Abstract
Conotruncal and ventricular septal congenital heart anomalies result from defects in formation and division of the embryonic outflow tract. Cardiac remodeling during outflow tract and ventricular septation converts the tubular embryonic heart into a parallel circulatory system with an independent left ventricular outlet and right ventricular inlet. Tbx3 encodes a T-box containing transcription factor expressed in the developing conduction system of the heart. Mutations in TBX3 cause Ulnar-Mammary syndrome. Here we show that mice lacking Tbx3 develop severe outflow tract defects, including connection of both the aorta and pulmonary trunk with the right ventricle, in addition to aortic arch artery anomalies and abnormal communication between the right atrium and left ventricle. Alignment defects are preceded by a delay in caudal displacement of the arterial pole during aortic arch artery formation. Embryonic anterior/posterior patterning and cardiac chamber development are unaffected in Tbx3 mutant embryos. However, the contribution of second heart field derived progenitor cells to the arterial pole of the heart is impaired. Tbx3 is expressed in pharyngeal epithelia and neural crest cells in the pharyngeal region suggesting an indirect role in second heart field deployment. Loss of Tbx3 affects multiple signaling pathways regulating second heart field proliferation and outflow tract morphogenesis, including fibroblast growth factor signaling, leading to a failure of normal heart tube extension and consequent atrioventricular and ventriculoarterial alignment defects.
Keywords: Tbx3, outflow tract, congenital heart defect, neural crest
Introduction
Morphogenesis of the arterial pole of the heart involves formation and subsequent division of the myocardial outflow tract (OFT) to generate the ascending aorta and pulmonary trunk connected to the left and right ventricles, respectively 1. This complex process requires interactions between myocardial, endocardial and neural crest-derived (NC) cells. Defects in OFT morphogenesis in man and mouse models result in congenital heart anomalies including abnormal ventriculoarterial alignment such as double outlet right ventricle and overriding aorta, associated with ventricular septal defects. The myocardial wall of the OFT is derived from progenitor cells of the second heart field (SHF), a population of pharyngeal mesodermal cells expressing the transcription factors Isl1 and Tbx12. Contribution of SHF derived myocardium to the heart tube is essential for normal elongation of the heart tube and subsequent ventriculoarterial alignment 3. Division of the OFT occurs concomitantly with ventricular septation and is driven by cardiac NC cells 4. Prior to their entry into the OFT, NC cells are required in the pharyngeal region for normal addition of SHF cells to the distal heart tube 3,5.
T-box containing transcription factors control multiple aspects of embryonic development including morphogenesis and patterning of the heart; haploinsufficiency or mutation in a number of Tbx genes in man and mouse result in congenital heart defects (reviewed in 6,7). Tbx2 and Tbx3 are closely related paralogs of the T-box family with many areas of overlapping gene expression during development 8. They act as transcriptional repressors and have at least some target genes in common, including regulators of proliferation and senescence 6. Tbx2 null embryos display cardiac abnormalities, including atrioventricular canal (AVC) anomalies and defects in OFT alignment 9. Tbx3 null embryos die over a range of several days during midgestation with severe but variable yolk sac abnormalities in addition to hindlimb defects and mammary gland aplasia 10. TBX3 mutations underlie Ulnar-Mammary syndrome in man 11. The cause of lethality in Tbx3 mutant embryos is uncertain, although cardiac abnormalities could be a contributing factor. Tbx3 is expressed in the AVC and the sinoatrial and central components of the cardiac conduction system12. Tbx2 represses the transcriptional program of ventricular and atrial myocardium in the AVC, and gain and loss of function experiments have demonstrated that Tbx3 is required for sinoatrial node identity 9,13–16. In addition, a recent report has described abnormalities in cardiac looping in mice carrying a novel Tbx3 mutation (Tbx3neo/neo)17.
Here we show that Tbx3 is required for arterial pole morphogenesis. In Tbx3−/− embryos elongation of the arterial pole of the heart is perturbed resulting in double outlet right ventricle, where both the aorta and pulmonary trunk are aligned with the right ventricle. Tbx3 is expressed in pharyngeal epithelia and NC cells in the pharyngeal region and loss of Tbx3 function is associated with elevated proliferation and defective deployment of SHF cells. Our data suggest that Tbx3 regulates multiple signaling pathways required for normal SHF development and OFT elongation.
Materials and Methods
Mice
The null alleles Tbx3tm1Pa and Tbx2tm1Pa (hereafter referred to as Tbx2− and Tbx3−) were maintained on a mixed genetic background 9,10. Genotyping details are provided in the online data supplement. Connexin40eGFP and Mlc1v-nlacZ-24 transgenic mice were genotyped as described 18,19. Mouse care and procedures were in accordance with institutional and US and French national guidelines.
Histology and immunochemistry
Details of procedures are provided in the online data supplement. Primary antibody concentrations were: Tbx3 (1/200, Santa Cruz Biotechnology), β-galactosidase (1/300, Cappel), MF20 (1/50, DSHB), Islet-1 (clone 40.2D6: 1/100, DSHB), rat anti-BrdU (1/100, Immunologics), AP-2α (clone 3B5; 1/50, DSHB), phospho-ERK (1/100, Cell Signaling).
In situ hybridization
Whole mount in situ hybridization was performed as previously described 19. For each experiment a minimum of 3 embryos of each genotype was scored. Details of riboprobes used are provided in the online data supplement.
Cell proliferation analysis
Cell proliferation was evaluated by bromodeoxyuridine (BrdU) incorporation. Pregnant females were injected intraperitoneally on E9 with 10μM of BrdU (Sigma) per 100g of body weight 1.5hrs prior to embryo harvest. Embryos were sectioned, followed by immunochemical detection of BrdU-incorporated cells.
Quantitative RT-PCR
Details of procedures and primer sequences are provided in the online data supplement.
Results
Outflow tract and atrioventricular alignment defects in Tbx3−/− embryos
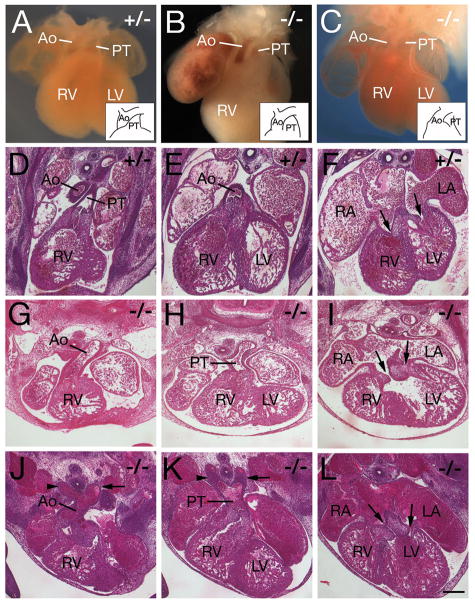
Tbx3−/− embryos surviving to E12.5 display a defect in leftward positioning and ventricular alignment of the ascending aorta (Figure 1, Table I). 12/12 embryos scored at E12.5 and E13.5 displayed double outlet right ventricle (DORV) such that the ascending aorta was aligned with the right rather than left ventricle. Ventricular septation, normally complete by E13.5, was incomplete in Tbx3−/− hearts at E13.5 (6/6). Two types of DORV were observed in Tbx3−/− embryos. In the first type, the ascending aorta is dextraposed such that the aorta and pulmonary trunk emerge from the right ventricle in a side-by-side configuration (5/12 embryos; Figure 1B). In the second type the aorta is positioned ventrally, a configuration resembling transposition of the great arteries (7/12 embryos; Figure 1C, G, J); histological analysis revealed that the pulmonary trunk is contiguous with cushion mesenchyme in the inner curvature of the OFT (Figure 1H, K). In addition to defects in alignment of the ascending aorta, aortic arch artery anomalies were observed in Tbx3−/− embryos, including persistence of the right 4th (9/12 embryos; Table 1 and Figure 1J) and right 6th (5/12; Table 1 and Figure 1K) arch arteries. Atrioventricular alignment defects were also observed in Tbx3−/− hearts; the right atrium frequently connected with the left rather than right ventricle, a configuration termed double inlet left ventricle (9/12 hearts; Figure 1I, L). Tbx3−/− hearts therefore display defective ventriculoarterial and atrioventricular alignment such that both great arteries emerge from the right ventricle and both atria connect with the left ventricle.
Figure 1.
Cardiac defects in Tbx3 mutant embryos. A-C, wholemount views; D-L, paraffin sections counterstained with haemotoxylin and eosin. (A) Tbx3+/− E12.5 heart showing the pulmonary trunk (PT) positioned ventrally and the ascending aorta (Ao) dorsally. (B) Tbx3−/− E13.5 heart showing the Ao and PT aligned side by side above the right ventricle. (C) Tbx3−/− E12.5 heart with a ventrally positioned Ao and dorsally positioned PT (transposition-type DORV). Insets in A-C schematize the position of the Ao and PT. (D-F) Tbx3+/− E12.5 heart showing the PT aligned with the right ventricle (D), the ascending Ao with the left ventricle (E) and left and right atria with left and right ventricles, respectively (arrows in F). (G-I) Tbx3−/− E12.5 heart showing transposition-type DORV with a ventrally positioned Ao aligned with the right ventricle (G), dorsally positioned PT (H) and a connection between both atria and the left ventricle (arrows in I). (J-L) Tbx3−/− E12.5 heart with a ventrally positioned Ao aligned with the right ventricle (J), dorsally positioned PT (K) and DILV (arrows in L). Note the abnormal persistence of the right 4th (arrowhead in J) and right 6th (arrowhead in K) aortic arch arteries, in addition to left arch arteries (arrows in J, K). RV, right ventricle; LV, left ventricle; RA, right atrium; LA, left atrium. Scale bar (D-L): 200μm.
Table 1.
Incidence of heart defects in Tbx3−/− embryos at E12.5 and E13.5
| Defect | Tbx3−/−a |
|---|---|
| Double outlet right ventricle | 5/12 |
| Double outlet right ventricle with transposition | 7/12 |
| Double inlet left ventricle | 9/12 |
| Persistent right 4th pharyngeal arch artery | 9/12 |
| Persistent right 6th pharyngeal arch artery | 5/12 |
Data from 6 E12.5 and 6 E13.5 Tbx3−/− embryos; no abnormalities were observed in Tbx3+/+ and Tbx3+/− littermates.
Tbx3 is required for complete cardiac looping
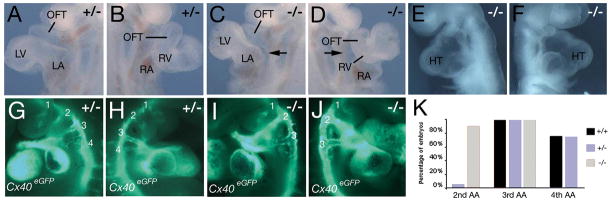
Cardiac looping is a prerequisite for correct alignment of the future cardiac chambers. Looping is a multistep process involving ventral then rightward looping of the elongating heart tube, followed by displacement of the inflow region of the heart dorsal to the ventricular segment, a process termed convergence 20. We observed a high incidence of Tbx3−/− embryos in which convergence was delayed or defective compared to somite matched Tbx3+/− and Tbx3+/+ littermates at E9.5 (Figure 2A-D, Table S1). In normal hearts, convergence positions the atria immediately posterior to the OFT (Figure 2A, B). In contrast, in Tbx3−/− embryos anterior displacement of the atria is delayed or defective, resulting in a gap between the OFT and atria (observed in 18/25 embryos; Figure 2C, D). This was accompanied in several cases by hypoplasia of the right ventricle and OFT of variable severity (Figure 2D, 5C). 4/25 Tbx3−/− embryos displayed a more severe phenotype in which a distended thin-walled heart tube looped ventrally and rightward looping and convergence were blocked (Figure 2E, F). In addition to a defect in looping we noted an overall developmental delay at this stage: the average somite number was 20.4±3.5 (n=32) for Tbx3+/+, 20.1±4.5 (n=48) for Tbx3+/−, and 16.6±4.4 (n=29) for Tbx3−/− embryos (p<0.001, Student’s t-test). Within litters, 27/29 (93%) of Tbx3−/− embryos were at or below the median somite number for the litter (n=14 litters).
Figure 2.
Looping defects and delayed aortic arch artery formation in Tbx3 mutant embryos. Left (A, C, E) and right (B, D, F) views of E9.5 Tbx3+/− (A, B) and Tbx3−/− (C-F) embryos. A convergence defect can be observed as a gap between the OFT and atria not present in normal embryos (arrows). Note the hypoplastic OFT and right ventricle (RV) in the Tbx3−/− embryo (D, compared to B). (E, F) Severely affected Tbx3−/− embryo showing an overall developmental delay. Note the thin walled dilated heart tube with a ventral loop. (G-K) Cx40eGFP expression shown in left (G, I) and right (H, J) views of Tbx3+/−; Cx40eGFP+/− (G, H) and Tbx3−/−; Cx40eGFP+/− (I, J) embryos at E10.5 (35 and 34 somites respectively). eGFP fluorescence in arterial endothelial cells shows that the aortic sac is connected to the 3rd and 4th arch arteries in the Tbx3+/− embryo (G, H). In contrast, the 2nd arch arteries are still connected to the aortic sac and the 4th arch artery is not yet apparent in the Tbx3−/− embryo (I, J). (K) Histogram showing the percentage of somite matched embryos in which the aortic sac is connected to the 2nd, 3rd and 4th aortic arch artery (AA) at E10.5. LA, left ventricle; LA left atrium; RA, right atrium.
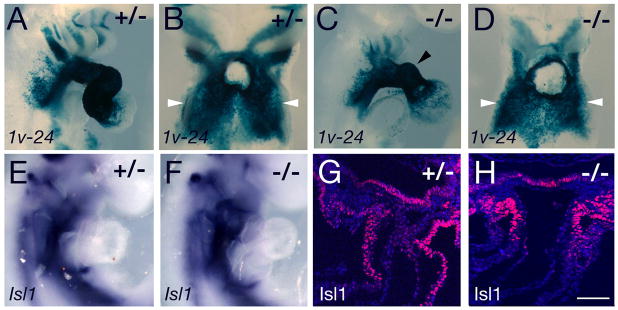
Figure 5.
Second heart field and outflow tract defects in Tbx3 mutant embryos. (A-D) X-gal stained E9.5 Mlc1v-nlacZ-24 transgenic embryos in right lateral (A, C) and ventral views with the heart removed (B, D). SHF cells in the dorsal pericardial wall are indicated by white arrowheads. Note the hypoplastic OFT and right ventricle in the embryo in panel C (arrowhead) and reduced transgene expression in the second arch. (E, F) Isl1 transcripts accumulate normally in the pharyngeal region of Tbx3−/− embryos. (G, H) Isl1 protein is observed in pharyngeal mesoderm and OFT myocardium of Tbx3+/− and Tbx3−/− hearts. Scale bar: 100μm.
Convergence occurs concomitantly with caudal displacement of the OFT in the pharyngeal region as bilateral arch arteries form sequentially in an anterior to posterior progression 20. The tubular heart is initially connected to the 1st arch arteries, subsequently to the 1st and 2nd and then to the 3rd, 4th and 6th arch arteries, during which process the connection with the 1st and 2nd arch arteries is lost. We scored arch artery connections in Tbx3−/− embryos at E10.5 using a Connexin40-eGFP allele expressed in arterial endothelial cells 18. A delay in caudal displacement of the OFT was observed such that whereas in Tbx3+/− and Tbx3+/+ embryos the OFT was connected to the 3rd and 4th arch arteries and the connection with the 2nd arch artery no longer existed (Figure 2G, H), the OFT of somite-matched mutant embryos maintained a connection with the 2nd arch artery (Figure 2I, J). This phenotype was observed in 9/10 mutant embryos (Figure 2K). Subsequently, posterior arch arteries are present in Tbx3−/− embryos at E11.5 and E12.5 (Figure 1; data not shown).
Molecular patterning of Tbx3−/− hearts
The above phenotypic analysis suggests that OFT defects in Tbx3−/− embryos arise as a result of incomplete looping and a delay in caudal displacement of the OFT. The anterior limit of two genes expressed at defined levels along the embryonic AP axis, Hoxb1 and Raldh2, was unaltered in Tbx3−/− embryos suggesting that overall anterior-posterior patterning of the pharyngeal region is not perturbed (Figure S1A-D). Nkx2-5 and Tbx5 are expressed normally in Tbx3−/− hearts at E9.5 (Figure S1E-L) suggesting that Tbx3 is not required for global cardiac patterning.
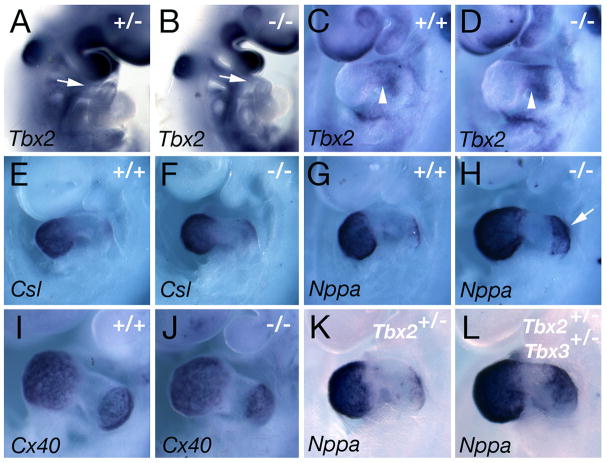
Tbx2 is closely related to Tbx3 and is required to repress the expression of the chamber-specific genes Csl, Cx40, and Nppa in AVC myocardium at E9.59,13,14. As Tbx3 is also expressed in AVC myocardium 8,12, patterning of the prospective chambers and AVC was analyzed in Tbx3−/− mutant embryos. Expression of Tbx2 in the OFT and AVC is unaffected in Tbx3−/− hearts (Figure 3A-D). Similarly, Tbx3 expression is normal in Tbx2−/− hearts at E9.5 suggesting that the AVC expression domains of Tbx2 and Tbx3 are not inter-dependent 9. Expression of Csl, Nppa and Cx40 is not expanded in the AVC of Tbx3−/− embryos (Figure 3E-J), revealing that AVC specification proceeds normally in the absence of Tbx3. Precocious expression of Nppa was observed in atrial myocardium of somite matched Tbx3−/− embryos at E9.5 (Fig 3G, H); however, no differences were observed at E10 (data not shown). As Tbx2 and Tbx3 interact genetically during mammary gland development 21, we explored a potential interaction of Tbx2 and Tbx3 during cardiac development. Nppa, Csl and Cx40 transcripts did not accumulate in AVC myocardium of Tbx2+/−; Tbx3+/− double heterozygous mutant embryos at E9.5 (Fig 3K, L; data not shown).
Figure 3.
Molecular patterning in Tbx3−/− embryos. Whole-mount views of E9.5 Tbx3+/−(A), Tbx3+/+ (C, E, G, I), Tbx3−/− (B, D, F, H, J), Tbx2+/− (K) and Tbx2+/−; Tbx3+/− (L) embryos after in situ hybridization. Tbx2 is expressed normally in the OFT (A, B, arrows) and AVC (C,D, arrowheads) of Tbx3−/− embryos. Csl (E, F), Nppa (G, H) and Cx40 (I, J) are expressed in working atrial and ventricular myocardium and do not accumulate in the AVC of Tbx3−/− hearts. Note that Nppa is precociously upregulated in atrial myocardium of Tbx3−/− hearts (arrow). (K, L) Tbx2+/− and Tbx2+/−; Tbx3+/− double heterozygous embryos do not accumulate Nppa transcripts in the AVC.
Tbx3 controls outflow tract elongation
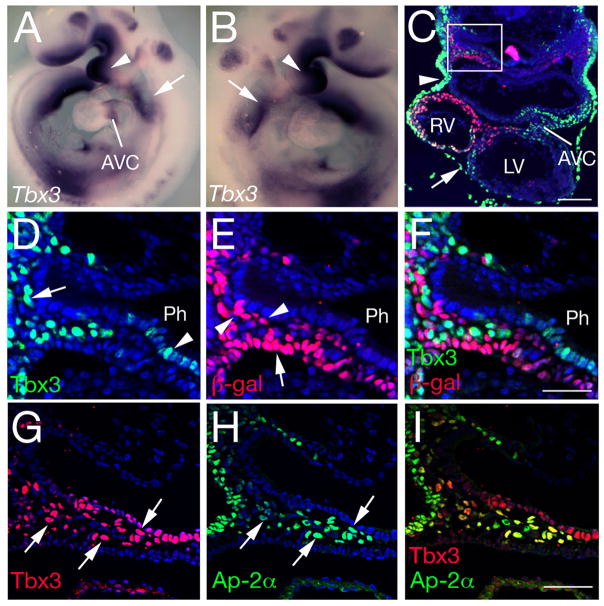
In addition to AVC myocardium, Tbx3 transcripts are observed in the pharyngeal region at E9.5 (Figure 4A, B) 8,12. Analysis of Tbx3 protein distribution revealed that the caudal pharyngeal region expression domain includes ectoderm, pericardium, ventral pharyngeal endoderm and mesenchymal cells (Figure 4C-F). Tbx3 distribution was compared to that of nuclear localized β-galactosidase in embryos carrying the Mlc1v-nlacZ-24 transgene, expressed in pharyngeal mesoderm and OFT myocardium as a result of integration upstream of the gene encoding Fibroblast growth factor 1019. Within the heart, Tbx3 positive nuclei are observed in AVC myocardium whereas β-galactosidase positive nuclei are found in myocardium of the right ventricle and OFT. A reciprocal distribution of Tbx3 and β-galactosidase positive nuclei was observed in pharyngeal mesenchyme (Figure 4D-F), such that most cells expressed one or other epitope but not both. These results suggest that Tbx3 is expressed in NC cells adjacent to the SHF. Immunohistochemistry with AP-2α revealed that Tbx3 and Ap-2α positive nuclei colocalize in mesenchymal cells in the caudal pharynx, confirming that these cells are NC-derived (Figure 4G-I). The contribution of NC cells to the OFT was investigated in Tbx3−/− mutant hearts. Crabp1 expressing and Ap-2α positive NC cells were observed in the pharyngeal region of Tbx3−/− embryos (Figure S2A-D). Subsequently, PlexinA2 expressing cells were observed in the distal OFT cushions of Tbx3−/− embryos in a pattern similar to that in control hearts, revealing that NC cells colonize the Tbx3−/− OFT (Figure S2E-H).
Figure 4.
Tbx3 expression in the pharyngeal region. (A, B) Tbx3 in situ hybridization at E9.5. Tbx3 is expressed in the first branchial arch (arrowhead), caudal pharynx (arrow) and atrioventricular canal (AVC). (C-F) Immunohistochemistry with anti-Tbx3 (green) and anti-β-galactosidase (red) antibodies in a transverse section through the caudal pharynx and heart of an E9.5 embryo carrying the Mlc1v-nlacZ-24 transgene. Tbx3 protein is observed in the AVC, ectoderm (arrowhead), pericardium (arrow), pharyngeal endoderm and NC cells (box in C); β-galactosidase is observed in right ventricular myocardium and the dorsal pericardial wall. (D-F) High magnification of the boxed region in C showing the reciprocal distribution of Tbx3 and β-galactosidase in caudal pharyngeal mesenchyme. Tbx3 is observed in pharyngeal endoderm (arrowhead in D) and NC derived mesenchyme (arrow). β-galactosidase is observed in pharyngeal mesoderm (arrowheads in E) including the SHF (arrow). (G-I) Immunohistochemistry with anti-Tbx3 (red) and anti-AP-2α (green) antibodies. Ap-2α colocalises with Tbx3 in pharyngeal ectoderm and NC derived mesenchyme (arrowheads). Ph, pharynx. Scale bars (C) 100μm; (D-I): 50μm.
The OFT defects observed in Tbx3−/− embryos suggest that Tbx3 might act indirectly on SHF deployment. Expression of the Mlc1v-nlacZ-24 transgene in SHF cells in the dorsal pericardial wall was observed in Tbx3+/− and Tbx3−/− embryos (Fig 5B, D), even in embryos with severe OFT and right ventricular hypoplasia (Fig 5C, D). Isl1 transcripts accumulate normally in the SHF of Tbx3−/− embryos (Fig 5E, F) and Isl1 protein was observed in pharyngeal mesoderm and the distal OFT of Tbx3−/− embryos; however, less Isl1 positive cells were observed in the OFT of hypoplastic hearts (Fig 5G, H). Quantitative analysis at E10.5 demonstrated a significant reduction in OFT length in Tbx3−/− compared to Tbx3+/+ (p<0.05) and Tbx3+/− (p<0.01) embryos; in contrast, the mutant OFT was significantly broader than that of control littermates (Figure S3). These data reveal a failure of normal OFT elongation and morphogenesis in the absence of Tbx3.
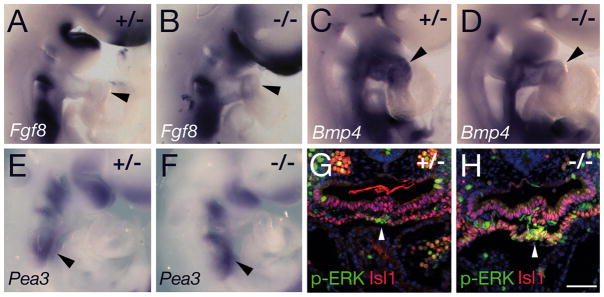
Signaling molecules required for OFT elongation were evaluated in Tbx3−/− embryos. Fgf8 transcripts are normally detectable in the SHF and OFT and were slightly elevated in the distal OFT of Tbx3−/− embryos (Figure 6A, B) 22,23. Bmp4 is expressed in the distal OFT and has been implicated in recruitment of SHF cells to the arterial pole of the heart 24. A slight reduction in Bmp4 transcript levels was observed in the OFT of Tbx3−/− embryos (Figure 6C, D). Quantitative RT-PCR revealed a significant increase in Fgf8 (1.5-fold) and Fgf10 (2-fold) transcript levels in the distal OFT and ventral pharynx of Tbx3−/− embryos; in contrast, Bmp4 transcript levels were reduced (0.6-fold; Figure S4). Downstream mediators of FGF signaling were evaluated. Pea3 transcript levels were slightly elevated in the caudal pharynx and an increase in phospho-ERK was observed in SHF cells in ventral pharyngeal mesoderm of Tbx3−/− embryos, consistent with elevated FGF signaling (Figure 6E-H). Shh in ventral pharyngeal endoderm regulates addition of SHF cells to the heart tube25; a reduction in Shh expression dorsal to the heart was observed in Tbx3−/− embryos (Figure S5A, B). Pitx2 is required in the SHF for normal OFT development 26. While Pitx2 transcripts are maintained in the first arch and in pharyngeal mesoderm, expression in the OFT was reduced in Tbx3−/− embryos (Fig S5C, D). Expression of Wnt11, which functions downstream of Pitx2 in OFT development 27, was slightly reduced in Tbx3−/− embryos (Figure S5E, F); TGFβ2, downstream of Wnt1127, was also reduced in the OFT and pharyngeal region of Tbx3−/− embryos (Figure S5G, H) 17. Loss of Tbx3 is thus associated with altered gene expression affecting multiple signaling pathways implicated in SHF and OFT development.
Figure 6.
Analysis of signaling pathways in Tbx3 mutant embryos at E9.5. (A, B) In situ hybridization showing elevated levels of Fgf8 transcript accumulation in the OFT of Tbx3−/− (B) relative to Tbx3+/− (A) embryos (arrowheads). (C, D) Bmp4 transcripts are reduced in the OFT of Tbx3−/− (D) relative to Tbx3+/− (C) hearts (arrowheads). (E, F) Pea3 transcripts are slightly upregulated in the caudal pharynx of Tbx3−/− embryos (F, arrowhead). (G, H) Fluorescent immunohistochemistry showing elevated levels of nuclear phospho-ERK (green) in Isl1-positive ventral pharyngeal mesoderm (red) of Tbx3−/− (H, arrowhead) compared to Tbx3+/− embryos (G). Scale bar (G, H): 100μm.
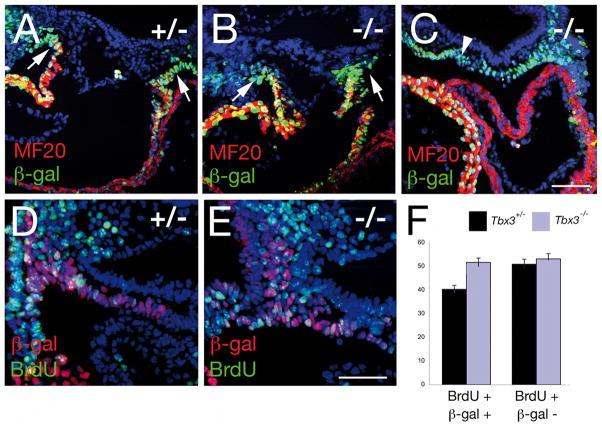
To further analyze SHF development in Tbx3−/− embryos we evaluated differentiation, cell death and proliferation within pharyngeal mesoderm at E9.5. We found no evidence of precocious or delayed myocardial differentiation. Normal accumulation of sarcomeric myosin heavy chain was observed in the distal OFT of Tbx3−/− embryos and not in adjacent splanchnic mesodermal cells (Fig 7A-C). No significant differences in cell survival between Tbx3+/− and Tbx3−/− embryos were observed in pharyngeal mesoderm using Caspase3 immunohistochemistry (data not shown). However, a significant increase in proliferation of pharyngeal mesoderm was observed in Tbx3−/− embryos (Fig 7D-F). Using a BrdU incorporation assay in Mlc1v-nlacZ-24 transgenic embryos, we observed a 20% increase in the percentage of BrdU positive lacZ-positive SHF cells in Tbx3−/− versus Tbx3+/− embryos versus (Fig 7F, based on counts from 3 Tbx3+/− and 3 Tbx3−/− embryos; p<0.001, Student’s t-test). In contrast, no difference was detected in BrdU incorporation in adjacent β-galactosidase negative nuclei (Fig 7F).
Figure 7.
Properties of the second heart field in Tbx3 mutant embryos. (A-C) Immunohistochemistry with MF20 anti-sarcomeric myosin and anti-β-galactosidase antibodies in paraffin sections of E9.5 Tbx3+/− (A) and Tbx3−/− (B, C) embryos carrying the Mlc1v-nlacZ-24 transgene. (A-C) No differentiated cardiomyocytes are observed in mesoderm adjacent to the OFT (arrows) or in the dorsal pericardial wall (arrowheads) of Tbx3+/− or Tbx3−/− embryos. Immunohistochemistry with anti-BrdU and anti-β-galactosidase antibodies in paraffin sections of E9.5 Tbx3+/− (D) and Tbx3−/− (E) embryos carrying the Mlc1v-nlacZ-24 transgene and treated with BrdU in utero. (F) Histogram showing the percentage of β-galactosidase positive and negative nuclei that are BrdU positive. There is a 20% increase in BrdU positive β-galactosidase positive nuclei in Tbx3−/− compared to Tbx3+/− embryos (p<0.001, Student’s t-test). Nuclei are labeled with Hoechst (blue). Scale bar (A-C): 100μm; (D, E): 50μm.
Discussion
The coordinated development of different cell types is critical for arterial pole morphogenesis. In particular, interactions between SHF and NC cells orchestrate OFT elongation and septation 5. Our results demonstrate that Tbx3 is required for normal arterial pole extension: in the absence of Tbx3 the heart tube fails to elongate correctly, the convergence step of looping is perturbed and resulting alignment anomalies include DORV, transposition of the great arteries and double inlet left ventricle, common congenital heart defects in man. Tbx3 is expressed in pharyngeal endoderm and NC cells closely associated with the SHF. Our data suggest that Tbx3 controls OFT development indirectly, through regulation of intercellular signaling pathways coordinating proliferation and deployment of the SHF.
Tbx3 and the related gene Tbx2 are coexpressed in AVC myocardium. Tbx2 plays a role in repressing a chamber myocardial phenotype 9,14. Here we show that Tbx3−/− and Tbx2+/−; Tbx3+/− embryos have normal AVC patterning, suggesting that Tbx2 plays the major role in this process. Zebrafish Tbx2 and Tbx3 homologues have recently been shown to redundantly control looping of the fish heart by regulating proliferation in the AVC 17. The same study proposed that looping defects in Tbx3Neo/Neo mutant mouse embryos are caused by a failure to reduce proliferation in future AVC myocardium. Here we show that OFT alignment defects in Tbx3−/− hearts result from incomplete looping, though our results suggest an alternative etiology of the looping defect. We propose that failure to elongate the heart tube underlies the convergence and caudal OFT displacement defects in Tbx3−/− embryos, reflecting a role for Tbx3 in the regulation of SHF deployment. Furthermore, we propose that this regulation is indirect and may be mediated by NC cells or pharyngeal endoderm. A subset of Tbx3−/− embryos exhibit general growth failure and have severely affected hearts where both rightward looping and convergence are blocked, suggesting the existence of an earlier, potentially distinct, role for Tbx3 in heart tube formation. Arch artery anomalies are observed in a subset of Tbx3−/− embryos at E12.5, revealing a later role for Tbx3 in asymmetric arch artery remodeling. The lack of survival of Tbx3−/− embryos to fetal stages precludes investigation of whether this phenotype is linked to the earlier developmental delay.
The addition of SHF cells to the arterial pole of the heart is coordinated by signals from adjacent cell-types, including pharyngeal endoderm and NC cells. The signals and upstream regulators mediating such effects, however, largely remain to be identified. Tbx3 is expressed in pharyngeal endoderm and NC cells in the pharyngeal region rather than in the SHF itself. Impaired NC function in the absence of Tbx3 could result in defective proliferation and deployment of the SHF. In the chick, NC in the pharyngeal region is required for normal SHF development in addition to OFT septation 3–5. NC ablation results in myocardial hypoplasia and alignment defects associated with a reduced contribution of SHF cells to the elongating heart tube 3,5. FGF signaling is elevated in the pharynx of NC ablated embryos, leading to hyperpoliferation and defective differentiation of the SHF 28. Reduction in FGF signaling has been shown to partially rescue the effects of NC ablation on the SHF 29. Precise levels of FGF signaling are critical for SHF deployment as pharmacological or genetic reduction of FGF signaling in the absence of NC ablation also leads to defective SHF development in chick and mouse 22,23,28–30.
Our results suggest that transcriptional targets of Tbx3 function in signaling pathways that regulate SHF deployment. In NC cells Tbx3 target genes may modulate FGF signaling in the pharyngeal region and thus regulate SHF exposure to FGF ligands, similar to the situation in NC ablated chick embryos. Elevated Pea3 transcript and phospho-ERK levels in the caudal pharyngeal region of Tbx3−/− embryos provide evidence for enhanced FGF signaling, and are likely to contribute to the increased proliferation observed in pharyngeal mesoderm. Perturbation of the balance between proliferation and differentiation may underlie the defects in SHF deployment and OFT morphogenesis resulting in a shorter, broader OFT. Loss of Tbx3 also affects other signaling pathways known to regulate OFT elongation: BMP signaling promotes SHF accretion at the arterial pole and Bmp4 expression in the distal OFT of Tbx3−/− embryos is decreased. Indeed, elevated Fgf8 and decreased Bmp4 expression suggest that differentiation of the SHF may be impaired in mutant embryos. The Pitx2/Wnt11/TGFβ2 axis, required for normal OFT development 27, is downregulated in the absence of Tbx3. Finally, altered Shh expression suggests that endodermal signaling is also impaired in mutant embryos. Loss of Tbx3 thus alters the balance of signaling molecules in the caudal pharynx, revealing a pleiotropic role for Tbx3 in the control of pharyngeal development and SHF deployment. Ongoing experiments aim to identify Tbx3 target genes and dissect the downstream signaling pathways required for SHF and OFT development. In addition, tissue-specific inactivation of Tbx3 will address the relative importance of Tbx3 expression in NC versus pharyngeal epithelia for heart tube elongation and the possible role of epigenetic effects secondary to haemodynamic changes. Although not part of the classically defined Ulnar Mammary syndrome phenotype, congenital heart defects have been reported in Ulnar Mammary patients 31; our results identify TBX3 as a candidate gene for human congenital heart defects affecting the arterial pole of the heart.
Supplementary Material
Acknowledgments
We thank Elana Ernstoff for technical assistance and Lucile Miquerol for Connexin40eGFP mice.
Sources of funding
This work was supported by the Inserm Avenir program, the Fondation de France, the Fondation pour la Recherche Medicale, the European Community’s Sixth Framework Programme contract (‘HeartRepair’) LSHM-CT-2005-018630 (RGK) and a grant from the National Institutes of Health RO1 HD033082 (VEP).
Footnotes
Disclosures
None
References
- 1.Sugishita Y, Watanabe M, Fisher SA. The development of the embryonic outflow tract provides novel insights into cardiac differentiation and remodeling. Trends in Cardiovascular Medicine. 2004;14:235–241. doi: 10.1016/j.tcm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nature Reviews. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 3.Yelbuz TM, Waldo KL, Zhang X, Zdanowicz M, Parker J, Creazzo TL, Johnson GA, Kirby ML. Myocardial volume and organization are changed by failure of addition of secondary heart field myocardium to the cardiac outflow tract. Developmental Dynamics. 2003;228:152–160. doi: 10.1002/dvdy.10364. [DOI] [PubMed] [Google Scholar]
- 4.Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Seminars in Cell & Developmental Biology. 2007;18:101–110. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldo KL, Hutson MR, Stadt HA, Zdanowicz M, Zdanowicz J, Kirby ML. Cardiac neural crest is necessary for normal addition of the myocardium to the arterial pole from the secondary heart field. Developmental Biology. 2005;281:66–77. doi: 10.1016/j.ydbio.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Naiche LA, Harrelson Z, Kelly RG, Papaioannou VE. T-box genes in vertebrate development. Annual Review of Genetics. 2005;39:219–239. doi: 10.1146/annurev.genet.39.073003.105925. [DOI] [PubMed] [Google Scholar]
- 7.Hoogaars WM, Barnett P, Moorman AF, Christoffels VM. T-box factors determine cardiac design. Cell Mol Life Sci. 2007;64:646–660. doi: 10.1007/s00018-007-6518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Developmental Dynamics. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Harrelson Z, Kelly RG, Goldin SN, Gibson-Brown JJ, Bollag RJ, Silver LM, Papaioannou VE. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development. 2004;131:5041–5052. doi: 10.1242/dev.01378. [DOI] [PubMed] [Google Scholar]
- 10.Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- 11.Bamshad M, Lin RC, Law DJ, Watkins WC, Krakowiak PA, Moore ME, Franceschini P, Lala R, Holmes LB, Gebuhr TC, Bruneau BG, Schinzel A, Seidman JG, Seidman CE, Jorde LB. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nature Genetics. 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- 12.Hoogaars WM, Tessari A, Moorman AF, de Boer PA, Hagoort J, Soufan AT, Campione M, Christoffels VM. The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart. Cardiovascular Research. 2004;62:489–499. doi: 10.1016/j.cardiores.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Habets PE, Moorman AF, Clout DE, van Roon MA, Lingbeek M, van Lohuizen M, Campione M, Christoffels VM. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes & Development. 2002;16:1234–1246. doi: 10.1101/gad.222902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christoffels VM, Hoogaars WM, Tessari A, Clout DE, Moorman AF, Campione M. T-box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers. Developmental Dynamics. 2004;229:763–770. doi: 10.1002/dvdy.10487. [DOI] [PubMed] [Google Scholar]
- 15.Mommersteeg MT, Hoogaars WM, Prall OW, de Gier-de Vries C, Wiese C, Clout DE, Papaioannou VE, Brown NA, Harvey RP, Moorman AF, Christoffels VM. Molecular pathway for the localized formation of the sinoatrial node. Circulation Research. 2007;100:354–362. doi: 10.1161/01.RES.0000258019.74591.b3. [DOI] [PubMed] [Google Scholar]
- 16.Hoogaars WM, Engel A, Brons JF, Verkerk AO, de Lange FJ, Wong LY, Bakker ML, Clout DE, Wakker V, Barnett P, Ravesloot JH, Moorman AF, Verheijck EE, Christoffels VM. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes & Development. 2007;21:1098–1112. doi: 10.1101/gad.416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro I, Kawakami Y, Buscher D, Raya A, Rodriguez-Leon J, Morita M, Rodriguez Esteban C, Izpisua Belmonte JC. Tbx2 and Tbx3 regulate the dynamics of cell proliferation during heart remodeling. PLoS ONE. 2007;2:e398. doi: 10.1371/journal.pone.0000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miquerol L, Meysen S, Mangoni M, Bois P, van Rijen HV, Abran P, Jongsma H, Nargeot J, Gros D. Architectural and functional asymmetry of the His-Purkinje system of the murine heart. Cardiovascular Research. 2004;63:77–86. doi: 10.1016/j.cardiores.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Developmental Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 20.Kirby ML, Waldo KL. Neural crest and cardiovascular patterning. Circulation Research. 1995;77:211–215. doi: 10.1161/01.res.77.2.211. [DOI] [PubMed] [Google Scholar]
- 21.Jerome-Majewska LA, Jenkins GP, Ernstoff E, Zindy F, Sherr CJ, Papaioannou VE. Tbx3, the ulnar-mammary syndrome gene, and Tbx2 interact in mammary gland development through a p19Arf/p53-independent pathway. Developmental Dynamics. 2005;234:922–933. doi: 10.1002/dvdy.20575. [DOI] [PubMed] [Google Scholar]
- 22.Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. Fgf8 is required for anterior heart field development. Development. 2006;133:2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- 23.Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 25.Goddeeris MM, Schwartz R, Klingensmith J, Meyers EN. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development. 2007;134:1593–1604. doi: 10.1242/dev.02824. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Liu W, Palie J, Lu MF, Brown NA, Martin JF. Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development. 2002;129:5081–5091. doi: 10.1242/dev.129.21.5081. [DOI] [PubMed] [Google Scholar]
- 27.Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nature Genetics. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrell MJ, Burch JL, Wallis K, Rowley L, Kumiski D, Stadt H, Godt RE, Creazzo TL, Kirby ML. FGF-8 in the ventral pharynx alters development of myocardial calcium transients after neural crest ablation. The Journal of Clinical Investigation. 2001;107:1509–1517. doi: 10.1172/JCI9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutson MR, Zhang P, Stadt HA, Sato AK, Li YX, Burch J, Creazzo TL, Kirby ML. Cardiac arterial pole alignment is sensitive to FGF8 signaling in the pharynx. Developmental Biology. 2006;295:486–497. doi: 10.1016/j.ydbio.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 30.Vitelli F, Taddei I, Morishima M, Meyers EN, Lindsay EA, Baldini A. A genetic link between Tbx1 and fibroblast growth factor signaling. Development. 2002 Oct;129:4605–4611. doi: 10.1242/dev.129.19.4605. [DOI] [PubMed] [Google Scholar]
- 31.Meneghini V, Odent S, Platonova N, Egeo A, Merlo GR. Novel TBX3 mutation data in families with Ulnar-Mammary syndrome indicate a genotype-phenotype relationship: mutations that do not disrupt the T-domain are associated with less severe limb defects. European Journal of Medical Genetics. 2006;49:151–158. doi: 10.1016/j.ejmg.2005.04.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.