Abstract
Glutamine plays a key role in intestinal growth and maintenance of gut function, and as we have shown protects the postischemic gut (Kozar RA, Scultz SG, Bick RJ, Poindexter BJ, Desoigne R, Weisbrodt NW, Haber MM, Moore FA. Shock 21: 433–437, 2004). However, the precise mechanisms of the gut protective effects of glutamine have not been well elucidated. In the present study, RNA microarray was performed to obtain differentially expressed genes in intestinal epithelial IEC-6 cells following either 2 mM or 10 mM glutamine. The result demonstrated that specificity protein 3 (Sp3) mRNA expression was downregulated 3.1-fold. PCR and Western blot confirmed that Sp3 expression was decreased by glutamine in a time- and dose-dependent fashion. To investigate the role of Sp3, Sp3 gene siRNA silencing was performed and apoptosis was assessed. Silencing of Sp3 demonstrated a significant increase in Bcl-2 and decrease in Bax protein expression, as well as a decrease in caspase-3, -8, and -9 protein expression and activity. The protein expression of apoptosis-related proteins after hypoxia/reoxygenation was similar to that of normoxia and correlated with a decrease in DNA fragmentation. Importantly, the addition of glutamine to Sp3-silenced cells did not further lessen apoptosis, suggesting that Sp3 plays a major role in the inhibitory effect of glutamine on apoptosis. This novel finding may explain in part the gut-protective effects of glutamine.
Keywords: Bcl-2, Bax, caspase, DNA fragmentation, hypoxia/reoxygenation, specificity protein 3
glutamine is involved in many processes vital to cell function and integrity. It is the most abundant free amino acid in the body and is involved in metabolic and biochemical processes in a number of organs and cell types, including the gut (29), where it plays an essential role in promoting and maintaining epithelial cell function (65). Glutamine has protective effects on intestinal mucosa by decreasing bacteremia and epithelial cell apoptosis (15, 31), enhancing gut barrier function (8, 45, 46, 47), and influencing gut immune response (9, 18). We and others have shown that enteral glutamine possesses protective effects to the postischemic gut (25, 26, 51). With the use of a rodent model of gut ischemia/reperfusion, glutamine preserved mucosal integrity, absorptive capacity, ATP, and the actin cytoskeleton of intestinal villi, which is key to maintaining gut barrier function (25, 26). Glutamine was also reported to exert a protective effect on the gut in rodent models of lipopolysaccharide and endotoxin (33, 59).
Specificity proteins (Sp) are a family of highly conserved zinc-finger transcription factors that bind to GC-rich consensus elements (43). Nine Sp proteins have been discovered, among which Sp1-Sp4 are the best-studied members (63). Among these Sp proteins, Sp1 and Sp3 are structurally similar and are known to interact with other proteins that help with their recruitment or stabilization of their DNA binding to activate or repress the expression of target genes (28, 44, 54, 56, 62). Sp3, specifically, has one long isoform and two short isoforms, which are products of differential translational initiation (32). Sp3 overexpression induces apoptosis in colon cancer cells (14, 58). It has also been identified as a marker of tumor aggressiveness and inducer of apoptosis via the caspase pathway in a number of tumor cell lines (14).
The aim of the present study was to further understand how glutamine protects intestinal epithelial cells. We performed microarray analysis of the small intestinal epithelial cell line, IEC-6 cells, and identified for the first time that Sp3 was modulated by glutamine. Our data suggest that glutamine decreased apoptosis under normoxia and oxidant-stressed conditions, a process that is mediated by inhibition of Sp3.
MATERIALS AND METHODS
Cell line, chemicals, and reagents.
The rat small intestinal cell line (CRL-1592), IEC-6, was purchased from the American Type Culture Collection and was maintained in DMEM supplemented with 10% (vol/vol) heat-inactivated FBS and 2 mM glutamine. Cell viability before experimentation was 100%.
Antibodies against caspase-3 (catalog no. 9665) and -9 (catalog no. 9508), phospho-Bad (Ser112) (catalog no. 5284), Bad (catalog no. 5292), Bax (catalog no. 2772), Bim (catalog no. 2819), Bok (catalog no. 4521), and β-actin (catalog no. 4967) were from Cell Signaling Technology (Danvers, MA). Antibody against caspase-8 (catalog no. ab15552) was obtained from Abcam (Cambridge, MA). Antibodies against Bcl-2 (catalog no. sc-783), Bid (catalog no. sc-11423), natural born killer (Bik) (catalog no. sc-30552), Sp1 (catalog no. sc-14027), and Sp3 (catalog no. sc-664) were from Santa Cruz Biotechnology (Santa Cruz, CA). Caspase-3/CPP32 Fluorometric Assay Kit (catalog no. K105-100), Caspase-8/FLICE Fluorometric Assay Kit (catalog no. K112-100), Caspase-9 Fluorometric Assay Kit (catalog no. K118-100), and Nuclear/Cytosol Fractionation Kit (catalog no. K266-100) were bought from BioVision Research Products (Mountain View, CA). Cycloheximide (CHX) (catalog no. 01810-1G) was ordered from Sigma-Aldrich (St. Louis, MO). Recombinant rat TNF (catalog no. 555109) was from BD Biosciences (Franklin Lakes, NJ). Cell Death Detection ELISAPLUS (catalog no. 11774425001) was from Roche Diagnostics (Indianapolis, IN). Silencer siRNA Starter Kit (catalog no. AM1640) was from Ambion (Austin, TX). Enhanced chemiluminescence (ECL) anti-rabbit IgG, horseradish peroxidase-linked whole antibody (from donkey) (catalog no. NA9340V), ECL anti-mouse IgG, horseradish peroxidase-linked whole antibody (from sheep) (catalog no. NA931V), and ECL plus Western blotting detection system (catalog no. RPN2132) were ordered from GE Healthcare (Piscataway, NJ).
Transcript profiling with Sentrix Beadchip Array.
The effects of various concentrations of glutamine have been studied in vitro and in vivo. Glutamine concentrations in vitro have been as high as 20 mM (22, 30, 40, 50), 40 mM (46), and even 80 mM (11). In our previous animal studies, glutamine was administered directly to small bowel IEC in concentrations ranging from 10 to 60 mM (25, 48, 51). Glutamine in commercially available enteral formulas is ∼60 mM and, when used as an isolated nutrient, can be as high as 500 mM. However, in the present study, the effects of 2 mM (basal concentration, as control) and 10 mM glutamine on IEC-6 cells were compared on the basis of previous studies that suggested that 2 mM and 10 mM glutamine cover the range of physiological and pharmacological concentrations in intestinal cells (12, 35, 36).
Confluent cells were incubated in serum-free media devoid of glutamine overnight and then treated with either 2 mM (basal concentration, as control) or 10 mM glutamine for 24 h under basal conditions. Cells were harvested and total RNA isolated using RNAzol Bee (Tel-Test, Friendswood, TX). The yield, purity, and integrity of the RNA samples were determined by spectrophotometry and electrophoresis. Three expression profiles (n = 3) for each experimental condition were generated. First-strand cDNA was synthesized, and in vitro transcription was then performed and biotinylated cRNA synthesized by amplification with dNTP mix containing biotin-dUTP and T7 RNA polymerase. An aliquot of 750 ng of amplified products were loaded onto Illumina Sentrix Beadchip Array Rat ref12-v1, hybridized at 58°C in an Illumina Hybridization Oven (catalog no. 198361; Illumina, San Diego, CA) for 17 h, washed, and incubated with straptavidin-Cy3 to detect biotin-labeled cRNA on the arrays. Arrays were dried and scanned with Bead Array Reader (Illumina). Data were analyzed using BeadStudio software (Illumina).
Quantitative real-time PCR.
Total RNA was prepared using Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed using an iScript Select cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) following the manufacturers' instruction. Primers for Sp3 (ID: Rn01485868_g1) and β-actin (ID: Rn00667869-m1) were obtained from Applied Biosystems (Austin, TX). Real-time PCR assays were performed in 96-well optical plates on an ABI Prism 7000 Sequence Detection System with SYBR Green PCR Master Mix (Applied Biosystems). Sp3 mRNA expression was normalized against that of β-actin. The value for control cells was set to 1, and the value for the various treatments was presented as a fraction of this number. Experiments were performed in triplicate.
RNA silencing of Sp3.
Interference transfections were performed when cells reached ∼70% confluence after 24 h of growth with the silencer siRNA Starter Kit according to the manufacturer's instructions. The Sp3 siRNA duplex oligonucleotide sense sequence was as follows: 5′-GUUCUCAGACAAUGACUGCUU-3′. The Ambion negative control siRNA no. 1 (catalog no. 4611; Ambion, Austin, TX) (scrambled siRNA) was used for negative control.
Western blot analysis.
To perform Western blot analysis, whole cell lysates were prepared by lysing cells with RIPA buffer (Sigma, Milwaukee, WI) containing protease inhibitors (Sigma), or according to the manufacturer's protocol nuclear and cytoplasmic protein fractions were extracted from cells using Nuclear/Cytosol Fractionation Kit. The whole cell lysates or nuclear proteins were electrophoresed on a Criterion precast gel (Bio-Rad Laboratories) and were then transferred onto a nitrocellulose membrane and blocked for 1 h in 5% nonfat dried milk in TBS with 0.1% Tween 20 and then incubated overnight at 4°C with the primary antibody. Membranes were then washed three times and incubated for 1 h at room temperature with ECL anti-rabbit IgG, horseradish peroxidase-linked whole antibody (from donkey), or ECL anti-mouse IgG, horseradish peroxidase-linked whole antibody (from sheep), developed with ECL plus Western blotting detection system.
Determination of caspase activation by fluorometric protease assay.
After siRNA transfection for 24 h, cells were grown in FBS-deprived medium overnight and then treated for 4 h with 20 ng/ml of TNF and 25 μg/ml of CHX (2), known inducers of apoptosis. Activities of caspases were then analyzed. Hypoxia/reoxygenation was used as model of oxidant stress. After incubation in FBS-deprived medium overnight, cells were incubated in a hypoxic chamber with 1% O2-5% CO2-94.5% N2 for 4 h and then cultured under normoxic conditions with 20 ng/ml of TNF and 25 μg/ml of CHX for an additional 4-h period. At the end of each treatment, activities of caspase-3, -8, and -9 were tested with caspase-3/CPP32 fluorometric assay kit, caspase-8/FLICE fluorometric assay kit, and caspase-9 fluorometric assay kit, respectively, according to the manufacturer's instructions. Briefly, cells were harvested and collected by centrifugation. The pelleted cells were lysed in lysis buffer. Lysates were incubated for 1 h at 37°C with the specific fluorescent substrate. Fluorescence derived from release of 7-amino-4-trifluoromethyl coumarin was followed using a spectrofluorometer at 400-nm excitation and 505-nm emission.
DNA fragmentation measurement.
After overnight incubation in FBS-free medium with or without 10 mM glutamine, untreated or siRNA-transfected cells were treated with 20 ng/ml of TNF and 25 μg/ml of CHX, for an additional 4-h period. For hypoxia/reoxygenation experiments, cells were incubated in a hypoxic chamber with 1% O2-5% CO2-94.5% N2 for 4 h, and cells were then cultured under normoxic conditions in presence of 20 ng/ml of TNF and 25 μg/ml of CHX for 4 h. The presence of cytoplasmic nucleosomes in cells was determined using a Cell Death Detection ELISA kit. The method is a quantitative sandwich enzyme immunoassay that uses antibodies directed against DNA and histone, respectively. Briefly, after treatment, floating cells were discarded, and the attached cells were washed twice with PBS and lysed according to the manufacturer's instructions. Then the mono- and oligonucleosomes in the cytoplasmic fraction of cell lysates were captured onto the ELISA plate, and the immunocomplex was detected photometrically at 405/490 nm in a microplate reader.
Data analysis.
All experiments were repeated at least three times. Statistical analysis was performed by unpaired Student's t-test or one-way ANOVA, and individual group means were compared using Tukey's multiple-group comparison test. P values <0.05 were considered significant. Data are expressed as means ± SE.
RESULTS
Microarray gene analysis.
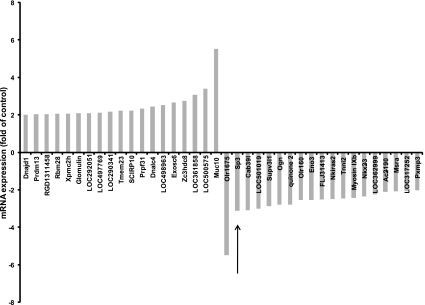
Microarray analysis revealed that the mRNA expression levels of 38 genes were found upregulated or downregulated at least twofold after treatment with 10 mM compared with 2 mM glutamine under basal conditions (Fig. 1). Among these genes, the most differentially expressed genes were muc10 and olr1675, which were upregulated or downregulated 5.52- and 5.50-fold, respectively. The mRNA expression of Sp3 was downregulated 3.12-fold by glutamine. Sp3 belongs to the Sp family of transcription factors, which controls expression of genes implicated in a diverse range of cellular genes. There are to date no reports on the regulation of Sp3 by glutamine, thus prompting our further investigation.
Fig. 1.
Microarray analysis. Intestinal epithelial IEC-6 cells were incubated in serum-free media overnight, were then treated with 2 mM or 10 mM glutamine for 24 h, and then evaluated by microarray analysis. The mRNA expression of specificity protein 3 (Sp3) (arrow) was 3.12-fold higher in 10 mM glutamine-treated cells than in 2 mM glutamine-treated cells.
Sp3 expression was regulated by glutamine.
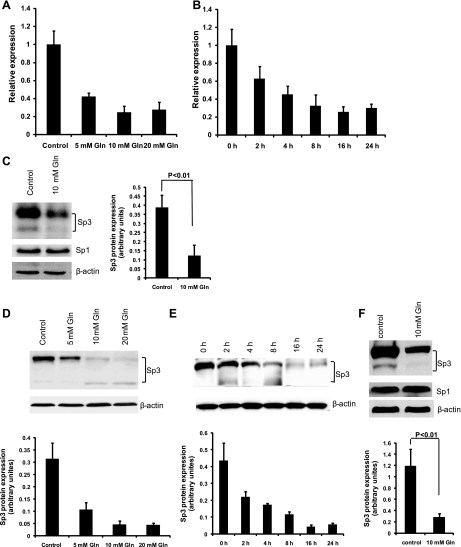
Quantitative real-time PCR was employed to validate the microarray result. Consistent with the microarray results, quantitative real-time PCR confirmed that Sp3 mRNA expression was reduced by 3.6-fold after treatment of 10 mM glutamine for 24 h compared with control (Fig. 2A). Western blot analysis was performed to evaluate the protein expression of Sp3. After treatment with 10 mM glutamine under basal conditions, Sp3 protein expression was decreased (Fig. 2C). Moreover, dose-response and time-course experiments showed that treatment with 10 mM glutamine for 16 h produced maximal Sp3 repression in both mRNA (Fig. 2, A and B) and protein levels (Fig. 2, D and E). To examine whether these observations were also present after oxidant stress, experiments were repeated after hypoxia and reoxygenation and demonstrated that glutamine similarly downregulated Sp3 expression (Fig. 2E). Because Sp1 and Sp3 compete for common target sequences, expression of Sp1 was also measured. Glutamine had no effect on Sp1 expression under either normoxia or oxidant-stressed conditions (Fig. 2, C and F).
Fig. 2.
Glutamine (Gln) downregulated Sp3 expression under normoxia and hypoxia/reoxygenation conditions. A: glutamine suppressed Sp3 mRNA expression in a dose-dependent fashion. After incubation in serum-free media overnight, cells were treated with indicated concentrations of glutamine for 24 h, and quantitative real-time PCR was performed for evaluation of Sp3 mRNA expression. B: glutamine suppressed Sp3 mRNA expression in a time-dependent fashion. After incubation in serum-free media overnight, cells were treated with 10 mM glutamine for indicated time points, and quantitative real-time PCR was performed for evaluation of Sp3 mRNA expression. C: glutamine suppressed Sp3 protein expression. After incubation in serum-free media overnight, cells were treated with 2 mM or 10 mM glutamine for 24 h, and Western blot was performed for evaluation of Sp1 and Sp3 protein expression. D: glutamine suppressed Sp3 protein expression in a dose-dependent fashion. After incubation in serum-free media overnight, cells were treated with indicated concentrations of glutamine for 24 h, and Western blot was performed for evaluation of Sp3 protein expression. E: glutamine suppressed Sp3 protein expression in a time-dependent fashion. After incubation in serum-free media overnight, cells were treated with 10 mM glutamine for indicated time points, and Western blot was performed for evaluation of Sp3 protein expression. F: glutamine also suppressed Sp3 expression after hypoxia/reoxygenation. After incubation in serum-free media with 2 mM or 10 mM glutamine overnight, cells were subjected to a hypoxic chamber with 1% O2-5% CO2-94.5% N2 for 4 h and then reoxygenated for 4 h, and Western blot was performed for evaluation of Sp3 protein expression. The densitometric analysis for Western blot corresponds to the means ± SE of 3 independent experiments.
Apoptosis was reduced by inhibition of Sp3.
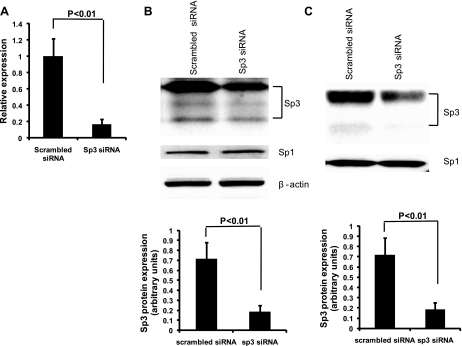
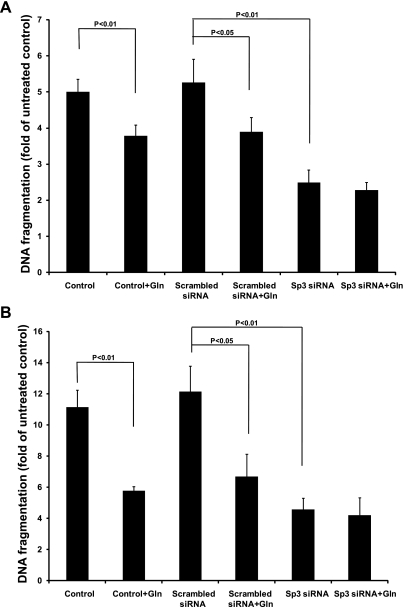
Sp3 has been shown to induce apoptosis, whereas glutamine may inhibit apoptosis (4, 14). We therefore hypothesized that a mechanism by which glutamine protects against intestinal apoptosis is via inhibition of Sp3. Small-interference RNA technology was used to silence Sp3 expression, and then quantitative real-time PCR performed to evaluate the efficiency of small RNA interference. Results demonstrated that mRNA expression of Sp3 was decreased by 82.9% after transfection of Sp3 siRNA for 48 h (Fig. 3A). Western blot revealed that Sp3 protein was reduced by Sp3 siRNA in the cytoplasm by 74.1% (Fig. 3B) and in the nucleus by 75.2% (Fig. 3C). However, Sp1 protein expression was not affected by Sp3 siRNA in either the cytoplasmic (Fig. 3B) or nuclear fractions (Fig. 3C). Under basal conditions, DNA fragmentation was similar between control and scrambled siRNA (5.01 ± 0.33 and 5.27 ± 0.63, respectively) but reduced by glutamine alone (control + glutamine; 3.79 ± 0.29) and scrambled siRNA plus glutamine (3.90 ± 0.39, respectively) (Fig. 4A). Silencing of Sp3 further reduced DNA fragmentation (2.49 ± 0.34). Similar results were observed when cells were subjected to hypoxia/reoxygenation (Fig. 4B). Importantly, the addition of glutamine to Sp3-silenced cells did not further lessen apoptosis under either basal or stressed conditions, suggesting that Sp3 plays a major role in the inhibitory effect of glutamine on apoptosis (Fig. 4, A and B).
Fig. 3.
Sp3 protein expression was silenced by siRNA. A: Sp3 mRNA expression of Sp3 was decreased by 82.9% after treatment with Sp3 siRNA. After transfection with Sp3 siRNA for 48 h, total RNA was extracted, and then quantitative real-time PCR was performed. B: cytosolic Sp3 protein, but not Sp1 protein, was decreased by 74.1% after treatment of siRNA. After transfection with Sp3 siRNA for 48 h, cytoplasmic protein fractions were extracted, and then Western blot was formed. C: nuclear Sp3 protein, but not Sp1 protein, was decreased by 75.2% after treatment of siRNA. After transfection with Sp3 siRNA for 48 h, nuclear protein fractions were extracted, and then Western blot was performed.
Fig. 4.
Apoptosis was reduced by inhibition of Sp3 under normoxia and hypoxia/reoxygenation. A: after overnight incubation in FBS-free medium with 2 mM (control) or 10 mM glutamine, untransfected or siRNA transfected cells were treated with 20 ng/ml of TNF and 25 μg/ml of cycloheximide (CHX) for 4 h. DNA fragmentation was determined using a Cell Death Detection ELISA kit. B: after overnight incubation in FBS-free medium with 2 mM (control) or 10 mM glutamine, untransfected or siRNA-transfected cells were incubated in a hypoxic chamber with 1% O2-5% CO2-94.5% N2 for 4 h, and then cultured under normoxic conditions in presence of 20 ng/ml of TNF and 25 μg/ml of CHX for an additional 4 h. DNA fragmentation in cells was determined using a Cell Death Detection ELISA. Cells treated with neither siRNA nor inducers of apoptosis and grown in basal conditions served as untreated control. Statistical significance was evaluated by one-way ANOVA with post hoc Tukey's test.
Inhibition of Sp3 reduced caspase expression and activity.
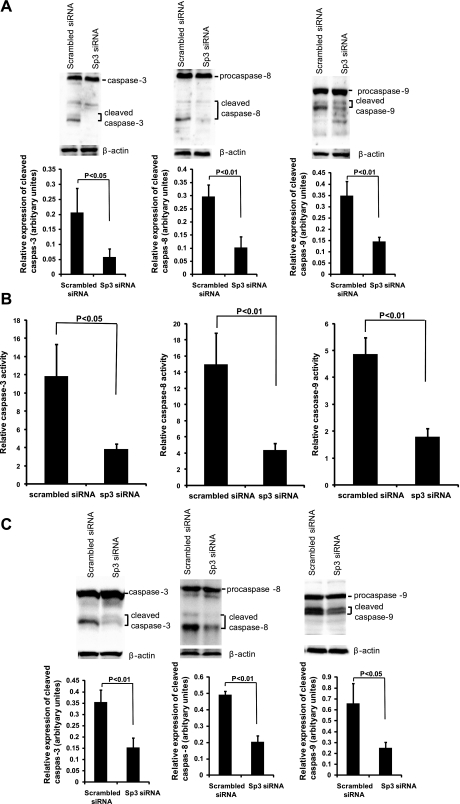
Caspases are a class of cysteine proteases that play a pivotal role in the execution of apoptosis. Caspase-3 is a key executioner of apoptosis, whose activation is mediated by the initiator caspases, caspase-8 and caspase-9 (10, 27, 57). Therefore, we sought to determine whether these caspases contribute to the protective effect of glutamine on apoptosis. Activations of caspases-3, -8, and -9 in Sp3-silenced IEC-6 cells were analyzed by two different and distinct methods: immunoblotting for evaluation of procaspases and cleaved caspase expression levels and caspase fluorometric assays for analysis of enzyme activity. Immunoblotting revealed that the cleaved caspases-3, -8, and -9 were inhibited after silencing of Sp3 compared with those treated with scrambled siRNA after treatment with inducers of apoptosis, TNF and CHX (Fig. 5A). The results from the caspase fluorometric assay further confirmed these findings, with activities of caspase-8, -9, and -3 inhibited by Sp3 siRNA (Fig. 5B). A similar observation was observed after hypoxia and reoxygenation (Fig. 5C).
Fig. 5.
Caspases were reduced by Sp3 silencing. A: after overnight incubation in FBS-free medium and treatment with 20 ng/ml of TNF and 25 μg/ml of CHX for 4 h, siRNA-transfected IEC-6 cells were analyzed for the expression of caspase-3, -8, and -9 by Western blot. B: after overnight incubation in FBS-free medium and treatment with 20 ng/ml of TNF and 25 μg/ml of CHX for 4 h, siRNA-transfected IEC-6 cells were analyzed for caspase-3, -8, and -9 activities by fluorometric protease assay. Statistical significance was evaluated by Student's t-test. C: after overnight incubation in FBS-free medium, siRNA-transfected IEC-6 cells were incubated in a hypoxic chamber with 1% O2-5% CO2-94.5% N2 for 4 h and then cultured under normoxic conditions with 20 ng/ml of TNF and 25 μg/ml of CHX for an additional 4 h. Western blot analysis for activities of caspase-3, -8, and -9 was performed.
Bcl-2 family proteins were regulated by Sp3.
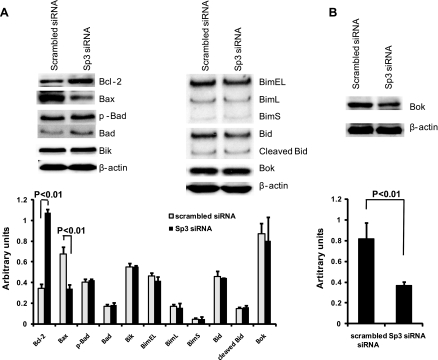
To further investigate the mechanisms by which Sp3 mediates apoptosis, Bcl-2 family protein expression was detected by Western blot. Under normoxia with 20 ng/ml of TNF and 25 μg/ml of CHX treatment for 4 h and after Sp3 silencing, prosurvival Bcl-2 protein expression was increased, whereas the proapoptotic protein, Bax, was inhibited. The expression levels of other Bcl-2 family proteins including Bad, Bik, Bim, Bid, and Bok remained unchanged (Fig. 6A). Similar to DNA fragmentation and caspases, results after hypoxia and reoxygenation followed those of normoxia (data not shown), except that expression of the proapoptotic protein, Bok, was decreased (Fig. 6B).
Fig. 6.
Bcl-2 family proteins were regulated by Sp3. A: after overnight incubation in FBS-free medium and treatment with 20 ng/ml of TNF and 25 μg/ml of CHX for 4 h, siRNA-transfected IEC-6 cells were analyzed for apoptosis-related proteins by Western blot. The proapoptotic protein Bcl-2 was increased after silencing of Sp3, whereas the antiapoptotic protein Bax was decreased. B: after overnight incubation in FBS-free medium, siRNA-transfected IEC-6 cells were incubated in a hypoxic chamber with 1% O2-5% CO2-94.5% N2 for 4 h and then cultured under normoxic conditions with 20 ng/ml of TNF and 25 μg/ml of CHX for an additional 4 h. Western blot analysis revealed an increase in the proapoptotic protein, Bok. The densitometric analysis corresponds to the means ± SE of 3 independent experiments.
DISCUSSION
In this study, we utilized microarray technology and identified that glutamine is a novel mediator of Sp3 in IEC-6 cells, with an inverse relationship between glutamine concentration and Sp3 expression demonstrated. Because both glutamine and Sp3 have been linked to apoptosis (1, 41, 61), we hypothesized that a mechanism by which glutamine protects against intestinal apoptosis is via inhibition of Sp3.
Glutamine has been shown to be antiapoptotic in the intestine (4). Previous studies have shown that glutamine protects human intestinal HT-29 cells and rat intestinal IEC-18 cells from cytokine-induced apoptosis (15) and NH2Cl (61). Many different mechanisms have been proposed to explain the protective effects of glutamine against apoptosis. Glutamine can activate mammalian target of rapamycin signaling and increase the expression of ornithine decarboxylase to promote intestinal restitution (55). Evans et al. (16) demonstrated that glutamine prevented cytokine-induced apoptosis in intestinal epithelial HT-29 cells via the pyrimidine pathway. The protective effect of glutamine on gut mucosa may also be related to the induction of cytoprotective proteins, such as the heat shock protein family (7, 49, 59, 60).
More directly, glutamine can upregulate the expression of antiapoptotic proteins, Bcl-2 and CD45RO, and downregulate the expression of proapoptotic proteins, Fas and Fas ligand, in the human T lymphocyte cell line Jurkat (5). In addition, glutamine can inhibit both caspase-3 in intestine (13) and caspase-8 activities in activated T cells (5), whereas glutamine starvation induces apoptosis by caspase-3 and caspase-8 sequential activation (41). Indeed, our results demonstrate that, when Sp3 was silenced, both DNA fragmentation and caspase-3, -8, and -9 expression and activity were significantly inhibited. Importantly, the addition of glutamine to Sp3-silenced cells did not further lessen apoptosis, suggesting that Sp3 plays a major role in the inhibitory effect of glutamine on apoptosis.
The Bcl-2 family plays a central role in apoptosis. The family can be separated into several groups on the basis of function and sequence homology including the following: anti-apoptotic Bcl-2 family proteins Bcl-2, Bcl-w, Bcl-xL, A1, and Mcl-1; Bcl-2 family effector proteins Bak and Bax; direct activator BH3-only proteins Bid and Bim; and sensitizers/derepressors BH3-only proteins Bad, Bik, Bmf, Hrk, Noxa, and Puma (6). In the present study, we found that silencing of Sp3 increased Bcl-2 expression and decreased Bax expression under both normoxia and after hypoxia/reoxygenation, suggesting that the protection against apoptosis by Sp3 inhibition was through the caspase-mediated apoptosis pathway and mediated by apoptosis-related proteins. Chang et al. (5) reported that glutamine enhanced expression of Bcl-2 in the human T lymphocyte cell line Jurkat (5), suggesting that Sp3 may mediate the regulation of Bcl-2 by glutamine.
We also showed that Sp3 silencing after hypoxia/reoxygenation decreased Bok expression. Several studies have shown that oxygen-glucose deprivation/reoxygenation causes apoptosis and enhances the expressions of Bax, Bok, and caspase-3 in rat pheochromocytoma cells, PC12, and in retinal ganglion cells (24, 39, 53).
Although Sp1 and Sp3 are structurally similar, they can possess strikingly different functions (32). Sp1 is well known as a transcriptional activator, whereas Sp3 can be either a transcriptional activator or repressor of Sp1-mediated transcription (34). The difference in the position of the inhibitory domain between the two proteins is believed to be a major reason for the distinct functions (32, 52). We found that glutamine inhibited Sp3 but not Sp1. Sumoylation of transcription factors, including Sp3 but not Sp1, has negative effects on its activity and may represent the mechanism by which glutamine represses Sp3. There is a single lysine residue within the inhibitory domain that is the target for conjugation of the small ubiquitin-related posttranslational modifier (54).
The findings of this study may have important clinical implications. During times of glutamine depletion, such as critical illness or injury, Sp3 expression may go unchecked, leading to intestinal mucosal damage. Glutamine supplementation, however, may protect the postischemic gut by repressing Sp3 expression and inhibiting apoptosis. A number of laboratory studies have confirmed the gut-protective effects of enteral glutamine administered to the postischemic gut (26, 38). Additionally, clinical studies have demonstrated that enteral glutamine administered to trauma, burn, and critically ill patients decreases morbidity in most but not all studies (3, 17, 19, 23, 42, 64). We have shown that the early use of glutamine during active shock resuscitation is safe (37). Lastly, the REDOX trial includes enteral glutamine administration to critically ill patients in shock, and preliminary results appear promising (20, 21).
In conclusion, we identified for the first time that glutamine represses Sp3 expression. Our results suggest that Sp3 plays a major role in the inhibitory effect of glutamine on apoptosis under both normoxia and oxidant-stressed conditions in small bowel IEC.
GRANTS
This work was supported by National Institutes of Health (RO1 GM077282).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Abdelrahim M, Smith R, 3rd, Burghardt R, Safe S. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer Res 64: 6740–6749, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya S, Ray RM, Johnson LR. Prevention of TNF-α-induced apoptosis in polyamine-depleted IEC-6 cells is mediated through the activation of ERK1/2. Am J Physiol Gastrointest Liver Physiol 286: G479–G490, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Brantley S, Pierce J. Effects of enteral glutamine on trauma patients (Abstract). Nutr Clin Pract 15: S13, 2000. [Google Scholar]
- 4.Brasse-Lagnel CG, Lavoinne AM, Husson AS. Amino acid regulation of mammalian gene expression in the intestine. Biochimie 92: 729–735, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Chang WK, Yang KD, Chuang H, Jan JT, Shaio MF. Glutamine protects activated human T cells from apoptosis by up-regulating glutathione and Bcl-2 levels. Clin Immunol 104: 151–160, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL2 family reunion. Mol Cell 37: 299–310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coëffier M, Le Pessot F, Leplingard A, Marion R, Lerebours E, Ducrotté P, Déchelotte P. Acute enteral glutamine infusion enhances heme oxygenase-1 expression in human duodenal mucosa. J Nutr 132: 2570–2573, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Coëffier M, Claeyssens S, Hecketsweiler B, Lavoinne A, Ducrotté P, Déchelotte P. Enteral glutamine stimulates protein synthesis and decreases ubiquitin mRNA level in human gut mucosa. Am J Physiol Gastrointest Liver Physiol 285: G266–G273, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Coëffier M, Marion R, Ducrotté P, Déchelotte P. Modulating effect of glutamine on IL-1beta-induced cytokine production by human gut. Clin Nutr 22: 407–413, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunol Rev 193: 10–21, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Deldicque L, Sanchez Canedo C, Horman S, De Potter I, Bertrand L, Hue L, Francaux M. Antagonistic effects of leucine and glutamine on the mTOR pathway in myogenic C2C12 cells. Amino Acids 35: 147–155, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Deniel N, Marion-Letellier R, Charlionet R, Tron F, Leprince J, Vaudry H, Ducrotté P, Déchelotte P, Thébault S. Glutamine regulates the human epithelial intestinal HCT-8 cell proteome under apoptotic conditions. Mol Cell Proteomics 6: 1671–1679, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Erbil Y, Oztezcan S, Giriş M, Barbaros U, Olgaç V, Bilge H, Küçücük H, Toker G. The effect of glutamine on radiation-induced organ damage. Life Sci 78: 376–382, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Essafi-Benkhadir K, Grosso S, Puissant A, Robert G, Essafi M, Deckert M, Chamorey E, Dassonville O, Milano G, Auberger P, Pagès G. Dual role of Sp3 transcription factor as an inducer of apoptosis and a marker of tumour aggressiveness. PLoS One 4: e4478, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans ME, Jones DP, Ziegler TR. Glutamine prevents cytokine-induced apoptosis in human colonic epithelial cells. J Nutr 133: 3065–3071, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Evans ME, Jones DP, Ziegler TR. Glutamine inhibits cytokine-induced apoptosis in human colonic epithelial cells via the pyrimidine pathway. Am J Physiol Gastrointest Liver Physiol 289: G388–G396, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Garrel D, Patenaude J, Nedelec B, Samson L, Dorais J, Champoux J, D'Elia M, Bernier J. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: a prospective, controlled, randomized clinical trial. Crit Care Med 31: 2444–2449, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Grimble RF. Immunonutrition. Curr Opin Gastroenterol 21: 216–222, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hall JC, Dobb G, Hall J, de Sousa R, Brennan L, McCauley R. A prospective randomized trial of enteral glutamine in critical illness. Intensive Care Med 29: 1710–1716, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Heyland DK, Dhaliwal R, Day AG, Muscedere J, Drover J, Suchner U, Cook D, Canadian Critical Care Trials Group Reducing Deaths due to Oxidative Stress (The REDOXS Study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proc Nutr Soc 65: 250–263, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Heyland DK, Dhaliwalm R, Day A, Drover J, Cote H, Wischmeyer P. Optimizing the dose of glutamine dipeptides and antioxidants in critically ill patients: a phase I dose-finding study. JPEN J Parenter Enteral Nutr 31: 109–118, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Hinshaw DB, Burger JM. Protective effect of glutamine on endothelial cell ATP in oxidant injury. J Surg Res 49: 222–227, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Houdijk AP, Rijnsburger ER, Jansen J, Wesdorp RI, Weiss JK, McCamish MA, Teerlink T, Meuwissen SG, Haarman HJ, Thijs LG, van Leeuwen PA. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet 352: 772–776, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Koubi D, Jiang H, Zhang L, Tang W, Kuo J, Rodriguez AI, Hunter TJ, Seidman MD, Corcoran GB, Levine RA. Role of Bcl-2 family of proteins in mediating apoptotic death of PC12 cells exposed to oxygen and glucose deprivation. Neurochem Int 46: 73–81, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kozar RA, Schultz SG, Hassoun HT, Desoignie R, Weisbrodt NW, Haber MM, Moore FA. The type of sodium-coupled solute modulates small bowel mucosal injury, transport function, and ATP after ischemia/reperfusion injury in rats. Gastroenterology 123: 810–816, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Kozar RA, Schultz SG, Bick RJ, Poindexter BJ, DeSoignie R, Moore FA. Enteral glutamine but not alanine maintains small bowel barrier function after ischemia/reperfusion injury in rats. Shock 21: 433–437, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell 138: 838–854, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kypriotou M, Beauchef G, Chadjichristos C, Widom R, Renard E, Jimenez SA, Korn J, Maquart FX, Oddos T, Von Stetten O, Pujol JP, Galéra P. Human collagen Krox up-regulates type I collagen expression in normal and scleroderma fibroblasts through interaction with Sp1 and Sp3 transcription factors. J Biol Chem 282: 32000–32014, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Labow BI, Souba WW. Glutamine. World J Surg 24: 1503–1513, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Lagranha CJ, Doi SQ, Pithon-Curi TC, Curi R, Sellitti DF. Glutamine enhances glucose-induced mesangial cell proliferation. Amino Acids 34: 683–685, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Larson SD, Li J, Chung DH, Evers BM. Molecular mechanisms contributing to glutamine-mediated intestinal cell survival. Am J Physiol Gastrointest Liver Physiol 293: G1262–G1271, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, He S, Sun JM, Davie JR. Gene regulation by Sp1 and Sp3. Biochem Cell Biol 82: 460–471, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Li N, Liboni K, Fang MZ, Samuelson D, Lewis P, Patel R, Neu J. Glutamine decreases lipopolysaccharide-induced intestinal inflammation in infant rats. Am J Physiol Gastrointest Liver Physiol 286: G914–G921, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Majello B, De Luca P, Lania L. Sp3 is a bifunctional transcription regulator with modular independent activation and repression domains. J Biol Chem 272: 4021–4026, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Marion R, Coëffier MM, Gargala G, Ducrotté P, Déchelotte PP. Glutamine and CXC chemokines IL-8, Mig, IP-10 and I-TAC in human intestinal epithelial cells. Clin Nutr 23: 579–585, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Marion R, Coëffier M, Leplingard A, Favennec L, Ducrotté P, Déchelotte P. Cytokine-stimulated nitric oxide production and inducible NO-synthase mRNA level in human intestinal cells: lack of modulation by glutamine. Clin Nutr 22: 523–528, 2003 [DOI] [PubMed] [Google Scholar]
- 37.McQuiggan M, Kozar R, Sailors RM, Ahn C, McKinley B, Moore F. Enteral glutamine during active shock resuscitation is safe and enhances tolerance of enteral feeding. JPEN J Parenter Enteral Nutr 32: 28–35, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Medeiros AC, Chacon DA, Sales VS, Egito ES, Brandão-Neto J, Pinheiro LA, Carvalho MR. Glucan and glutamine reduce bacterial translocation in rats subjected to intestinal ischemia-reperfusion. J Invest Surg 19: 39–46, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Nakajima Y, Shimazawa M, Mishima S, Hara H. Neuroprotective effects of Brazilian green propolis and its main constituents against oxygen-glucose deprivation stress, with a gene-expression analysis. Phytother Res 23: 1431–1438, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Nakamura E, Hagen SJ. Role of glutamine and arginase in protection against ammonia-induced cell death in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol 283: G1264–G1275, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Papaconstantinou HT, Chung DH, Zhang W, Ansari NH, Hellmich MR, Townsend CM, Jr, Ko TC. Prevention of mucosal atrophy: role of glutamine and caspases in apoptosis in intestinal epithelial cells. J Gastrointest Surg 4: 416–423, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Peng X, Yan H, You Z, Wang P, Wang S. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 30: 135–139, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Philipsen S, Suske G. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res 27: 2991–3000, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray SK, Leiter AB. The basic helix-loop-helix transcription factor NeuroD1 facilitates interaction of Sp1 with the secretin gene enhancer. Mol Cell Biol 27: 7839–7847, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reeds PJ, Burrin DG. Glutamine and the bowel. J Nutr 131, Suppl 9: 2505S–2508S, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Rhoads JM, Argenzio RA, Chen W, Rippe RA, Westwick JK, Cox AD, Berschneider HM, Brenner DA. L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol Gastrointest Liver Physiol 272: G943–G953, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Rhoads JM, Argenzio RA, Chen W, Graves LM, Licato LL, Blikslager AT, Smith J, Gatzy J, Brenner DA. Glutamine metabolism stimulates intestinal cell MAPKs by a cAMP-inhibitable, Raf-independent mechanism. Gastroenterology 118: 90–100, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Robinson EK, Kelly DP, Mercer DW, Kozar RA. Differential effects of luminal arginine and glutamine on metalloproteinase production in the postischemic gut. JPEN J Parenter Enteral Nutr 32: 433–438, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ropeleski MJ, Riehm J, Baer KA, Musch MW, Chang EB. Anti-apoptotic effects of L-glutamine-mediated transcriptional modulation of the heat shock protein 72 during heat shock. Gastroenterology 129: 170–184, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Santoso JT, Lucci JA, 3rd, Coleman RL, Hatch S, Wong P, Miller D, Mathis JM. Does glutamine supplementation increase radioresistance in squamous cell carcinoma of the cervix? Gynecol Oncol 71: 359–563, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Sato N, Moore FA, Kone BC, Zou L, Smith MA, Childs MA, Moore-Olufemi S, Schultz SG, Kozar RA. Differential induction of PPAR-γ by luminal glutamine and iNOS by luminal arginine in the rodent postischemic small bowel. Am J Physiol Gastrointest Liver Physiol 290: G616–G623, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Suske G. The Sp-family of transcription factors. Gene 238: 291–300, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Tabakman R, Jiang H, Levine RA, Kohen R, Lazarovici P. Apoptotic characteristics of cell death and the neuroprotective effect of homocarnosine on pheochromocytoma PC12 cells exposed to ischemia. J Neurosci Res 75: 499–507, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Valin A, Gill G. Regulation of the dual-function transcription factor Sp3 by SUMO. Biochem Soc Trans 35: 1393–1396, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Wang JY. Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids 33: 241–252, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Wang W, Dong L, Saville B, Safe S. Transcriptional activation of E2F1 gene expression by 17beta-estradiol in MCF-7 cells is regulated by NF-Y-Sp1/estrogen receptor interactions. Mol Endocrinol 13: 1373–1387, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Wang ZB, Liu YQ, Cui YF. Pathways to caspase activation. Cell Biol Int 29: 489–496, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Wilson AJ, Chueh AC, Tögel L, Corner GA, Ahmed N, Goel S, Byun DS, Nasser S, Houston MA, Jhawer M, Smartt HJ, Murray LB, Nicholas C, Heerdt BG, Arango D, Augenlicht LH, Mariadason JM. Apoptotic sensitivity of colon cancer cells to histone deacetylase inhibitors is mediated by an Sp1/Sp3-activated transcriptional program involving immediate-early gene induction. Cancer Res 70: 609–620, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wischmeyer PE. Glutamine and heat shock protein expression. Nutrition 18: 225–228, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol 90: 2403–2410, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Wischmeyer PE, Musch MW, Madonna MB, Thisted R, Chang EB. Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Physiol Gastrointest Liver Physiol 272: G879–G884, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Zelko IN, Mueller MR, Folz RJ. Transcription factors sp1 and sp3 regulate expression of human extracellular superoxide dismutase in lung fibroblasts. Am J Respir Cell Mol Biol 39: 243–251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao C, Meng A. Sp1-like transcription factors are regulators of embryonic development in vertebrates. Dev Growth Differ 47: 201–211, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Zhou YP, Jiang ZM, Sun YH, Wang XR, Ma EL, Wilmore D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double-blind, controlled clinical trial. JPEN J Parenter Enteral Nutr 27: 241–245, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Ziegler TR, Bazargan N, Leader LM, Martindale RG. Glutamine and the gastrointestinal tract. Curr Opin Clin Nutr Metab Care 3: 355–362, 2000 [DOI] [PubMed] [Google Scholar]