DATA
Data from two randomized controlled trials in Honduras provide evidence for a causal association between lactation and maternal weight loss. Dewey et al42 conducted two studies randomizing mother–infant dyads to exclusive breast-feeding through 6 months or introduction of supplemental food with continued partial breast-feeding from 4 to 6 months. In the first cohort, they randomized healthy, low-income, primiparous mothers of infants >2500 g at birth. The second cohort enrolled primiparous mothers of infants who weighed 1500 to 2500 g at birth. The authors assessed maternal weight, infant weight, and infant breast milk and solid food intake to estimate the metabolic burden of exclusive breast-feeding. Maternal dietary intake was not assessed.
In the normal-birth-weight group, exclusive breast-feeding through 6 months was associated with a 0.6-kg greater decrease in maternal weight from 4 to 6 months compared with complementary feeding (−0.7 ± 1.5 versus −0.1 ± 1.7 kg, p <0.05). Mothers who were exclusive breast-feeding expended a cumulative additional 5520 kcal in energy over the 2-month period, compared with mothers who supplemented. In the low-birth-weight group, exclusive breast-feeding was associated with a nonsignificant 0.2-kg greater weight loss (−0.3 ± 1.6 versus −0.1 ± 1.7, p >0.05), with an estimated additional energy expenditure of 2700 kcal. The lower maternal energy expenditure in the low-birth-weight group may reflect increased infant intake of complementary foods or less overall energy intake in smaller infants. Interestingly, the difference in maternal weight loss, in grams of fat at 9 kcal/g, corresponds closely with the estimated excess energy expenditure needed to support exclusive breast-feeding (5400 kcal for the normal-birth-weight group, 1800 kcal for the low-birth-weight group).
These findings from a randomized, controlled trial provide evidence that greater intensity of lactation from 4 to 6 months postpartum causes greater weight loss among mothers of normal-birth-weight infants in a developing country. It is unclear whether these data are generalizable to more obesogenic environments.
HOW DOES LACTATION AFFECT GLUCOSE HOMEOSTASIS?
Mothers experience increases in insulin resistance and glucose intolerance during pregnancy. Data from animal studies and human populations suggest that lactation plays a role in reestablishing glucose homeostasis after delivery.
Animal Studies
In animal studies, lactation decreases both glucose levels and insulin resistance during the postpartum period.43,44 Lactation-related hormones also affect insulin production. In a recent study, Karnik et al45 examined the effects of prolactin on pregnancy-associated β-cell proliferation in the mouse. The authors found that prolactin stimulates β cell proliferation by down-regulating the expression of menin. Menin is the protein product of the MenI gene, which regulates islet cell proliferation through a histone-methylation pathway. In pregnant animals, transgenic expression of menin suppressed β-cell proliferation and led to impaired glucose tolerance. The role of prolactin in regulating β-cell mass after birth is not well characterized, but prolactin levels remain elevated during lactation, suggesting that the hormone may play a role in regulating insulin secretion and glucose homeostasis. Repeated pregnancies without lactation appear to disrupt glycemic regulation. In their study of three cycles of pregnancy without lactation, Zhong et al15 found deterioration in glucose regulation in animals that had not lactated, with higher fasting glucose levels and higher rates of spontaneous abortion in the third pregnancy, compared with animals that had lactated with each gestation.
Human Studies
Human observational studies suggest that lactation affects insulin and glucose homeostasis. Kjos et al46 studied glucose tolerance in 809 primarily Latina women previously diagnosed with gestational diabetes. In follow-up testing at 4 to 12 weeks postpartum, women who were breast-feeding had improved glucose tolerance and lower fasting glucose than women who were bottle-feeding, with adjustment for maternal age, BMI, and insulin use during pregnancy. A smaller study by McManus et al47 assessed postpartum metabolic function in 26 Caucasian women (14 lactating, 12 nonlactating) with gestational diabetes. At 3 months postpartum, there were no significant differences in insulin sensitivity, glucose effectiveness, or visceral fat or subcutaneous fat. The lactating group did have a higher disposition index, which indicates more efficient β-cell function, adjusted for insulin resistance. These findings are interesting in light of Karnik et al’s study45 linking prolactin with β-cell proliferation, as they suggest that lactation may result in improvement in β-cell function.
In a nondiabetic population, Butte et al18 found significant differences in metabolic parameters between lactating and nonlactating women at 3 and 6 months postpartum, independent of BMI. In the lactating group, insulin levels and insulin:glucose ratios were significantly lower, and carbohydrate utilization and total energy expenditure were higher. Diniz and Da Costa48 examined the relation between breast-feeding and glucose homeostasis in a cross-sectional study at 12 to 18 months postpartum. They found that breast-feeding history was inversely associated with insulin resistance, independent of adiposity. It is unclear whether these results reflect a lasting effect of lactation, because 60% of participants were still breast-feeding.
It is not clear how long the apparent benefit of lactation on glucose homeostasis persists after weaning. We studied glucose metabolism at 3 years postpartum among 177 women in Project Viva,49 a prospective, longitudinal study of maternal and infant health. In this group of mothers who had nursed their infant for an average of 6.4 months and had not had an intervening pregnancy in the past 3 years, we found no association between lactation duration and homeostasis model assessment of insulin resistance or fasting glucose.
On the other hand, authors have found an inverse association between lactation and the fasting glucose,6 as well as type 2 diabetes,4,7 in three large cohort studies. In the Nurses’ Health Studies, we found that lifetime lactation was inversely associated with development of type 2 diabetes, independent of BMI. The long-term effect of lactation on women with gestational diabetes is less clear. In a subgroup analysis in the Nurses’ Health Study II,4 lactation among gestational diabetics was associated with a borderline-significant reduction in covariate-adjusted risk (hazard ratio [HR] 0.89 per year of lactation, 95% confidence interval [CI] 0.78 to 1.00), but this association was markedly attenuated with adjustment for BMI (HR 0.96, 95% CI 0.84 to 1.09). These results suggest that weight loss may mediate the association between lactation and incident disease risk in this high-risk group. It is also plausible that breast-feeding women with a history of gestational diabetes mellitus were more motivated to engage in other healthy behaviors such as intentional weight loss.
HOW DOES LACTATION AFFECT LIPID HOMEOSTASIS?
Lactation requires mobilization of lipids for milk synthesis, and this process involves substantial changes in lipid metabolism during breast-feeding. In cohort studies, lactation has been associated with a reduced risk of hyperlipidemia and cardiovascular disease.
Human Studies
During human pregnancy, triglyceride and total cholesterol levels increase. These levels fall after delivery, particularly in lactating women. Several authors have conducted longitudinal studies of lipid levels in lactating women. Darmady and Postle50 followed 34 women from prior to conception through pregnancy and the post-partum period and found that triglyceride levels retuned to baseline 13 weeks earlier in lactating than in non-lactating women (p <0.001). Other authors have identified a protective association between lactation and high-density lipoprotein (HDL) metabolism at 6 weeks46,51–56 and 3 months52 postpartum. In women who continue breast-feeding for 1 year, higher levels of HDL appear to persist until weaning.53
When we examined lipid metabolism at 3 years postpartum among 177 women in Project Viva,49 we found no consistent association between lactation duration and lipid levels. By contrast, in a recent study of 109 women in the CARDIA cohort, Gunderson et al54 documented lasting differences in lipid metabolism between women who had given birth and had or had not breast-fed. Lipid levels were compared over a 3-year interval from prior to conception until after weaning. Mothers who breast-fed had lower low-density lipoprotein levels than those that had not, and those who breast-fed for more than 3 months had more favorable changes in HDL than those who breast-fed for less than 3 months, adjusting for demographic and lifestyle covariates.
These breast-feeding-associated differences in lipid metabolism may impact long-term cardiovascular risk. In cohort studies, lifetime lactation has been inversely associated with prevalence of the metabolic syndrome,6 hyperlipidemia and hypertension,7 and incidence of myocardial infarction.5
CAVEATS: RESIDUAL CONFOUNDING AND REVERSE CAUSATION
Observational studies suggest that lactation is associated with weight loss, improved glucose metabolism, more favorable lipid profiles, and reduced risk of metabolic disease. These findings must be interpreted with caution, however, because women who breast-feed are more likely to engage in other healthy behaviors.55 Secular trends may also influence observed associations between breast-feeding and health outcomes. For example, during the 1970s, breast-feeding rates differed markedly by education. In 1974, 65% of women with a bachelor’s degree initiated breast-feeding, compared with 14% of women with less than a high school education.56 More recently, breast-feeding rates have increased in the United States, but significant disparities by race, income, and education persist.57 It is therefore crucial for studies of breast-feeding and long-term metabolic outcomes to consider the role of residual confounding by socioeconomic status in any observed associations.
Observational studies of lactation and adiposity also raise concerns of reverse causation. Obese or overweight women are less likely to initiate breast-feeding and lactate for shorter durations than normal BMI women.58–62 A study in the early postpartum period documented diminished prolactin response to suckling among obese women,63 suggesting that excess adiposity interferes with hormonal establishment of lactation. Excess gestational weight gain is also associated with early cessation of lactation.64,65 If obesity causes breast-feeding failure, then successful lactation may simply be a marker for low prepregnancy BMI, appropriate weight gain, and a less obesogenic metabolism, rather than a mechanism for reducing risk.
To distinguish between these hypotheses, researchers need to collect data on a mother’s prepregnancy and postpartum metabolic risk, infant feeding intentions during pregnancy, attitudinal and physiological reasons for lactation success or failure, introduction of supplemental foods, reasons for weaning, and postpartum lifestyle risk factors such as diet and exercise. To our knowledge, no existing study has collected sufficiently comprehensive data to distinguish the “reset hypothesis,” in which lactation helps women to regain their prepregnant weight and metabolic and cardiovascular risk status, from a “preset hypothesis,” in which prelactation metabolic risk factors determine lactation initiation and duration as well as later-life metabolic risk. Until such studies are completed, either the “reset hypothesis” or the “preset hypothesis” could explain the apparent association between lifetime lactation and metabolic risk.4,6,7,54
CONCLUSION
For maternal metabolism, pregnancy ends not with birth, but with weaning. Data from animal models and human studies suggest that lactation is associated with favorable changes in adiposity, glycemic control, and lipid homeostasis that persist long after weaning. Observational studies from human populations must be interpreted with caution, because breast-feeding is associated with other beneficial health behaviors that may confound observed associations. It is reassuring, however, that Dewey et al42 found greater maternal weight loss with exclusive breast-feeding than with partial breast-feeding in a randomized, controlled trial. These results provide support for a causal association between lactation and weight change.
Dewey et al’s study provides a model for future research to delineate the role of lactation in resetting maternal metabolism after pregnancy. It is ethically problematic to randomize mothers to formula-feeding or breast-feeding, but it is feasible to assign women to interventions that prolong lactation.66 To test the independent effect of lactation on maternal metabolism, randomized trials are needed of intensive breast-feeding support versus usual care, with longitudinal follow-up of maternal anthropometry and biomarkers of glucose and lipid metabolism. Studies showing that intensive breast-feeding support can improve metabolic outcomes would provide strong evidence that lactation is a modifiable risk factor for maternal metabolic disease.
Eighty-five percent of U.S. women give birth to at least one child,67 and 42% of women breast-feed for at least 6 months.57 If randomized trials provide evidence that lactation affects metabolism, then efforts to increase breast-feeding duration could impact disease risk for more than half of the female population. Once proven, breast-feeding promotion interventions could significantly reduce the burden of metabolic disease among young- and middle-aged women.
Acknowledgments
The authors thank Eduardo Villamor for his suggestions in preparing this manuscript.
References
- 1.Gunderson EP, Murtaugh MA, Lewis CE, et al. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA) Int J Obes Relat Metab Disord. 2004;28:525–535. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368:1164–1170. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- 3.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005;106:1349–1356. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 4.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 5.Stuebe AM, Michels KB, Willett WC, Manson JE, Rich-Edwards J. Duration of lactation and incidence of myocardial infarction. Am J Obstet Gynecol. doi: 10.1016/j.ajog.2008.10.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ram KT, Bobby P, Hailpern SM, et al. Duration of lactation is associated with lower prevalence of the metabolic syndrome in midlife-SWAN, the study of women’s health across the nation. Am J Obstet Gynecol. 2008;198:268.e261–268.e266. doi: 10.1016/j.ajog.2007.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz EB, Stuebe AM, Allison MA, et al. Impact of lactation on risk factors for cardiovascular disease. J Gen Intern Med. 2008;23(Suppl 2):321–322. [Google Scholar]
- 8.Ellison PT. On Fertile Ground. Cambridge: Harvard University Press; 2001. Metabolizing for Two; pp. 94–97. [Google Scholar]
- 9.Dall SR, Boyd IL. Evolution of mammals: lactation helps mothers to cope with unreliable food supplies. Proc Biol Sci. 2004;271:2049–2057. doi: 10.1098/rspb.2004.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Einstein FH, Fishman S, Muzumdar RH, et al. Accretion of visceral fat and hepatic insulin resistance in pregnant rats. Am J Physiol Endocrinol Metab. 2008;294:E451–E455. doi: 10.1152/ajpendo.00570.2007. [DOI] [PubMed] [Google Scholar]
- 11.Hamosh M, Clary TR, Chernick SS, Scow RO. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta. 1970;210:473–482. doi: 10.1016/0005-2760(70)90044-5. [DOI] [PubMed] [Google Scholar]
- 12.Steingrimsdottir L, Brasel JA, Greenwood MR. Diet, pregnancy, and lactation: effects on adipose tissue, lipoprotein lipase, and fat cell size. Metabolism. 1980;29:837–841. doi: 10.1016/0026-0495(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 13.Moore BJ, Brasel JA. One cycle of reproduction consisting of pregnancy, lactation or no lactation, and recovery: effects on carcass composition in ad libitum-fed and food-restricted rats. J Nutr. 1984;114:1548–1559. doi: 10.1093/jn/114.9.1548. [DOI] [PubMed] [Google Scholar]
- 14.Moore BJ, Olsen JL, Marks F, Brasel JA. The effects of high fat feeding during one cycle of reproduction consisting of pregnancy, lactation and recovery on body composition and fat pad cellularity in the rat. J Nutr. 1984;114:1566–1573. doi: 10.1093/jn/114.9.1566. [DOI] [PubMed] [Google Scholar]
- 15.Zhong S, Almario R, Dubrinsky M, et al. Repeated pregnancy without lactation: effects on maternal glycemic control, pregnancy outcome, carcass composition, and fat distribution in rats. Metabolism. 1990;39:1127–1132. doi: 10.1016/0026-0495(90)90083-o. [DOI] [PubMed] [Google Scholar]
- 16.Butte NF, Garza C, Stuff JE, Smith EO, Nichols BL. Effect of maternal diet and body composition on lactational performance. Am J Clin Nutr. 1984;39:296–306. doi: 10.1093/ajcn/39.2.296. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg GR, Prentice AM, Coward WA, et al. Longitudinal assessment of the components of energy balance in well-nourished lactating women. Am J Clin Nutr. 1991;54:788–798. doi: 10.1093/ajcn/54.5.788. [DOI] [PubMed] [Google Scholar]
- 18.Butte NF, Hopkinson JM, Mehta N, Moon JK, Smith EO. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am J Clin Nutr. 1999;69:299–307. doi: 10.1093/ajcn/69.2.299. [DOI] [PubMed] [Google Scholar]
- 19.van Raaij JM, Schonk CM, Vermaat-Miedema SH, Peek ME, Hautvast JG. Energy cost of lactation, and energy balances of well-nourished Dutch lactating women: reappraisal of the extra energy requirements of lactation. Am J Clin Nutr. 1991;53:612–619. doi: 10.1093/ajcn/53.3.612. [DOI] [PubMed] [Google Scholar]
- 20.Brewer MM, Bates MR, Vannoy LP. Postpartum changes in maternal weight and body fat depots in lactating vs nonlactating women. Am J Clin Nutr. 1989;49:259–265. doi: 10.1093/ajcn/49.2.259. [DOI] [PubMed] [Google Scholar]
- 21.Sadurskis A, Kabir N, Wager J, Forsum E. Energy metabolism, body composition, and milk production in healthy Swedish women during lactation. Am J Clin Nutr. 1988;48:44–49. doi: 10.1093/ajcn/48.1.44. [DOI] [PubMed] [Google Scholar]
- 22.Dewey KG, Heinig MJ, Nommsen LA. Maternal weight-loss patterns during prolonged lactation. Am J Clin Nutr. 1993;58:162–166. doi: 10.1093/ajcn/58.2.162. [DOI] [PubMed] [Google Scholar]
- 23.Ohlin A, Rossner S. Maternal body weight development after pregnancy. Int J Obes. 1990;14:159–173. [PubMed] [Google Scholar]
- 24.Ohlin A, Rossner S. Factors related to body weight changes during and after pregnancy: the Stockholm Pregnancy and Weight Development Study. Obes Res. 1996;4:271–276. doi: 10.1002/j.1550-8528.1996.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 25.Janney CA, Zhang D, Sowers M. Lactation and weight retention. Am J Clin Nutr. 1997;66:1116–1124. doi: 10.1093/ajcn/66.5.1116. [DOI] [PubMed] [Google Scholar]
- 26.Valeggia CR, Ellison PT. Impact of breastfeeding on anthropometric changes in peri-urban Toba women (Argentina) Am J Hum Biol. 2003;15:717–724. doi: 10.1002/ajhb.10202. [DOI] [PubMed] [Google Scholar]
- 27.Quandt SA. Changes in maternal postpartum adiposity and infant feeding patterns. Am J Phys Anthropol. 1983;60:455–461. doi: 10.1002/ajpa.1330600407. [DOI] [PubMed] [Google Scholar]
- 28.Kac G, Benicio MH, Velasquez-Melendez G, Valente JG, Struchiner CJ. Breastfeeding and postpartum weight retention in a cohort of Brazilian women. Am J Clin Nutr. 2004;79:487–493. doi: 10.1093/ajcn/79.3.487. [DOI] [PubMed] [Google Scholar]
- 29.Soltani H, Fraser R. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr. 2000;84:95–101. doi: 10.1017/s0007114500001276. [DOI] [PubMed] [Google Scholar]
- 30.Chou TW, Chan GM, Moyer-Mileur L. Postpartum body composition changes in lactating and non-lactating primiparas. Nutrition. 1999;15:481–484. doi: 10.1016/s0899-9007(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 31.Kramer FM, Stunkard AJ, Marshall KA, McKinney S, Liebschutz J. Breast-feeding reduces maternal lower-body fat. J Am Diet Assoc. 1993;93:429–433. doi: 10.1016/0002-8223(93)92289-a. [DOI] [PubMed] [Google Scholar]
- 32.Martinez H, Allen LH, Lung’aho M, Chavez A, Pelto GH. Maternal fatness in Mexican women predicts body composition changes in pregnancy and lactation. Adv Exp Med Biol. 1994;352:99–107. doi: 10.1007/978-1-4899-2575-6_7. [DOI] [PubMed] [Google Scholar]
- 33.Forsum E, Sadurskis A, Wager J. Estimation of body fat in healthy Swedish women during pregnancy and lactation. Am J Clin Nutr. 1989;50:465–473. doi: 10.1093/ajcn/50.3.465. [DOI] [PubMed] [Google Scholar]
- 34.Sidebottom AC, Brown JE, Jacobs DR., Jr Pregnancy-related changes in body fat. Eur J Obstet Gynecol Reprod Biol. 2001;94:216–223. doi: 10.1016/s0301-2115(00)00329-8. [DOI] [PubMed] [Google Scholar]
- 35.Sohlstrom A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr. 1995;61:287–295. doi: 10.1093/ajcn/61.2.287. [DOI] [PubMed] [Google Scholar]
- 36.Rebuffe-Scrive M, Enk L, Crona N, et al. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J Clin Invest. 1985;75:1973–1976. doi: 10.1172/JCI111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lassek WD, Gaulin SJ. Changes in body fat distribution in relation to parity in American women: a covert form of maternal depletion. Am J Phys Anthropol. 2006;131:295–302. doi: 10.1002/ajpa.20394. [DOI] [PubMed] [Google Scholar]
- 38.Gigante DP, Victora CG, Barros FC. Breast-feeding has a limited long-term effect on anthropometry and body composition of Brazilian mothers. J Nutr. 2001;131:78–84. doi: 10.1093/jn/131.1.78. [DOI] [PubMed] [Google Scholar]
- 39.Sichieri R, Field AE, Rich-Edwards J, Willett WC. Prospective assessment of exclusive breastfeeding in relation to weight change in women. Int J Obes Relat Metab Disord. 2003;27:815–820. doi: 10.1038/sj.ijo.0802285. [DOI] [PubMed] [Google Scholar]
- 40.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol. 2002;100:245–252. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- 41.Rush D, Lumey LH, Ravelli AC, Myers B. The indirect association of lactation with subsequent perimenopausal body weight. Eur J Clin Nutr. 1996;50:12–16. [PubMed] [Google Scholar]
- 42.Dewey KG, Cohen RJ, Brown KH, Rivera LL. Effects of exclusive breastfeeding for four versus six months on maternal nutritional status and infant motor development: results of two randomized trials in Honduras. J Nutr. 2001;131:262–267. doi: 10.1093/jn/131.2.262. [DOI] [PubMed] [Google Scholar]
- 43.Jones RG, Ilic V, Williamson DH. Physiological significance of altered insulin metabolism in the conscious rat during lactation. Biochem J. 1984;220:455–460. doi: 10.1042/bj2200455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnol AF, Leturque A, Ferre P, Kande J, Girard J. Increased insulin sensitivity and responsiveness during lactation in rats. Am J Physiol. 1986;251(5 Pt 1):E537–E541. doi: 10.1152/ajpendo.1986.251.5.E537. [DOI] [PubMed] [Google Scholar]
- 45.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 46.Kjos SL, Henry O, Lee RM, Buchanan TA, Mishell DR., Jr The effect of lactation on glucose and lipid metabolism in women with recent gestational diabetes. Obstet Gynecol. 1993;82:451–455. [PubMed] [Google Scholar]
- 47.McManus RM, Cunningham I, Watson A, Harker L, Finegood DT. Beta-cell function and visceral fat in lactating women with a history of gestational diabetes. Metabolism. 2001;50:715–719. doi: 10.1053/meta.2001.23304. [DOI] [PubMed] [Google Scholar]
- 48.Diniz JM, Da Costa TH. Independent of body adiposity, breast-feeding has a protective effect on glucose metabolism in young adult women. Br J Nutr. 2004;92:905–912. doi: 10.1079/bjn20041288. [DOI] [PubMed] [Google Scholar]
- 49.Stuebe A, Gillman M, Kleinman K, Rifas-Shiman S, Rich-Edwards J. Duration of lactation and maternal metabolism at 3 years postpartum. Am J Obstet Gynecol. 2007;197:S128. doi: 10.1089/jwh.2009.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darmady JM, Postle AD. Lipid metabolism in pregnancy. Br J Obstet Gynaecol. 1982;89:211–215. doi: 10.1111/j.1471-0528.1982.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 51.Knopp RH, Walden CE, Wahl PW, et al. Effect of postpartum lactation on lipoprotein lipids and apoproteins. J Clin Endocrinol Metab. 1985;60:542–547. doi: 10.1210/jcem-60-3-542. [DOI] [PubMed] [Google Scholar]
- 52.Erkkola R, Viikari J, Irjala K, Solakivi-Jaakkola T. One-year follow-up of lipoprotein metabolism after pregnancy. Biol Res Pregnancy Perinatol. 1986;7:47–51. [PubMed] [Google Scholar]
- 53.Kallio MJ, Siimes MA, Perheentupa J, Salmenpera L, Miettinen TA. Serum cholesterol and lipoprotein concentrations in mothers during and after prolonged exclusive lactation. Metabolism. 1992;41:1327–1330. doi: 10.1016/0026-0495(92)90103-h. [DOI] [PubMed] [Google Scholar]
- 54.Gunderson EP, Lewis CE, Wei GS, et al. Lactation and changes in maternal metabolic risk factors. Obstet Gynecol. 2007;109:729–738. doi: 10.1097/01.AOG.0000252831.06695.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pesa JA, Shelton MM. Health-enhancing behaviors correlated with breastfeeding among a national sample of mothers. Public Health Nurs. 1999;16:120–124. doi: 10.1046/j.1525-1446.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- 56.National Center for Health Statistics. Health, United States, 2005, With Chartbook on Trends in the Health of Americans. Hyattsville, MD: 2005. Table 18. Breastfeeding by mothers 15–44 years of age by year of baby’s birth, according to selected characteristics of mother: United States, average annual 1972–74 to 1999–2001. [Google Scholar]
- 57.Centers for Disease Control and Prevention. [Accessed July 21, 2008];Breastfeeding Practices—Results from the National Immunization Survey. Web site: http://www.cdc.gov/breastfeeding/data/NIS_data/data_2004.htm.
- 58.Oddy WH, Li J, Landsborough L, et al. The association of maternal overweight and obesity with breastfeeding duration. J Pediatr. 2006;149:185–191. doi: 10.1016/j.jpeds.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Donath SM, Amir LH. Does maternal obesity adversely affect breastfeeding initiation and duration? Breastfeed Rev. 2000;8:29–33. [PubMed] [Google Scholar]
- 60.Hilson JA, Rasmussen KM, Kjolhede CL. Maternal obesity and breast-feeding success in a rural population of white women. Am J Clin Nutr. 1997;66:1371–1378. doi: 10.1093/ajcn/66.6.1371. [DOI] [PubMed] [Google Scholar]
- 61.Hilson JA, Rasmussen KM, Kjolhede CL. High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. J Hum Lact. 2004;20:18–29. doi: 10.1177/0890334403261345. [DOI] [PubMed] [Google Scholar]
- 62.Baker JL, Michaelsen KF, Sorensen TI, Rasmussen KM. High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. Am J Clin Nutr. 2007;86:404–411. doi: 10.1093/ajcn/86.2.404. [DOI] [PubMed] [Google Scholar]
- 63.Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113:e465–e471. doi: 10.1542/peds.113.5.e465. [DOI] [PubMed] [Google Scholar]
- 64.Li R, Jewell S, Grummer-Strawn L. Maternal obesity and breast-feeding practices. Am J Clin Nutr. 2003;77:931–936. doi: 10.1093/ajcn/77.4.931. [DOI] [PubMed] [Google Scholar]
- 65.Hilson JA, Rasmussen KM, Kjolhede CL. Excessive weight gain during pregnancy is associated with earlier termination of breast-feeding among white women. J Nutr. 2006;136:140–146. doi: 10.1093/jn/136.1.140. [DOI] [PubMed] [Google Scholar]
- 66.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413–420. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 67.National Center for Health Statistics. Data From the National Survey of Family Growth. Hyattsville, MD: Public Health Service; 2006. Fertility, Family Planning, and Reproductive Health of U.S. Women: Data from the 2002 National Survey of Family Growth; p. 174. [PubMed] [Google Scholar]


