Abstract
Stearoyl-CoA desaturase-1 (SCD-1) is the rate-limiting enzyme in the biosynthesis of monounsaturated fatty acids (MUFA), which are required for efficient neutral lipid esterification. In the present investigation, we demonstrate that loss of SCD-1 activity increases free cholesterol (FC) content and induces Xbp-1 splicing. We assessed the small molecule SCD-1 inhibitor A939572 on [14C]stearate incorporation into neutral lipids and found its incorporation into triglyceride was unaffected, whereas labeled cholesteryl ester (CE) content was notably diminished. Using either A939572 or liver knockout mice (LKO), we show that loss of SCD-1 activity increases FC levels and activates the liver X receptor (LXR) pathway. Using adenoviral delivery of an active form of X-box binding protein-1 (Xbp-1; Xbp-1s), we show increased sterol synthesis only when cells lack the ability to generate MUFA. The results of the cell-based model were confirmed in LKO mice where fasting-refeeding decreased CE, increased FC, and increased Xbp-1s. On the basis of the present data, we conclude that SCD-1 activity is required for efficient cholesterol esterification to MUFA and that loss of its activity increases Xbp-1s-mediated FC synthesis. It is likely that the accumulation of FC enhances Xbp-1 splicing, induces LXR transcriptional activity, and increases ABCA1 (ATP-binding cassette transporter A1) expression to maintain cholesterol homeostasis.
Keywords: cell stress, fasting-refeeding, cholesterol metabolism, acyl-coenzyme A:cholesterol acyltransferase-1, X-box binding protein-1, caveolin-1
stearoyl-coa desaturase-1 (SCD-1) is an iron-containing integral membrane protein of the endoplasmic reticulum (ER), where it introduces the first cis-double bond in the Δ9 position of stearate (18:0) and palmitate (16:0) to generate oleate (18:1n9) and palmitoleate (16:1n7), respectively. Recently, evidence has emerged indicating that SCD-1 is not only involved in, but is required for, cell proliferation and survival. Two independent investigations have used RNAi-mediated knockdown of SCD-1, and both reported a decrease in cell proliferation. Scaglia and Igal (29), using SV40-transformed human lung fibroblasts, found that the desaturation index was reduced, that lipid profiles were shifted toward an increased incorporation of stearate into triglyceride, and that supplementation with exogenous oleate was insufficient to rescue survival. Morgan-Lappe et al. (26) further demonstrated that SCD-1 knockdown effectively induced apoptosis via caspase-3 activity in a siRNA screeen. These results suggest that SCD-1 is required for cell survival; however, the mechanism by which SCD-1 promotes viability is unknown.
Although the exact mechanism by which SCD-1 regulates cell survival is not fully understood, it was shown that increased SCD-1 activity enhances the retention of intracellular cholesterol in vivo and in vitro (25, 35). It is hypothesized that this effect is achieved via increased acyl-CoA:cholesterol acyltransferase-1 (ACAT1)-mediated cholesterol esterification. Furthermore, we have shown that SCD-1 deficiency in animal models decreases monounsaturated fatty acid (MUFA) synthesis (12, 25). Excessive free cholesterol (FC) is cytotoxic in the ER, and in the absence of esterification it is rapidly effluxed (11). Conversely, an increase in cholesteryl-oleate synthesis would reduce the requirement for ATP-binding cassette transporter A1 (ABCA1)-mediated FC efflux and enhance viability by minimizing cholesterol depletion. Thus, in the presence of SCD-1 and ACAT1 activity, cholesteryl ester (CE) synthesis would increase, whereas a deficiency in either SCD-1 or ACAT1 would likely induce cholesterol efflux. It is logical to assume that SCD-1 activity may enhance cell viability by providing substrate for ACAT-mediated CE synthesis and reducing FC-mediated lipotoxicity.
Modest increases in ER free cholesterol levels are known to cause a rapid induction of the unfolded protein response and induce ER stress (11). Recently, the IRE-1α-mediated activation of the stress-induced X-box binding protein-1 (Xbp-1) transcription factor was shown to induce lipogenesis and sterol synthesis (22). Xbp-1 mRNA contains an unconventional intron that, when transcribed, leads to early termination and a nonfunctional 33-kDa protein. However, when a 26 nucleotide intron is excised via IRE-1α, the result is a frame shift in translation that eliminates a stop codon and generates a 54-kDa active bZIP transcription factor (4). IRE-1α is a cell stress signaling factor and induces the unfolded protein response, largely through induction of Xbp-1s. In the present investigation, we provide evidence that loss of SCD-1 activity prevents efficient cholesterol esterification with MUFA. Furthermore, loss of SCD-1 activity induces Xbp-1 splicing, increases cellular FC levels, and increases LXRα and ABCA1 expression.
MATERIALS AND METHODS
Animals and diets.
Mice were housed and bred in a pathogen-free barrier facility of the Department of Biochemistry at the University of Wisconsin-Madison. The breeding and handling of animals was in accordance with protocols approved by the Animal Care Research Committee of the University. Generation of liver specific SCD-1-deficient mice has been previously described (24), and the animals were maintained on a 12:12-h light-dark cycle with free access to water and a standard chow diet (Purina 5008). For fasting-refeeding studies, a high-sucrose, very-low-fat (HSVLF) diet was used (Harlan Teklad, TD03045) to induce lipogenesis and cholesterolgenesis. For the fasting-refeeding studies, 8- to 10-wk-old male and female mice were either fasted for 24 h (fasted group) or fasted for 24 h followed by refeeding the HSVLF diet for 12 h (refed group). For long-term activation of de novo hepatic lipogenesis, animals were placed on the HSVLF diet for 10 days. All animals were euthanized by isoflurane overdose, and tissues and plasma were rapidly removed, snap-frozen in liquid nitrogen, and stored at −80°C.
Cell culture.
The human breast cancer cell lines MCF-7 and MDA-MB-231 were maintained in DMEM with 10% FBS and 1% penicillin-streptomycin at 37°C and 5% CO2. For most fatty acid treatments, cells were grown in standard culture conditions until confluence, after which the medium was replaced with serum-free medium to eliminate exogenous free fatty acids (FFA). FBS contains 18:1; therefore, for all fatty acid treatments, BSA-conjugated fatty acids (3:1 molar ratio BSA/FFA) were used to treat cells with serum-free DMEM unless otherwise indicated.
Cell viability and proliferation.
Cell viability was determined using 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT; Sigma). Cells (1 × 104) were plated in triplicate onto 96-well tissue culture plates and allowed to adhere overnight. The next morning, cells were serum starved and then treated with varying concentrations of inhibitors and/or BSA-fatty acid complexes in serum-free medium for the indicated times.
Protein expression and analysis.
Total protein concentration was measured using the Bradford method prior to Western blotting. Antibodies to α-actin (Santa Cruz Biotechnology) were used at 1:400 dilution, and the mouse mAb against caveolin-1 (BD Biosciences) and ABCA1 (27) were used at 1:1,000. Horseradish peroxidase-conjugated secondary antibodies were detected using enhanced chemiluminescence.
Fluorescence microscopy and image quantitation.
Approximately 105 cells/ml were plated onto 24-well tissue culture plates and grown overnight with indicated treatments including methyl-β-cyclodextrin (MβCD; Sigma). Cells were then washed with PBS and grown in the indicated conditions. Cells were then washed twice with PBS, fixed with 3% paraformaldehyde in PBS for 1 h at 37°C, and then quenched with 1.5 mg/ml glycine for 10 min at room temperature. Cells were then stained with either filipin (Sigma) or Cholera Toxin B (CTB; Molecular Probes Alexa fluor 555) and Hoechst nuclear stain (Sigma). The cells were visualized at 430 nm (filipin or Hoechst) or 555 nm (CTB) by use of an inverted fluorescent microscope at ×10 magnification for filipin due to rapid photobleaching or ×60 for CTB. Filipin images were captured, and fluorescence was estimated by measuring 10 randomly chosen fields per image using ImageJ (rsbweb.nih.gov/ij/).
Inhibition of SCD activity.
The small molecule inhibitor of SCD-1 {4-(2-chlorophenoxy)-N-[3-(methylcarbamoyl)-phenyl]piperidine-1-carboxamide} (A939572) was from Biofine International (Vancouver, BC, Canada). A 64 mM stock of A939572 was dissolved in DMSO and stored at −20°C, and working concentrations (1 μM) were diluted in serum-free DMEM prior to assay. Vehicle (control) -treated cells consisted of diluent DMSO in serum-free DMEM.
Lipid extraction, TLC, and cellular desaturase activity assay.
Cells were treated with 100 μM stearate containing 0.5 μCi [14C]stearic acid conjugated to BSA for 18 h. Total cellular lipids were extracted according to the Folch method (14) and resolved on a silica gel plate using hexane-diethyl ether-acetic acid (90:30:1) as the developing solvent. A live cell-based desaturation assay was employed to assess the ability of A939572 to repress SCD-1 activity. Briefly, cells were treated with 100 μM [14C]stearate (0.5 μCi) in DMEM containing 1% FBS for 24 h and lysed in 10 N NaOH for 30 min. For SCD-1 activity measurements, total cellular lipids were extracted with hexane after acidification with HCl and separated by TLC on silver nitrate-impregnated silica gel plates using a chloroform-methanol-acetic acid-water (90:8:1:0.8) solvent system. Radioactive spots were read using a Packard InstantImager with the upper band corresponding to [14C]stearate and the lower band to [14C]oleate. For FC measurements, N2-dried lipids were resuspended in 0.01 ml of CHCl3 + (0.09 ml isopropanol + 0.1% Triton X-100) and analyzed using a commercially available kit (Wako Diagnostics, 435-35801).
Collection of RNA and quantification of gene expression.
Total RNA was collected from cells by use of Tri reagent (Molecular Research), and reverse-strand cDNA synthesis was conducted using 1 ng RNA (Applied Biosystems). Primers were designed to amplify 100- to 200-bp segments that span exon-exon borders of the target genes to exclude amplifying any genomic DNA that might have been carried over. Xbp-1 processing was assessed as previously described (13).
Statistical analyses.
Statistical analyses were performed using SAS software (SAS v. 8.2; SAS Institute, Cary, NC). The a priori α-level was set at P < 0.05 for all planned comparisons. All values represent means ± SE of three independent experiments; * denotes a significant difference from the respective control (i.e., within groups), and # represents a significant within-treatment (i.e., between groups) difference (P < 0.05).
RESULTS
A939572 inhibits SCD-1 activity and suppresses cell growth.
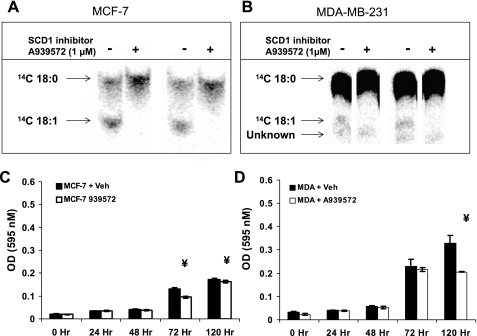
Recently, the piperidine-aryl urea-based inhibitors of SCD-1 activity have become available for use in biological assays (37). The ability of these small molecule inhibitors to cross cell membranes and rapidly and efficiently inhibit the activity of SCD-1 at low doses has enabled us to determine the role of de novo desaturation in cell viability. First, we assessed the ability of A939572 to repress oleic acid generation in MCF-7 cells, which have a high level SCD-1 gene expression, protein, and activity (7) (Fig. 1A). The cells were treated with 100 μM [14C]stearate bound to BSA in DMEM plus 1% FBS for 4 h. We found that 1 μM A939572 was sufficient to completely repress endogenous oleate synthesis in both MCF-7 and MDA-MB-231 (Fig. 1B), indicating that the inhibitor was effective in preventing de novo MUFA generation.
Fig. 1.
The small molecule A939572 (A939) represses stearoyl-CoA desaturase-1 (SCD-1) activity and cell growth. MCF-7 (A) and MDA-MB-231 (MDA) cell lines (B) were used to establish the ability of the SCD-1 small molecule inhibitor to repress desaturase activity. Cells were treated with 14C-labeled 18:0 for 4 h (A) or 24 h (B), after which total lipids were extracted and separated by TLC. Results are from 2 independent experiments analyzed in parallel. MCF-7 (C) and MDA cells (D) were grown in standard culture conditions containing 10% FBS supplemented with 1 μM A939572, and cell growth was assessed by MTT assay. Both cell lines showed significant impairment in growth during the exponential phase in the presence of inhibitor. Cells showed significant impairment in growth during the exponential phase (e.g., >72 h) in the presence of inhibitor.
After establishing the effectiveness of the inhibitor, we next determined its ability to repress cell growth in culture. MCF-7 (Fig. 1C) and MDA-MB-231 cells (Fig. 1D) were plated into 96-well culture plates at a density of 103 cells/ml DMEM + 10% FBS either with 1 μM A939572 or without inhibitor (vehicle), and serum-containing medium was replaced every 48 h. Cell density was measured using the MTT assay from wells in triplicate and indicated that during the exponential growth phase (∼72 h) MCF-7 displayed modest sensitivity to SCD-1 inhibition and that MDA-MB-231 cell growth was repressed at 72 h and failed to increase at 120 h. It is important to note that, while MDA-MB-231 cells display less SCD-1 activity than MCF-7 cells, they demonstrate greater growth retardation upon loss of activity.
Loss of SCD-1 activity suppresses CE biosynthesis.
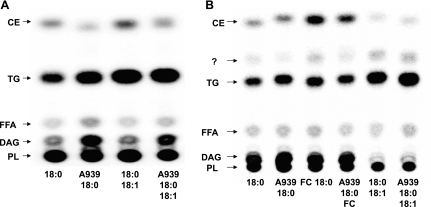
To gain insight into the mechanism by which SCD-1 inhibition suppresses cell growth, we treated both MCF-7 and MDA-MB-231 cells with [14C]stearate for 18 h. After treating the cells with labeled fatty acid, we separated total cellular lipids by TLC and visualized [14C]stearate incorporation relative to known standards (standards were visualized using fluorescein and UV light, and then their positions were marked on the TLC plates) (Fig. 2, A and B). In both cell lines, there was a large degree of label incorporation into phospholipids regardless of whether SCD-1 activity was present or not. In the absence of either SCD-1 activity or exogenous oleate, there appeared to be an accumulation of diacylglycerol that was diminished either by the presence of SCD-1 activity (non-inhibitor-treated MCF-7 cells vs. non-inhibitor-treated MDA) or by exogenously added SCD-1 product (oleate in MDA-MB-231 cells). It is possible that what we have labeled as diacylglycerol may in fact be FC, although it is highly unlikely. In order for the 14C label to be incorporated into FC, labeled stearate would have to be metabolized into acetate and then converted into cholesterol. Surprisingly, triglyceride (TG) content appeared to be unaffected by loss of SCD-1 activity, and although we did not determine fatty acid composition, it is likely that an increase in stearate incorporation was responsible for maintaining TG synthesis in the absence of SCD-1 activitiy. However, it is possible that redistribution of oleate from neutral lipid stores may have contributed to TG synthesis in these cells. Last, we saw a clear reduction in the CE fraction in both MCF-7 and MDA-MB-231 cells. ACAT activity has been shown to proceed in the presence of both SFA and MUFA in purified microsomal fractions; yet the speed of the reaction increases with long-chain MUFA (30). This suggests that SCD-1-mediated synthesis of oleate is an important step in the synthesis of cholesteryl-oleate, and these data suggest that loss of SCD-1 activity severely represses CE formation in these cells.
Fig. 2.
Inhibition of SCD-1 suppresses cholesteryl ester (CE) formation. TLC separation of total neutral lipids indicates that loss of SCD-1 activity specifically suppresses [14C]stearate incorporation into CE. MCF-7 (A) and MDA cells (B) were treated with 50 μM [14C]stearic acid (18:0), ± 50 μM oleaic acid (18:1), and ± 100 μg/ml free cholesterol (FC) for 18 h, after which total lipids were extracted and separated, and incorporation of 14C was visualized by radiography. Loss of SCD-1 activity increased diacylglycerol (DAG) content, whereas phospholipid (PL) and triglyceride (TG) formation were largely unaffected in both cell lines. To visualize the CE band, MDA cells were supplemented with 50 μM FC in addition to indicated free fatty acid (FFA) treatments. Both cell lines showed a decreased intensity of 14C incorporation into CE, suggesting that loss of SCD-1 activity specifically suppresses cholesterol esterification.
SCD inhibition increases FC and ABCA1 expression.
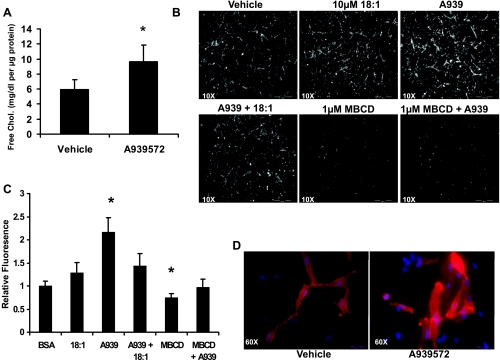
LDL receptor-mediated uptake of cholesterol, mainly in the form of CE, is still dependent on ACAT-mediated esterification with MUFA. This is because CE targeted to lysosomes is deesterified by cholesterol esterase and thus requires reesterification in the ER (18). On the basis of these facts, we assumed that, if cholesterol esterification to MUFA was prevented in the absence of SCD-1 activity, then free cholesterol should therefore accumulate. We quantitatively measured total FC in MDA-MB-231 cells (Fig. 3A) by enzymatic assay and found that FC levels increased 63% when SCD activity was inhibited (5.9 ± 1.3 to 9.7 ± 2.3 μg/ml, vehicle vs. A939572). The cells were also treated with the FC-specific probe filipin with a combination of treatments including A939572, 25 μM oleate, and/or MβCD, and filipin staining intensity was quantified as described above using ImageJ software (Fig. 3B). There was no independent effect of oleate treatment on filipin staining intensity, whereas A939572 treatment increased fluorescence more than twofold (2.2,27 0.3; Fig. 3C). When the cells were cotreated with 18:1 + A939572, the increase in FC level was prevented, with similar results obtained with MβCD + A939572 cotreatment. The filipin staining data clearly show that, in the absence of SCD-1 activity (i.e., losss of MUFA synthesis), FC levels rise. However, when SCD-1 substrate (oleate) is added back, the increase is FC is completely prevented. Similarly, when the cells are pretreated with a cholesterol chelating agent to deplete total cholesterol, the effect of SCD-1 inhibition is lost. Therefore, we conclude that the effect of A939572 is due in large part to the loss of MUFA synthesis and the increase in FC levels, indicating that the small molecule inhibitor is fairly specific for SCD-1.
Fig. 3.
Inhibition of SCD-1 activity increases FC levels in MDA-MB-231 cells. In light of the fact that SCD-1 inhibition prevented cholesterol esterification with MUFA, we measured total cellular FC levels. A: cells were grown for 18 h in serum-free medium with or without 1 μM A939572, and FC levels were assessed by enzymatic assay. Inhibitor treatment caused a significant increase to total FC levels in culture. B: alternatively, cells were grown as in A and supplemented with indicated fatty acids, fixed, and stained with the FC probe filipin and visualized using fluorescence microscopy. C: inhibitor treatement increased filipin staining intensity and was reversed by cotreatment with 10 μm oleate or methyl-β-cyclodextrin (MβCD) treatment. Average fluorescence intensity was quantified from 10 randomly chosen fields from the images in B. D: excessive FC can alter membrane composition, and we saw that staining with the lipid raft probe cholera toxin B (red) and Hoescht (blue) increased with A939572 treatment. At ×10, scale bar represents 500 μm; at ×60, scale bar represents 50 μm.
To accurately assess the effects of SCD-1 inhibition in culture, all treatments must be carried out in serum-free medium, as FBS contains oleate in FFA form. As a consequence, cellular ABCA1-mediated cholesterol efflux will be blocked at the membrane, as there is no FC acceptor in the medium. The CTB stain is useful for staining lipid raft or caveolin-1 (Cav-1)-enriched segments of the membrane, which is where ABCA1-mediated cholesterol efflux occurs (16, 23). When the cells were stained with CTB to visualize lipid raft content, we found that fluorescence intensity increased with SCD-1 inhibitor treatment, suggesting that cellular FC was localized or accumulating in the cell membrane (Fig. 3D).
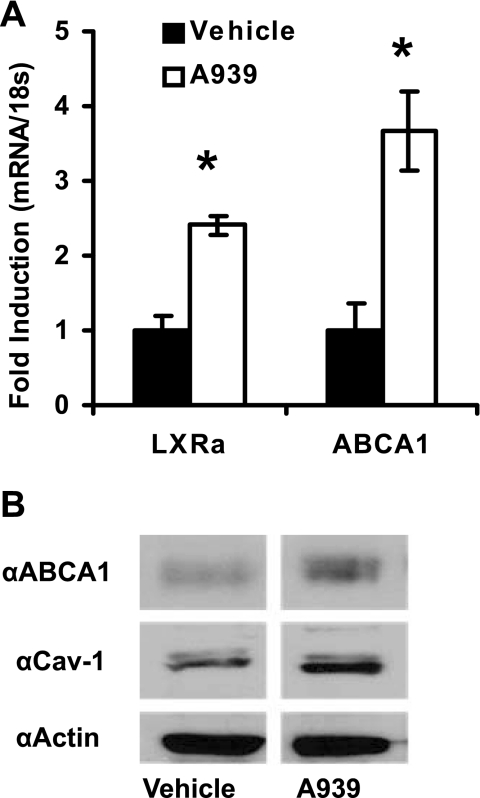
With the addition of the SCD-1 inhibitor, both LXRα and ABCA1 expression increased 2.4- and 3.7-fold, respectively (Fig. 4A). ABCA1 is responsible for a large portion of active transport of cellular phospholipids and FC from the cytoplasm to the circulation via lipid-poor apoA-I. In addition to regulated gene expression, several modes of control of ABCA1 have been described, including membrane-to-cytosol shuttling and protein stability and degradation (3). Therefore, to confirm that the rise in ABCA1 gene expression resulted in translated protein, we assessed its levels via Western blot under the same treatments. In agreement with the gene expression results, ABCA1 protein levels increased with SCD inhibition (Fig. 4B). The activation of ABCA1-mediated cholesterol efflux has been shown to require the cell membrane protein Cav-1 during the transition from the inactive cytosolic pool to the membrane lipid raft localized, actively effluxing, pool (6, 16, 23). For this reason, we also measured total Cav-1 levels. Despite the fact that gene expression did not change (data not shown), Cav-1 protein levels were elevated over vehicle-treated cells, suggesting that not only are the genes for cholesterol efflux increasing with SCD inhibition, but membrane remodeling may be occurring as well.
Fig. 4.
liver X receptor (LXR) target gene expression increases with A939572 treatment. The changes in LXRα and its target genes were assessed after inhibitor treatment. A: loss of SCD-1 activity in MDA cells caused a significant increase in LXRα and ATP-binding cassette transporter A1 (ABCA1) expression. Results are expressed as fold change over noninhibitor (vehicle)-treated cells. B: increase in ABCA1 was confirmed by Western blotting, which also shows that total caveolin-1 (Cav-1) protein is increased, suggesting that both cholesterol efflux and lipid raft content are increased with loss of SCD-1 activity.
Loss of SCD activity increases xbp-1 splicing and cholesterol synthesis.
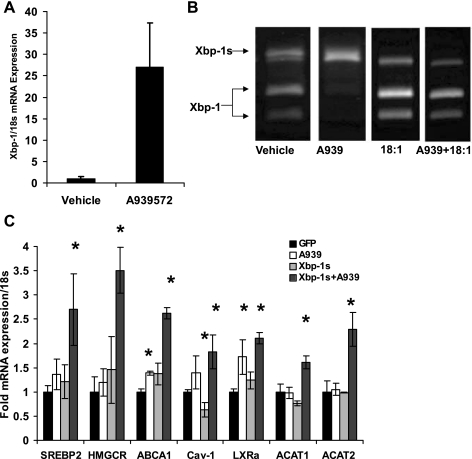
On the basis of our results, we sought to determine whether the increase in FC as a result of SCD-1 inhibition was activating ER stress and whether it was responsible for cholesterol synthesis. Figure 5A shows that inhibitor treatment led to a 27-fold induction of total Xbp-1 message.
Fig. 5.
Loss of SCD-1 activity increases the transcriptionally active form of X-box binding protein-1 (Xbp-1s)-mediated induction of cholesterol synthesis. Modest increases in FC can induce cell stress; therefore, we assessed the degree of Xbp-1 mRNA induction with SCD-1 inhibition. A: cell stress transcription factor Xbp-1 mRNA was significantly increased with 1 μM A939572 treatment for 18 h. B: Xbp-1s was assessed by resistance to PstI digest and showed that loss of endogenous MUFA synthesis increased splicing, whereas replenishment with 10 μM oleate was sufficient to restore normal function. C: the constitutively active Xbp-1s adenovirus (10 GFU/ml) was used to assess the ability of endoplasmic reticulum (ER) stress to induce cholesterolgenesis in the presence or absence of SCD-1 activity. In GFP-, A939572-, or Xbp-1s-treated cells, there was no effect on cholesterol synthesis and only a slight inductin of LXRα and ABCA1. In the absence of SCD-1 activity, Xbp-1s increased cholesterol synthesis gene expression [stearoly regulatory element-binding protein-2 (SREBP-2), HMG-CoA reductase (HMGCR), acyl-CoA:cholesterol acyltransferase (ACAT)1, and -2] and LXR target genes (LXRα, ABCA1, and Cav-1).
The 26-nucleotide intron contains a PstI restriction site; thus, unspliced Xbp-1 (Xbp-1u) PCR products can be digested with PstI to yield two products (290 and 183 bp). Spliced Xbp-1 (Xbp-1s) lacks the PstI restriction site, and therefore it will yield a single PCR band at 447 bp (13). By use of this method, vehicle-treated MDA-MB-231 cells display both Xbp-1u and Xbp-1s in roughly equal quantities (Fig. 5B). When cells were treated with 1 μM A939572 for 18 h, Xbp-1u completely disappeared, whereas Xbp-1s was the principal cDNA isoform. Once again, to address the specificity of the small molecule inhibitor, the cells were cotreated with oleate in the presence or absence of A939572. At 25 μM, oleate alone increased Xbp-1u relative to Xbp-1s, and in the absence of SCD-1 activity exogenous oleate was able to reduce Xbp-1 splicing. These results, combined with those in Fig. 5A, suggest that loss of SCD-1 activity not only increases Xbp-1 content but activates the transcription factor as well.
Membrane biogenesis is an integral aspect of the unfolded protein response (UPR) and has been shown to require cholesterol (21, 34). In light of that fact, we sought to determine whether Xbp-1s could induce sterol synthesis gene expression. MDA-MB-231 cells were grown in standard culture conditions (i.e., DMEM + 10% FBS) with or without A939572. By treating the cells with Xbp-1s adenovirus in the presence or absence of SCD-1 activity while providing exogenous oleate, we could effectively determine the independent effect of Xbp-1s activation in the absence of complete activation of the UPR. We found no effect of Xbp-1s on sterol synthesis genes in the presence of SCD-1 activity, nor was there an independent effect of A939572 (Fig. 5C). However, when the cells were cotreated with Xbp-1s and A939572, both SREBP2 and its target gene HMG-CoA reductase (HMGCR) increased significantly (2.7 ± 0.7- and 3.5 ± 0.5-fold, respectively). We also found that there was a slight yet significant elevation in LXRα (1.7 ± 0.3-fold vs. GFP) expression and its target gene ABCA1 (1.4 ± 0.04-fold vs. GFP) with A939572 alone. Xbp-1s alone caused a minor, nonsignificant increase in both ABCA1 and LXRα while significantly reducing Cav-1 expression (0.6 ± 0.2-fold vs. GFP). Finally, when cells were cotreated with A939572 + Xbp-1s, LXRα, ABCA1, Cav-1, ACAT1, and ACAT2 expression all increased significantly over GFP-treated cells (2.1 ± 0.1, 2.6 ± 0.1, 1.8 ± 0.4, 1.6 ± 0.1, 2.3 ± 0.3, respectively). These results show that activation of Xbp-1s induces sterol synthesis only in the absence of SCD-1 activity, possibly by increasing cholesterol synthesis without a concomitant increase in MUFA for esterification.
Fasting-refeeding increases FC levels and xbp-1s in SCD-1 LKO mice.
To confirm our in vitro results using A939572, we chose to use a mouse model of liver-specific SCD-1 deficiency. We have previously reported that liver-specific deletion (LKO) of SCD-1 impairs lipogenesis and increases plasma cholesterol when mice are fed an HSVLF diet for 10 days (24). Additionally, in global SCD-1-deficient mice, 10-day feeding of the HSVLF diet was shown to induce cell stress and Xbp-1 processing (13). Others have shown that during fasting-refeeding, which robustly activates lipogenesis, there is also a concomitant increase in sterol synthesis (19, 36). On the basis of this fact, we surmised that if LKO mice could in fact activate lipogenesis and cholesterolgenesis, it would likely induce Xbp-1 splicing because any de novo synthesis of cholesterol would either be esterified to saturated fatty acid (SFA) or it would remain as FC.
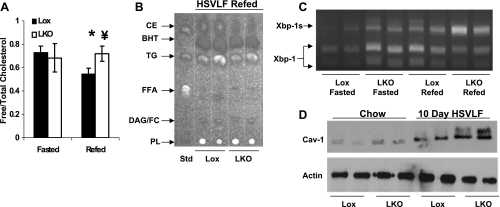
Eight- to ten-week-old Lox control and LKO mice were fasted for 24 h (0800–0800; Fasted group) or fasted for 24 h (2000–2000) and then refed the HSVLF diet for 12 h (2000–0800; Refed group) to induce cholesterolgenesis. The timing of the 24-h fast for the fasted group was staggered such that both groups were killed between 0800 and 1000. To confirm the induction of cholesterol synthesis, we measured free cholesterol in liver of Lox and LKO fasted and refed mice. We found that in the fasted state there was no significant difference between groups. With refeeding, Lox animals decreased significantly (0.7 ± 0.05 vs. 0.5 ± 0.05, P = 0.01), whereas LKO animals failed to decrease (0.7 ± 0.12 vs. 0.7 ± 0.06, P = 0.4; Fig. 6A). The significant decrease in Lox animals with refeeding and the failure to decrease in LKO under the same conditions resulted in a significantly different FC level between groups (P = 0.01). This was also confirmed by TLC separation of hepatic neutral lipids (Fig. 6B), where the upper CE band in refed Lox mice is clearly larger than in LKO animals.
Fig. 6.
SCD-1 liver knockout (LKO) mice exhibit decreased CE content and Xbp-1s with fasting-refeeding. Lox control and LKO mice were fasted for 24 h (Fasted) or fasted for 24 h and then refed a high-sucrose, very-low-fat diet (HSVLF) for 12 h (Refed) prior to collecting liver for cholesterol and mRNA measurements. A: FC was determined from hepatic total lipids, which illustrated that refeeding in LKO animals fails to reduce FC below fasting conditions. B: TLC-separated hepatic neutral lipids reveals that CE formation is impaired in LKO mice. C: increased cholesterolgenesis during fasting and refeeding can be accounted for by an increase in the degree of Xbp-1s in LKO animals during fasting that increases with refeeding. D: animals were fed the HSVLF diet for 10 days, after which liver expression of Cav-1 was compared with chow-fed controls. BHT, butylated hydroxytoluene.
We demonstrated above how loss of SCD-1 activity induces Xbp-1 splicing by increasing FC, and similarly, when we assessed the degree of Xbp-1 activation, we found that LKO mice appeared to have higher Xbp-1s content under fasting conditions (Fig. 6C). Both groups increased the Xbp-1s/Xbp-1u ratio with refeeding as expected, but with the higher total Xbp-1 mRNA content in the LKO animals to begin with, the amount of active Xbp-1 in the refed state was much higher compared with Lox mice. Last, we assessed changes in membrane composition by Cav-1 expression following 10-day feeding of the HSVLF diet in both Lox and LKO mice (Fig. 6D). There appeared to be a slight increase in total Cav-1 expression in chow-fed LKO mice compared with Lox controls. Both groups increased Cav-1 expression when placed on the HSVLF diet for 10 days, but the extent of increase was far greater in the LKO animals. This would suggest that chronic feeding of the diet may be causing changes in membrane composition in lipid rafts (15, 16).
DISCUSSION
The present investigation was designed to determine the effect of SCD-1 inhibition on cell growth where we found a striking effect on CE synthesis. We were able to confirm the relationship between CE synthesis and SCD-1 activity in MCF-7 and MDA-MB-231 cell lines using the small molecule inhibitor A939572. The ability to effectively repress SCD-1 activity in cells allowed us to determine the mechanism by which endogenous desaturase activity promoted viability. We found that treatment of cells with 1 μM A939572 SCD-1 inhibitor was sufficient to repress desaturase activity and significantly impair cell growth even in the presence of exogenous MUFA.
We observed that A939572 treatment of MDA-MB-231 and MCF-7 cells caused a significant reduction in cell proliferation at 72 (MCF-7) and 120 h (MCF-7 and MDA-MB-231). At earlier time points there was no observable effect of inhibition, yet when the cells entered the exponential phase of growth, proliferation was retarded in both cell lines. However, in subsequent experiments, side-by-side comparisons of cell viability and sensitivity to either SCD-1 inhibition or SFA treatment showed that MDA-MB-231 cells were far more susceptible to loss of SCD-1 activity (data not shown). This is likely due to the fact that MCF-7 cells have been shown to possess a much greater degree of fatty acid oxidation than MDA-MB-231 and therefore would not store FFA or TG to the same extent (1, 38). MDA-MB-231 cells are more glycolytic, and yet they are still a lipogenic cell line. Thus, loss of SCD-1 activity may induce SFA toxicity due, in part, to the fact that they are unable to increase β-oxidation while accumulating lipids.
We initially explored the effect of inhibitor treatment on fatty acid-mediated induction of lipogenesis by assessing changes in TG synthesis in the presence of SFA (data not shown). Despite the fact that diacylglycerol levels were elevated, loss of SCD-1 activity did not prevent induction of lipogenic gene expression, nor did it prevent [14C]stearate incorporation into TG. It did, however, severely repress CE synthesis in high-ACAT1-expressing cells (MDA-MB-231) (2). It is known that the ACAT reaction proceeds at a much faster rate with 18:1 as the substrate, and while it does still proceed, 16:0 or 18:0 are less preferred substrates (18). Additionally, qualitative differences in cholesteryl-MUFA vs. cholesteryl-SFA will likely have detrimental effects on cholesterol solubility (32, 33) and will likely induce efflux, as we and others (20) have shown.
It is important to note that total CE synthesis is not blocked by the loss of SCD-1 activity, whereas oleate synthesis is completely ablated. As a result, the cells retain the ability to generate CE that is likely esterified to stearate. Therefore, when the cells are not given exogenous FC and retain SCD-1 activity (Fig. 2B, lane 1), a portion of the [14C]stearate would likely be unsaturated and used as substrate for β-oxidation, thus disappearing from the system and/or accumulating as TG, as seen in the image. In the absence of exogenous FC and SCD-1 activity (e.g., with A939572), the inability of the cells to desaturate [14C]stearate would alter the distribution of SFA and its subsequent incorporation into CE and TG. In our experiments, MDA-MB-231 cells were far more susceptible to apoptosis following loss of SCD-1 activity compared with MCF-7 cells (data not shown), and the latter seemed to be able to tolerate loss of activity to a far greater extent. It is tempting to speculate that because MDA-MB-231 cells have far less stored 18:1 to use for mobilization via TG hydrolysis, whereas MCF-7 cells own a far greater capacity to shift oleate from TG to CE and thus use [14C]stearate for β-oxidation (28). Last, in the case of the difference between lanes 1 and 2 vs. lanes 3 and 4 of Fig. 2B, we speculate that, in the absence of excess (or at least larger) quantities of FC, MDA-MB-231 cells can sequester exogenous FA into CE, at least in part, by increasing [14C]stearate conversion into [14C]oleate. However, in the absence of excess FC, [14C]stearate incorporation into TG might be favored simply due to the fact that total FC levels may not be sufficient to enable detoxification. In either case, it is quite likely that incorporation of SFA into either fraction is highly detrimental to cell viability, leading to Xbp-1 splicing and eventually apoptosis.
Previous reports have shown that loss of SCD-1 in mouse liver reduces cholesterol esterification (8, 25) and that forced overexpression of SCD-1 in CHO cells is sufficient to increase CE formation (35). While overexpression of SCD-1 or addition of exogenous MUFA in macrophages has been shown to repress ABCA1-mediated cholesterol efflux, loss of SCD-1 has been shown to increase ABCA1 activity (35). We found the change in total FC levels to parallel that of ABCA1 and Cav-1, and, on the basis of the current data, we can conclude that inhibition of SCD-1 activity via the small molecule inhibitor A939572 causes a significant rise in FC levels that is associated with an increase in cholesterol efflux gene and protein expression.
The potential for changes in FC content of the cells following SCD inhibition led us to speculate that the LXR pathway might be responsible for the observed phenotype. Recently, the LXR pathway was reported to repress cancer cell proliferation, due in part to increased ABCA1 expression that was independent of SREBP-1c (9, 10, 17). When we treated breast cancer cells with the SCD-1 inhibitor, we observed an increase in FC that was associated with an increase in ABCA1 and LXRα expression. We saw an elevation in FC levels, an increase in Cav-1 and ABCA1 protein levels, and an increase in cellular membrane lipid raft content under these treatments. Thus, it would seem that inhibition of proliferation by LXR activation may be mediated by cholesterol depletion and membrane remodeling.
The current data provide new mechanistic insight into the potential link between SCD-1 and Cav-1. Recently, Antalis et al. (2) reported that SCD-1 expression was negatively correlated with Cav-1 expression. This phenomenon is potentially significant because 1) FC has been shown to increase Cav-1 expression, and 2) Cav-1 helps stabilize ABCA1 membrane attachment to aid in cholesterol efflux. Thus, increased Cav-1 expression may be a downstream consequence of low/absent SCD-1 activity. Cav-1 is thought to be cardioprotective in atherogenic-prone mice (15), whereas SCD-1 deficiency has recently been shown to increase lesion formation (5). It would be interesting to know whether Cav-1 expression is also upregulated in the SCD-1 × LDLR KO model as well.
We also saw that loss of SCD-1 activity increased FC, which can induce cell stress and Xbp-1 processing (13). Xbp-1s can not only induce lipogenic gene expression in hepatocytes (22), but it is also required for adipogenesis (31). We found that SCD-1 inhibition induced Xbp-1 splicing, which activated cholesterol synthesis genes. It could be expected that under normal circumstances (e.g., SCD-1 activity) the induction of cell stress/UPR would require cholesterol synthesis to aid in ER membrane biogenesis. However, in the absence of SCD-1 activity, the induction of SREBP-2 and HMGCR would lead to accumulation of FC, which could become cytotoxic. However, by increasing FC conversion into oxysterols, which serve as LXR ligands, the subsequent activation of ABCA1 and Cav-1 expression would remove FC and/or sequester it into lipid rafts.
Taken together, the data presented herein suggest that desaturase activity is required for efficient synthesis and retention of CE and that loss of SCD-1 activity induces Xbp-1 splicing and cholesterol efflux. We have shown here in cancer cells that SCD-1 inhibition suppresses cell viability. Several reports have linked SCD-1 activity with cancer proliferation, and on the basis of our results it is likely that killing of cancer cells could be occurring by one of two mechanisms. First, increased FC levels can cause a shift, or redistribution in cellular CE, away from storage and may consume limited supplies of oleate. Furthermore, the oleate-to-FC ratio may be important to cell viability and membrane fluidity, potentially causing the observed increase in Xbp-1 splicing. Second, LXR activation will increase cholesterol efflux and may deplete membrane biosynthetic constituents. If ABCA1 expression persists in the absence of increased cholesterol uptake or synthesis, the ability of the cells to proliferate and thrive will be severely impaired. It is beyond the scope of the current investigation to determine which of these mechanisms is responsible; however, future investigations may provide additional insight into these events.
Finally, we were able to confirm our in vitro models using the liver-specific SCD-1 KO mouse. These animals have been shown to lack the ability to maintain lipogenesis while on a 10-day HSVLF diet. In the current investigation, we show that with a single feeding of a high-sucrose diet there does not appear to be any impairment in cholesterolgenesis. What we did observe was an apparent inability to reduce hepatic FC content during refeeding either by failing to esterify due to lack of MUFA or by Xbp-1s-mediated induction of cholesterolgenesis. Within the current investigation, we are unable to determine which is direct and which is indirect; that is, does FC induce ER stress or does the ER stress induce cholesterol synthesis? Based on our results, it is likely that each plays a causative role in mediating the phenotype observed in vivo and in vitro. It is logical to assume that under normal lipogenic conditions the induction of Xbp-1s would increase lipid and sterol synthesis to maintain adequate storage and secretory function. While others have shown that Xbp-1s is necessary for the induction of lipogenesis, we have clearly demonstrated that in the absence of SCD-1 activity Xbp-1s also increases cholesterol synthesis. These data suggest that in the absence of SCD-1 activity the lack of oleate most likely increases Xbp-1 splicing, which increases FC in amounts that exceed available MUFA for efficient esterification. Future studies aimed at defining the role of cholesteryl-oleate in cell stress will be necessary to clearly delineate the role(s) of SCD-1 on enabling cholesterol synthesis and maintaining hepatic sterol homeostasis.
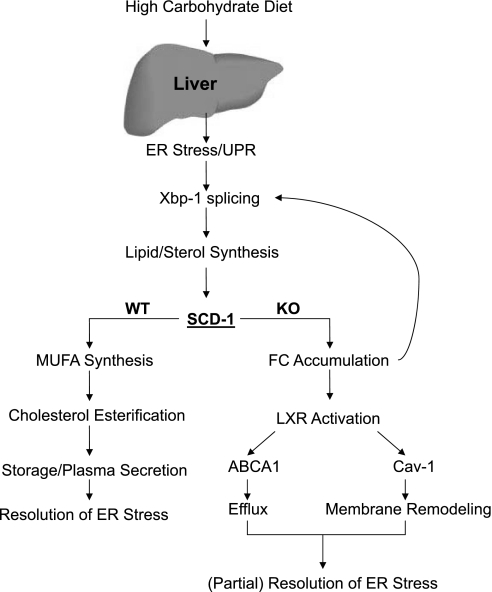
The recent observation that Xbp-1 splicing is required for the induction of lipogenesis places the UPR upstream of lipogenesis and cholesterol biosynthesis. Furthermore, SCD-1 was shown to be an Xbp-1 target gene, and our data suggest that SCD-1 expression may be necessary for proper functioning of Xbp-1-mediated induction and resolution of ER stress. Figure 7 depicts the proposed model in which normal animals activate the UPR/Xbp-1 pathway following a high-sucrose diet. The transcriptional activation of lipogenesis via Xbp-1s leads to MUFA synthesis only when SCD-1 activity is present, where the MUFA is then used to form both TG and CE for storage and release into the circulation, likely as VLDL and/or chylomicrons, and resolving ER stress.
Fig. 7.
Proposed model. After consuming a high-carbohydrate diet, activation of liver ER stress or the unfolded protein response (UPR) is required to induce lipid synthesis. SCD-1 is a target gene of Xbp-1s and increases MUFA synthesis to enable efficient TG and CE formation, subsequent VLDL formation, and eventual resolution of ER stress. However, in the absence of SCD-1, the inability to generate MUFA diminishes both TG and CE, with FC accumulating and further increasing ER stress/Xbp-1 splicing. FC conversion to oxysterols will lead to LXR activation, cholesterol efflux, and membrane remodeling in part through Cav-1 expression.
In the absence of SCD-1 activity, the UPR pathway is activated as in Lox mice and leads to lipid synthesis, but the inability to generate MUFA prevents efficient esterification. In the absence of proper TG and CE biosynthesis, both storage (TG) and secretion (CE) are impaired, and FC accumulation will lead to further activation of cell stress and Xbp-1s. The accumulated FC can be cleared from the hepatocyte via oxidation, which activates LXR, increases cholesterol efflux, and promotes membrane remodeling via Cav-1 to (partially) resolve ER stress.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-62388 (J. M. Ntambi) and DK-007665 (C. M. Paton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Aja S, Landree LE, Kleman AM, Medghalchi SM, Vadlamudi A, McFadden JM, Aplasca A, Hyun J, Plummer E, Daniels K, Kemm M, Townsend CA, Thupari JN, Kuhajda FP, Moran TH, Ronnett GV. Pharmacological stimulation of brain carnitine palmitoyl-transferase-1 decreases food intake and body weight. Am J Physiol Regul Integr Comp Physiol 294: R352–R361, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Antalis C, Arnold T, Rasool T, Lee B, Buhman K, Siddiqui R. High ACAT1 expression in estrogen receptor negative basal-like breast cancer cells is associated with LDL-induced proliferation. Breast Cancer Res Treat 122: 661–670, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Attie AD. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem Sci 32: 172–179, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Back SH, Lee K, Vink E, Kaufman RJ. Cytoplasmic IRE1alpha-mediated XBP1 mRNA splicing in the absence of nuclear processing and endoplasmic reticulum stress. J Biol Chem 281: 18691–18706, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen T, Zhu X, Duong MN, Wibley AL, Shah R, Davis MA, Kelley K, Wilson MD, Kent C, Parks JS, Rudel LL. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation 118: 1467–1475, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao WT, Tsai SH, Lin YC, Lin WW, Yang VC. Cellular localization and interaction of ABCA1 and caveolin-1 in aortic endothelial cells after HDL incubation. Biochem Biophys Res Commun 332: 743–749, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Choi Y, Park Y, Storkson JM, Pariza MW, Ntambi JM. Inhibition of stearoyl-CoA desaturase activity by the cis-9,trans-11 isomer and the trans-10,cis-12 isomer of conjugated linoleic acid in MDA-MB-231 and MCF-7 human breast cancer cells. Biochem Biophys Res Commun 294: 785–790, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol 26: 6786–6798, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi J, Chen RY, Liao S. Inhibition of tumor growth and progression of lncap prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res 66: 6482–6486, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chuu CP, Kokontis J, Hiipakka R, Liao S. Modulation of liver X receptor signaling as novel therapy for prostate cancer. J Biomed Sci 14: 543–553, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5: 781–792, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Flowers MT, Groen AK, Oler AT, Keller MP, Choi Y, Schueler KL, Richards OC, Lan H, Miyazaki M, Kuipers F, Kendziorski CM, Ntambi JM, Attie AD. Cholestasis and hypercholesterolemia in SCD1-deficient mice fed a low-fat, high-carbohydrate diet. J Lipid Res 47: 2668–2680, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Flowers MT, Keller MP, Choi Y, Lan H, Kendziorski C, Ntambi JM, Attie AD. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiol Genomics 33: 361–372, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 15.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol 24: 98–105, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Fu Y, Hoang A, Escher G, Parton RG, Krozowski Z, Sviridov D. Expression of caveolin-1 enhances cholesterol efflux in hepatic cells. J Biol Chem 279: 14140–14146, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Fukuchi J, Kokontis JM, Hiipakka RA, Chuu Cp, Liao S. Antiproliferative effect of liver X receptor agonists on LNCaP human prostate cancer cells. Cancer Res 64: 7686–7689, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Goldstein JL, Dana SE, Faust JR, Beaudet AL, Brown MS. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem 250: 8487–8495, 1975 [PubMed] [Google Scholar]
- 19.Im SS, Hammond LE, Yousef L, Nugas-Selby C, Shin DJ, Seo YK, Fong LG, Young SG, Osborne TF. Sterol regulatory element binding protein 1a regulates hepatic fatty acid partitioning by activating acetyl coenzyme A carboxylase 2. Mol Cell Biol 29: 4864–4872, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langer C, Huang Y, Cullen P, Wiesenh++tter B, Mahley RW, Assmann G, von Eckardstein A. Endogenous apolipoprotein E modulates cholesterol efflux and cholesteryl ester hydrolysis mediated by high-density lipoprotein-3 and lipid-free apolipoproteins in mouse peritoneal macrophages. J Mol Med 78: 217–227, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320: 1492–1496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YC, Ma C, Hsu WC, Lo HF, Yang VC. Molecular interaction between caveolin-1 and ABCA1 on high-density lipoprotein-mediated cholesterol efflux in aortic endothelial cells. Cardiovasc Res 75: 575–583, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab 6: 484–496, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem 275: 30132–30138, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Morgan-Lappe SE, Tucker LA, Huang X, Zhang Q, Sarthy AV, Zakula D, Vernetti L, Schurdak M, Wang J, Fesik SW. Identification of Ras-related nuclear protein, targeting protein for Xenopus kinesin-like protein 2, and stearoyl-CoA desaturase 1 as promising cancer targets from an RNAi-based screen. Cancer Res 67: 4390–4398, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Mulligan JD, Flowers MT, Tebon A, Bitgood JJ, Wellington C, Hayden MR, Attie AD. ABCA1 is essential for efficient basolateral cholesterol efflux during the absorption of dietary cholesterol in chickens. J Biol Chem 278: 13356–13366, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Przybytkowski E, Joly E, Nolan CJ, Hardy S, Francoeur AM, Langelier Y, Prentki M. Upregulation of cellular triacylglycerol-free fatty acid cycling by oleate is associated with long-term serum-free survival of human breast cancer cells. Biochem Cell Biol 85: 301–310, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Scaglia N, Igal RA. Stearoyl-CoA desaturase is involved in the control of proliferation, anchorage-independent growth, and survival in human transformed cells. J Biol Chem 280: 25339–25349, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Seo T, Oelkers PM, Giattina MR, Worgall TS, Sturley SL, Deckelbaum RJ. Differential modulation of ACAT1 and ACAT2 transcription and activity by long chain free fatty acids in cultured cells. Biochemistry 40: 4756–4762, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Sha H, He Y, Chen H, Wang C, Zenno A, Shi H, Yang X, Zhang X, Qi L. The IRE1[alpha]-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab 9: 556–564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smaby JM, Schmid PC, Brockman HL. Phospholipid structure and the packing of cholesteryl oleate at the lipid/water interface. Biochemistry 23: 1955–1958, 1984 [Google Scholar]
- 33.Snow J, Phillips MC. Phase behavior of cholesteryl ester dispersions which model the inclusions of foam cells. Biochemistry 29: 2464–2471, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, Brewer JW. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem 282: 7024–7034, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Hao M, Luo Y, Liang Cp Silver DL, Cheng C, Maxfield FR, Tall AR. Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J Biol Chem 278: 5813–5820, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Xie X, Liao H, Dang H, Pang W, Guan Y, Wang X, Shyy JYJ, Zhu Y, Sladek FM. Down-regulation of hepatic HNF4(alpha) gene expression during hyperinsulinemia via SREBPs. Mol Endocrinol 23: 434–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin Z, Zhao H, Serby MD, Liu B, Liu M, Szczepankiewicz BG, Nelson LTJ, Smith HT, Suhar TS, Janis RS, Cao N, Camp HS, Collins CA, Sham HL, Surowy TK, Liu G. Discovery of piperidine-aryl urea-based stearoyl-CoA desaturase 1 inhibitors. Bioorg Med Chem Lett 18: 4298–4302, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Zhou W, Simpson PJ, McFadden JM, Townsend CA, Medghalchi SM, Vadlamudi A, Pinn ML, Ronnett GV, Kuhajda FP. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res 63: 7330–7337, 2003 [PubMed] [Google Scholar]