Abstract
In the stomach, strictly regulated cell adherens junctions are crucial in determining epithelial cell differentiation. Sonic Hedgehog (Shh) regulates epithelial cell differentiation in the adult stomach. We sought to identify whether Shh plays a role in regulating adherens junction protein E-cadherin as a mechanism for epithelial cell differentiation. Mouse nontumorigenic gastric epithelial (IMGE-5) cells treated with Hedgehog signaling inhibitor cyclopamine and anti-Shh 5E1 antibody or transduced with short hairpin RNA against Skinny Hedgehog (IMGE-5Ski) were cultured. A mouse model expressing a parietal cell-specific deletion of Shh (HKCre/ShhKO) was used to identify further changes in adherens and tight junctions. Inhibition of Hedgehog signaling in IMGE-5 cells caused loss of E-cadherin expression accompanied by disruption of F-actin cortical expression and relocalization of zonula occludens-1 (ZO-1). Loss of E-cadherin was also associated with increased proliferation in IMGE-5Ski cells and increased expression of the mucous neck cell lineage marker MUC6. Compared with membrane-expressed E-cadherin and ZO-1 protein in controls, dissociation of E-cadherin/β-catenin and ZO-1/occludin protein complexes was observed in HKCre/ShhKO mice. In conclusion, we demonstrate that Hedgehog signaling regulates E-cadherin expression that is required for the maintenance of F-actin cortical expression and stability of tight junction protein ZO-1.
Keywords: IMGE-5 cells; epithelial-to-mesenchymal-transition, zonula occludens-1
sonic hedgehog (Shh) is a known regulator of epithelial cell function and differentiation in the adult stomach (31–33, 36, 38), but we are only beginning to understand the underlying mechanisms. Loss of Shh expression during Helicobacter pylori infection is strongly correlated with the disruption of normal epithelial cell differentiation (31, 33). Recent work from our laboratory using a mouse model expressing a parietal cell-specific deletion of Shh demonstrated that loss of Shh triggered a number of molecular events, which were consistent with epithelial-to-mesenchymal transition (EMT) of gastric epithelial cells (21, 38). Such changes included increased Snail and loss of E-cadherin expression, translocation of β catenin, and activation of the Wnt pathway (38). Snail is a known repressor of E-cadherin (21), and destruction of adherens junctions plays a central role in the development of EMT, by which epithelial cells lose their polarity (16).
The integrity of adherens and tight junctions is a crucial factor determining epithelial morphology, physiological function, and differentiation (4, 15, 29, 40). The adherens junction is morphologically associated with actin filaments (25). E-cadherin directly binds to β-catenin, which in turn binds to actin filament binding protein α-catenin (39). The adherens junction cadherin/β-catenin/α-catenin protein complex is morphologically associated with actin filaments (25). In the stomach, expression of tight junction protein zonula occludens-1 (ZO-1) is required for maintaining the organization of the epithelium that is necessary for the cell migration and maturation of cell lineages such as the chief cells (6, 40). The disruption of the tight junction and apical junctional complex is characteristic of a number of diseases including cancer (reviewed in Ref. 24). Tight junctions, together with adherens junctions and desmosomes, form the junctional complex (9). Tight junctions form a network of scaffolding proteins that appear as sites of fusion between the outer plasma membrane of adjacent cells of a polarized epithelium (reviewed in Ref. 24). Extensive studies using cultures of Madin-Darby canine kidney (MDCK) cells demonstrate that the stability of tight junctions is dependent on the formation and maintenance of adherens junctions and the actin cytoskeleton (39). Consistent with this notion, it would be predicted that loss of E-cadherin and disorganization of the actin cytoskeleton would result in the dissociation of membrane-expressed ZO-1 contributing to the development of EMT. However, the mechanisms regulating adherens and tight junctions in the adult stomach are not clearly understood.
Shh is synthesized as a 45-kDa precursor protein that subsequently undergoes either an autocatalytic or protease cleavage to yield a 26-kDa carboxy-terminal fragment and a 19-kDa amino-terminal fragment (7). The 19-kDa fragment is further modified by a membrane bound O-acyltransferase commonly known as Skinny Hedgehog (Ski), which covalently links a molecule of palmitate to the 19-kDa fragment (7). Shh signaling in vertebrates is relayed via the seven-span transmembrane receptor Smoothened (Smo) (10, 34). Shh does not directly bind to Smo, but instead indirectly controls the activity of Smo through binding to a second receptor Patched (Ptch). In the absence of Shh, Ptch inhibits Smo. Binding of Shh to Ptch results in the removal of the inhibition of Ptch on Smo and subsequently enables the passage of the signaling cascade and the activation of transcription factor Gli (27, 35). Transduction of the Hedgehog signal into the cytoplasm leads to activation of the Glioblastoma (Gli) family of transcription factors (10, 34) (Fig. 1). In the stomach, Gli induces transcription of a number of signaling targets such as Wnt (38) as a mechanism of regulating cell proliferation, H+-K+-ATPase expression regulating gastric parietal cell function (32). Evidence from the dental epithelium shows that E-cadherin and ZO-1 are also targets of Gli controlling cell size and polarity (11), but in the stomach whether these adherens and tight junction proteins are Hedgehog signaling targets is unknown.
Fig. 1.
Schematic diagram of the Hedgehog signaling pathway. Sonic Hedgehog (Shh) is synthesized as a 45-kDa precursor protein that undergoes cleavage to yield a 26-kDa carboxy-terminal fragment and a 19-kDa amino-terminal fragment. The 19-kDa fragment is further modified by membrane bound O-acyltransferase Skinny Hedgehog (Ski), which covalently links a molecule of palmitate to the 19-kDa fragment. In the absence of Shh, Ptch inhibits Smo. Binding of Shh to Ptch results in the removal of the inhibition of Ptch on Smo and enables the passage of the signaling cascade and the activation of transcription factor Gli. The Hedgehog signaling pathway was blocked by treating mouse nontumorigenic gastric epithelial (IMGE-5) cells with Smo inhibitor cyclopamine (1), anti-Shh 5E1 antibody (2), or knockdown of Ski by transducing Ski short hairpin RNA (shRNA) into IMGE-5 cells by using a lentiviral vector (3) to inhibit the synthesis of biologically active Shh protein secreted from IMGE-5.
The present study sought to identify whether Shh signaling regulates adherens junctions as a mechanism for epithelial cell differentiation and maintenance of tissue integrity. Effects of Shh on adherens and tight junctions were studied in mouse nontumorigenic gastric epithelial (IMGE-5) cells treated with Hedgehog signaling inhibitor cyclopamine (1 in Fig. 1) and anti-Shh 5E1 antibody (2 in Fig. 1) or transduced with short hairpin RNA (shRNA) against Skinny Hedgehog (IMGE-5Ski) (3 in Fig. 1) and mice expressing a parietal cell-specific Shh (HKCre/ShhKO) gene disruption. Using these approaches, the present study demonstrates that Hedgehog signaling regulates E-cadherin expression that is required for the maintenance of F-actin cortical expression and stability of tight junction protein ZO-1.
MATERIALS AND METHODS
Cell line culture conditions for treated IMGE-5 cells.
IMGE-5 cells were grown initially under permissive conditions that included DMEM (Fisher Scientific) containing 10% FCS (Fisher Scientific), 1% penicillin-streptomycin (Fisher Scientific), and 1 U/ml IFN-γ (Sigma Aldrich) at 33°C. Once cells reached confluency they were seeded onto 12-well polyester permeable membranes (Corning Life Sciences), transferred into medium without IFN-γ, and incubated at 39°C and grown under nonpermissive conditions for 48 h (13, 14). Once confluent after 48 h, vehicle (0.1% DMSO/PBS), cyclopamine (Smo inhibitor, 10 μM, Sigma Aldrich), or anti-Shh 5E1 antibody (10 μg/ml, Developmental Studies Hybridoma Bank University of Iowa) was added to culture medium at time 0 h. After 24 h of treatment vehicle- and cyclopamine-treated cells were washed and fresh medium was added without vehicle or cyclopamine. For the wells treated with 5E1 antibody, medium was changed every 24 h with fresh 5E1 antibody. Monolayer formation was monitored by using an epithelial-volt-Ohmmeter (EVOM, World Precision Instruments) that measured the specific transepithelial electrical resistance (TEER). The approximate resistance reading of the solution was 64 ± 1.49 Ω·cm2, which was subtracted from the final TEER reading measured from the cultured cells. Transwell permeable membranes were collected from cultured IMGE-5 cells at 0, 6, 12, 24, 48, and 72 h, fixed, and immunostained for E-cadherin, ZO-1, and F-actin as detailed under Immunofluorescence. In a separate series of experiments, total protein was isolated from vehicle-, cyclopamine-, and 5E1-treated IMGE-5 cells at 0, 6, 12, 24, 48, and 72 h, and E-cadherin, β-catenin, occludin, and ZO-1 expression were measured by immunoprecipitation and Western blot analysis (see Immunoprecipitation and Western blot below). Total RNA was extracted from vehicle-, cyclopamine-, and 5E1-treated IMGE-5 cells at 0, 6, 12, 24, 48, and 72 h and analyzed for Ptch, Smo, and Gli mRNA expression by quantitative RT-PCR (qRT-PCR; refer to qRT-PCR below).
Two-color fluorescence cell viability assay.
To identify potential cytotoxicity of vehicle, cyclopamine, and anti-Shh 5E1 antibody treatments on IMGE-5 cells, a two-color fluorescence cell viability flow cytometric assay with the fluorescent probes ethidium homodimer (EthD-1) and calcein AM were used (Invitrogen, Carlsbad, CA). Live cells were identified by the presence of intracellular esterase activity, determined by the enzymatic conversion of calcein AM to calcein with green fluorescence. EthD-1 enters cells via damaged membranes and produced a red fluorescence upon binding to nucleic acid of dead cells. Cells were harvested at 24 h after treatment and cell viability assayed by flow cytometry according to the manufacturer's protocol by using a FACSCalibur system (Becton Dickinson).
Transduction of IMGE-5 cells.
To identify the role of Hedgehog signaling in the regulation of adherens and tight junctions, the effect of knockdown of Ski, an acetyltransferase that functions as the Hedgehog palmitoyl-transferase (7), was determined by transducing Ski shRNA into IMGE-5 cells by use of a lentiviral vector. A PIKO.1 base lentiviral vector expressing a short hairpin sequence targeting the transcript of either the mouse and human Ski (Ski3, CGTGAGCACCATGTTCAGTTT) or mouse Ski (Ski8, AACGGGCCCATCCTTAACTTC) genes was transduced into IMGE-5 cells (IMGE-5Ski3 and IMGE-5Ski8). As a control, IMGE-5 cells were also transduced with a nonsilencing shRNA PIKO.1 lentiviral vector (IMGE-5Scram). The shRNA lentivirus was made by using HEK293T cells seeded at a density of 6 × 105 cells in a 100-mm petri dish. When cells were 70% confluent, viral packaging constructs and shRNA were transfected by using Lipofectamine 2000 Reagent (Invitrogen) in the following concentrations: 3 μg shRNA, 2.25 μg psPAX2 packaging plasmid, and 750 ng pMD2.G envelope plasmid (kindly donated by Dr. David Robbins). Between 16 and 20 h after transfection, medium was removed and replaced with fresh RPMI medium containing 10% FBS. After 24 h viral medium from cells was removed and filtered through a 0.45-μm filter. Polybrene (1,000×, 8 mg/ml) was added to the medium a final 1× concentration on cells and used for transduction of IMGE-5 cells.
IMGE-5 cells were cultured under permissive conditions (DMEM containing 10% FCS, 1% penicillin-streptomycin, 1 U/ml IFN-γ at 33°C) until 80% confluent in six-well plates (Corning Life Sciences). Once IMGE-5 cells reached 80% confluency, 1 ml of culture medium was removed and 1 ml was replaced with viral medium. After 48 h of incubation, transduced cell lines were kept in permissive condition during selection through the use of DMEM culture medium (10% FCS, 1% penicillin-streptomycin, 1 U/ml IFN-γ) at 33°C with the addition of 10 μg/ml puromycin. The effective puromycin dose needed to eliminate nontransduced cells was determined through a puromycin kill curve that tested concentrations from 0 to 50 μg/ml (data not shown). Once cells reached confluency they were seeded onto 12-well polyester permeable membranes and transferred into medium without IFN-γ or puromycin, incubated at 39°C, and grown under nonpermissive conditions for 48 h prior to Western blot analysis, qRT-PCR, TEER measurements, and immunofluorescence staining.
Animal analyses.
A mouse model expressing a parietal cell-specific deletion of Shh (HKCre/ShhKO) was generated by using transgenic animals bearing loxP sites flanking exon 2 of the Shh gene (Shh loxP, C57Bl/6, 129/Sv background) (kindly donated from Dr. J. A. Whitsett, Department of Pediatrics, University of Cincinnati Children's Hospital Medical Center with permission from Dr. A. P. McMahon, Harvard University) and mice expressing a Cre transgene under the control of the H+-K+-ATPase β subunit promoter (HKCre, C57Bl/6, 129/Sv background, kindly donated by Dr. J. Gordon, Washington University, St. Louis) as previously described (38). Age-matched Shh loxP (homozygous for the loxP sites without the Cre transgene) and HKCre littermates were used as control groups. Mice were analyzed at 8 mo of age. All mouse studies were approved by the University of Cincinnati Institutional Animal Care and Use Committee that maintains an American Association of Assessment and Accreditation of Laboratory Animal Care facility.
A longitudinal section of the stomach (spanning both the fundic and antral regions) was fixed in 4% paraformaldehyde-PBS and paraffin embedded, and 4-μm sections were prepared. Sections were stained for hematoxylin and eosin. For immunofluorescence of mouse gastric tissue, antigen retrieval was performed after deparaffinization by heating the slides for 10 min at 100°C in 0.01 M sodium citrate (Antigen Unmasking Solution, Vector Laboratories, Burlingame, CA). Nonspecific antigenic sites were blocked with 5% BSA in Tris-buffered saline-0.1% Tween 80 (TBS-T) for 30 min before incubating with either 1 μg H+-K+-ATPase β-subunit (Affinity BioReagents) antibody followed by a 1-h incubation with a 1:100 dilution of donkey anti-mouse Alexa Fluor 488 secondary antibody (Invitrogen/Molecular Probes) or a 20 μg/ml Ulex europaeus (UEAI) FITC conjugate (Sigma Aldrich). Sections were then counterstained with a 1:50 dilution of mouse anti-ZO-1 antibody (Invitrogen/Molecular Probes) at 4°C for 16 h followed by a 1-h incubation with a 1:100 dilution of donkey anti-mouse Alexa Fluor 633 secondary antibody (Invitrogen/Molecular Probes). All tissue sections were rinsed and coverslips were mounted by use of Prolong Gold Antifade Reagent Mounting Medium (Invitrogen/Molecular Probes). The slides were allowed to dry at 4°C and viewed under a fluorescence microscope (Olympus BX60 with Diagnostic Instruments “Spot” Camera) or analyzed via a Zeiss LSM510 META confocal microscope.
A Mem-PER eukaryotic membrane protein extraction kit was used for isolating cytoplasmic- and membrane-associated proteins using a reagent-based procedure according (Thermo Scientific). According to the manufacturer's protocol, tissue was homogenized and subjected to a series of reagent-based and centrifugation extractions to yield cytoplasmic- and membrane-associated proteins. Extracted proteins were then immunoprecipitated and analyzed for E-cadherin, β-catenin, occludin, and ZO-1 expression by Western blot (see Immunoprecipitation and Western blot analysis).
Immunofluorescence.
IMGE-5 cells grown of permeable membranes were fixed in 4% paraformaldehyde for 20 min, blocked with 2% normal donkey serum for 30 min, and immunostained with a 1:100 dilution of mouse anti-E-cadherin (BD Biosciences), anti-β-catenin (BD Biosciences), or rabbit anti-ZO-1 antibody (Zymed Laboratories, South San Francisco, CA) for 1 h at room temperature, followed by a 1-h incubation with a 1:100 dilution of anti-mouse or anti-rabbit Alexa Fluor 488 secondary antibody. Cells were also immunostained with 5 μg/μl Alexa Fluor 633 Phalloidin (Invitrogen, Molecular Probes) according to the manufacturer's protocol. Cells were counterstained with 1:1,000 dilution of either TO-PRO-3 (Alexa Fluor 633, Invitrogen) for 20 min.
Membranes were rinsed and coverslips were mounted by use of Prolong Gold antifade reagent mounting medium (Invitrogen/Molecular Probes). The slides were allowed to dry at 4°C and were viewed under a fluorescence microscope (Olympus BX60 with Diagnostic Instruments “Spot” Camera) or analyzed with a Zeiss LSM510 META confocal microscope.
Immunoprecipitation.
Cell lysates were prepared using M-PER mammalian protein extraction reagent (Thermo Scientific) supplemented with a protease inhibitor (Roche) according to the manufacturer's protocol. Cell and tissue lysates (100 μg total protein) were immunoprecipitated with rat anti-E-cadherin (2 μg, Santa Cruz Biotechnology) or rabbit anti-occludin (5 μg, Invitrogen) for 2 h at 4°C. Protein A/G agarose beads (40 μl, Santa Cruz Biotechnology) were added and samples incubated at 4°C for a further 16 h. After 16 h of incubation, immunoprecipitates were washed three times with PBS and resuspended in 40 μl of Laemmli loading buffer (Bio-Rad Laboratories, Hercules, CA) and analyzed by Western blot.
Shh secreted into the medium of collected cultured IMGE-5 cells was immunoprecipitated with anti-Shh 5E1 antibody (2 μg) at 4°C for 16 h. Protein A/G agarose beads (40 μl, Santa Cruz Biotechnology) were added and samples were incubated at 4°C for a further 2 h. After 2 h of incubation, immunoprecipitates were washed three times using PBS and resuspended in 40 μl of Laemmli loading buffer (Bio-Rad Laboratories) and analyzed by Western blot.
Western blot analysis.
Immunoprecipitates were loaded on a 4–20% sodium dodecyl sulfate-polyacrylamide gradient gel (Invitrogen). Proteins were transferred to membranes (Hybond-C Extra Nitrocellulose, Fisher Scientific) that were then blocked with Detector Block (KPL, Gaithersburg, MD) for 1 h at room temperature. Membranes were then incubated with either a 1:100 dilution of goat anti-Shh (N-19, sc-1194, Santa Cruz Biotechnology), 1:2,000 dilution of mouse anti-E-cadherin (BD Bioscience), 1:2,000 dilution mouse anti-β-catenin (BD Biosciences Pharmingen), 1:1,000 dilution of mouse anti-occludin (Invitrogen), or a 1:1,000 dilution of mouse anti-ZO-1 (Invitrogen) for 16 h at 4°C. Membranes were then incubated for 1 h at room temperature with a 1:1,000 dilution of Alexa Fluor 680 anti-goat or anti-mouse antibody (Invitrogen/Molecular Probes). Proteins were visualized and quantified by using the Odyssey Infrared Imaging System software.
qRT-PCR.
Total RNA was isolated using Trizol Reagent. The High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used for cDNA synthesis from 100 ng of RNA following the recommended protocol. Predesigned real-time PCR assays were purchased for the following genes (Applied Biosystems): Ptch1 (Mm00970977_m1), Smo (Mm01162711_g1), Gli1 (Mm0049645_m1), Atp4a/HK-ATPase (Mm01176574_g1), Muc5ac (Mm_01276711_mH), Muc6 (Mm00725185_g1), pepsinogen C (PgC, Mm01278038_m1), cyclin B1 (Mm01322149_mH) and mouse GAPDH (20×) (4352932-0803020). PCR amplifications were performed in a total volume of 20 μl, containing 20× TaqMan Expression Assay primers, 2× TaqMan Universal Master Mix (Applied Biosystems, TaqMan Gene Expression Systems), and cDNA template. Each PCR amplification was performed in duplicate wells in a StepOne real-time PCR system (Applied Biosystems), under the following conditions: 50°C 2 min, 95°C 10 min, 95°C 15 s (denature), and 60°C 1 min (anneal/extend) for 40 cycles. Fold change was calculated as (Ct − Ct high) = ntarget, 2ntarget/2nGAPDH = fold change where Ct is threshold cycle. The results were expressed as average fold change in gene expression relative to the control group, and GAPDH was used as an internal control. The amplification efficiencies for each gene were determined by calculating the linear regression of ΔCt with different cDNA dilutions according to Livak and Schmittgen (22).
Flow cytometry and cell cycle progression.
Transduced IMGE-5Scram, IMGE-5Ski3, and IMGE-5Ski8 cells were cultured in six-well plates until 80% confluent, harvested, and fixed in ethanol for 15 min at −20°C. Cells were stained with propidium iodide for 45 min for measurement of DNA content using the FACSCalibur system (Becton Dickinson). The measured values of peak fluorescence per total number of cells were obtained using the program CellQuest Pro (Becton Dickson). The percentage of cells from the population in each phase of the cell cycle was calculated from the peak fluorescence measurements through analysis with ModFit LT software.
Statistical analysis.
The significance of the results was tested by either a one-way ANOVA or unpaired t-test, except for Fig. 4, A–C, which was analyzed by two-way ANOVA, by using commercially available software (GraphPad Prism, GraphPad Software, San Diego, CA). A P value <0.05 was considered significant.
Fig. 4.
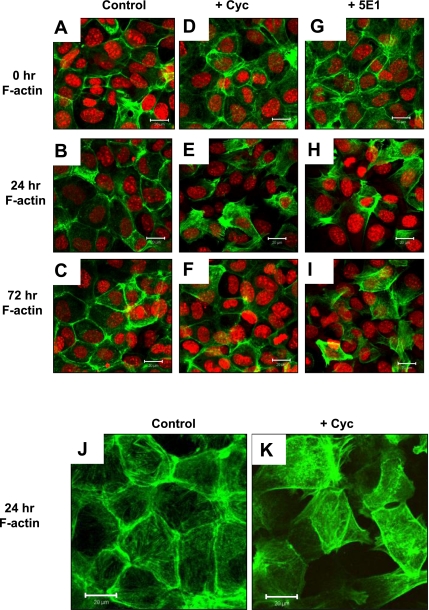
F-actin expression pattern of IMGE-5 cells treated with vehicle, cyclopamine, and anti-Shh 5E1 antibody. Permeable membranes collected from vehicle (control)-treated (A–C), cyclopamine (Cyc)-treated (D–F), and anti-Shh 5E1 antibody (5E1)-treated (G–I) IMGE-5 cells at 0, 24, and 72 h immunostained for F-actin (Alexa Fluor 488, green) and nuclear marker ToPRO (red). Size bar = 20 μm. J: images captured at a higher magnification of control- and Cyc-treated IMGE-5 cells immunostained for F-actin. Size bar = 20 μm.
RESULTS
Inhibition of Hedgehog signaling disrupts IMGE-5 cell monolayer formation.
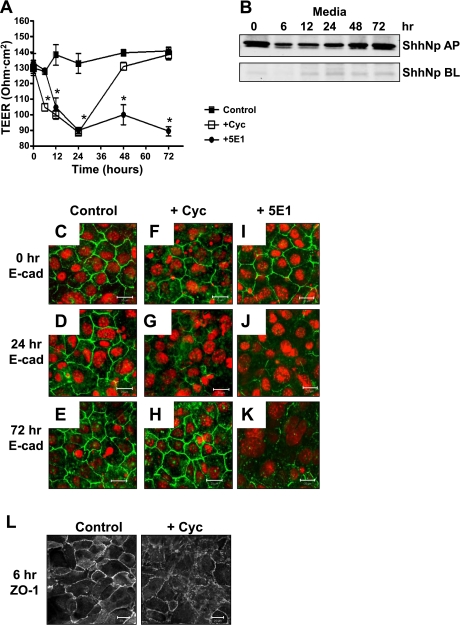
To study the role of Hedgehog signaling as a regulator of adherens and tight junction protein expression, a nontumorigenic gastric epithelial cell line (IMGE-5) was used that was derived from H-2Kb-tsA58 transgenic mice as previously developed by Hollande et al. (13). IMGE-5 cells cultured on permeable membranes were treated with vehicle, cyclopamine, or anti-Shh antibody 5E1. TEER was measured to monitor polarized monolayer formation (Fig. 2A). Specifically, cells were cultured with vehicle or cyclopamine where treatment started at time 0 h (Fig. 2A). TEER readings were collected every 6 h for the first 24 h and showed that, compared with the vehicle-treated cells (Control), cyclopamine (+Cyc) induced a significant decrease in TEER within 6 h of treatment (Fig. 2A). After 24 h cells were washed and wells were replaced with culture medium without vehicle or cyclopamine. In the absence of cyclopamine, TEER readings steadily increased over the subsequent 48 h (Fig. 2A).
Fig. 2.
Changes in E-cadherin and zonula occludens-1 (ZO-1) expression with inhibition of Hedgehog signaling in IMGE-5-treated cells. A: transepithelial electrical resistance (TEER) of vehicle (control)-, cyclopamine (Cyc)-, or anti-Shh 5E1 antibody-treated IMGE-5 cells grown on permeable membranes. Cells were cultured with vehicle or cyclopamine where treatment started at time 0 h. After 24 h cells were washed and wells were replaced with culture medium without vehicle or cyclopamine. Cells cultured in anti-Shh 5E1 antibody were treated for the entire 72 h. Data shown are means ± SE from 6 individual experiments. *P < 0.05 compared with vehicle (control)-treated group. B: media were collected from both apical (AP) and basolateral (BL) compartments of cyclopamine-treated IMGE-5 cells cultured on permeable supports at 0, 6, 12, 24, 48, and 72 h, immunoprecipitated using anti-Shh 5E1 antibody, and analyzed for expression of processed 19-kDa Shh protein (ShhNp) by Western blot. Permeable membranes collected from vehicle (control)-treated (C–E), cyclopamine-treated (F–H), and 5E1-treated (I–K) IMGE-5 cells at 0, 24 and 72 h immunostained for E-cadherin (E-cad, Alexa Fluor 488, green) and nuclear marker ToPRO (red). Size bar = 20 μm. L: permeable membranes collected from vehicle (control)-treated and cyclopamine-treated IMGE-5 cells at 24 h immunostained for ZO-1. Size bar = 20 μm.
Medium was collected from the apical and basolateral compartments of cyclopamine-treated IMGE-5 cells cultured on permeable membranes between 0 and 72 h. Medium was immunoprecipitated using anti-Shh antibody 5E1, and secretion of Shh was measured by Western blot analysis (Fig. 2B). Processed 19-kDa Shh protein (ShhNp) was secreted predominantly in the apical compartment of cultured IMGE-5 cells (Fig. 2B). Based on a standard curve generated using 0 and 50 ng of recombinant mouse Shh protein (data not shown), the concentration of ShhNp secreted by IMGE-5 cells was ∼50 ng. Therefore, to test the role of secreted Shh as a regulator in the formation of a polarized monolayer, IMGE-5 cells cultured on permeable membranes were treated with anti-Shh antibody 5E1 starting at time 0 h (Fig. 2A). IMGE-5 cells remained cultured in 5E1 antibody and there was a significant decrease in TEER within 6 h of treatment that was sustained over the subsequent 72 h (Fig. 2A).
Inhibition of the Hedgehog signaling results in loss of E-cadherin and relocalization of ZO-1.
Recent studies from our laboratory have shown that in the adult stomach E-cadherin may be a target of the Hedgehog signaling pathway (38). Localization of the adherens junction protein E-cadherin was investigated by immunofluorescent microscopy of polarized gastric IMGE-5 cells (Fig. 2C). E-cadherin expression in vehicle-treated cells remained membrane bound and continuous throughout the 72-h time period (Fig. 2, C–E). Within 24 h of cyclopamine treatment, E-cadherin expression was disrupted and appeared discontinuous (Fig. 2, F–H). After 24 h cells were washed and wells replaced with culture medium without vehicle or cyclopamine. In the absence of cyclopamine, E-cadherin expression was restored after 24 h and appeared localized to the cell membrane by 72 h (Fig. 2H). E-cadherin expression remained disrupted from 6 to 72 h after 5E1 antibody treatment (Fig. 2, I–K).
The tight junction is an important structure in the development of cell polarity and is disappears during loss of E-cadherin and EMT (16). Moreover, the adherens junction protein E-cadherin is required for tight junction formation (39). Consistent with the disruption of E-cadherin expression within 6 h of cyclopamine treatment was the loss of normal ZO-1 membrane localization (Fig. 2L).
To determine whether the loss of E-cadherin from the membrane in response to cyclopamine was caused by reduced protein expression or specific relocalization of the protein, we next quantified changes in extractable E-cadherin from vehicle-, cyclopamine-, and 5E1-treated IMGE-5 cells at 0 to 72 h in the same experiment detailed in Fig. 1A (Fig. 3, A–D). Compared with the vehicle-treated cells, there was a significant decrease in extractable E-cadherin expression in response to cyclopamine and 5E1 antibody within 6 h of treatment (Fig. 3, A–D). Whereas E-cadherin expression significantly increased 48 h after cyclopamine treatment (Fig. 3, B and D), E-cadherin protein expression remained suppressed in IMGE-5 cells that were continuously treated with anti-Shh 5E1 antibody (Fig. 3, C and D). E-cadherin directly binds to peripheral membrane protein β-catenin (reviewed in Ref. 25). β-Catenin coimmunoprecipitated with E-cadherin in vehicle-treated cells at all time points (Fig. 3, A and D). However, the amount of β-catenin coimmunoprecipitating with E-cadherin was significantly decreased within 6 h of cyclopamine treatment and then increased with reexpression of E-cadherin at 48 and 72 h (Fig. 3, B and D). A sustained loss of E-cadherin with immunoneutralization of secreted Shh in the 5E1-treated cells resulted in loss of coimmunoprecipitation with β-catenin 6 to 72 h after treatment (Fig. 3, C and D).
Fig. 3.
Effect of cyclopamine on E-cadherin and ZO-1 expression in IMGE-5 cells. Cell lysates were collected from vehicle-treated (control; A and E), cyclopamine-treated (Cyc; B and F), and anti-Shh 5E1 antibody-treated (5E1; C and G) IMGE-5 cells cultured on permeable supports at 0, 6, 12, 24, 48, and 72 h. A–C: lysates were immunoprecipitated (IP) using anti-E-cadherin (E-cad) antibody and immunoblotted (WB) for E-cad and β-catenin (β-cat) protein expression. D: E-cad protein expression was quantified by the Odyssey Infrared Imaging System software. Data are shown as means ± SE for 3 individual experiments and expressed as E-cad (pixels/mm2). E–G: lysates were immunoprecipitated by use of anti-occludin (Occl) antibody and immunoblotted for Occl and ZO-1 protein expression. H: ZO-1 protein expression was quantified by the Odyssey Infrared Imaging System software. Data are shown as means ± SE for 3 individual experiments and expressed as ZO-1 (pixels/mm2).
In contrast to loss of E-cadherin protein expression, extractable tight junction protein occludin expression was unchanged in cyclopamine- (Fig. 3, F and H) and 5E1-treated cells (Fig. 3, G and H) compared with vehicle-treated cells (Fig. 3, E and H). ZO-1 coimmunoprecipitated with occludin in vehicle-treated cells at all time points (Fig. 3, E and H). The amount of ZO-1 coimmunoprecipitating with occludin was significantly decreased within 6 h of cyclopamine treatment that then increased at 48 and 72 h (Fig. 3, F and H). A sustained loss of ZO-1 coimmunoprecipitation with occludin was observed with immunoneutralization of secreted Shh in the 5E1-treated cells 6 to 72 h after treatment (Fig. 3, G and H). Collectively, Fig. 2 demonstrates that inhibition of Hedgehog signaling results in a loss of E-cadherin expression and relocalization of ZO-1.
Loss of E-cadherin is associated with disruption of F-actin and relocalization of ZO-1.
The structural integrity and polarization of epithelial cells is maintained by E-cadherin binding to β-catenin and a network of actin filaments. Loss of E-cadherin and remodeling of the actin cytoskeleton are hallmarks of epithelial-to-mesenchymal transition and loss (8). Changes in the F-actin expression pattern in IMGE-5 treated cells was determined by immunofluorescence. Although vehicle-treated cells showed cortical actin staining (Fig. 4, A–C), cyclopamine- (Fig. 4, D–F), and 5E-1-treated cells (Fig. 4, G–I) displayed elongated actin stress fibers. Higher power images captured vehicle- and cyclopamine-treated cells are shown in Fig. 4, J and K, and show the disruption of the F-actin expression pattern in cyclopamine-treated cells. Given the close interplay between actin and E-cadherin/β-catenin during adherens junction formation (37), it is likely that the loss of E-cadherin, observed with inhibition of Hedgehog signaling, results in the disruption of F-actin cortical expression and relocalization of ZO-1.
Total RNA was extracted from vehicle- and cyclopamine-treated IMGE-5 cells at 0, 6, 12, 24, 48, and 72 h. Changes in Hedgehog signaling genes Ptch1, Smo, and Gli1 were analyzed by qRT-PCR (Fig. 5, A–C). Compared with the vehicle-treated cells, cyclopamine treatment induced a significant reduction in Ptch1, Smo, and Gli1 expression within 24 h of treatment compared with mRNA expression at time 0 h (Fig. 5, A–C). After 6 h of cyclopamine treatment Smo and Gli significantly decreased, whereas Ptch mRNA expression remained similar to the vehicle-treated group (Fig. 5A). Ptch1, Smo, and Gli1 expression was restored 24 h after the removal of cyclopamine from the medium (Fig. 5, A–C). Although Ptch gene expression appeared to increase above the vehicle-treated group at 48 and 72 h, these differences were not significantly different (Fig. 5A). The time course of inhibition of Hedgehog signaling was consistent with the loss of E-cadherin, disruption of F-actin, and relocalization of ZO-1 between 6 and 24 h. Increased Hedgehog signaling observed 24 h after the removal of cyclopamine from the culture medium correlated with the restoration of adherens and tight junction protein expression.
Fig. 5.
Changes in the Hedgehog signaling pathway in response to vehicle and cyclopamine in the IMGE-5 cells. Quantitative RT-PCR was performed on RNA prepared from vehicle- and cyclopamine-treated IMGE-5 cells grown on permeable membranes at 0, 6, 12, 24, 48, and 72 h. Shown is the average (Av.) fold change of Ptch1 (A), Smo (B), and Gli1 (C) mRNA expression related to vehicle-treated cells. Data shown are means ± SE from 3 individual experiments. *P < 0.05 compared with response at 0 h.
Two-color fluorescence cell viability assay measured the cytotoxicity of cyclopamine on cultured IMGE-5 cells.
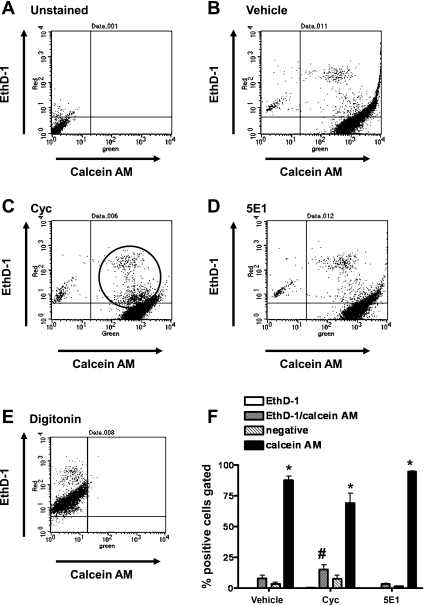
To identify potential cytotoxicity of cyclopamine and anti-Shh 5E1 antibody treatments on IMGE-5 cells, a two-color fluorescence cell viability flow cytometric assay with the fluorescent probes ethidium homodimer (EthD-1) and calcein AM was used. Live cells were identified by the presence of intracellular esterase activity, determined by the enzymatic conversion of calcein AM to calcein with green fluorescence. EthD-1 enters cells via damaged membranes and produces a red fluorescence upon binding to nucleic acid of dead cells. Cells were harvested at 24 h after treatment, and cell viability was assayed by flow cytometry (Fig. 6). In vehicle-treated IMGE-5 cells 90.5 ± 1.52% of total cells stained positive for calcein AM, directly reflecting the percentage of live cells in the culture (Fig. 6, B and F). Similar numbers of live cells were observed (92.7 ± 1.10%) in IMGE-5 cells cocultured with anti-Shh 5E1 antibody (Fig. 6, D and F). Cyclopamine caused a decrease in the total number of live, calcein AM-positive stained cells (88.7 ± 0.44%, Fig. 6, C and F). A significant increase in cells costained with calcein AM/EthD-1 (Fig. 6, C and F) was also observed in cyclopamine-treated compared with vehicle-treated cells (Fig. 6, B and F). These data suggest that cyclopamine induces apoptosis in IMGE-5 cells within 24 h of treatment. The occurrence of cell death in cyclopamine-treated cells could be a consequence of the disruption of epithelial integrity and an indicator of the decreased TEER measurements in Fig. 2A. Indeed, in a number of tissues decreased E-cadherin expression induces apoptosis (2, 3, 28). Therefore, we decided to take an alternative approach to confirm our findings observed in Figs. 2–5.
Fig. 6.
Analysis of cell viability of IMGE-5 cells treated with vehicle, cyclopamine, and anti-Shh 5E1 antibody by flow cytometry. A 2-color fluorescence cell viability flow cytometric assay with the fluorescent probes ethidium homodimer (EthD-1, dead cells) and calcein AM (live cells) were used. Distribution of Eth-D1-positive and calcein AM-positive IMGE-5 cells unstained (A) or treated with vehicle (B), cyclopamine (Cyc; C), anti-Shh 5E1 antibody (5E1; D) or digitonin (E). Shown is the light scatter analysis of Eht-D1- and calcein AM-gated cells. F: summary of data expressed as percentage of EthD1-, EthD-1/calcein AM-, and calcein AM-positive cells from 3 individual experiments. *P < 0.05 compared with EthD-1-positive cells, #P < 0.05 compared with vehicle-treated group.
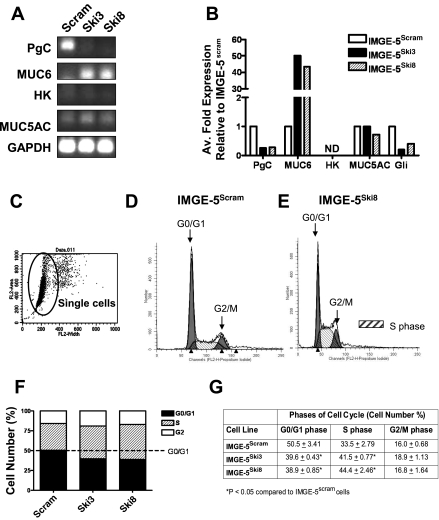
IMGE-5 cells transduced with Ski shRNA exhibit increased proliferation and changes in cell lineage markers.
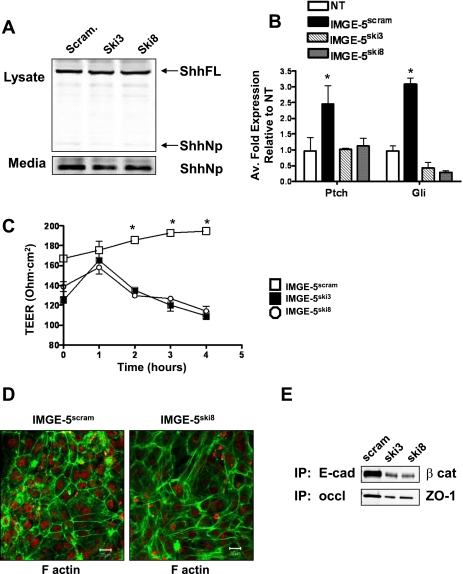
Ski is an acetyltransferase that functions as the Hedgehog palmitoyl-transferase where loss of Ski results in Hedgehog protein that is not amino-terminally modified, resulting in the loss or reduction of Hedgehog signaling activity (7). To further identify the role of Hedgehog signaling in the regulation of adherens and tight junction formation, the effect of knockdown of Ski on E-cadherin, ZO-1, and F-actin expression was determined by transducing Ski shRNA into IMGE-5 cells with use of a lentiviral vector. A PIKO.1 base lentiviral vector expressing a short hairpin sequence targeting the transcript of either the mouse and human Ski (Ski3) or mouse Ski (Ski8) genes was transduced into IMGE-5 cells (IMGE-5Ski3 and IMGE-5Ski8). As a control, IMGE-5 cells were also transduced with the PIKO.1 lentiviral vector (IMGE-5Scram). There was no change in protein expression of the precursor (ShhFL) 45-kDa or processed 19-kDa Shh proteins in IMGE-5Ski cells compared with IMGE-5Scram cells (Fig. 7A). As a measure of Hedgehog-dependent signaling, we assayed the ability of Shh secreted from IMGE-5Ski and IMGE-5Scram cells to induce Gli and Ptch mRNA expression in the Hedgehog responsive C3H10T1/2 cell line (Fig. 7B) (17). Conditioned medium was collected from IMGE-5Ski and IMGE-5Scram cells cultured for 72 h and incubated with C3H10T1/2 cells for 5 days. Total RNA was extracted and changes in Gli1 and Ptch were assayed by qRT-PCR. C3H10T1/2 cells cultured in conditioned medium collected from IMGE-5Scram cells showed a significant increase in Gli and Ptch mRNA expression compared with cells not treated with conditioned medium (Fig. 7B). C3H10T1/2 cells cultured in conditioned medium collected from IMGE-5Ski cells showed no induction in Gli or Ptch gene expression (Fig. 7B), showing that Shh secreted from IMGE-5Ski cells is not biologically active.
Fig. 7.
Biological activity of IMGE-5 cells transduced with shRNA targeting Ski. A PIKO.1 base lentiviral vector expressing a shRNA targeting the transcript of either the mouse and human Ski (Ski3) or mouse Ski (Ski8) genes was transduced into IMGE-5 cells (IMGE-5Ski3 and IMGE-5Ski8). As controls IMGE-5 cells were also transduced with PIKO.1 lentiviral vector (IMGE-5Scram). A: cell lysates and media were collected from IMGE-5Scram, IMGE-5Ski3, and IMGE-5Ski8 cells, and expression of full length 45-kDa Shh (ShhFL) and processed 19-kDa Shh (ShhNp) proteins analyzed by Western blot. B: quantitative RT-PCR was performed on RNA prepared from C3H10T1/2 cells treated with conditioned medium collected from IMGE-5Scram, IMGE-5Ski3, and IMGE-5Ski8 cells. Shown is the average fold change of Ptch and Gli mRNA expression related to untreated cells (NT). Data shown are means ± SE from 3 individual experiments. *P < 0.05 compared with response NT group. C: TEER of IMGE-5Scram, IMGE-5Ski3, and IMGE-5Ski8 cells grown on permeable membranes. Data shown are means ± SE from 6 individual experiments. *P < 0.05 compared with IMGE-5Ski3 and IMGE-5Ski8 cells. D: cell lysates collected from IMGE-5Scram, IMGE-5Ski3, and IMGE-5Ski8 cells were immunoprecipitated by use of anti-E-cadherin or anti-occludin antibody and immunoblotted for β-catenin or ZO-1 protein expression. E: permeable membranes collected from IMGE-5Scram and IMGE-5Ski8 cells immunostained for F-actin (Alexa Fluor 488, green) and nuclear marker ToPRO (red). Size bar = 20 μm. Scram, scramble.
IMGE-5Ski and IMGE-5Scram cells were cultured on permeable membranes and TEER was measured. Compared with IMGE-5Scram cells, IMGE-5Ski3 and IMGE-5Ski8 cells failed to form a polarized monolayer (Fig. 7C). Similar to that observed with inhibition of Hedgehog signaling in response to cyclopamine treatment (Fig. 3), loss of bioactive secreted Shh from cultured IMGE-5Ski cells resulted in a reduction of E-cadherin expression and β-catenin coimmunoprecipitation and a reduction of ZO-1 protein that coimmunoprecipitated with occludin (Fig. 7E). Loss of E-cadherin that was observed in IMGE-5Ski cells correlated with the disruption of F-actin cortical expression (Fig. 7D).
The integrity of adherens junctions plays a central role in the development of EMT, by which epithelial cells lose their polarity (16), and also epithelial cell differentiation in the adult stomach (38). The expression of the major gastric epithelial cell lineage markers PgC (zymogenic cells), mucin 6 (MUC6, mucous neck cells), H+-K+-ATPase (HK, parietal cells), and mucin 5AC (MUC5AC, surface mucous pit cells) was determined by qRT-PCR in IMGE-5Ski and IMGE-5Scram cells. Cell lineage markers PgC and MUC5AC were only expressed in IMGE-5Scram cells (Fig. 8, A and B). Interestingly, relative to mRNA expression quantified in the IMGE-5Scram cells, IMGE-5Ski cells had significantly reduced PgC expression and increased mucous neck cell MUC6 expression (Fig. 8, A and B). The parietal cell marker HK was not detected in either IMGE-5Ski and IMGE-5Scram cells (Fig. 8, A and B). Collectively, these data suggest that inhibition of Hedgehog signaling via the loss of bioactive secreted Shh results in disruption of the normal differentiation of mouse nontumorigenic gastric epithelial IMGE-5 cells.
Fig. 8.
Changes in differentiation and cell cycle progression in IMGE-5Ski cells. A: quantitative RT-PCR was performed on RNA prepared from IMGE-5Scram, IMGE-5Ski3, and IMGE-5Ski8 cells. Transcripts for pepsinogen C (PgC), mucin 6 (MUC6), HK-ATPase (HK), mucin 5AC (MUC5AC), and GAPDH. B: average fold change in gene expression for PgC, MUC6, HK, MUC5AC, and Gli relative to IMGE-5Scram cells. C: cells were stained with propidium iodide and cell cycle analyzed by flow cytometry on the single-cell population. Flow cytometry graphs generated from cell cycle phase analysis by MODFIT software showing changes in distribution of G0/G1, S, and G2/M phases from IMGE-5Scram (D) and IMGE-5Ski8 (E) cells. F: flow cytometry graph generated from cell cycle phase analysis by MODFIT software showing changes in distribution of G0/G1, S, and G2/M phases from IMGE-5Scram, IMGE-5Ski3, and IMGE-5Ski8 cells. G: mean ± SE values from 3 individual experiments of IMGE-5Scram, IMGE-5Ski3, and IMGE-5Ski8 cells. ND, not detected.
Published data from our laboratory demonstrate that loss of Shh in the adult stomach causes a delay in the differentiation of zymogen cells from mucous neck cells (38). As the mucous neck cells migrate toward the base of the gastric gland, these cells differentiate into the pepsinogen-expressing zymogen cells (18). The aberrant differentiation of the zymogenic cell lineage typically results in the coexpression of mucous neck cell markers such as MUC5AC with intrinsic factor or pepsinogen (38). In mouse models of disrupted zymogen cell differentiation there is typically an increase in proliferation (6, 38). Cell cycle phases were analyzed by flow cytometry using propidium iodide staining of IMGE-5Ski and IMGE-5Scram cells. The analysis was performed only on the selected single cell population (Fig. 8C). Figure 8, D and E, represents plots showing the changes in the cell cycle phases. The G0/G1 and G2/M phases are indicated as the two major peaks with arrows (Fig. 8, D and E). The shaded blue area between the major peaks (G1/G1, G2/M phases) is the S phase (Fig. 8, D and E). Analysis of the raw fluorescence data showed a significant increase in the percentage of cells in S phase, and reduced the percentage of cells in G0/G1 phase with in IMGE-5Ski cells compared with IMGE-5Scram cells (Fig. 8, F and G). Therefore, inhibition of Hedgehog signaling induces an increase in proliferation and deviation from the typical cell lineage marker expression.
Disruption of membrane-expressed E-cadherin and ZO-1 proteins in HKCre/ShhKO mouse stomachs.
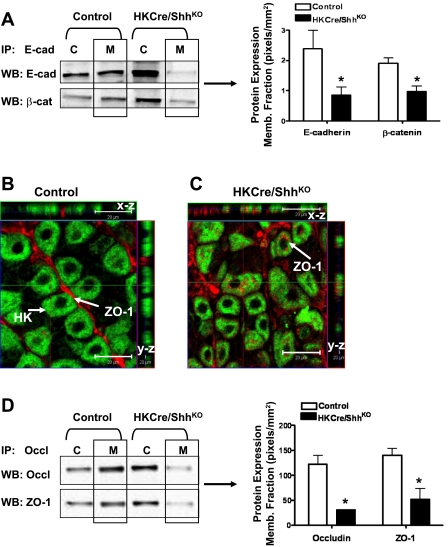
Recent work from our laboratory using a mouse model expressing a parietal cell-specific deletion of Shh (HKCre/ShhKO) demonstrated that loss of Shh triggered a number of molecular events, which were consistent with EMT of gastric epithelial cells that included loss of E cadherin expression (38). We also observed that HKCre/ShhKO mice developed hyperproliferation of the surface pit mucous cells and delayed differentiation of the zymogen cell lineage (38). Consistent with our published observations, HKCre/ShhKO mice developed a phenotype similar to foveolar hyperplasia with an expansion of the surface epithelium into the gland and base regions of the stomach (Fig. 9B) compared with the normal gastric epithelium in control animals (Fig. 9A). Immunofluorescence using a lectin specific for surface mucous cells (UEAI) was used to show the expansion of the pit region (Fig. 9, C and D). Compared with the fundic mucosa of control mice (Fig. 9C) HKCre/ShhKO animals had an expanded surface epithelium that stained positive for UEAI (Fig. 9D).
Fig. 9.
Histological evaluation of control and HKCre/ShhKO mouse stomachs. Hematoxylin and eosin stains of stomachs collected from control (A) and HKCre/ShhKO (B) mice. Immunofluorescence staining for surface pit mucous cell marker Ulex europaeus (UEAI, Alexa Fluor 488, green) and ZO-1 (Alexa Fluor 633, red) in 8-mo-old control (C) and HKCre/ShhKO (D) mice. Representative of n = 8 per group.
The expression and relocalization of E-cadherin and ZO-1 were further studied by immunoprecipitation and Western blot analysis using stomachs collected from HKCre/ShhKO mice. Stomach tissue was used to isolate of total cytoplasmic and membrane protein isolation. Protein was then immunoprecipitated by use of anti-E-cadherin antibody and analyzed for extractable E-cadherin expression and coimmunoprecipitated β-catenin expression (Fig. 10A). Compared with the control mice HKCre/ShhKO animals exhibited significantly decreased E-cadherin expression in the membrane protein extracts that correlated with decreased β-catenin coimmunoprecipitated expression (Fig. 10A). ZO-1 expression in the mouse stomachs was analyzed by confocal microscopy. In addition to the x-y image, a series of optical sections were then taken from identical x-z and y-z planes to specifically localize ZO-1 expression to the membrane or cytoplasm (Fig. 10, B and C). ZO-1 expression in control mice localized to the apical membrane of the gastric epithelial cells lining the luminal space between glandular units (Fig. 10B). In the HKCre/ShhKO animals, ZO-1 localization was localized to the cell surfaces and also intracellular spaces (Fig. 10C). To identify the relocalization of ZO-1 further, stomach tissue was subjected to isolation of total cytoplasmic and membrane protein isolation. Protein was then immunoprecipitated using anti-occludin antibody and analyzed for extractable occludin expression and coimmunoprecipitated ZO-1 expression (Fig. 10D). Compared with the control mice HKCre/ShhKO animals exhibited significantly decreased occludin expression in the membrane protein extracts that correlated with decreased ZO-1 coimmunoprecipitated expression (Fig. 10D). Findings observed in Fig. 9D are different than the localization of ZO-1 observed in the IMGE-5 cyclopamine and anti-Shh antibody-treated cells (Fig. 3E–G). In the IMGE-5 experiments we observe that there is a significant dissociation between occludin and ZO-1 whereas in the HKCre/ShhKO mouse tissue there is a shift in both occludin and ZO-1 expression from the membrane to the cytoplasmic fractions. This discrepancy suggests that in the IMGE-5 cells occludin may remain membrane bound whereas in the mouse tissue membrane-bound occludin is disrupted and may be accounted for by differences in cell and tissue systems. Consistent with that observed in the IMGE-5 cultured cells, in vivo loss of Shh is associated with loss of E-cadherin, which may be a plausible mechanism for the hyperproliferation and loss of epithelial cell differentiation previously reported in this model (38).
Fig. 10.
Expression of E-cadherin and ZO-1 in control and HKCre/ShhKO mouse stomachs. A: lysates were collected from control and HKCre/ShhKO mouse stomachs, and cytoplasmic (C) and membrane (M) protein extracts were prepared, immunoprecipitated by use of anti-E-cadherin antibody, and immunoblotted for E-cadherin and β-catenin protein expression. E-cad and β-cat protein expression was quantified by the Odyssey Infrared Imaging System software. Data are shown as means ± SE for 4 mice/group and expressed as pixels/mm2. Confocal images of immunofluorescence staining for H+-K+-ATPase (HK, Alexa Fluor 488, green) and ZO-1 (Alexa Fluor 633, red) in 8-mo-old (B) control and (C) HKCre/ShhKO mice. Representative of n = 8 per group. Arrows in B show apical-expressed ZO-1. Arrows in C show ZO-1 predominantly expressed in cytoplasm. Optical sections spaced 1 μm in the z-axis of section (x-z and y-z). The green line in the x-y image is the location of the x-z slice. The red line in the x-y image is the location of the y-z slice. D: lysates were collected from control and HKCre/ShhKO mouse stomachs and cytoplasmic and membrane protein extracts prepared, and immunoprecipitated using anti-occludin (Occl) antibody and immunoblotted for Occl and ZO-1 protein expression. Occludin and ZO-1 protein expression was quantified by the Odyssey Infrared Imaging System software. Data are shown as means ± SE for 4 individual mice/group and expressed as pixels/mm2.
DISCUSSION
In vitro studies using IMGE-5 cells demonstrated that the stability of the adherens junctions was crucial for the maintenance of the actin cytoskeleton and tight junction protein ZO-1. The Hedgehog signaling pathway was blocked by treating IMGE-5 cells with Smo inhibitor cyclopamine or by blocking biologically active Shh protein secreted from IMGE-5 cells by using anti-Shh antibody or knockdown of Ski. In all cases, inhibition of Hedgehog signaling resulted in decreased E-cadherin expression and dissociation of the E-cadherin/β-catenin protein complex and F-actin cortical expression. E-cadherin directly binds to β-catenin, which in turn binds to actin filament binding protein α-catenin (39). The adherens junction cadherin/β-catenin/α-catenin protein complex is morphologically associated with actin filaments (25). In gastric cancer cells loss of E-cadherin and disruption of the actin cytoskeleton facilitates translocation of β-catenin that promotes migration and proliferation of gastric carcinoma cells (20). A study using primary mouse tubular epithelial cells isolated from the renal cortex of TGF-β1 knockout mice to model EMT in vitro demonstrated that restoration of cortical actin expression contributed to the reversal of EMT (8). Our published work demonstrates that the loss Hedgehog signaling in the stomach results in loss of E-cadherin and the development of EMT (38). The present study extends our understanding of the development of EMT by demonstrating that Hedgehog signaling regulates E-cadherin and that adherens junction protein expression is crucial for the maintenance of the actin cytoskeleton and epithelial cell polarization.
Loss of ZO-1 membrane distribution was reflected by similar changes in TEER whereby cyclopamine- and 5E1-treated IMGE-5 and IMGE-5Ski transduced cells failed to maintain a stable TEER. These findings are consistent with the knowledge that the adherens junction protein E-cadherin is required for tight junction formation (39). Extensive studies using MDCK cell cultures clearly show that stability of tight junctions was dependent on the formation and maintenance of adherens junctions (12, 39). Consistent with this notion, it would be predicted that loss of E-cadherin and disorganization of the actin cytoskeleton result in the dissociation of membrane-expressed ZO-1 as observed in the IMGE-5 cells and HKCre/ShhKO mouse stomachs. Indeed, in treated and transduced IMGE-5 cells and HKCre/ShhKO mouse stomachs there was a significant decrease in ZO-1 coimmunoprecipitation with the integral membrane tight junction protein occludin that is supporting evidence for the relocalization of ZO-1 to the cytoplasm (13, 14). Disruption of the tight junction and apical junctional complex is characteristic of a number of diseases including cancer (reviewed in Ref. 24), loss of intestinal barrier function as in celiac disease (30), and the pathogenesis of H. pylori explained by the disruption of tight junctions within the gastric epithelium (1, 19), but the mechanisms regulating tight junction formation in the stomach are largely unknown. We advance these findings by demonstrating that Hedgehog signaling regulates E-cadherin expression, which is required for the maintenance of F-actin cortical expression and stability of tight junction protein ZO-1 in gastric epithelial cells. In the stomach, the expression pattern of tight junction scaffolding protein ZO-1 determines epithelial cell organization and differentiation (40). Our studies uncover a plausible mechanism by which Shh acts as a regulator of gastric epithelial cell function and differentiation in the adult stomach and thus warrants further investigation.
The HKCre/ShhKO mice may be used to elucidate the mechanism by which Shh signaling regulates cell-cell adhesion. Recent studies from our laboratory using the HKCre/ShhKO demonstrated that the loss of Shh triggers a number of molecular changes (38) that were consistent with EMT of gastric epithelial cells (16, 21). Such molecular changes included loss of E cadherin expression and translocation of β-catenin to the nucleus (38). Nuclear translocation of β-catenin binds to the DNA-binding proteins Tcf/Lef1, which subsequently regulates target genes including cyclin D1 (26) that are important in proliferation (23). Consistent with our published data, the HKCre/ShhKO mice also exhibited hyperproliferation of the surface mucous cells (38). Hyperproliferation of surface mucous cells often disrupts the differentiation of other cell lineages such as the zymogen cells. The HKCre/ShhKO mice have a delay in the differentiation of zymogen cells from mucous neck cells whereby zymogen cells located in the base of the gland frequently coexpressed mucous neck cell markers (38). Intriguingly, disruption of the Hedgehog signaling pathway in the transduced IMGE-5Ski cells resulted in the loss of zymogen cell marker pepsinogen and expression of mucous neck cell marker MUC6 compared with the IMGE-5Scram controls. Expression of MUC6 in IMGE-5Ski cells was accompanied by an increase in cell proliferation and dissociation of the E-cadherin/β-catenin complex. In support of our findings, Bredemeyer et al. (6) discovered that in a model knocking out the cytoskeleton-regulating gene Cd2ap in Mist1−/− mice zymogen cells reorganize their cytoskeleton as they differentiate to form a polarized epithelium. Our data also suggest that aberrant E-cadherin expression causes changes in zymogenic cell morphology and differentiation.
The present study demonstrates that adherens junction protein E-cadherin is a downstream target of the Hedgehog signaling pathway. Shh regulates critical pathways in epithelial cell differentiation, but we are only beginning to understand the underlying mechanisms (31–33, 36, 38). The integrity of adherens junctions is a crucial factor determining epithelial morphology, physiological function, and differentiation, but also tumor growth, invasiveness, and metastasis (4, 5, 15, 29, 40). Thus understanding the role of Hedgehog signaling as a regulator of the adherens junctions advances our knowledge by which Shh may regulate gastric epithelial cell physiology and pathophysiology.
GRANTS
This work was supported by the American Cancer Society Research Scholar Award 119072-RSG-10-167-01-MPC (Y. Zavros) and in part by the Digestive Health Center Cincinnati Children's Medical Health Center (DHC: Bench to Bedside Research in Pediatric Digestive Disease) Pilot and Feasibility Project award CHTF/SUB DK078392 (Y. Zavros).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the assistance Monica DeLay, manager of the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children's Hospital Medical Center, supported in part by National Institutes of Health (NIH) AR-47363. All flow cytometric data were acquired by using equipment maintained by the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children's Hospital Medical Center, supported in part by NIH AR-47363.
REFERENCES
- 1.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical junctional complex by Helicobacter pylori CagA. Science 300: 1430–1434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben Chedly H, Boutinaud M, Bernier-Dodier P, Marnet P, Lacasse P. Disruption of cell junctions induces apoptosis and reduces synthetic activity in lactating goat mammary gland. J Dairy Sci 93: 2938–2951, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev 115: 53–62, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Braga V. Cell-cell adhesion and signalling. Curr Opin Cell Biol 14: 546–556, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol 137: 1421–1431, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bredemeyer A, Geahlen J, Weis V, Huh W, Zinselmeyer B, Srivatsan S, Miller M, Shaw A, Mills J. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol 325: 211–224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamoun Z, Mann R, Nellen D, von Kessler D, Bellotto M, Beachy P, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science 293: 2080–2084, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Das S, Becker B, Hoffmann F, Mertz J. Complete reversal of epithelial to mesenchymal transition requires inhibition of both ZEB expression and the Rho pathway. BMC Cell Biol 10: 1–18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 17: 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev 10: 301–312, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Gritli-Linde A, Bei M, Maas R, Zhang X, Linde A, McMahon A. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 129: 5323–5337, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol 107: 1575–1587, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollande F, Blanc E, Bali J, Whitehead R, Pelegrin A, Baldwin G, Choquet A. HGF regulates tight junctions in new nontumorigenic gastric epithelial cell line. Am J Physiol Gastrointest Liver Physiol 280: G910–G921, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hollande F, Lee D, Choquet A, Roche S, Baldwin G. Adherens junctions and tight junctions are regulated via different pathways by progastrin in epithelial cells. J Cell Sci 116: 1187–1197, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Hopkins A, Walsh S, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci 116: 725–742, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci 116: 1959–1967, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by Sonic Hedgehog in pluripotent mesenchymal cells. Oncogene 21: 8196–8205, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 236: 259–279, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Krueger SHT, Kuester D, Kalinski T, Peitz U, Roessner A. Helicobacter pylori alters the distribution of ZO-1 and p120ctn in primary human gastric epithelial cells. Pathol Res Pract 203: 433–444, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Li A, Zhou T, Guo L, Si J. Collagen type I regulates beta-catenin tyrosine phosphorylation and nuclear translocation to promote migration and proliferation of gastric carcinoma cells. Oncol Rep 23: 1247–1255, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Li XDW, Nail CD, Bailey SK, Kraus MH, Ruppert JM, Lobo-Ruppert SM. Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene 25: 609–621, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Luo GQ, Li JH, Wen JF, Zhou YH, Hu YB, Zhou JH. Effect and mechanism of the Twist gene on invasion and metastasis of gastric carcinoma cells. World J Gastroenterol 14: 2487–2493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin T, Jiang W. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta 1788: 872–891, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol 1: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303: 1483–1487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 287: 795–801, 1980 [DOI] [PubMed] [Google Scholar]
- 28.Peluso J, Pappalardo A, Fernandez G. E-cadherin-mediated cell contact prevents apoptosis of spontaneously immortalized granulosa cells by regulating Akt kinase activity. Biol Reprod 64: 1183–1190, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Samarin S, Ivanov A, Flatau G, Parkos C, Nusrat A. Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol Biol Cell 18: 3429–3439, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sander G, Cummins A, Henshall T, Powell B. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett 579: 4851–4855, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Shiotani A, Iishi H, Uedo N, Ishiguro S, Tatsuta M, Nakae Y, Kumamoto M, Merchant JL. Evidence that loss of sonic hedgehog is an indicator of Helicobater pylori-induced atrophic gastritis progressing to gastric cancer. Am J Gastroenterol 100: 581–587, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem 280: 15700–15708, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki H, Minegishi Y, Nomoto Y, Ota T, Masaoka T, van den Brink GR, Hibi T. Down-regulation of a morphogen (sonic hedgehog) gradient in the gastric epithelium of Helicobacter pylori-infected Mongolian gerbils. J Pathol 206: 186–197, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature 418: 892–896, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev 87: 1343–1375, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Van den Brink GR, Hardwick JC, Nielsen C, Xu C, ten Kate FJ, Glickman J, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut 51: 628–633, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol 13: 76–84, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology 138: 550–561, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada A, Irie K, Fukuhara A, Ooshio T, Takai Y. Requirement of the actin cytoskeleton for the association of nectins with other cell adhesion molecules at adherens and tight junctions in MDCK cells. Genes Cells 9: 843–855, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Zhu L, Hatakeyama J, Zhang B, Makdisi J, Ender C, Forte JG. Novel insights of the gastric gland organization revealed by chief cell specific expression of moesin. Am J Physiol Gastrointest Liver Physiol 296: G185–G195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]