Abstract
Lazaroids have been reported to attenuate preservation and reperfusion injury. In this study, we examined whether lazaroids can improve the outcome after 48-hr canine liver preservation and transplantation. Adult female beagle dogs were randomized into 4 dosage groups (5 animals each). Lazaroid U-74389G was intravenously administered at a dose of 0 mg/kg, 6 mg/kg, 10 mg/kg, or 15 mg/kg to donors 30 min before harvesting and also to recipients 30 min before revascularization. Control animals (0 mg/kg) were given the lazaroid vehicle. The liver grafts were orthotopically transplanted after 48 hr of hypothermic preservation in UW solution. Lazaroid treatment significantly improved outcome after transplantation. Five-day animal survival increased from 0% in the control to 60% in the 6 mg/kg group, 100% in the 10 mg/kg group, and 80% in the 15 mg/kg group. Lazaroid protected the hepatocytes from damage during preservation, and enhanced energy charge and hepatic blood flow after reperfusion. Histological alterations were significantly less severe in the lazaroid-treated groups. The area of necrotic hepatocytes decreased from 43.7±17.7 in the control to 13.5±3.0 in the lazaroid 10 mg/kg group. These results indicate that lazaroid U-74389G has potential for improvement of clinical liver preservation.
Lazaroids are a group of new synthetic 21-aminosteroids, which inhibit iron-dependent lipid peroxidation without glu-cocorticoid and mineral ocorticoid actions (1). In addition to potent antioxidant properties, lazaroids have been shown to suppress cytokine production (2), adhesion molecule expression (3), and neutrophile activation and infiltration (4). From these unique biological features, lazaroids were first reported to ameliorate ischemia and reperfusion injury of the central nervous system (5–7). Later the effects of lazaroids were confirmed in other tissues and organs in small and large animal experiments (8–15).
In this study, we examined whether lazaroids can improve outcome after 48-hr liver preservation and orthotopic transplantation in beagle dogs. Lazaroid U-74389G was selected from the lazaroid compounds and tested at three different doses. Animals receiving vehicle alone were used as the control.
MATERIALS AND METHODS
Animals
Adult female beagle dogs, weighing 9 kg to 13 kg, were used as liver donors and recipients. After overnight fasting, the animals were anesthetized with thiopental-sodium (25 mg/kg) for induction, and maintained with fluorane, nitrous oxide, and oxygen by positive pressure mechanical ventilation. Heart rate, arrythmia, arterial blood pressure, central venous pressure, and esophageal temperature were monitored during surgery. Blood gas and electrolytes were measured frequently and corrected if necessary.
Operative procedures
Hepatic homograft procurement and orthotopic liver transplantation were performed using our standard laboratory method (16, 17). In brief, the hepatic ligaments, the distal common bile duct, the portal vein, and the hepatic artery were dissected, and the liver was flushed with 1.5 L cold University of Wisconsin (UW) solution (ViaSpan, DuPont Merck Pharmaceutical Company, Wilmington, DE) via catheters inserted into the splenic vein (1 L) and the inferior abdominal aorta (0.5 L). The biliary tract was irrigated via cholecystostomy with 100 ml of normal saline. After removal, the graft was placed in a sterile plastic bag containing 0.5 L of UW solution, and kept at 4°C for 48 hr (until transplantation). The recipient liver was dissected and removed with the use of veno-venous bypass during the anhepatic phase. The graft was revascularized by end-to-end anastomoses of the suprahepatic vena cava, the infrahepatic vena cava, the portal trunk, and the abdominal aorta. Prior to completion of the infrahepatic vena cava anastomosis, the graft was perfused with 250 ml of cold lactated Ringer’s solution through the portal vein to remove air and UW solution in the graft. The first 50 ml of effluent from the flushing procedure was collected from the infrahepatic vena cava and stored at −70°C for later biochemical analysis. After obtaining hemostasis, the transplant was completed by cholecystoduodenostomy for biliary reconstruction.
Electrolyte solution (Plasmalyte, 1.5–2.0 L) and blood (limited to 1500 ml) collected from donor dogs were transfused to maintain recipient hemodynamics. Cephalosporin 1 g was given to animals intraoperatively and continued daily for 5 days. Animals were allowed to eat and drink from the following morning. Oral cyclosporine 20 mg/kg was administered daily for postoperative immunosuppression until the surviving animals were sacrificed at 14 days.
Experimental groups
Lazaroid U-74389G was supplied by The Upjohn Co. (Kalamazoo, MI) and dissolved in a citrate buffer vehicle (pH 3.0) at a concentration of 1.5 mg/ml. The dissolved lazaroid was administered intravenously to donors 30 min before harvesting and to recipients 30 min before graft revascularization. Animals were randomized into four groups: lazaroid U-74389G at 0 mg/kg (Laz [15]). The 6 mg/kg (Laz [6]), 10 mg/kg (Laz [10]), or 15 mg/kg (Laz [15]). The 6 mg/kg dose was the dose that The Upjohn Company recommended. Control animals (Laz [0]) were given the same amount of vehicle as the Laz 10 group.
Assessments
Liver enzyme levels, including glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), and lactate dehydrogenase (LDH), from the effluent of the final flushing solution and from postoperatively collected blood samples were measured using a Technicon RA500 autoanalyzer (Bayer, Tarrytown, NY). Wedge biopsy samples of liver tissues were collected before graft procurement, at the end of the 48-hr preservation period, and 1 hr after graft reperfusion. The biopsy specimens were halved: one section was immediately frozen in liquid nitrogen for biochemical analysis and the other was fixed in buffered formalin for histopathologic study.
Liver tissues taken for biochemical analysis were analyzed for protein concentration, adenine nucleotides, purine catabolites, malondialdehyde, and myeloperoxidase. Protein concentration of the homogenate was measured using the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA) method described by Bradford et al. (18). Adenine nucleotides (AN) and purine catabolites (PC) were measured using a Waters HPLC system (19) (Waters Chromatography Division/Millipore Corp., Milford, MA; Model 510 pumps, Model 484 absorbance module, and Model 717 WISP system). Concentrations of AN and PC were monitored at 254 nm (Waters 484, Tunable Absorbance Detector). Energy charge (EC) was calculated using the equation (ATP + [1/2] ADP/(ATP + ADP + AMP) (20). Malondialdehyde (MDA) concentration was estimated by thiobarbituric acid reaction by fluorospectrophotometry (21) (excitation wavelength 515 nm; emission wavelength 553 nm; Shimadzu fluorospectrophotometer, Model RF5000U, Shimadzu Corp., Kyoto, Japan). Myeloperoxidase (MPO) activity assay was measured using the fluorospectrophoto-metric method of Krawisz et al. (22). One unit of MPO activity was defined as the concentration that caused a 1.0 change in optical density at 460 nm for 1 min at 22°C.
Liver sections taken for histopathology were stained by hematoxylin and eosin and examined by a single pathologist without knowing the groups and the timing of tissue sampling. In addition, the area of necrotic hepatocytes was quantified by a morphometric method using an eyepiece with 135 intersections. Morphometry was performed on five randomly selected periportal fields per section at a magnification of 400×.
Measurement of hepatic blood flow was performed before liver harvesting and one hr after graft reperfusion using an ultrasonic Doppler flowmeter (Transonic T201D, Transonic Systems Inc., Ithaca, NY) and a laser Doppler flowmeter (Transonic ALF21). Flow values for both machines were expressed as ml/min/100 g liver tissue.
Statistics
Values are expressed as the mean ± SD. Analysis of variance between groups was performed using the Kruskal-Wallis H test. When the analysis of variance showed a significant difference (P<0.05), the Mann-Whitney U test was used to determine the P values between each group. Animal survival was determined using the Kaplan-Meier method, and the log-rank test was used to determine significance.
RESULTS
Clinical observations
Intravenous administration of U-74389G or vehicle alone caused no important hemodynamic changes in either donors or recipients. There was mild but transient acidosis after infusion in all animals. Cold ischemia time (49.2±1.2 hr), warm ischemia time (53±13 min), and graft weight loss during cold storage (15.3±6.8%), were not statistically significantly different in the experimental groups.
Survival
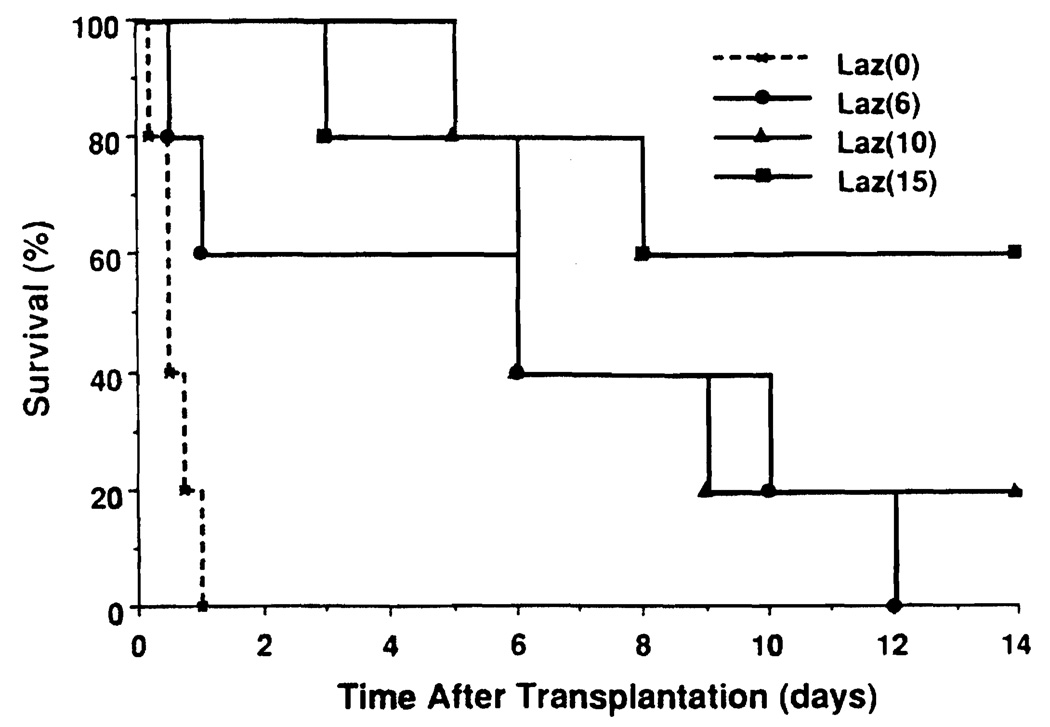
Lazaroid U-74389G significantly improved animal survival and reduced the incidence of graft failure during the early postoperative period (Fig. 1). While all animals in the Laz 0 group died of graft failure, only 2 animals in the Laz 6 group and no animals in the Laz 10 or Laz 15 group died of graft failure one day after transplantation. Animals dying of graft failure had pulmonary edema and various amounts of serosanguinous ascites at autopsy. One Laz 15 animal died of intussusception on postoperative day 3. Five-day animal survival was 0% in the Laz 0 group, 60% in the Laz 6 group, 100% in the Laz 10 group, and 80% in the Laz 15 group. However, after 5 days only 4 animals (1 in the Laz 10 and 3 in the Laz 15 group) survived for 14 days. The remaining 8 animals were lost to vascular thrombosis (n=3), cholangitis (n=2), intussusception (n=1), peritonitis (n=1), or bleeding duodenal ulcer (n=1). The difference in animal survival in the treated groups was not statistically significant.
FIGURE 1.
Animal survival after liver transplantation. The Laz 0 group was inferior to the Laz 6 group (P<6.03), the Laz 10 group (P<0.01), and the Laz 15 group (P<0.01). Treated groups were not statistically significantly different.
Liver enzyme release
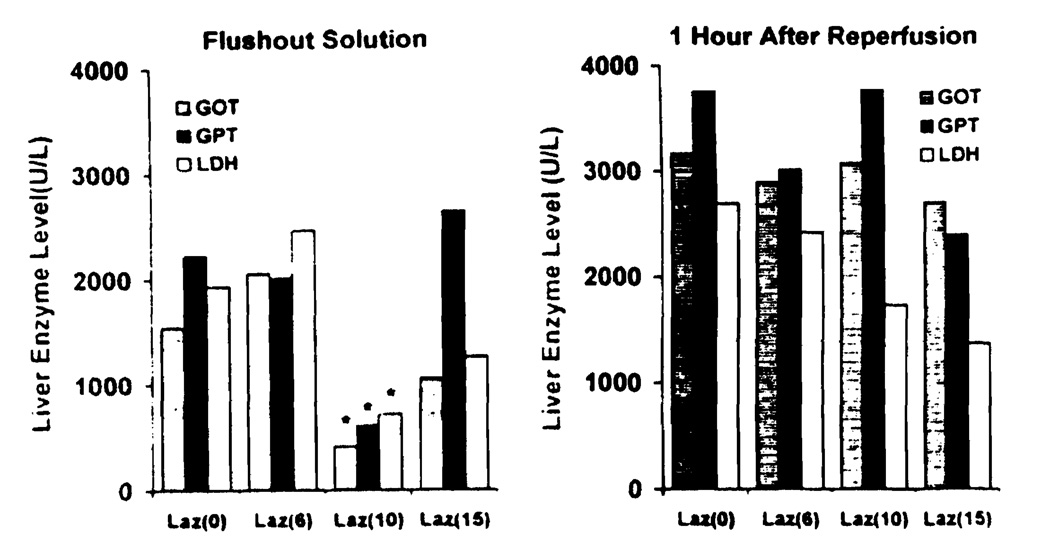
Liver enzymes (GOT, GPT, LDH) released into the effluent after final flushing were significantly lower in the Laz 10 group than the other three groups (Fig. 2). Similarly, LDH blood levels 1 hr after reperfusion were lower in animals receiving higher doses of U-74389G, but the difference was not significant. Liver enzymes of the surviving animals did not return to normal 14 days after transplantation.
FIGURE 2.
Liver enzymes in the final flush effluent and at 1 hr after reperfusion (*P<0.05 versus the other three groups).
Biochemistry
At higher doses (Laz 10 and Laz 15), lazaroid U-74389G retarded the degradation of adenine nucleotides to purine catabolites during preservation, and enhanced energy resynthesis after graft revascularization (Table 1). Cold storage induced a universal decline in adenine nucleotides and a corresponding increase in purine catabolites in all groups. The tissue concentration of total adenine nucleotides was significantly higher in the Laz 10 group than the Laz 0 group, and the tissue concentration of total purine catabolites was significantly lower in the Laz 15 group than the Laz 0 group. At 1 hr after reperfusion the energy charge of Laz 10 livers returned to normal levels, while the energy charge of the other 3 groups was significantly below normal. Although lazaroid U-74389G has been known to inhibit lipid peroxidation, MDA levels at the end of preservation and at 1 hr after reperfusion showed no significant difference among the groups. Levels increased equally during preservation and declined to below normal after reperfusion. Although MPO levels were undetectable after the neutrophils were washed from the liver by the preservation solution, they rose significantly, and they were higher than normal after reperfusion. Neutrophil accumulation in livers given vehicle alone was double that of the Laz 15 group animals.
TABLE 1.
Biochemistry of liver tissues at the end of preservation and at 1 hr after reperfusion
| Groups | ECa | TAN (nmol/g liver) |
ATP (nmol/g liver) |
ADP (nmol/g liver) |
AMP (nmol/g liver) |
TPC (nmol/g liver) |
HX (nmol/g liver) |
MDA (nmol/mg protein) |
MPO (U/min/mg protein) |
|---|---|---|---|---|---|---|---|---|---|
| Normal | 1.13±0.02 | 3798.7±206.3 | 2578.08±116.40 | 861.74±126.14 | 358.83±29.65 | 128.3±53.9 | 73.35±38.77 | 0.72±0.13 | 0.54±0.31 |
| At end of 48-hr preservation | |||||||||
| Laz(0) | 0.44±0.07b | 2919.7±225.3b | 182.52±54.75b | 539.50±64.46b | 2197.69±285.04b | 3042.3±223.2b | 1845.75±270.98b | 1.23±0.39b | 0.00±0.00b |
| Laz(6) | 0.42±0.11b | 3332.6±488.4b | 207.74±117.50b | 607.16±177.92b | 2517.72±405.37b | 2914.5±250.0b | 1871.04±243.78b | 1.07±0.27b | 0.00±0.01b |
| Laz(10) | 0.44±0.12b | 3392.4±343.6b,c | 229.42±124.58b | 626.06±107.45b | 2536.95±482.33b | 2919.3±180.4b | 1928.24±196.39b | 1.15±0.39b | 0.13±0.16b |
| Laz(15) | 0.38±0.11b | 3258.8±317.4b | 165.11±95.96b | 549.41±174.25b | 2544.31±263.21b | 2724.9±81.4b,c | 1854.67±265.12b | 1.18±0.23b | 0.00±0.00b |
| At 1 hr after reperfusion | |||||||||
| Laz(0) | 1.06±0.03b | 2179.5±683.4b | 1300.7±492.0b | 516.26±194.98b | 362.51±91.37 | 168.14±87.44 | 114.04±81.97 | 0.33±0.04b | 4.30±1.73b |
| Laz(6) | 10.8±0.02b | 2373.0±540.0b | 1469.4±332.5b | 546.54±153.25b | 357.09±73.76 | 102.01±29.22 | 55.77±13.60 | 0.35±0.04b | 3.41±1.78b |
| Laz(10) | 1.11±0.05 | 2501.5±599.3b | 1483.1±340.0b | 649.63±248.72 | 368.81±81.57 | 163.53±119.96 | 101.60±109.55 | 0.37±0.09b | 2.90±2.28b |
| Laz(15) | 1.04±0.09b | 2122.7±223.3b | 1118.9±368.6b | 545.12±98.75b | 458.64±164.31 | 412.67±697.64 | 307.80±573.24 | 0.49±0.28b | 2.85±2.37b |
EC: Energy charge; TAN: total adenine nucleotides; ATP: adenine triphosphate; ADP: adenine diphosphate; AMP: adenine monophosphate; TPC: total purine catabolites; HX: hypoxanthine; MDA: malondialdehyde; MDO: myeloperoxidase.
P<0.05 versus normal value.
P<0.05 versus Laz(0) group.
Hepatic blood flow
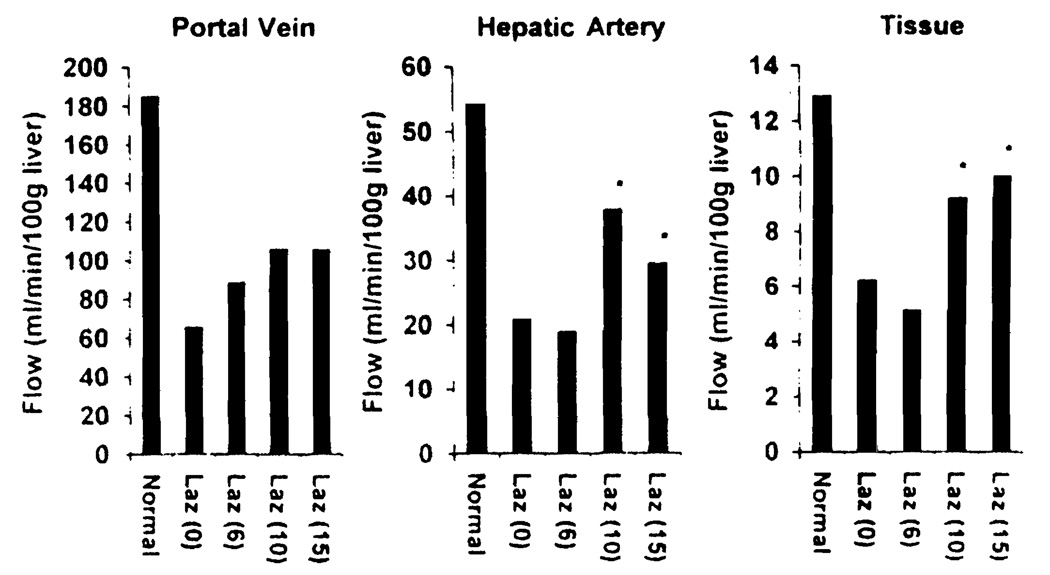
Upon portal unclamping, heterogeneous reperfusion and the occurrence of outflow block were always seen in livers treated by vehicle alone. Lazaroid U-74389G ameliorated these disturbances and improved hepatic blood flow, particularly at the higher doses (Fig. 3). Portal blood flow, hepatic arterial blood flow, and hepatic tissue blood flow of normal livers were measured at 186±80 ml/min/100 g liver, 53±24 ml/min/100 g liver, and 13±2 ml/min/100 g liver, respectively. At 1 hr after reperfusion, these three estimates in Laz 0 and Laz 6 livers decreased to one-third of the normal values, whereas both hepatic arterial blood flow and hepatic tissue flow in Laz 10 and Laz 15 groups were significantly higher than those of the other two groups.
FIGURE 3.
Hepatic blood flow at 1 hr after reperfusion (P<0.05 versus Laz 0 and Laz 6 groups).
Histopathology
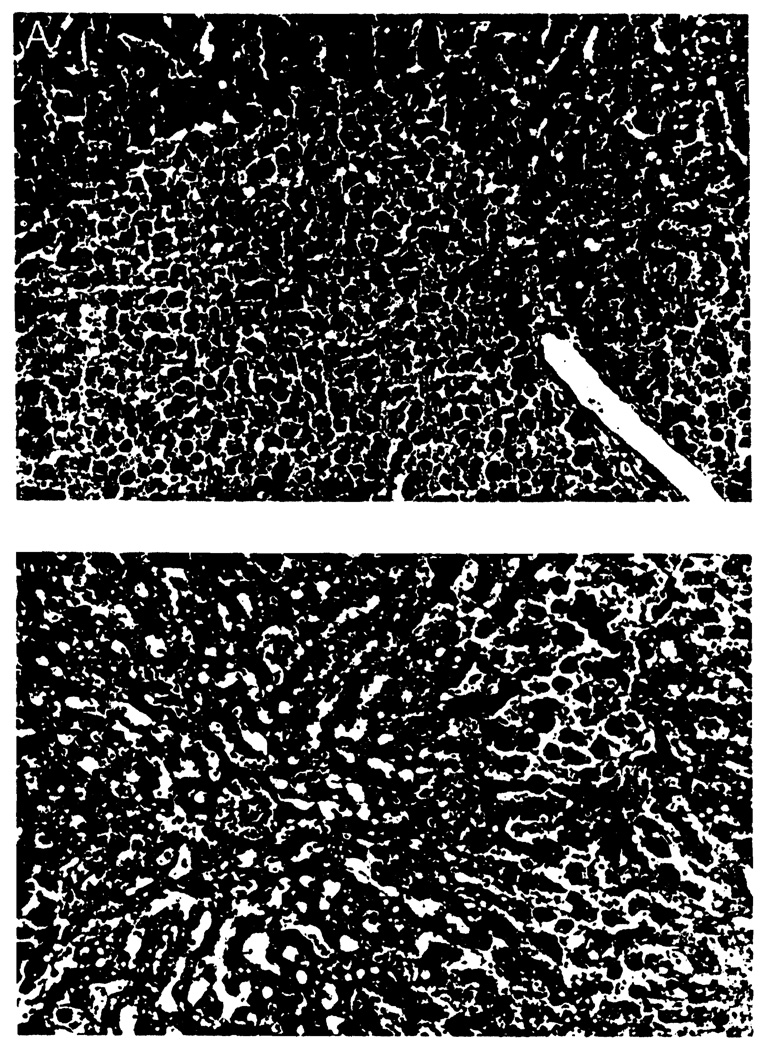
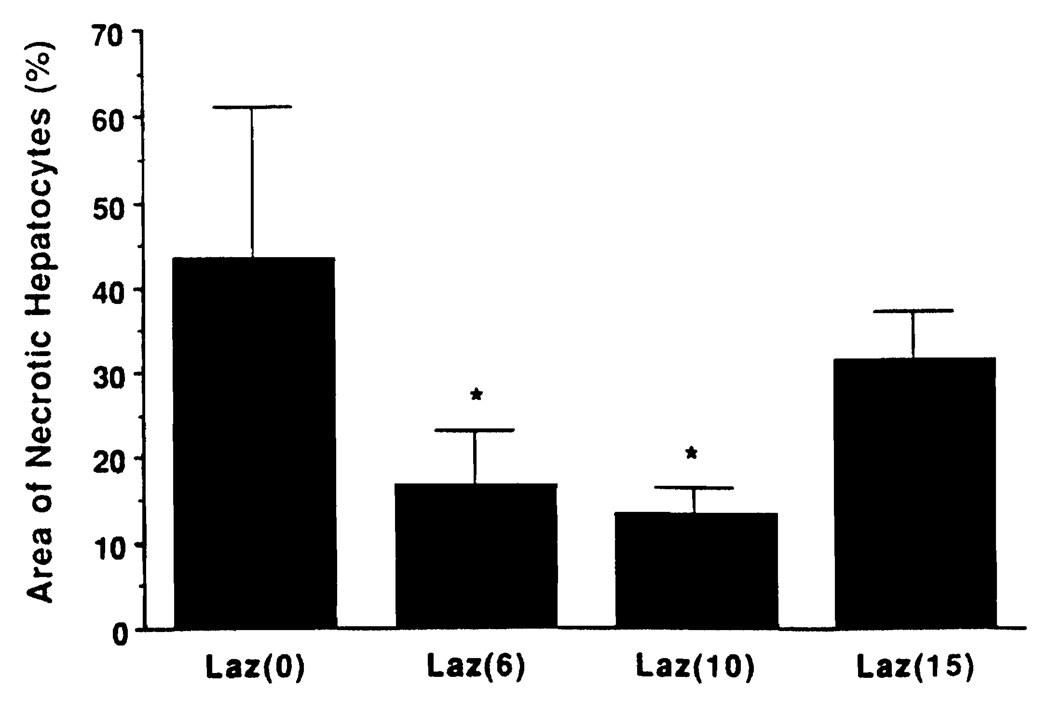
No morphological difference could be detected between the groups prior to revascularization. After reperfusion, livers from the animals receiving no treatment (Laz 0 group) showed severe structural abnormalities, such as diffuse hepatocyte necrosis, disarray of hepatocyte cords, disaggregation of hepatocytes, and hepatic congestion (Fig. 4A). In contrast, the treated livers, particularly from the Laz 10 group animals, had rather well-preserved hepatic architecture with small foci of the necrotic area and/or single-cell necrosis (Fig. 4B). The necrotic hepatocyte area was measured at 43.7±17.7% in the Laz 0 group, 16.8±6.3% in the Laz 6 group, 13.5±3.0% in the Laz 10 group, and 31.5±5.7% in the Laz 15 group (Fig. 5). The necrotic area of Laz 6 and Laz 10 was significantly less than the other two groups.
FIGURE 4.
(A) Control group (C4) liver 48 hr after preservation with UW solution; treatment with citrate buffer. The liver parenchyma showed significant necrotic damage that occupied more than 50% of hepatic tissue. There was loss of hepatic plate structure and disintegration of hepatocytes. (H&E; × 100.(B) Experimental group (No. 10R) liver 48 hr after preservation with UW solution and treatment with lazaroid 10 mg/kg of the donor and recipient. The liver parenchyma showed small foci of necrotic damage that occupied 10–15% of hepatic tissue. (H&E; ×100).
FIGURE 5.
Morphometry of necrotic hepatocytes. (*)P<0.05 versus Laz 0 and Laz 15 groups.
DISCUSSION
This study demonstrated that lazaroid U-74389G significantly improved the outcome of beagle dogs after 48-hr liver preservation and transplantation. Lazaroid U-74389G not only ameliorated hepatocyte damage during hypothermic storage, but also attenuated reperfusion injury after graft revascularization. Restoration of energy charge and hepatic blood flow were greatly enhanced by lazaroid treatment. Donor and recipient treatment with lazaroid U-74389G prevented graft failure and significantly improved animal survival. Of the three doses studied in this experiment, 10 mg/kg of lazaroid U-74389G was found to be the most effective.
The poor outcome of the Laz 0 group is consistent with previous studies of 48-hr liver preservation in our laboratory (23,24). While UW solution has proven to be superior to other preservation solutions (25, 26), we have not been able to effectively preserve the canine liver for 48 hr. In contrast to the flawless results after 24-hr liver preservation, grafts preserved for 48 hr consistently failed, showing extensive damage to the hepatocytes and microvasculature (23). However, these results are contrary to other reports (27, 28), which showed consistently better results with 48-hr canine liver preservation. The discordant results may be attributable to differences in the experimental model. Other investigators used larger mongrel dogs and laboratory-made fresh UW solution, and pretreated the liver donors with phentolamine or chloropromazine and hydrocortisone. In our laboratory, we attempt to mimic clinical practice by using commercially available UW solution and by not pretreating the donor. Of factors that differ in the two models, the use of laboratory-made fresh UW solution appears to be the most important. Reduced glutathione (GSH) in UW solution spontaneously converts to its oxidized form (GSSG) over time, which causes it to become less effective for prolonged liver preservation (29). This suggests that antioxidants are crucial for preservation of hepatic homografts.
Liver damage from ischemia and reperfusion has largely been explained by the generation of superoxide radicals (30, 31). Superoxide anions and hydrogen peroxides are produced by the xanthine oxydase enzyme system via the conversion of hypoxanthine to xanthine. Highly toxic hydroxyl radicals are generated in the presence of ferrous iron (Fe++) via the Harber-Weiss reaction or Fenton reduction. Other superoxide anion species are produced by the NADPH-dependent oxydase system. These superoxide radicals interact with lipids, proteins, nucleic acids, and cell membranes, and cause lipid peroxidation in hepatocytes, endothelial cells, Kupffer cells, and infiltrating inflammatory cells. Lipid peroxides, in turn, impair cellular and subcellular function, and often lead to cell death. Lipid peroxides also stimulate cytokine production, mediate arachidonic acid metabolism, induce expression of adhesion molecules, and activate leukocyte adherence and infiltration. Although cells are equipped with defensive endogenous antioxidants, supplemental exogenous antioxidants are needed to protect cells from increased free radical generation (32).
Lazaroids are a novel form of antioxidant that inhibits iron-dependent lipid peroxidation, and they were developed in studies of the effect of large doses of methylpredonisolone on central nervous ischemia. Compared with a large dose of methylpredonisolone, lazaroids were reported to be 10,000 times more potent in inhibiting lipid peroxidation (1). Although the mechanism of action is still unclear, attenuation of ischemia and reperfusion injury by lazaroid was found in the central nervous system of rats (5, 6) and dogs (7), and later in patients (33). The protective effect was subsequently confirmed in the heart (8, 9), lung (10), kidney (14, 15), and intestine (13) after ischemia (warm and cold) and reperfusion. Recent reports have shown that lazaroid compounds maintain endothelial cell viability in a dose-dependent manner (11,12). Pretreatment of the donor with lazaroid reduced liver enzyme release and phospholipase A2 release after 24-hr preservation in an ex vivo isolated pig liver perfusion model (12). They also showed that lazaroid pretreatment of rats receiving 24-hr-preserved livers allowed survival to increase from 30% without treatment to 90% with treatment. These results, except for changes in MDA, are consistent with the findings obtained in our study. Cosenza et al. found that postreperfusion MDA increased in only vehicle-treated livers, while our study showed that MDA increased in all experimental groups during preservation and decreased to below normal after reperfusion. Ferguson et al. (34) reported MDA changes in rat livers that were similar to our results. A more specific and sensitive analytical method is required to elucidate these differences, since the measurement of MDA to estimate lipid peroxidation is rather crude.
In conclusion, lazaroids are effective for prolonging the preservation period of hepatic homografts, with a potential for clinical use. However, before embarking on a clinical trial, several issues need to be considered. First, despite successful 48-hr canine liver preservation, the agent appears to be useful for improving graft viability within the current preservation time frame (35). It is apparent from the present study that the longer the preservation time, the greater the incidence of vascular and biliary complications. Second, it should be determined whether lazaroid treatment is needed for both donor and recipient, and when the drug should be administered. Third, it is especially important to determine the proper clinical dose, since lazaroids have a U-shaped dose-response curve (33) and have different pharmacokinetics between male and female (36). This last question is currently under investigation in multicenter trials with central nervous injuries.
Footnotes
Presented at the 21st Annual Meeting of the American Society of Transplant Surgeons, May 17–19, 1995, Chicago, IL
This work was supported by Research grants from the Veterans Administration and Project Grant DK-29961 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Braughler JM, Pregenzer JF, Chase RL, Duncan LA, Jacobsen EJ, McCall JM. Novel 21-amino steroids as potent inhibitors of iron-dependent lipid peroxidation. J Biol Chem. 1987;262:10, 438. [PubMed] [Google Scholar]
- 2.Shenkar R, Abraham E. Effects of treatment with the 21-aminosteroid, U7438F, on pulmonary cytokine expression following hemorrhage and resuscitation. Crit Care Med. 1995;23:132. doi: 10.1097/00003246-199501000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Meyer RJ, Juarez RA, Holden WE. 21-aminosteroids protect endothelial cells against injury by neutrophils. Am Rev Respir Dis. 1992;145:A571. [Google Scholar]
- 4.Gadaleta D, Verma M, Davis JM. Inhibition of neutrophil leukotriene generation by the 21-aminosteroid, U-74389F. J Surg Res. 1994;57:233. doi: 10.1006/jsre.1994.1137. [DOI] [PubMed] [Google Scholar]
- 5.Hall ED, Pazara KE, Braughler JM. 21-aminosteroid lipid peroxidation inhibitor U74006F protects against cerebral ischemia in gerbils. Stroke. 1988;19:997. doi: 10.1161/01.str.19.8.997. [DOI] [PubMed] [Google Scholar]
- 6.Park CK, Hall ED. Dose-response analysis of the effect of 21-aminosteroid tirilazad mesylate (U-74006F) upon neurological outcome and ischemic brain damage in permanent focal cerebral ischemia. Brain Res. 1994;645:157. doi: 10.1016/0006-8993(94)91649-7. [DOI] [PubMed] [Google Scholar]
- 7.Perkins WJ, Milde LN, Milde JH, Michenfelder JD. Pretreatment with U74006F improves neurologic outcome following complete cerebral ischemia in dogs. Stroke. 1991;22:902. doi: 10.1161/01.str.22.7.902. [DOI] [PubMed] [Google Scholar]
- 8.Holzgrefe HH, Buchanan LV, Gibson JK. Effects of U74006F, a novel inhibitor of lipid peroxidation, in stunned reperfused canine myocardium. J Cardiovasc Phmacol. 1990;15:239. doi: 10.1097/00005344-199002000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Hendry PJ, Anstadt MP, Plunkett MD, Amato MT, Menius JA, Jr, Lowe JE. Improved donor myocardial recovery with a new lazaroid lipid antiperoxidant in the isolated canine heart. J Heart Lung Transplant. 1992;11:636. [PubMed] [Google Scholar]
- 10.Aeba R, Killinger WA, Keenan RJ, et al. Lazaroid U74500A as an additive to University of Wisconsin solution for pulmonary grafts in the rat transplant model. J Thorac Cardiovasc Surg. 1992;104:1333. [PubMed] [Google Scholar]
- 11.Killinger WA, Jr, Dorofi DB, Keagy BA, Johnson G., Jr Improvement of endothelial cell viability at 4 degrees C by addition of lazaroid U74500A to preservation solutions. Transplantation. 1992;53:983. doi: 10.1097/00007890-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Cosenza CA, Cramer DV, Cunneen SA, Tuso PJ, Wang HK, Makowka L. Protective effect of the lazaroid U74006F in cold ischemia-reperfusion injury of the liver. Hepatology. 1994;19:418. [PubMed] [Google Scholar]
- 13.Katz SM, Sun S, Schechner RS, Tellis VA, Alt ER, Greenstein SM. Improved small intestinal preservation after lazaroid U74389G treatment and cold storage in University of Wisconsin solution. Transplantation. 1995;59:694. doi: 10.1097/00007890-199503150-00009. [DOI] [PubMed] [Google Scholar]
- 14.Stanley JJ, Goldblum JR, Frank TS, Zelenock GB, D'Alecy LG. Attenuation of renal reperfusion injury in rats by the 21-aminosteroid U74006F. J Vasc Surg. 1993;17:685. [PubMed] [Google Scholar]
- 15.Shackleton CR, Ettinger SL, Scudamore CH, Toleikia PF, Keown PA. Effect of a 21-minosteroid, U74006F, on lipid peroxidation and glomerulotubular function following experimental renal ischemia. J Surg Res. 1994;57:433. doi: 10.1006/jsre.1994.1166. [DOI] [PubMed] [Google Scholar]
- 16.Todo S, Kam I, Lynch S, Starzl TE. Animal research in liver transplantation—with special reference to the dog. Semin Liver Dis. 1985;5:309. doi: 10.1055/s-2008-1040626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todo S, Podesta L, Ueda Y, et al. A comparison of UW with other solutions for liver preservation in dogs. Clin Transplant. 1989;3:253. [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Wynants J, Van Belle H. Single-run high performance liquid chromatography of nucleotides, nucleosides, and major purine bases and its application to different tissue extracts. Anal Biochem. 1985;144:258. doi: 10.1016/0003-2697(85)90114-9. [DOI] [PubMed] [Google Scholar]
- 20.Hamamoto I, Takaya S, Todo S. Can adenine nucleotides predict primary nonfunction of the human liver homograft? Transplant International. 1994;7:89. doi: 10.1007/bf00336468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Krawisz JE, Sharon P, Stenson WR. Quantitative assay for acute intestinal inflammation based on myeloperoxidse activity. Gastroenterology. 1984;87:1334. [PubMed] [Google Scholar]
- 23.Furukawa H, Wu YM, Zhu Y, Suzuki T, Todo S, Starzl TE. Disturbance of microcirculation associated with prolonged preservation of dog livers under UW solution. Transplant Proc. 1993;25:1591. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, Zeng Q, Suzuki T, et al. Successful 48-hour preservation of the canine liver by modified simple hypothermic storage with UW solution. Transplant Proc. 1995;27:732. [PubMed] [Google Scholar]
- 25.Belzer FO, Southard JH. Principles of solid organ preservation by cold storage. Transplantation. 1988;45:673. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711. [PMC free article] [PubMed] [Google Scholar]
- 27.Jamieson NV, Sundberg R, Lindell S, et al. Preservation of the canine liver for 24—48 hours using simple cold storage with UW solution. Transplantation. 1988;46:517. doi: 10.1097/00007890-198810000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Sumimoto R, Lindell SL, Southard JH, Belzer FO. A comparison of histidine-lactobionate and UW solution in 48-hour dog liver preservation. Transplantation. 1992;54:610. doi: 10.1097/00007890-199210000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Boudjema K, Van Gulik TM, Lindell SL, Vreugdenhil PS, Southard JH, Belzer FO. Effect of oxidized and reduced glutathione in liver preservation. Transplantation. 1990;50:948. doi: 10.1097/00007890-199012000-00009. [DOI] [PubMed] [Google Scholar]
- 30.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 31.Clavien PA, Harvey PRC, Strasberg SM. Preservation and reperfusion injuries in liver allografts. Transplantation. 1992;53:957. doi: 10.1097/00007890-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Goode HF, Webster NR, Howdle PD, et al. Reperfusion injury, antioxidants and hemodynamics during orthotopic liver transplantation. Hepatology. 1994;19:354. [PubMed] [Google Scholar]
- 33.Haley EC, Jr, Kassell NF, Alves WM, et al. Phase II trial of tirilazad in aneurysmal subarachnoid hemorrhage: a report of the cooperative aneurysm study. J Neurosurg. 1995;82:786. doi: 10.3171/jns.1995.82.5.0786. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson DM, Gores GJ, Ludwig J, Krom RAF. UW solution protects against reperfusion injury by inhibiting lipid peroxidation. Transplant Proc. 1991;23:1552. [PubMed] [Google Scholar]
- 35.Furukawa H, Todo S, Imventarza O, et al. Effect of cold ischemia time on the early outcome of human heptic allografts preserved with UW solution. Transplantation. 1991;51:1000. doi: 10.1097/00007890-199105000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hulst LK, Fleishaker JC, Peters GR, Harry JD, Wright M, Ward P. Effect of age and gender on tirilazad pharmacokinetics in humans. Clin Pharmacol Ther. 1994;55:378. doi: 10.1038/clpt.1994.45. [DOI] [PubMed] [Google Scholar]