Abstract
Glucagon-like peptide-2 (GLP-2) is a nutrient-dependent, proglucagon-derived hormone that is a proposed treatment for human short bowel syndrome (SBS). The objective was to determine how the timing, duration, and cessation of GLP-2 administration affect intestinal adaptation and enterocyte kinetics in a rat model of human SBS that results in intestinal failure requiring total parenteral nutrition (TPN). Rats underwent 60% jejunoileal resection plus cecectomy and jugular vein cannulation and were maintained exclusively with TPN for 18 days in these treatments: TPN control (no GLP-2); sustained GLP-2 (1–18 days); early GLP-2 (1–7 days, killed at 7 or 18 days); and delayed GLP-2 (12–18 days). Body weight gain was similar across groups, and plasma bioactive GLP-2 was significantly increased with coinfusion of GLP-2 (100 μg·kg−1·day−1) with TPN. GLP-2-treated rats showed significant increases in duodenum and jejunum mucosal dry mass, protein, DNA, and sucrase activity compared with TPN control. The increased jejunum cellularity reflected significantly decreased apoptosis and increased crypt mitosis and crypt fission due to GLP-2. When GLP-2 infusion stopped at 7 days, these effects were reversed at 18 days. Sustained GLP-2 infusion significantly increased duodenum length and decreased 18-day mortality to 0% from 37.5% deaths in TPN control (P = 0.08). Colon proglucagon expression quantified by real-time RT-qPCR was increased in TPN controls and attenuated by GLP-2 infusion; jejunal expression of the GLP-2 receptor did not differ among groups. In summary, early, sustained GLP-2 infusion reduces mortality, induces crypt fission, and is required for intestinal adaptation, whereas cessation of GLP-2 reverses gains in mucosal cellularity in a rat model of intestinal failure.
Keywords: short bowel syndrome, proglucagon, apoptosis, crypt fission, parenteral nutrition
glucagon-like peptide-2 (GLP-2) is a 33-amino acid intestinotrophic hormone derived from tissue-specific posttranslational processing of proglucagon in the endocrine L cells of the ileum and colon (10). Ingestion of nutrients, especially lipid and carbohydrate in humans, stimulates GLP-2 secretion, and GLP-2 is inactivated by dipeptidyl peptidase IV cleavage, with a biological half-life of ∼7 min (8). GLP-2 maintains gastrointestinal homeostasis by inhibiting gastric acid secretion and motility, upregulating intestinal blood flow, stimulating nutrient absorption, and reducing intestinal permeability (8, 10, 16). Moreover, GLP-2 is considered a key mediator of intestinal adaptive growth through stimulation of epithelial cell proliferation and inhibition of apoptosis, leading to an enhanced absorptive surface area (1, 3, 9, 28). Clinical studies evaluating native GLP-2 and (Gly2)GLP-2 (teduglutide), a degradation-resistant analog of GLP-2, demonstrate improved intestinal morphology and absorption in humans with short bowel syndrome (SBS) who have reduced endogenous GLP-2 secretion and are dependent on total (TPN) or partial parenteral nutrition (PN) (11, 20–24). The intestinotrophic effects of GLP-2 are not sustained after discontinuation of therapy in adults with SBS (23). Thus questions remain about the optimal timing and duration of GLP-2 therapy, which can be addressed in suitable animal models.
Rodent models have shown that exogenous GLP-2 augments the adaptive response following resection of the proximal mid-small bowel when there is residual ileum and colon in continuity (3, 25, 28, 38). After proximal mid-small bowel resection, oral feeding sustains somatic growth, and there is upregulation of endogenous GLP-2 secretion from residual ileum and colon, resulting in significant intestinal adaptive growth without administration of GLP-2 (3). While useful as a physiological tool, proximal mid-small bowel resection that permits oral feeding does not mimic the human condition of extreme SBS, resulting in intestinal failure where ileum and colon are often resected, endogenous synthesis of GLP-2 is minimal, and TPN or PN is required to sustain life (7, 40). Our rat SBS model of distal small bowel resection (60% jejunoileal resection + cecectomy with jejunocolic anastomosis) reliably mimics extreme human SBS requiring TPN because minimal adaptation of residual small bowel occurs. Interestingly, colon adapts, but this is not sufficient to maintain nutritional status (13, 15, 27, 31). Ingestion of food after this distal small bowel resection is not sufficient to induce functional intestinal adaptation (14, 29, 31, 37) and results in significant malnutrition and weight loss, termed intestinal failure, such that TPN or PN is required for survival. This model is clinically relevant, as humans with extreme SBS are more likely to benefit from treatment with exogenous GLP-2 and have been the cohort studied in human trials of GLP-2 and teduglutide.
Our previous research has established the time course of the intestinal and hormonal response for the SBS model of distal bowel resection used in the present study (60% jejunoileal resection + cecectomy with jejunocolic anastomosis). The model shows an absence of adaptive growth in the residual small intestine up to 12 days postresection, with peak endogenous plasma GLP-2 levels that are not significantly different from those of animals receiving transection control surgery at 4–7 days postresection (27). Thus the present study uses a model of distal small bowel resection that mimics the human condition of extreme SBS with intestinal failure, where there is little endogenous GLP-2, and is suitable to ask questions about the cellular events underlying the mitogenic effect of GLP-2 therapy. Our objective was to determine how the timing, duration, and cessation of GLP-2 administration affect intestinal adaptation and enterocyte kinetics in a rat model of SBS that results in intestinal failure requiring TPN.
MATERIALS AND METHODS
Animals and experimental design.
The animal facilities and protocols reported were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan, Madison, WI), initially weighing 150–200 g, were housed in individual, stainless steel cages with unlimited access to water in a room maintained at 22°C on a 12:12-h light-dark cycle. All rats were acclimated to the facility for 6 days while being fed a semipurified diet ad libitum (4).
A total of 39 animals underwent 60% jejunoileal resection + cecectomy with jejunocolic anastomosis and placement of a jugular venous catheter and were randomized to the following five treatments: TPN control (no GLP-2); sustained GLP-2 (1–18 days); early GLP-2 (1–7 days, killed at 7 or 18 days); and delayed GLP-2 (12–18 days). We chose not to include a transection control arm and included only animals receiving resection + exclusive TPN, given our evidence of the absence of intestinal adaptation and minimal changes in endogenous GLP-2 levels in this model compared with transected animals (27). Animals were killed on postresection day 18, except for the early GLP-2 group, one-half of which were killed at day 7. A total of 4 of 39 rats undergoing surgery died. This included 3 animals from the TPN control group that died on postsurgery days 8, 12, and 15 of complications not directly related to surgery, and 1 animal from the early GLP-2 18-day group that died on day 3 from excess blood loss during surgery. GLP-2-treated animals received 100 μg·kg body wt−1·day−1 human GLP-2 (33 amino acids, preproglucagon 126–158, CA Peptide Research, Napa, CA), which was coinfused continuously with TPN solution. A nonsurgical group of rats fed a semipurified diet ad libitum (4) was included for reference (oral, n = 8).
To prepare the bowel for resection, rats were fed a low-residue, semi-elemental liquid diet (Vital, donated by Ross Products Division, Abbott Laboratories, Columbus, OH) ad libitum for 3 days and then fasted for 18 h before surgery. At surgery, animals were anesthetized by inhalation of isoflurane (IsoFlo; Abbot Laboratories, North Chicago, IL) via an anesthetic machine. After anesthesia, rats underwent 60% jejunoileal resection + cecectomy, as described previously (13). Briefly, small intestine was resected from 40 cm distal to the ligament of Treitz to 1 cm distal to the cecum. The remaining jejunum was measured with a 40-cm length of silk suture placed along the bowel to ensure that animals had an equivalent amount of residual jejunum. Bowel continuity was reestablished with an end-to-end jejunocolic anastomosis using 6–0 silk suture. Animals received 5 ml of intraperitoneal saline for fluid resuscitation. The peritoneum was closed with absorbable suture, and the abdominal skin incision was closed with wound clips. After abdominal closure, an intravenous catheter was placed in the superior vena cava via the internal jugular vein (4). Rats received oxymorphone (0.18 mg/kg body wt) every 6 h for 24 h after surgery for analgesia, and ampicillin (200 mg/kg body wt) was administered before surgery and every 12 h for 48 h postoperatively as perioperative prophylaxis (3).
All resected rats were maintained exclusively with an isonitrogenous and isoenergetic TPN regimen for the duration of the study and provided water ad libitum. Nutritionally complete TPN solution was prepared aseptically as a total nutrient admixture using commercial preparations of amino acids, dextrose, 20% lipid emulsion, electrolytes, vitamins, trace elements, and a stock solution of choline to meet requirements of the rat (4). Infusion of TPN solution was initiated using a Harvard syringe pump (Harvard Apparatus, Holliston, MA) at 1.0 ml/h immediately following surgery (day 0), advanced to 1.67 ml/h on day 1, and maintained at full strength infusion of 2.5 ml/h, providing 250 kcal/kg body wt for 7 or 18 days. The TPN solution had a caloric density of ∼1 kcal/ml and provided 1.5 g N·kg−1·day−1 and 32% of nonprotein energy from Intralipid (Kabi Pharmacia, Clayton, NC). Animals were weighed every 3 days. At 7 or 18 days postsurgery, rats were anesthetized with isofluorane and killed by exsanguinations within 10 min of stopping the continuous infusion feeding, at which time the entire small and large intestine were removed for analysis.
Intestinal composition, histology, and sucrase activity.
After removal, residual duodenum, jejunum, and colon were flushed with ice-cold saline and placed on a chilled glass plate. The bowel was sectioned into duodenum, defined as pylorus to ligament of Treitz; jejunum, defined as ligament of Treitz to colon; and colon. Intestine 1 cm on either side of the anastomosis was discarded, and the length of duodenum, jejunum, and colon were measured after hanging each section with a constant weight. The first 2 cm of each section were used for measuring wet and dry mucosal mass. The 3rd cm of jejunum was fixed in 10% buffered formalin, transferred to 70% ethanol, paraffin embedded, cut into 5-μm sections, and stained with hematoxylin and eosin for histomorphology, as previously described (5). The next 2 cm of each section were used for determining concentrations of mucosal protein (bicinchoninic acid protein assay; Pierce Chemicals, Rockford, IL), DNA (30), and sucrase activity (2).
Jejunal apoptosis, mitosis, and crypt fission.
Conventional light microscopy of hematoxylin and eosin-stained jejunal specimens was used to quantitate crypt fission, apoptosis, based on condensed chromatin, nuclear fragmentation, and formation of apoptotic bodies, and mitosis, based on spindle formation, chromatin condensation, and actin cytoskeleton rearrangement (5). An experienced human pathologist (X. Chen), blinded to the treatment groups, examined the slides for the characteristic findings of mitotic and apoptotic cells (26, 36) and crypt fission. Fifty crypt and 50 villus well-oriented columns (i.e., one side of the crypt or villus in a longitudinal cross section) were assessed per animal for mitotic and apoptotic cells (5–10 animals per group). The crypt undergoing fission was defined as a bifurcating crypt with a fissure creating two flask-shaped bases and a shared single crypt-villus junction (6, 12). The number of crypt fission was counted in 100 crypts per animal. Data are presented two ways to normalize for the effects of the TPN control group to decrease and GLP-2 treatment to increase the total number of cells in the crypt or villus columns. First, data are presented as the mean number of mitotic or apoptotic cells per column (Supplemental Table 1; the online version of this article contains supplemental data). Second, data are presented as an apoptotic or mitotic index calculated as the total number of cells expressed as a percentage of the total number of cells in one side of a crypt or villus column.
Biochemical analyses.
Blood was collected in chilled tubes containing a final concentration of 1 mg/ml EDTA, 0.1 mM Diprotin A (MP Biomedicals, Aurora, OH), and 0.01 mM aprotinin (Calbiochem, La Jolla, CA). Plasma was isolated by centrifugation at 1,800 g for 15 min at 4°C and was stored at −70°C until GLP-2 measurement. Plasma bioactive GLP-2 was measured by RIA using an antibody specific to the NH2 terminus of GLP-2 (17). Plasma IGF-I was measured by RIA after separation of IGF binding proteins by HPLC, as previously reported (35).
Total RNA was extracted from intact colon and jejunum using the TRIzol reagent (Gibco BRL Life Technologies, Grand Island, NY). All RNA extracts were quantitated by absorbance at 260 nm, and quantity and integrity were confirmed by electrophoresis through 1.25% agarose/2.2 M formaldehyde gels and staining with ethidium bromide to visualize ribosomal RNA bands. Expression of proglucagon mRNA in the colon and GLP-2 receptor mRNA in the jejunum were measured in a two-step reverse transcriptase real-time PCR (RT-qPCR) using the SYBR Green detection method, as described previously (27, 34). Sequences for forward and reverse primers (Integrated DNA Technologies, Coralville, IA) were previously reported (27). Data were analyzed using 7000 system software (Applied Biosystems), and relative quantification was done using the ΔΔCt (cycle threshold) method with β-actin as the internal control and the orally fed group as reference control (32).
Statistical analyses.
Treatment groups were analyzed using general linear models; differences among the treatment groups were assessed by one-way ANOVA followed by the protected least significant differences technique (SAS version 8.2; SAS Institute, Cary, NC). Statistics were performed on log-transformed data for results showing unequal variances among groups. All data are presented as means ± SE; P < 0.05 was considered statistically significant.
RESULTS
Body weight and mortality.
There were no significant differences in initial body weight (200–230 g) among the five groups treated with TPN + resection on the day of surgery, and rats regained their presurgical body weight by 3–4 days after surgery. The mean gain in body weight for the first 7 days after surgery ranged from 4 to 12 ± 4 g and for the entire 18 days period from 23 to 31 ± 4 g without significant differences among the treatment groups for 18-day weight gain. The TPN control group showed an increased 18-day mortality rate compared with the groups given GLP-2 treatment. Three of eight rats randomized to the TPN control group or 37.5% died, whereas none of the seven rats assigned to each of the sustained or delayed GLP-2 groups died (P = 0.08, by log rank test).
Plasma GLP-2, colon proglucagon expression, and plasma IGF-I.
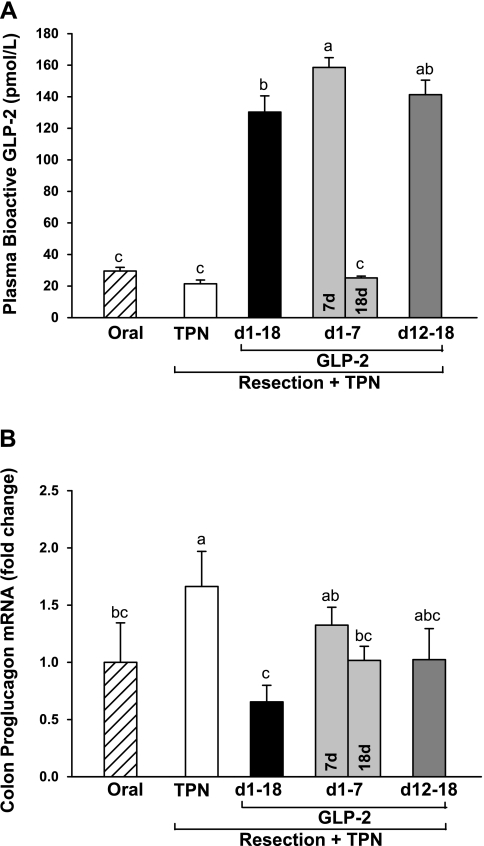
Plasma concentration of bioactive GLP-2 was significantly increased by approximately fivefold in the treatment groups receiving sustained, early (7 days), or delayed (12–18 days) GLP-2 treatment compared with oral reference and TPN control groups (Fig. 1). TPN control and oral reference groups (fed 90–120 min before sampling) showed similar concentrations of GLP-2 in plasma. Consistent with GLP-2 having a short half-life of ∼7 min (8), cessation of GLP-2 infusion after 7 days with euthanasia at 18 days resulted in plasma GLP-2 concentration not different from oral and TPN control. The early 7-day GLP-2 group showed significantly, ∼20% higher, plasma GLP-2 concentration compared with sustained GLP-2 treatment at 18 days, most likely due to a transient increase in endogenous GLP-2 production noted in this model at 4–7 days after resection (27).
Fig. 1.
Plasma concentration of bioactive glucagon-like peptide-2 (GLP-2; A) and proglucagon expression in colon (B) in rats subjected to distal small bowel resection (60% jejunoileal resection + cecectomy) and maintained with total parental nutrition (TPN) plus GLP-2 infusion. The treatment groups included the following: TPN control (no GLP-2, n = 5); sustained GLP-2 [1–18 days (d), n = 7]; early GLP-2 (1–7 days, killed at 7 days, n = 10, or 18 days, n = 8); and delayed GLP-2 (12–18 days, n = 8). The oral group includes nonsurgical reference animals fed ad libitum, n = 8. Values are means + SE. a,b,c Means with different superscripts are significantly different (P < 0.05).
Colon proglucagon expression was significantly increased in the TPN control group compared with oral reference (Fig. 1). This increase in endogenous proglucagon expression in colon was blunted in all of the GLP-2 treatment groups to the level noted in the oral reference group. Significantly greater proglucagon expression was noted in the early GLP-2, 7 days, compared with the sustained GLP-2 group consistent with higher plasma GLP-2 concentration at 7 days. Jejunal expression of the GLP-2 receptor showed considerable variation, and there were no significant differences among all groups (data not shown).
Plasma IGF-I concentration was significantly reduced in all TPN + resection groups by 20–25% compared with oral reference (oral, 40 ± 2; 18-day GLP-2 groups, 31–34 ± 2; and 7-day GLP-2, 25 ± 1 nmol IGF-I/l), as previously noted when comparing TPN and oral feeding (34). The early GLP-2 7-day group showed a significantly greater decrease in plasma IGF-I concentration compared with the 18-day resection treatment groups, suggesting that the stress of gut resection may impair IGF-I secretion in the early postsurgical period. The concentration of IGF-I in plasma was not correlated with the concentration of GLP-2.
Mucosal adaptive growth and sucrase activity.
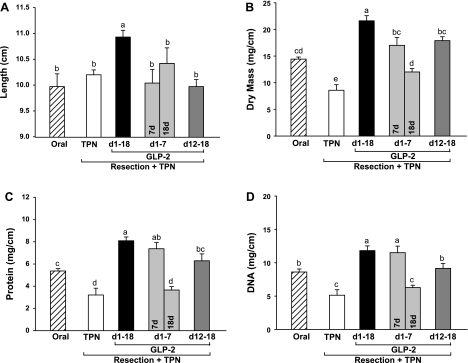
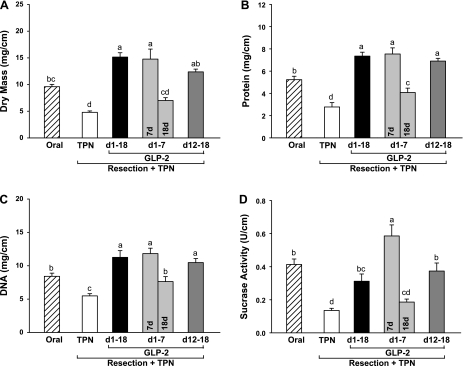
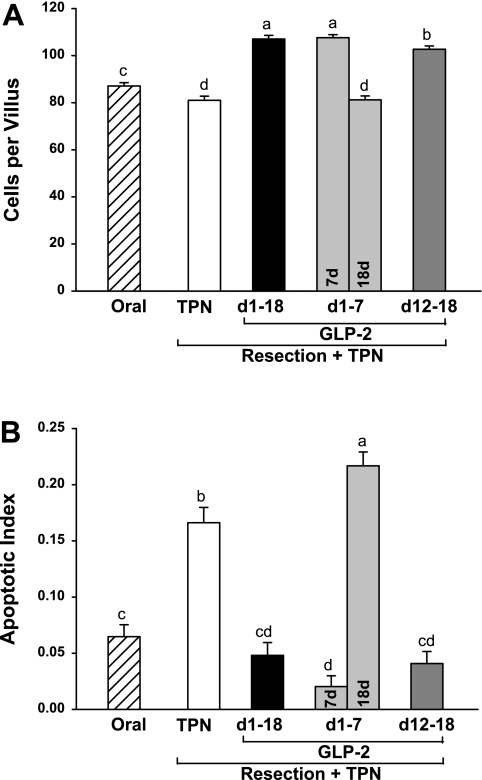
As expected in this model of intestinal failure (13, 27, 29, 31, 37), resected TPN control rats showed an absence of adaptive growth in residual duodenum and jejunum with significantly decreased mucosal dry mass and concentrations of mucosal protein and DNA compared with oral reference (Figs. 2 and 3). Sustained, early 7-day and delayed GLP-2 treatment reversed this mucosal atrophy, such that mucosal cellularity in duodenum and jejunum based on dry mass, protein, and DNA was significantly higher than observed in TPN control and, in some instances, oral reference animals. Strikingly, cessation of GLP-2 infusion at 7 days in the early GLP-2 group with euthanasia at 18 days reversed the mucosal hyperplasia induced by early treatment with GLP-2 to levels not different than observed in the TPN control group. Increased jejunal cellularity due to GLP-2 treatment was confirmed by a significantly greater number of cells per villus and crypt columns with GLP-2 treatment compared with TPN control (Figs. 4 and 5), as well as increases in villus height and crypt depth. These effects were reversed with cessation of GLP-2 infusion at 7 days. GLP-2 treatment did not alter colon mass or morphology (data not shown), as previously reported (13, 27, 29, 31).
Fig. 2.
Duodenum length (A), dry mucosal mass (B), and concentrations of mucosal protein (C) and DNA (D) in rats subjected to distal small bowel resection (60% jejunoileal resection + cecectomy) and maintained with TPN plus GLP-2 infusion. The oral group includes nonsurgical reference animals fed ad libitum. Values are means + SE. a,b,c,d Means with different superscripts are significantly different (P < 0.05).
Fig. 3.
Jejunum mucosa dry mass (A), concentrations of protein (B) and DNA (C), and sucrase activity (D) in rats subjected to distal small bowel resection (60% jejunoileal resection + cecectomy) and maintained with TPN plus GLP-2 infusion. The oral group includes nonsurgical reference animals fed ad libitum. Values are means + SE. a,b,c,d Means with different superscripts are significantly different (P < 0.05).
Fig. 4.
Jejunum villus cell number (A) and apoptotic index (B) in rats subjected to distal small bowel resection (60% jejunoileal resection + cecectomy) and maintained with TPN plus GLP-2 infusion. The apoptotic index is defined as the total number of apoptotic cells, determined by a pathologist based on morphological characteristics, expressed as a percentage of the total number of cells counted in 50 well-oriented villus columns. The oral group includes nonsurgical reference animals fed ad libitum. Values are means + SE. a,b,c,d Means with different superscripts are significantly different (P < 0.05).
Fig. 5.
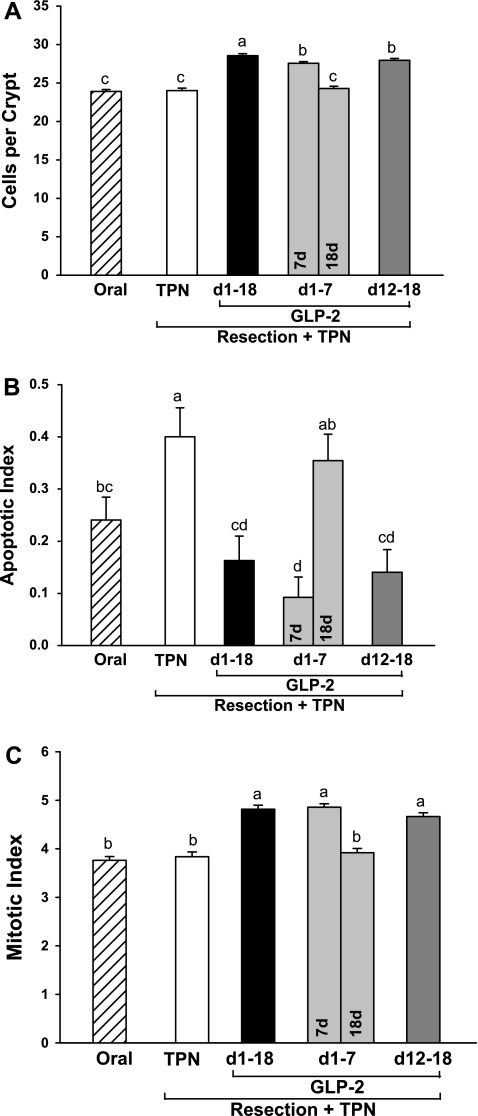
Jejunum crypt cell number (A), apoptotic index (B), and mitotic index (C) in rats subjected to distal small bowel resection (60% jejunoileal resection + cecectomy) and maintained with TPN plus GLP-2 infusion. The apoptotic and mitotic indexes are defined as the total number of apoptotic or mitotic cells, determined by a pathologist based on morphological characteristics, expressed as a percentage of the total number of cells counted in 50 well-oriented crypts. The oral group includes nonsurgical reference animals fed ad libitum. Values are means + SE. a,b,c,d Means with different superscripts are significantly different (P < 0.05).
Duodenal length was significantly increased in animals given sustained GLP-2 infusion for 18 days compared with TPN control, GLP-2 treatment, and oral reference groups (Fig. 2). The length of residual jejunum and colon showed a large variation, and there were no differences among groups.
Mucosal sucrase activity reflects the digestive capacity of the small intestine. Sucrase activity in duodenum and jejunum declined with TPN and was rescued by GLP-2 treatment, with the highest activity (U/cm mucosa) observed in the early GLP-2 7-day group (Fig. 3). Sucrase activity in the sustained and delayed GLP-2 treatment groups was significantly greater than TPN control and not different from oral reference. Cessation of GLP-2 treatment at 7 days in the early GLP-2 18-day group reversed the increase in sucrase activity to levels noted in TPN control.
Jejunal apoptosis, mitosis, and crypt fission.
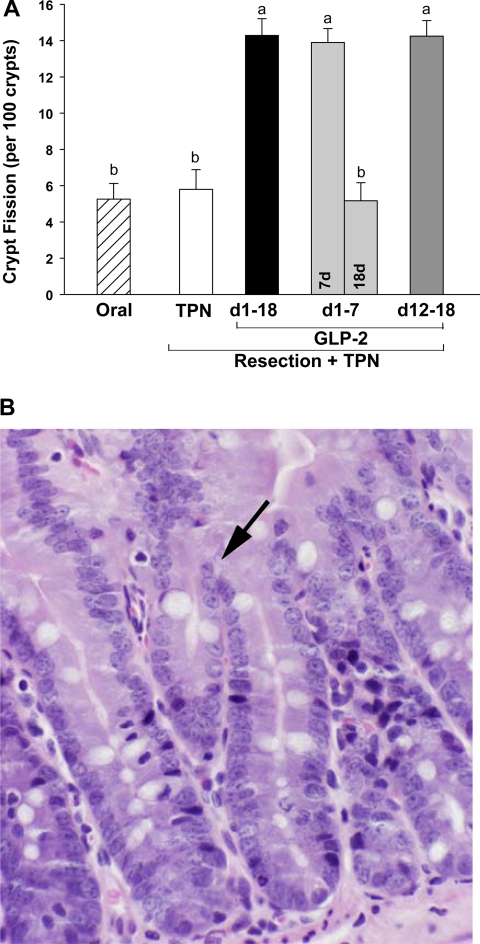
Increased jejunal cellularity due to GLP-2 treatment was consistently associated with a significantly decreased apoptotic index in both the crypt and villus compartments and an increased crypt mitotic index in residual jejunum compared with TPN control (Figs. 4 and 5). There was a dramatic and consistent 2.5-fold increase in the occurrence of crypt fission per 100 crypts with early 7-day, sustained, or delayed GLP-2 infusion compared with TPN control (Fig. 6). Consistent with the TPN control group showing an absence of adaptive growth in residual jejunum, the apoptotic index was increased 66% in the jejunum crypt and 140% in the jejunum villus compartments compared with the oral reference group. In contrast, the mitotic index for jejunum crypt and crypt fission were unchanged in the TPN control compared with the oral reference group.
Fig. 6.
The incidence of crypt fission in the jejunum in rats subjected to distal small bowel resection and maintained with TPN plus GLP-2 infusion (A) and a representative section (×100) from a GLP-2-treated animal with an arrow indicating crypt fission (B). The oral group includes nonsurgical reference animals fed ad libitum. Values are means + SE. a,b Means with different superscripts are significantly different (P < 0.05).
Strikingly and consistent with data for jejunum mass, protein, DNA, and cell number, cessation of GLP-2 infusion at 7 days in the early GLP-2 18-day group reversed the effects of GLP-2 to decrease apoptosis and increase mitosis and crypt fission, such that kinetic parameters were not different from TPN control levels. Thus cessation of GLP-2 infusion after 7 days produced a response not different from never infusing GLP-2. Moreover, in the jejunum villus compartment, the apoptotic index was significantly higher after cessation of GLP-2 at 7 days compared with TPN control, suggesting a persistent detrimental effect due to cessation of GLP-2 infusion.
Sustained (1–18 days) compared with delayed (12–18 days) infusion of GLP-2 after resection showed improvements in jejunum cellularity. The total number of cells in the crypt and villus compartments was significantly higher with sustained compared with delayed infusion of GLP-2. Differences in the apoptotic and mitotic indexes were not associated with the expansion of jejunum cellularity within this 18-day experiment.
DISCUSSION
GLP-2 is a proglucagon-derived intestinotrophic hormone synthesized in the ileum and colon. Humans with massive resection of the jejunum, ileum, and colon (often with an end jejunostomy), resulting in extreme SBS, have impaired GLP-2 secretion (20) and minimal adaptation of residual bowel, resulting in intestinal failure and the need for TPN or PN to survive. Administration of GLP-2 or teduglutide to humans with intestinal failure at least 12 mo from their most recent bowel resection has shown promising results to improve fluid and nutrient absorption (21–24). However, these effects are not sustained after GLP-2 is stopped (23), and questions remain about the optimal time to administer GLP-2 after resection and the effects of cessation of GLP-2 on enterocyte kinetics. In this study, we have used a well-characterized, TPN-dependent rat model of SBS with intestinal failure (13–15, 27, 31) that produces limited endogenous GLP-2 to determine effects of the timing, duration, and cessation of GLP-2 administration on intestinal adaptation and enterocyte kinetics. Results indicate that early, sustained GLP-2 infusion (1–18 days after resection) increases jejunal cellularity and absorptive surface area by increasing crypt fission and mitosis and decreasing apoptosis, and that these effects are reversed when GLP-2 infusion stops.
Infusion of GLP-2 for the first 7 days postresection in a model that mimics extreme human SBS increased cellularity of the duodenum and jejunum above that observed in oral control animals. However, when GLP-2 infusion stopped after 7 days, the intestinal adaptive growth was completely reversed when examined in animals euthanized at 18 days compared with 7 days. This resulted in a response not different from the absence of resection-induced intestinal adaptive growth noted in TPN control animals not given GLP-2. The cellular events underlying intestinal growth showed an identical response in that significant observations of GLP-2-induced crypt fission, increased crypt mitosis, and decreased apoptosis in both the crypt and villus compartments at 7 days were all reversed by 18 days with cessation of GLP-2 infusion for 10 days. This reversal of the mitogenic effects of GLP-2 can be attributed to the cessation of exogenous GLP-2, as our detailed time course study established that the endogenous GLP-2 response after resection does not differ from transection controls in this model (27). Previous studies assessing the effects of early vs. late administration of GLP-2 in resection models have not included the appropriate controls to assess intestinal status at the time GLP-2 infusion stops compared with a later time point preceded by earlier administration of GLP-2 (12, 25). Moreover, they have used models with residual ileum that contributes endogenous GLP-2 and confounds questions about the effects of exogenous GLP-2 (25). For example, oral feeding in rats with proximal mid-small bowel resection and residual ileum increases plasma GLP-2 concentration two- to threefold (3, 33) compared with transection control; whereas oral feeding does not increase plasma GLP-2 in the current resection model (27, 31). Thus, to our knowledge, this is the first explanation of the changes in enterocyte kinetics that accompany the loss of GLP-2's beneficial intestinal effects when administration stops, as noted by Jeppesen et al. (23) in GLP-2 clinical trials.
Studies in mice demonstrate that expansion of intestinal progenitors and putative stem cells occurs early, within 4 days following ileo-cecal resection, and precede increases in crypt fission, which is an important mechanism for increasing crypt number and mucosal mass (12, 39). In the present study, we observed increased crypt fission and mucosal mass with early (1–7 days), sustained (1–18 days), or delayed (12–18 days) GLP-2 infusion. Compared with the detailed study by Garrison et al. in mice (12), GLP-2-induced crypt fission and mucosal mass were observed over a longer period of time, 1–12 days in rats compared with 4–7 days in mice. Assuming that expansion of intestinal stem cells precedes crypt fission (12, 39), this also suggests a longer window of opportunity for early expansion of putative intestinal stem cells in the rat (1–12 days) compared with the mouse (4–7 days). Alternatively, the capacity for intestinal adaptation following intestinal resection in the mouse and rat models may differ and explain our observations. For example, the orally fed mouse resection model adapts on its own, as evidenced by mitogenic and morphogenic comparisons at 6 wk compared with 7 days postresections, suggesting endogenous production of GLP-2 or other growth factors (12). In contrast, the current rat model of SBS does not adapt in the absence of exogenous GLP-2, even with oral feeding (14, 27, 31). Further research is needed regarding the role of GLP-2 in expansion of putative stem cells during the adaptive phase following intestinal resection in various models, and how this relates to the potential for intestinal adaptation after bowel resections in humans.
Sustained infusion of GLP-2 during the entire 18 days period postresection produced greater improvements in intestinal adaptation compared with delayed infusion of GLP-2 from 12 to 18 days. Despite similar plasma levels of GLP-2, jejunal crypt and villus cell number reflecting intestinal absorptive capacity were modestly but significantly greater with sustained compared with delayed GLP-2 infusion. Moreover, duodenal length was significantly increased with sustained compared with delayed GLP-2 infusion, although the length of residual jejunum showed high variation and did not show differences. Given the importance of increased intestinal length in predicting absorptive capacity, the finding of greater duodenum length supports the greater intestinal adaptation induced by sustained GLP-2 infusion. Although proglucagon expression was decreased with GLP-2 infusion compared with TPN controls, it was not lower than observed in oral controls, and there were no differences in abundance of GLP-2 receptor mRNA among groups. Moreover, the increased colonic proglucagon expression induced by resection alone, i.e., TPN control group, in this model was not sufficient to increase circulating levels of GLP-2 to a level greater than 50 pM GLP-2, which we have observed is the threshold for intestinal adaptation (27, 31).
The decreased mortality in animals receiving sustained or delayed GLP-2 infusion compared with TPN controls for 18 days after surgery is noteworthy and consistent with empirical observations in our laboratory over a number of years. The deaths in the TPN control group were of unknown etiology and did not appear to be related to infusion problems or surgery, as they occurred at 8, 12, and 15 days after surgery. Possible explanations for the increased survival of animals given GLP-2 include reduced septic complications due to improved immune function, in association with the anti-inflammatory effects of GLP-2 in the intestinal mucosa (18), and improved postsurgical wound healing, given that GLP-2 increases intestinal blood flow due to increased nitric oxide synthesis in TPN-fed piglets (16). Moreover, Jeppesen and Hellstrom (19) recently demonstrated increased mucosal microcirculation in jejunal stoma of patients with SBS given GLP-2, consistent with the potential for GLP-2 to improve wound healing after intestinal resection. The basis for the survival benefit associated with GLP-2 administration deserves further investigation.
Increased mucosal sucrase activity due to GLP-2 infusion suggests improved digestive capacity in the absence of enteral nutrients. Interestingly, the highest sucrase activity was observed in the early GLP-2 group killed at 7 days. Given that sucrase activity decreased from 7 to 18 days with sustained infusion of GLP-2, greater sucrase activity with GLP-2 treatment at 7 days appears to reflect the time course of the sucrase response to GLP-2, and not a specific benefit of early treatment with GLP-2. Our previous research demonstrates that enteral nutrients provide a stronger stimulus than GLP-2 to increase sucrase activity (31). This response reflects the differential effects of GLP-2 to promote proliferation of enterocytes and enteral nutrients to promote maturation and differentiation of enterocytes with greater sucrase activity. The combination of supplemental enteral nutrients and GLP-2 infusion in this model synergistically increases mucosal growth and sucrase activity (31), demonstrating the importance of both hormonal and nutritional therapy to promote intestinal adaptation after bowel resection.
Continuous infusion of native GLP-2 was utilized in the present study. This contrasts with the approach used in clinical trials, where GLP-2 is administered three times daily by subcutaneous injection to mimic physiological, meal-induced increases in plasma GLP-2 concentration (21–23). We observe similar reversal of the benefits of GLP-2 with cessation of continuous infusion in the rat compared with cessation of intermittent injections in humans. To better understand the application of rodent models to human SBS, it will be important in future experiments to determine how different methods of administering native GLP-2 and teduglutide impact enterocyte kinetics.
In summary, this study provides novel information about how cessation of GLP-2 therapy reverses the mitogenic effects of GLP-2 in a rat model of SBS that produces minimal endogenous GLP-2, resulting in intestinal failure, as occurs in humans with extreme SBS. The data suggest that delayed administration of GLP-2 after resection promotes intestinal adaptation in the current rat model, as noted in humans treated with GLP-2. The greatest benefit to GLP-2 therapy, however, occurs with early and sustained GLP-2 “replacement” therapy, especially for those patients with resection of ileum and colon and limited capacity to secrete endogenous GLP-2.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-42835 and T32-DK-07665 and the C. Richard Fleming Grant awarded to M. C. Koopmann from the ASPEN Rhoads Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Michael J. Grahn, Sangita Murali, Wing Pun, and Patrick Solverson for expert technical assistance. We acknowledge the generous contribution of Vital formula from Abbott Nutrition.
REFERENCES
- 1.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology 146: 22–32, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem 7: 18–25, 1964 [DOI] [PubMed] [Google Scholar]
- 3.Dahly EM, Gillingham MB, Guo Z, Murali SG, Nelson DW, Holst JJ, Ney DM. Role of luminal nutrients and endogenous GLP-2 in intestinal adaptation to mid-small bowel resection. Am J Physiol Gastrointest Liver Physiol 284: G670–G682, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Dahly EM, Guo Z, Ney DM. Alterations in enterocyte proliferation and apoptosis accompany TPN-induced mucosal hypoplasia and IGF-I-induced hyperplasia in rats. J Nutr 132: 2010–2014, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Dahly EM, Guo Z, Ney DM. IGF-I augments resection-induced mucosal hyperplasia by altering enterocyte kinetics. Am J Physiol Regul Integr Comp Physiol 285: R800–R808, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Dekaney CM, Gulati AS, Garrison AP, Helmrath MA, Henning SJ. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol 297: G461–G470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiBaise JK, Young RJ, Vanderhoof JA. Intestinal rehabilitation and the short bowel syndrome: part 1. Am J Gastroenterol 99: 1386–1395, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Dube PE, Brubaker PL. Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. Am J Physiol Endocrinol Metab 293: E460–E465, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dube PE, Forse CL, Bahrami J, Brubaker PL. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology 131: 589–605, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Estall JL, Drucker DJ. Glucagon-like peptide-2. Annu Rev Nutr 26: 391–411, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Ferrone M, Scolapio JS. Teduglutide for the treatment of short bowel syndrome. Ann Pharmacother 40: 1105–1109, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Garrison AP, Dekaney CM, von Allmen DC, Lund PK, Henning SJ, Helmrath MA. Early but not late administration of glucagon-like peptide-2 following ileo-cecal resection augments putative intestinal stem cell expansion. Am J Physiol Gastrointest Liver Physiol 296: G643–G650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillingham MB, Dahly EM, Carey HV, Clark MD, Kritsch KR, Ney DM. Differential jejunal and colonic adaptation due to resection and IGF-I in parenterally fed rats. Am J Physiol Gastrointest Liver Physiol 278: G700–G709, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Gillingham MB, Dahly EM, Murali SG, Ney DM. IGF-I treatment facilitates transition from parenteral to enteral nutrition in rats with short bowel syndrome. Am J Physiol Regul Integr Comp Physiol 284: R363–R371, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Gillingham MB, Kritsch KR, Murali SG, Lund PK, Ney DM. Resection upregulates the IGF-I system of parenterally fed rats with jejunocolic anastomosis. Am J Physiol Gastrointest Liver Physiol 281: G1158–G1168, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, Burrin DG. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology 125: 136–147, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Hartmann B, Johnsen AH, Orskov C, Adelhorst K, Thim L, Holst JJ. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides 21: 73–80, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Ivory CP, Wallace LE, McCafferty DM, Sigalet DL. Interleukin-10-independent anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 295: G1202–G1210, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Jeppesen P, Hellstrom P. GLP-2 stimulates mucosal microcirculation measured by laser Doppler flow in jejunal stoma of patients with short bowel syndrome (Abstract). In: Digestive Diseases Week New Orleans, LA. Bethesda, MD: Digestive Diseases Week, 2010, no. T1765 [Google Scholar]
- 20.Jeppesen PB, Hartmann B, Hansen BS, Thulesen J, Holst JJ, Mortensen PB. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut 45: 559–563, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, Mortensen PB. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120: 806–815, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Jeppesen PB, Lund P, Gottschalck IB, Nielsen HB, Holst JJ, Mortensen J, Poulsen SS, Quistorff B, Mortensen PB. Short bowel patients treated for two years with glucagon-like peptide 2 (GLP-2): compliance, safety, and effects on quality of life. Gastroenterol Res Pract 2009: 425759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeppesen PB, Lund P, Gottschalck IB, Nielsen HB, Holst JJ, Mortensen J, Poulsen SS, Quistorff B, Mortensen PB. Short bowel patients treated for two years with glucagon-like peptide 2: effects on intestinal morphology and absorption, renal function, bone and body composition, and muscle function. Gastroenterol Res Pract 2009: 616054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeppesen PB, Sanguinetti EL, Buchman A, Howard L, Scolapio JS, Ziegler TR, Gregory J, Tappenden KA, Holst J, Mortensen PB. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut 54: 1224–1231, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaji T, Tanaka H, Redstone H, Wallace LE, Holst JJ, Sigalet DL. Temporal changes in the intestinal growth promoting effects of glucagon-like peptide 2 following intestinal resection. J Surg Res 152: 271–280, 2009 [DOI] [PubMed] [Google Scholar]
- 26.King KL, Cidlowski JA. Cell cycle and apoptosis: common pathways to life and death. J Cell Biochem 58: 175–180, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Koopmann MC, Liu X, Boehler CJ, Murali SG, Holst JJ, Ney DM. Colonic GLP-2 is not sufficient to promote jejunal adaptation in a PN-dependent rat model of human short bowel syndrome. JPEN J Parenter Enteral Nutr 33: 629–638; discussion 638–629, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koopmann MC, Nelson DW, Murali SG, Liu X, Brownfield MS, Holst JJ, Ney DM. Exogenous glucagon-like peptide-2 (GLP-2) augments GLP-2 receptor mRNA and maintains proglucagon mRNA levels in resected rats. JPEN J Parenter Enteral Nutr 32: 254–265, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kripke SA, De Paula JA, Berman JM, Fox AD, Rombeau JL, Settle RG. Experimental short-bowel syndrome: effect of an elemental diet supplemented with short-chain triglycerides. Am J Clin Nutr 53: 954–962, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 102: 344–352, 1980 [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Nelson DW, Holst JJ, Ney DM. Synergistic effect of supplemental enteral nutrients and exogenous glucagon-like peptide 2 on intestinal adaptation in a rat model of short bowel syndrome. Am J Clin Nutr 84: 1142–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Nelson DW, Liu X, Holst JJ, Raybould HE, Ney DM. Vagal afferents are essential for maximal resection-induced intestinal adaptive growth in orally fed rats. Am J Physiol Regul Integr Comp Physiol 291: R1256–R1264, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Nelson DW, Murali SG, Liu X, Koopmann MC, Holst JJ, Ney DM. Insulin-like growth factor I and glucagon-like peptide-2 responses to fasting followed by controlled or ad libitum refeeding in rats. Am J Physiol Regul Integr Comp Physiol 294: R1175–R1184, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Ney DM, Yang H, Smith SM, Unterman TG. High-calorie total parenteral nutrition reduces hepatic insulin-like growth factor-I mRNA and alters serum levels of insulin-like growth factor-binding protein-1, -3, -5, and -6 in the rat. Metabolism 44: 152–160, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Potten CS, Wilson JW, Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells 15: 82–93, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Scarpello JH, Cary BA, Sladen GE. Effects of ileal and caecal resection on the colon of the rat. Clin Sci Mol Med 54: 241–249, 1978 [DOI] [PubMed] [Google Scholar]
- 38.Scott RB, Kirk D, MacNaughton WK, Meddings JB. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol Gastrointest Liver Physiol 275: G911–G921, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Wright NA. Epithelial stem cell repertoire in the gut: clues to the origin of cell lineages, proliferative units and cancer. Int J Exp Pathol 81: 117–143, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Q, Kock ND. Intestinal adaptation following massive ileocecal resection in 20-day-old weanling rats. J Pediatr Gastroenterol Nutr 50: 16–21, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.