Abstract
BACKGROUND
Plasmodium falciparum-infected erythrocytes sequester in the placenta and elicit an inflammatory response that is harmful to both fetus and mother. Histologic measurements during placental malaria (PM) might provide surrogate endpoints for interventional trials, but existing histologic schemes capture limited complexity and are not consistently used among study sites.
METHODS
Using frozen section histology in Tanzania (high transmission area), we establish a novel grading scheme to separately quantify inflammation and pigment deposition during PM (n=102). To generalize this method, formalin-fixed paraffin-embedded placental samples from Karen women in Thailand (low transmission area) were selected from among those with documented antenatal parasitemia near term (n=18).
RESULTS
In the Tanzanian cohort, the inflammation and pigment deposition scores were independently associated with birth weight, and the inflammation score was associated with chemokine levels. In the smaller cohort from Thailand, both inflammation and pigment scores were associated with birth weight, and the pigment score had an inverse trend with the number of antenatal clinic visits.
CONCLUSIONS
This semiquantitative pathological grading scheme is simple to implement yet captures information that is associated with outcomes in Asia and Africa, and therefore should facilitate the comparison and standardization of results among clinical trials across areas of differing endemicity.
Keywords: Malaria, Plasmodium falciparum, pregnancy, placenta, histology
INTRODUCTION
Malaria during pregnancy is associated with morbidity and mortality for pregnant women and their newborns in tropical areas. During placental malaria (PM), Plasmodium falciparum-infected erythrocyes (IE) adhere to chondroitin sulfate A present on the trophoblast surface (1). Parasite sequestration results in a maternal inflammatory response that can be harmful to both the mother and the fetus. Histologic features of PM correlate with poor clinical outcomes and therefore might be useful as efficacy endpoints for interventional trials of prophylactic or treatment drugs.
Women living in different geographical areas experience different levels of malaria exposure, and their level of immunity prior to pregnancy influences disease course during infection. In areas of high stable transmission, such as Tanzania, women have significant clinical immunity prior to pregnancy, and PM is often asymptomatic but associated with severe maternal anemia and fetal growth retardation. In these areas, PM is most frequent and severe in first-time mothers because women develop specific immunity against placental forms of IE over successive pregnancies. In areas of low unstable transmission, such as the Thai-Burma border, malaria is symptomatic in women of all parities, and associated with high rates of fetal loss and maternal death (2). The approach to antenatal care and the sensitivity of the parasite to different antimalarials also differ and could contribute to differences in outcomes. In Tanzania, insecticide treated bed-nets (ITN) and intermittent preventive treatment in pregnancy (IPTp) are routinely used as preventive interventions but case-detection is passive (3), whereas preventive measures are not routine at the Thai-Burma border where case-detection is active and prompts early treatment.
Three key histologic features of an untreated PM episode were described by Garnham in 1938: parasites, inflammation and pigment deposition, which correspond to three distinct biologic timelines (4) (Fig 1). Infected erythrocytes accumulate in the intervillous space, and parasitemia changes rapidly over the course of the parasite life cycle and in response to treatment. Maternal inflammatory cells, predominately monocyte-macrophages, also accumulate in the intervillous space, and are estimated to accumulate over the course of days to weeks in susceptible women and can persist for a brief time following treatment (4). Malaria pigment accumulates in intervillous fibrin, likely originating from degenerating pigment-laden macrophages, and can persist for months following heavy infection during gestation, or may become undetectable with adequate treatment and no further reinfection (5, 6).
Figure 1.
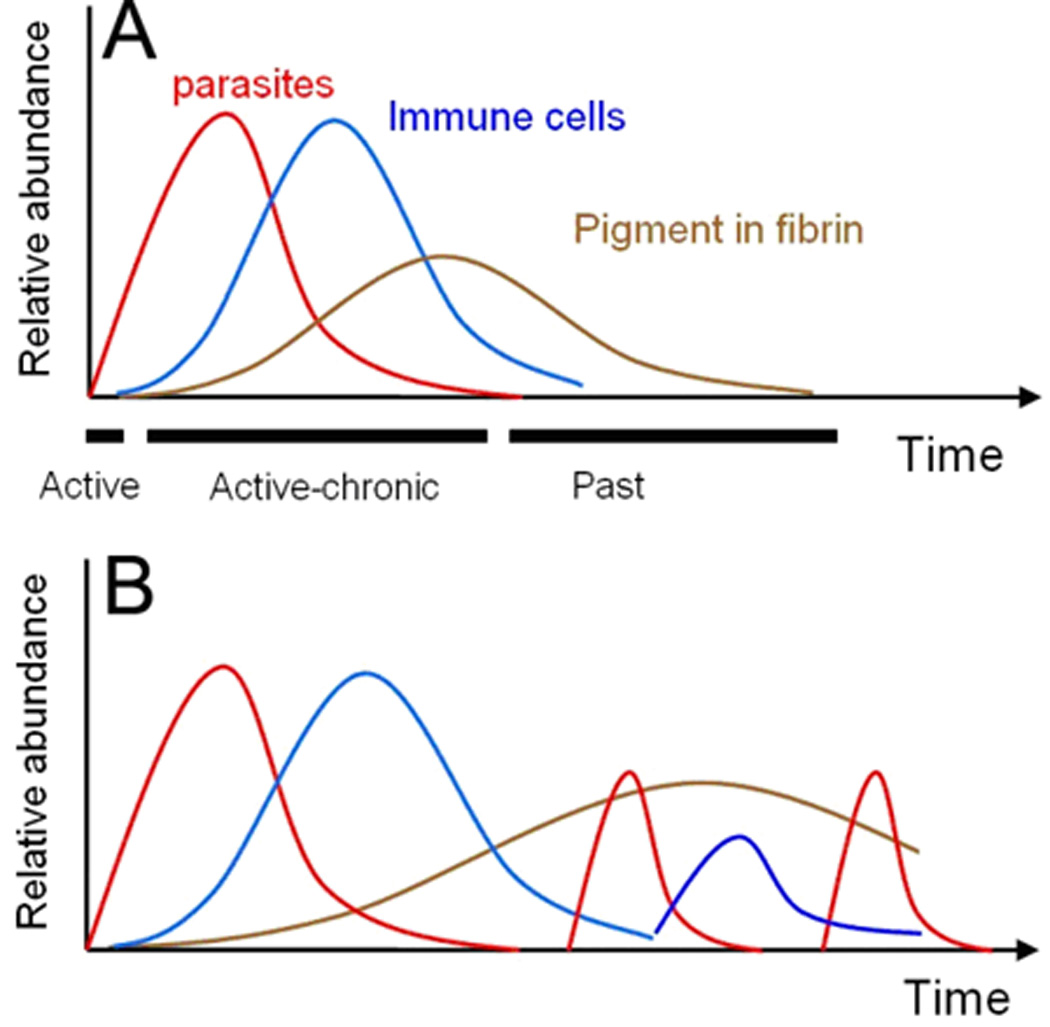
Schematics of A) a hypothetical single PM episode in a susceptible woman, and B) recrudescenses and reinfections in a single woman that can lead to varying degrees of parasitemia, inflammation and pigment, especially when the drugs for IPT or treatment are not effective.
In endemic areas, pathologic features of PM correspond to clinical outcomes. Maternal inflammation is mostly seen in first-time mothers, and is linked to decreased birth weight (7–9) and anemia (9). Pigment present in immune cells or fibrin has been associated with reduced birth weight (8, 10). The presence of pigment in women without active parasitemia (past infection) has been associated with decreased BW in some studies (9, 11), but not in others (8, 12, 13). Differences in outcomes associated with past infections may be related to the sensitivity of different methods for detecting parasites, especially during very low parasitemia. Along the Thai-Burma border, an area of low transmission, pathologic changes of PM were rare in women without evidence of recent infection (6).
A pathologic classification scheme for PM was developed by Bulmer in 1993 (14) to reflect the chronology of the infection. This scheme infers “active” or “acute” infections when parasites are present in the intervillous spaces with or without pigment in intervillous monocytes; “chronic” infections when parasites are present in the intervillous spaces along with pigment as deposits or in macrophages within fibrin; and “past-chronic” infections when pigment is present in the absence of parasites (Fig 1A). Subsequent modifications and additional grading schemes have been developed by Ismail (15) and Rogerson (9) similar in spirit to the Bulmer scheme, as well as by Davison to incorporate other features of placental injury including intervillous inflammatory cells (16).
The application of the existing PM grading schemes is limited because chronic PM is a broad category encompassing varying degrees of pathology, scoring criteria vary by study site, and in general, the degree of inflammation is not independently considered. In contrast, a scheme using two parameters, inflammation and fibrosis, is widely used in monitoring progression and treatment of chronic viral hepatitis (17). We have developed a two parameter semi-quantitative grading scheme that scores the degrees of inflammation and pigment deposition during PM. The proposed scheme is simple to perform, corresponds to pregnancy outcomes in two continents, and may improve the comparison and standardization of results among clinical trials across areas of differing endemicity.
METHODS
Study sites
Placental samples from study populations living in two areas of different malaria endemicity were analyzed. Informed consent was obtained at both sites. Women living in the high transmission area around Muheza, Tanzania, were enrolled in the Mother Offspring Malaria Studies Project during their delivery at the Muheza Designated District Hospital. These women received antenatal care according to Tanzanian national guidelines, including mostly passive malaria case detection and one or two presumptive antimalarial treatments. Women from refugee and migrant populations living in an area of sporadic transmission along the Thai-Burma received weekly antenatal care with active malaria screening at one of five clinics operated by the Shoklo Malaria Research Unit. Antenatal care was provided free of charge, and a subset of women enrolled in clinical trials. The demographics of the cohorts are presented in Table 1.
Table 1.
Pregnancy Malaria Epidemiology, Therapy, and Antenatal care in the Tanzania and Thailand cohorts.
| Africa, Tanzania, 2002–2005 | Asia, Thailand, 1995–2002 | |
|---|---|---|
| Malaria incidence during pregnancy |
34% of women reported treatment for acute malaria |
~5–30% with episodes during pregnancy (varies by site); < 1 P.falciparum infection per woman/year <1.5 P.vivax infection per woman/year |
| Cases of pregnancy malaria due to non- falciparum species |
< 1% | ~60% P.vivax |
| Case-detection method during pregnancy |
Passive (screening available at ANC) |
Active (weekly visits) and Passive (24 hour malaria screening available) |
| Placental malaria incidence at delivery |
12.6% all women, 19.4% of first-time mothers |
< 1% |
| Women Receiving Antenatal Care (ANC) |
36% with documented ANC attendance 83% with reported IPTp exposure |
>90% attend ANC in refugee camp |
| Average Number (Range) of Antenatal Visits |
4 (1–12), where documented by ANC card |
Weekly ANC >50% in first trimester Average 15–20 visits |
| Preventive Treatment | SP-IPTp | None as high levels of MDR- Pf resistant strains. SP not used in Thailand > 30 years. |
| Women Reporting IPTp Usage |
83% | N/A |
| Average Number (Range) of IPTp doses |
1 (0–3), where documented by ANC card |
N/A |
| Drugs used to treat malaria during pregnancy |
ACT, quinine | Quinine, mefloquine, artesunate monotherapy, ACT |
| Efficacy of available drugs |
Limited: High level resistance to first line therapy (SP) 2002– 2008; Currently efficacious: ACT (artemether- lumefantrine), quinine Severe malaria=IV quinine |
Limited: Quinine and mefloquine monotherapy, 1995–2002 Currently efficacious: 1st trimester = QC7 2nd&3rd trimester =ACT Severe malaria=IV artesunate |
| Bednet usage | 62%, any net 19%, treated net |
~90% treated net |
| Screening for anaemia: Hb or HCT |
At most ANC visits | Every 2nd week |
| Nutritional supplements |
Ferrous and folic acid at most ANC visits |
At each visit: Prophylactic: ferrous 200 mg daily and folic acid 5mg/wk Treatment: ferrous 400mg BID and folic acid 5mg daily |
| Antihelminthic policy | All women: stool and urine testing |
Anaemic women stool testing |
| Prevalence HIV in pregnant women |
5–7%, from (35). | <0.5%, from (33) |
SP: Sulfadoxine pyrimethamine.
IPTp: intermittent preventive treatment in pregnancy.
ACT: Artemisinin-based combination therapy currently (2009) artesunate+clindamycin 7 days.
QC7: Quinine and Clindamycin for 7 days.
Malaria Diagnosis and Sample Selection
In the Tanzanian cohort, PM was detected by microscopy of Giemsa-stained thick and thin smears of blood extracted from placental tissue by mechanical grinding. Placental parasite density was quantified as percent IE. The primary analysis is among PM-positive women in order to identify features that are specifically linked to poor outcomes within this group. In the cohort from the Thai-Burma border, malaria episodes were detected by peripheral blood smear at weekly antenatal clinic visits and at delivery, with additional examination of mother peripheral blood and placental blood collected by incision at delivery. Samples were selected from among those with peripheral blood parasitemia at least 2 weeks prior to delivery. Only women with recent infection were included to avoid examining negative histopathology samples (6), and to facilitate comparison with the Tanzanian cohort.
Histology
Paraffin-embedded blocks were previously generated from tissue samples collected on the Thai-Burma border (6), from which Giemsa-stained slides were made (Fig 2A–B). In Tanzania, placental tissue was placed in polyvinyl alcohol, snap frozen in liquid nitrogen, and stored at −80°C. Sections were made on a cryostat, air-dried, methanol-fixed and Giemsa-stained for 12 minutes (Fig 2C–E). The proposed grading scheme is detailed below in the results section. Placental malaria episodes were also classified as acute or chronic (14), with chronic PM defined by the presence of pigment in fibrin (greater than 1 in 50 high power fields).
Figure 2.
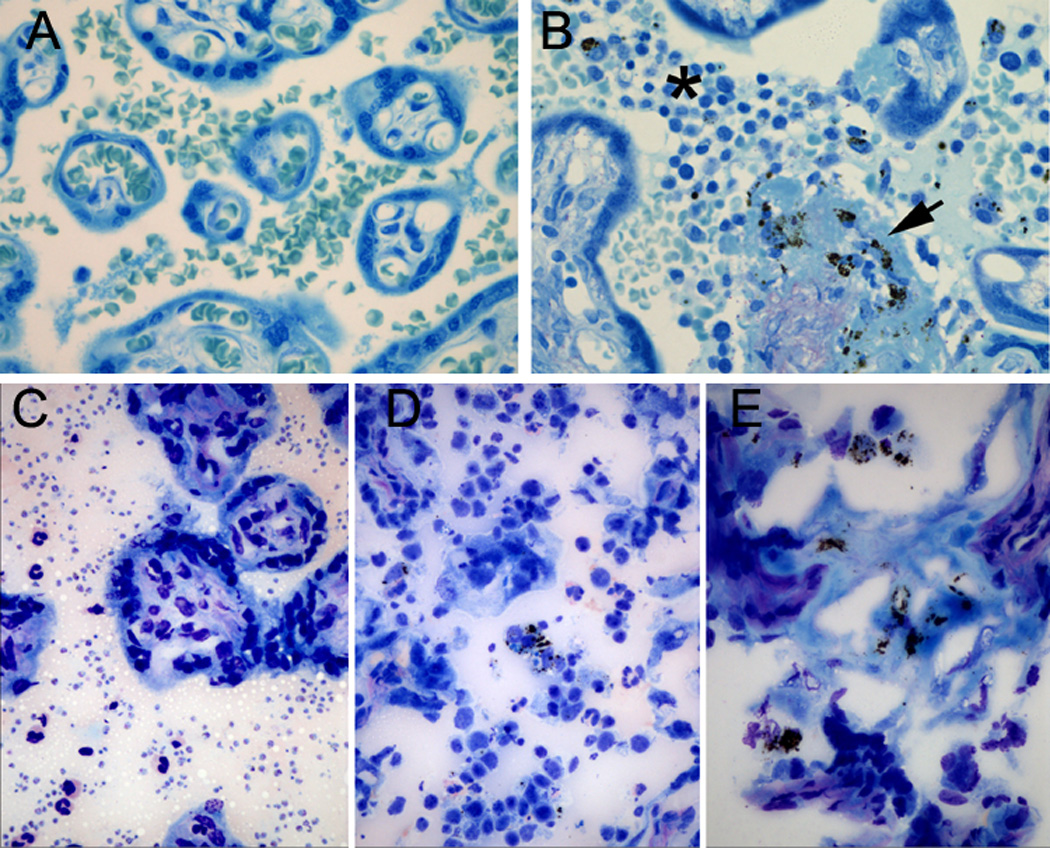
Formalin-fixed paraffin embedded placental tissue section from PM cases on the Thai-Burma border. A) Remote infection, with no histologic alteration. B) P. falciparum at delivery, with inflammatory infiltrate (asterisk) and pigment deposition in fibrin (arrow).. Fresh-frozen Giemsa-stained sections from PM cases in Tanzania. The features of placental malaria are readily identified: C) Parasites; D) Inflammation; and E) Pigment deposition in fibrin. 400× magnification.
Biomarker analysis
Quantitative PCR was performed as previously described (18). Briefly, total RNA was extracted from frozen placental cryosections using RNeasy mini kits (Qiagen) and realtime PCR was performed using SYBR Green Master Mix, an ABI Prism 7500 system (Applied Biosystems) and intron-spanning primers for CXCL13 and for KRT7 (a gene expressed by the trophoblast).
Statistical analysis
Analyses were performed using Statview and SAS (SAS Institute). Continuous and categorical variables were analyzed by Student’s t-test and Fisher's exact test. Bar graphs are presented as mean (standard error). Percent infected erythrocytes were log transformed prior to analysis. Logistic regression and analysis of variance (ANOVA) were used for multivariate analyses.
RESULTS
The Inflammation Score
The inflammation categories are qualitative and are intended to reflect distinct biologic entities (Fig 3A). “Minimal inflammation” (I) describes cases with no appreciable intervillous inflammation. Pigmented monocytes are rare, and the intervillous space white cell density is not increased above the level of peripheral white blood cells expected in transit. “Inflammation present” (II) describes cases with mononuclear cells sequestering in the intervillous space, particularly pigment-laden macrophages. This is a broad category describing the intermediate stage between no inflammation and massive intervillositis. Massive intervillositis (III) is a distinct entity where the intervillous space contains sheets of densely packed mononuclear cells (20–22). It was reported in 6.3% of PM cases in southern Tanzania (8), in 7 of 102 (6.9%) PM cases in MOMS Project in northeastern Tanzania, and in 1.7% (3/175) of women who had malaria during pregnancy on the Thai-Burma border (6). Massive intervillositis is a rare finding in the absence of malaria, where it is associated with recurrent miscarriage (20).
Figure 3.

Schematic demonstrating placental villi (v) for grading the histologic features of PM. A) Categories of maternal inflammation in the intervillous spaces (ivs): I- minimal; II-present; III- massive. B) Cut off values for categorizing malarial pigment deposition within intervillous fibrin (f). (% 60× high power fields).
The Pigment Deposition Score
The pigment deposition score is semiquantitative and can be rapidly assessed by quantifying the percentage of high power fields that are positive for pigment in fibrin within intervillous spaces (Fig 3B). The scoring method excludes pigment in erythrocytes or monocytes. Malaria pigment is golden brown after Giemsa stain, and can either be identified on paraffin or frozen sections. The use of a 60× objective is recommended, because pigment is present in fine granules, and at least 40 fields within the intervillous space are counted. The total field count excludes stromal tissue within the decidua, basal plate and stem villi. Although the total amount of fibrin may be variable, the total field count includes fields within intervillous spaces that do not have fibrin.
The categories are determined as follows: I (<10%), II (10–40%) and III (>40% of fields positive). The cut off values of 10% and 40% per high power field corresponded to the 50th and 90th percentiles respectively for both multiparous Tanzanian PM+ women and for the selected cohort from Thailand (all parities). Among first-time mothers with PM from Tanzania, these values corresponded to the 20th and 75th percentiles respectively.
A null (“0”) category could be considered for women if pigment is absent, particularly if the purpose of the study is to detect past infections during drug trials. In the MOMS Project, pigment was absent in 10% of PM-positive women, and these women had a trend towards increased birth weight and multiparity (data not shown). We elected to include these cases in category I for this study because the criteria for pigment exclusion would require a greater amount of tissue examined and would be problematic in formalin-fixed tissues with even a scant amount of formalin pigment deposition. In Tanzania, among PM-negative first-time mothers, histologic evidence of past infection was not associated with decreased birth weight (n=58 of 104; p=0.462).
The Tanzanian Cohort
Of 984 singleton deliveries in Muheza, Tanzania, 12% (124/984) had PM, as determined by placental blood smear. Tissue was available for frozen section histology on 82% (102/124). Based on the traditional histologic grading scheme, chronic PM was associated with a decrease in mean birth weight compared to acute PM that approached significance (0.21 kg, p-value 0.057). When the cohort was stratified by inflammation and pigment deposition scores using the new scheme (Fig 4), both scores were associated with birth weight. Pigment and inflammation scores were strongly related (p-value 0.005), but nevertheless were independently associated with birth weight by ANOVA (p=0.016 and 0.005, respectively; n=101). Inflammation and pigment scores of III were independently associated with low birth weight by logistic regression analysis (p=0.003 and 0.017, respectively; n=101).
Figure 4.
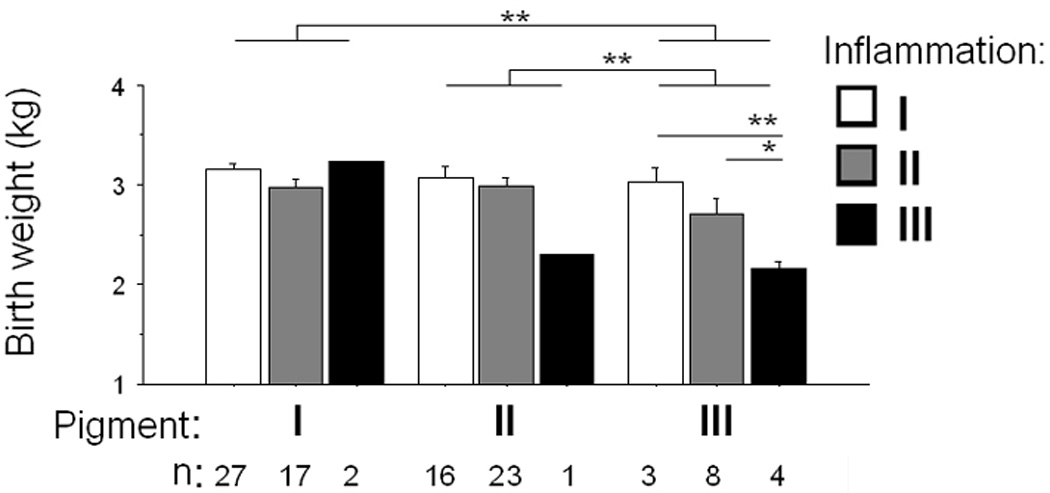
Inflammation and pigment scores in relation to birth weight in Tanzanian women of all parities. * p<0.05; ** p<0.01.
Primiparous women were analyzed as a subgroup because first-time mothers are at risk for poor outcomes (Fig 5). Based on the traditional grading scheme, chronic PM was not associated with a significant decrease in mean birth weight compared to acute PM (0.22 kg, p-value 0.46). Pigment and inflammation scores were related (p-value 0.042, n=47), and birth weight was inversely associated both with inflammation and with pigment scores, however by multivariate analysis neither pigment nor inflammation was independently associated with birth weight. Placental parasitemia was inversely associated with pigment deposition. Placental mRNA levels of CXCL13, a PM biomarker, were 200-fold higher in placental tissue with inflammation score III versus score I, and 16-fold higher with pigment scores of III versus I.
Figure 5.

Inflammation and pigment scores examined in Tanzanian first-time mothers. Relationship between A) birth weight and pigment score, B) birth weight and inflammation score, C) placental parasitemia and pigment score, and D) placental CXCL13 transcript levels (fold over cytokeratin 7 expression) and inflammation score. * p<0.05; ** p<0.01.
The Thai-Burma Cohort
Samples collected during a histopathologic study at the Thai-Burma border from 1995–1997 (6) were reviewed. Of the 175 placental samples from women with malaria episodes, 18 women had P. falciparum within the 2 weeks prior to delivery (Range 0–10 days, median 2 days) and were included for analysis. Inflammation and pigment scores I through III were observed. A single sample demonstrated both pigment and inflammation scores of III, and no inflammation was identified in 10 and no pigment was identified in 5 of 18 samples. Inflammation and pigment were not significantly related in this small sample size (p-value 0.28).
We conducted preliminary analyses of this small sample size to examine trends in relationships between scores and clinical outcomes. Pigment deposition together with inflammation by histology was associated with decreased birth weight (Fig 6). Estimated gestational age (EGA) did not significantly differ by inflammation or pigment category, although EGA did decrease with increasing pigment. The number of antenatal clinic visits (where women are screened and promptly treated for malaria) was inversely related with pigment, but this was not significant. CXCL13 was not examined in these samples because of formalin fixation.
Figure 6.

Inflammation and pigment scores in Karen women who had been diagnosed with P. falciparum malaria within 2 weeks of delivery. Relationships between: A) birth weight and pigment score; B) birth weight and inflammation score; C) estimated gestational age (EGA) and pigment score; and D) number of antenatal clinic (ANC) visits and pigment score. * p<0.05; ** p<0.01.
Comparison across study sites
A formal analysis across study sites is limited by differences in study design, malaria diagnosis, and histologic technique. Samples from the Thai-Burma border were compared to those from Tanzanian first-time mothers, the group that has the least immunity and is most susceptible to PM. Pigment deposition was significantly lower in the Thai-Burma cohort (p-value = 0.002), but the presence or degree of inflammation did not significantly differ (p-value = 0.267, n= 18 Karen versus 47 Tanzanian). The parasite densities in the placenta were not examined across sites due to different modalities of tissue processing and malaria diagnosis.
DISCUSSION
The clinical and epidemiologic features of PM vary widely with malaria transmission levels, as do control measures. We developed a 2-parameter grading scheme measuring inflammation and pigment deposition that is simple to use, and captures more information than earlier schemes. We find that the criteria for histologic classification are applicable in an African setting with high malaria transmission and an Asian setting with low transmission. Further, the scores are significantly related with pregnancy outcomes in a relatively large Tanzanian cohort, and have similar trends in a small study of Karen women in Thailand. Based on these preliminary findings, we propose that this scheme be further evaluated in clinical trials, where it may facilitate comparison and standardization of results between sites.
As a result of differences in exposure, immunity, and treatment, malaria episodes are complex. Chronic PM can result from several factors, including low host immunity and lack of effective antenatal control measures. Women can be inoculated multiple times during pregnancy, be treated or not treated adequately, and experience reinfections and recrudescences (23, 24) (Fig 1B). For example, in a recently published treatment study along the Thai-Burma border 253 women had a median number of 2 (range of 1 to 11) episodes of malaria (P.falciparum and P.vivax) detected and treated in pregnancy (25).
In this study, pigment deposition and inflammation were associated with decreased birth weight at two distant sites. In Tanzania, both pigment deposition and inflammation were independently associated with low birth weight in the overall cohort but not the primiparous subgroup. Inflammation by histology was strongly associated with CXCL13, a proposed biomarker of inflammatory PM (22), and has been linked to maternal peripheral blood levels of IL10 (27), suggesting utility for these or other markers to monitor placental inflammation prior to delivery.
On the Thai-Burma border, the relationship between pathologic features of malaria and outcome differs from areas of high stable transmission. In one study where all episodes of peripheral parasitemia were detected by active screening during the pregnancy and treated, antenatal P. falciparum or P. vivax infections were both associated with birth weight reduction (26), although the impact was greater with P. falciparum than with P. vivax. No placental pigment was observed in 33% (16/49) of women with documented P. falciparum infections during pregnancy (6). However, pathological changes were more likely to be observed when malaria was diagnosed in the month before delivery, and more pigment (in immune cells and fibrin) was observed when malaria was diagnosed in the week before delivery (6).
This is the first study to document conserved histopathologic features in pregnancy malaria between Africa and Asia. Although a formal analysis across study sites is precluded by differences in study design and histologic technique, samples from Tanzanian first-time mothers were compared to selected samples representing the small number of Karen women who experienced P. falciparum malaria within the last 2 weeks of pregnancy. This timeframe was chosen in order to maximize the likelihood of finding histologic changes, including the presence of pigment (6). The histologic degree of placental inflammation did not significantly differ between these two groups, however there was significantly decreased pigment deposition in the Karen women. This likely reflects the increased inoculation rate and limited treatment and antenatal care efficacy in Tanzania versus the effect of early detection and prompt treatment on the Thai-Burma border, since pigment deposition was inversely related to frequency of ANC visits among Karen women. These differences might also reflect the differing biologic timelines that regulate inflammation and pigment deposition during PM: inflammation changes more dynamically in response to parasitemia, whereas pigment persists over a longer period of time (Fig 1). Interpretation of the associations of inflammation and pigment with birth weight would have been improved if gestational age data were available for all the study sites, and would have allowed the association to be examined in growth-restricted term infants.
This study was not powered to analyze the result of HIV co-infection (n=4, Tanzanian cohort). Since HIV and malaria both affect birth weight and pregnancy outcome, HIV could alter the reported findings. Several studies have found an increased risk of placental malaria with HIV co-infection in endemic areas (28, 29), and that PM episodes are more likely to have greater parasitemia and increased pigment deposition by histology (28). Co-infected women exhibit altered humoral immune responses to placental IE (30) and immune cells isolated from the intervillous spaces of co-infected women exhibit decreased IL-12 production (31) and contain an expanded CD16+ monocyte subset (32). In areas of low transmission, such as the Thai-Burma border where HIV prevalence is low (33), the effect of HIV co-infection on PM episodes has not been determined.
Here, we demonstrated that the new grading scheme is applicable on both frozen and formalin-fixed paraffin-embedded (FFPE) sections. A direct comparison of frozen versus FFPE histology to detect the features of PM was not performed and would require both methods to be performed on the same sample population. FFPE tissue preserves fine details of cellular morphology and erythrocytes remain intact, whereas in frozen tissue cellular architecture is disrupted and erythrocytes lyse. The formation of formalin pigment or acid hematin during prolonged storage or during processing of FFPE tissue can obscure malarial hemozoin, often precluding analysis; formalin pigment formation is reduced with smaller tissue samples, generous amounts of buffered formalin, and prompt passage through graded alcohols following fixation. Frozen tissue is handled minimally, decreasing the concern of washing out intervillous contents. Frozen section histology is not routinely available in most rural tropical areas, however the infrastructural costs are significantly less than those for FFPE histology. Frozen sections have several advantages: no formalin pigment artifact, reduced laboratory infrastructure, and preserved tissue that yields high quality RNA (22, 23), antigens (22), protein and DNA for use in molecular analyses.
In the Tanzanian cohort, parasitemia was diagnosed by placental blood smear, and parasitemia per se is not included in the proposed grading scheme. Placental blood smears may not be available for all studies, with diagnosis and quantification of parasitemia relying on histologic sections or other ancillary tests. As described by Ewing (34), and in our experience (AM, MF), the identification of low level parasitemia (<1%) by histology is challenging due to routine dehydration and sectioning of erythrocytes and can be precluded by intraerythrocyte formalin pigment formation. Parasitemias above 5% may be readily detected and parasitemia quantified in histologic sections.
In this paper, PM refers strictly to the placental sequestration of P falciparum IE because it is not known whether all P. falciparum episodes during pregnancy have a placental phenotype, and there is no evidence at this time that P. vivax sequesters in the placenta. The mechanisms by which malaria episodes (both P. falciparum and vivax) in the first or second trimester lead to poor birth outcomes are not known, particularly in the absence of malaria-specific histologic features at term. Pathologic studies of miscarriages due to both species of malaria, or longitudinal studies that incorporate ultrasound measurements and biomarkers of infection would be of great interest.
In conclusion, we provide a semi-quantitative pathological grading scheme is simple to implement, specifically documents inflammation, and is associated with outcomes. Future clinical trials may benefit from this scheme for evaluation of histologic endpoints and for comparison across study sites.
ACKNOWLEDGEMENTS
Billie Davidson performed initial histology on the cohort of Karen women. Ronald S. Veazey coordinated the transfer of samples. Corinne Fligner contributed to the pathological analyses. Wonjong Moon contributed to demographic analyses of the Tanzanian cohort. This work was supported by a University of Washington House Staff Association grant and the Benjamin H Kean fellowship from the American Society of Tropical Medicine and Hygiene (to AM). The Shoklo Malaria Research Unit is part of the Wellcome-Mahidol University- Oxford Tropical Medicine Research Program funded by the Wellcome Trust of Great Britain. Part of this work was supported by PREMA-EU (Contract no ICA4-CT-2001-10012). The Mother Offspring Malaria Studies Project was supported by grants from the Bill and Melinda Gates Foundation (29202) and National Institutes of Health (R01AI52059) to PED.
Footnotes
CONFLICT OF INTEREST STATEMENT:
All authors declare no conflicts of interest.
STATEMENT OF PRIOR PRESENTATION:
This work was presented at the American Society of Tropical Medicine and Hygiene and at the United States and Canadian Academy of Pathology annual meetings in 2009 and 2010, respectively.
REFERENCES
- 1.Fried M, Duffy PE. Adherence of plasmodium falciparum to chondroitin sulfate a in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 2.Nosten F, et al. Malaria in pregnancy and the endemicity spectrum: What can we learn? Trends Parasitol. 2004;20:425–432. doi: 10.1016/j.pt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Roll Back Malaria, W. H. O. Malaria in pregnancy. [accessed dec. 2009]; Http://www.Rollbackmalaria.Org/cmc_upload/0/000/015/369/rbminfosheet_4.Htm.
- 4.Garnham PCC. The placenta in malaria with special reference to reticulo-endothelial immunity. Trans R Soc Trop Med Hyg. 1938:13–48. [Google Scholar]
- 5.McGready R, et al. Haemozoin as a marker of placental parasitization. Trans R Soc Trop Med Hyg. 2002;96:644–646. doi: 10.1016/s0035-9203(02)90339-1. [DOI] [PubMed] [Google Scholar]
- 6.McGready R, et al. The effects of plasmodium falciparum and p. Vivax infections on placental histopathology in an area of low malaria transmission. Am J Trop Med Hyg. 2004;70:398–407. [PubMed] [Google Scholar]
- 7.Leopardi O, et al. Malaric placentas. A quantitative study and clinico-pathological correlations. Pathol Res Pract. 1996;192:892–898. doi: 10.1016/S0344-0338(96)80068-9. discussion 899–900. [DOI] [PubMed] [Google Scholar]
- 8.Menendez C, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–1745. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 9.Rogerson SJ, et al. Placental monocyte infiltrates in response to plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–119. [PubMed] [Google Scholar]
- 10.Shulman CE, et al. Malaria in pregnancy: Adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Trop Med Int Health. 2001;6:770–778. doi: 10.1046/j.1365-3156.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 11.Watkinson M, Rushton DI. Plasmodial pigmentation of placenta and outcome of pregnancy in west african mothers. Br Med J (Clin Res Ed) 1983;287:251–254. doi: 10.1136/bmj.287.6387.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matteelli A, et al. Malarial infection and birthweight in urban zanzibar, tanzania. Ann Trop Med Parasitol. 1996;90:125–134. doi: 10.1080/00034983.1996.11813036. [DOI] [PubMed] [Google Scholar]
- 13.Muehlenbachs A, Mutabingwa TK, Fried M, Duffy PE. An unusual presentation of placental malaria: A single persisting nidus of sequestered parasites. Hum Pathol. 2007;38:520–523. doi: 10.1016/j.humpath.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Bulmer JN, et al. Placental malaria. I. Pathological classification. Histopathology. 1993;22:211–218. doi: 10.1111/j.1365-2559.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 15.Ismail MR, et al. Placental pathology in malaria: A histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31:85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 16.Davison BB, et al. Placental changes associated with fetal outcome in the plasmodium coatneyi/rhesus monkey model of malaria in pregnancy. Am J Trop Med Hyg. 2000;63:158–173. doi: 10.4269/ajtmh.2000.63.158. [DOI] [PubMed] [Google Scholar]
- 17.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Muehlenbachs A, et al. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J Immunol. 2007;179:557–565. doi: 10.4049/jimmunol.179.1.557. [DOI] [PubMed] [Google Scholar]
- 19.Muehlenbachs A, et al. Natural selection of flt1 alleles and their association with malaria resistance in utero. Proc Natl Acad Sci U S A. 2008;105:14488–14491. doi: 10.1073/pnas.0803657105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyd TK, Redline RW. Chronic histiocytic intervillositis: A placental lesion associated with recurrent reproductive loss. Hum Pathol. 2000;31:1389–1396. [PubMed] [Google Scholar]
- 21.Nebuloni M, et al. Malaria placental infection with massive chronic intervillositis in a gravida 4 woman. Hum Pathol. 2001;32:1022–1023. doi: 10.1053/hupa.2001.27603. [DOI] [PubMed] [Google Scholar]
- 22.Ordi J, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22:1006–1011. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Beck S, et al. Multiplicity of plasmodium falciparum infection in pregnancy. Am J Trop Med Hyg. 2001;65:631–636. doi: 10.4269/ajtmh.2001.65.631. [DOI] [PubMed] [Google Scholar]
- 24.Mutabingwa TK, et al. Randomized trial of artesunate+amodiaquine, sulfadoxine−pyrimethamine+amodiaquine, chlorproguanal-dapsone and sp for malaria in pregnancy in tanzania. PLoS One. 2009;4:e5138. doi: 10.1371/journal.pone.0005138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGready R, et al. A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated plasmodium falciparum treatment in pregnancy. PLoS Med. 2008;5:e253. doi: 10.1371/journal.pmed.0050253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nosten F, et al. Effects of plasmodium vivax malaria in pregnancy. Lancet. 1999;354:546–549. doi: 10.1016/s0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 27.Kabyemela ER, et al. Maternal peripheral blood level of il-10 as a marker for inflammatory placental malaria. Malar J. 2008;7:26. doi: 10.1186/1475-2875-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Eijk AM, et al. Hiv increases the risk of malaria in women of all gravidities in kisumu, kenya. Aids. 2003;17:595–603. doi: 10.1097/00002030-200303070-00015. [DOI] [PubMed] [Google Scholar]
- 29.Steketee RW, et al. Impairment of a pregnant woman's acquired ability to limit plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg. 1996;55:42–49. doi: 10.4269/ajtmh.1996.55.42. [DOI] [PubMed] [Google Scholar]
- 30.Mount AM, et al. Impairment of humoral immunity to plasmodium falciparum malaria in pregnancy by hiv infection. Lancet. 2004;363:1860–1867. doi: 10.1016/S0140-6736(04)16354-X. [DOI] [PubMed] [Google Scholar]
- 31.Chaisavaneeyakorn S, et al. Immunity to placental malaria. Iii. Impairment of interleukin(il)-12, not il-18, and interferon-inducible protein-10 responses in the placental intervillous blood of human immunodeficiency virus/malaria-coinfected women. J Infect Dis. 2002;185:127–131. doi: 10.1086/338013. [DOI] [PubMed] [Google Scholar]
- 32.Jaworowski A, et al. Cd16+ monocyte subset preferentially harbors hiv-1 and is expanded in pregnant malawian women with plasmodium falciparum malaria and hiv-1 infection. J Infect Dis. 2007;196:38–42. doi: 10.1086/518443. [DOI] [PubMed] [Google Scholar]
- 33.Plewes K, et al. Low seroprevalence of hiv and syphilis in pregnant women in refugee camps on the thai-burma border. Int J STD AIDS. 2008;19:833–837. doi: 10.1258/ijsa.2008.008034. [DOI] [PubMed] [Google Scholar]
- 34.Ewing J. Contribution to the pathological anatomy of malarial fever. J Exp Med. 1902;6:119–180. doi: 10.1084/jem.6.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somi GR, et al. Estimating and projecting hiv prevalence and aids deaths in tanzania using antenatal surveillance data. BMC Public Health. 2006;6:120. doi: 10.1186/1471-2458-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]


