Abstract
Maternal obesity (MO) has harmful effects on both fetal development and subsequent offspring health. The impact of MO on fetal myocardium development has received little attention. Fibrogenesis is regulated by the transforming growth factor-β (TGF-β)/p38 signaling pathway. Using the well-established model of MO in pregnant sheep, we evaluated the effect of MO on TGF-β/p38 and collagen accumulation in fetal myocardium. Nonpregnant ewes were assigned to a control diet [Con, fed 100% of National Research Council (NRC) nutrient recommendations] or obesogenic diet (OB, fed 150% of NRC recommendations) from 60 days before conception. Fetal ventricular muscle was sampled at 75 and 135 days of gestation (dG). At 75 dG, the expression of precursor TGF-β was 39.9 ± 9.9% higher (P < 0.05) in OB than Con fetal myocardium, consistent with the higher content of phosphorylated Smad3 in OB myocardium. The phosphorylation of p38 tended to be higher in OB myocardium (P = 0.08). In addition, enhanced Smad complexes were bound to Smad-binding elements in 75 dG OB fetal myocardium measured by DNA mobility shift assay (130.2 ± 26.0% higher, P < 0.05). Similar elevation of TGF-β signaling was observed in OB fetal myocardium at 135 dG. Total collagen concentration in OB was greater than Con fetal myocardium (2.42 ± 0.16 vs. 1.87 ± 0.04%, P < 0.05). Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 were higher in the Con group compared with OB sheep (43.86 ± 16.01 and 37.23 ± 7.97% respectively, P < 0.05). In summary, MO results in greater fetal heart connective tissue accumulation associated with an upregulated TGF-β/p38 signaling pathway at late gestation; such changes would be expected to negatively impact offspring heart function.
Keywords: collagen, fibrogenesis, transforming growth factor-β, maternal obesity, fetus, myocardium
the rising incidence of obesity is adversely affecting general health in the United States and other countries in epidemic proportions. According to a National Health and Nutrition Examination Survey (1999–2002), 29% of nonpregnant women between 20 and 39 years old are obese. Maternal obesity (MO) resulting from a high-energy diet is harmful to fetal development, predisposing offspring to hypertension, type II diabetes, dyslipidemia, and heart diseases (25, 29, 34).
Cardiac fibrosis impairs myocardial contractility and is associated with cardiac aging, restrictive cardiomyopathy, and many other cardiovascular anomalies (27). Transforming growth factor-β (TGF-β) promotes pathological fibrosis via activation of the Smad signaling pathway (7, 11). p38 promotes TGF-β signaling, which is necessary for development of fibrosis (10, 20). Fibrosis occurs in response to inflammation (15, 18). Our earlier study in the fetuses of MO ewes revealed changes characteristic of overt inflammatory response in skeletal muscle (38), associated with enhanced TGF-β signaling and collagen accumulation (14). TGF-β stimulates fibrosis partially via changing the expression of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) (9). Inflammation is known to trigger an array of cardiovascular diseases, such as atherosclerosis (3), cardiac remodeling, and myocardial fibrosis in hypertension (16). MO may induce similar changes in fetal myocardium; thus, we hypothesized that MO would increase TGF-β signaling and result in fibrogenesis in fetal myocardium.
MATERIALS AND METHODS
Care and use of animals.
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee. Multiparous Rambouillet/Columbia ewes were used. Ewes were bred to a single ram. Beginning 60 days before conception and continuing to the date of necropsy (first day of mating = day 0), ewes were individually fed a highly palatable diet either 100% (Con) of National Research Council recommendations for energy (26) (n = 6) or 150% (OB) of recommended energy requirements for early gestation (n = 6) as previously reported. Ewes were housed in individual pens in a temperature-controlled room (∼20°C). All ewes were weighed at weekly intervals, and rations were adjusted for weekly changes in metabolic body weight. Body condition was scored at monthly intervals to evaluate changes in fatness. A body condition score of one (emaciated) to nine (obese) was assigned by two trained observers after palpation of the transverse and vertical processes of the lumbar vertebrae (L2 through L5) and the region around the tail head (16). The maternal body weight and condition scores were reported previously (38).
Immediately before necropsy on day 75 and 135 of gestation, ewes were weighed and sedated with intravenous ketamine (10 mg/kg), and anesthesia was induced and maintained by isoflurane inhalation (1–2%). Ewes were exsanguinated while under general anesthesia, and fetuses were removed. No difference in body weight was observed between twins, so only one fetus of twins was used for analyses. The number of twin pregnancies and the sex ratio of fetuses between treatments in each gestation age were balanced (each treatment contained fetuses from 3 twin pregnancies and 2 single pregnancies, with 3 males and 2 females). The whole left ventricular myocardium was sampled. Surface tissues were trimmed, and a piece at the center of ventricular muscle was fixed in fresh paraformaldehyde before being embedded in paraffin. The remaining muscle was minced and snap-frozen in liquid nitrogen for biological analyses.
Antibodies.
Antibodies against tubulin (no. 2128), TGF-β (no. 3711), Smad2/3 (no. 3102), phospho-Smad2/3 at Ser423/425 (no. 9520), p38 (no. 9212) and phospho-p38 at Thr180/182 (no. 9211) were purchased from Cell Signaling (Danvers, MA).
Histological examination.
Ventricular samples were fixed in 4% (wt/vol) paraformaldehyde in phosphate buffer (0.12 M; pH 7.4), embedded in paraffin, and sectioned (10 μm thickness). Paraffin was removed via a series of incubations in xylene and ethanol solutions, and sections were stained with Masson trichrome; muscle fibers stain red, nuclei black, and collagen blue.
Western blot analyses.
Myocardium samples were washed with PBS and lysed in a buffer containing 50 mM HEPES (pH 7.4), 2% SDS, 1% Nonidet P-40, 10% glycerol, 2 mM phenylmethylsulfonyl fluoride, 10 mM sodium pyrophosphate, 10 mg/ml aprotinin, 10 mg/ml leupeptin, 2 mM Na3VO4, and 100 mM NaF. Soluble proteins were recovered after a 10-min centrifugation (10,000 g), and their concentrations were determined using the Bradford method (Bio-Rad Laboratories, Hercules, CA). Proteins in cell lysates were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were incubated in a blocking solution with 1:1 Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) and PBS for 1 h. Membranes were incubated overnight in a 1:1,000 to 1:500 dilution of primary antibodies and a 1:2,000 dilution of Tubulin in 1:1 Odyssey Blocking Buffer and PBS/T. Membranes were then incubated with IRDye 800CW Goat Anti-Rabbit Secondary Antibody and IRDye 680 Goat Anti-Mouse Secondary Antibody from LI-COR Biosciences (Lincoln, NE) at a 1:10,000 dilution for 1 h in 1:1 Odyssey Blocking Buffer and PBS/T with gentle agitation protecting from light. Membranes were visualized by an Odyssey Infrared Imaging System. Density of bands was quantified and then normalized according to the tubulin content.
Collagen concentration analysis.
Myocardium samples were ground and dried in a convection oven at 60°C, and the samples were weighed and hydrolyzed in 6 N HCl at 105°C for 16 h. An aliquot was removed for hydroxyproline determination using the method of Woessner (37). Collagen concentration (%dry wt) was calculated assuming collagen weighs 7.25 times the measured weight of hydroxyproline.
Real-time quantitative PCR.
Total mRNA was extracted from the fetal myocardium using TRI reagent (Sigma, St. Louis, MO) and reverse transcribed into cDNA by using a kit (Qiagen, Valencia, CA). RT-PCR was performed using an iQ5 RT-PCR detection system (Bio-Rad Laboratories). A SYBR Green RT-PCR kit from Bio-Rad Laboratories was used along with the following primers: Ovis aries collagen type I, forward 5′-GGTGACAGGAAGTCCCAGAA-3′ and reverse 5′-CTGTAGGTGAAGC GGCTGTT-3′; O. aries collagen type III, forward 5′-GGTCAGCCTGGCGTCATGGG-3′ and reverse 5′-GACCTCCAGGGCCACCTCGT-3′; O. aries MMP1, forward 5′-ACTTGTACCGGGTGGCAGCG-3′ and reverse 5′-GTTTGGGGGCCGACTGGCTG-3′; O. aries MMP2, forward 5′-TGGCCATGCAATGGGGCTGG-3′ and reverse 5′-TCAGGAGTGACGGGGCCGAG-3′; O. aries MMP9, forward 5′-CGACGGCATGCTCTGGTGCA-3′ and reverse 5′-CGCATTGCCGTCCTGGGTGT-3′; O. aries MMP13, forward 5′-ACGCCGGACAAATGTGACCCTT-3′ and reverse 5′-GCTGAGGATGCAGCCGCCAG-3′; O. aries TIMP1, forward 5′-CCCAGCGCCCAGAGAGGCTA-3′ and reverse 5′-TCTGTGGGTGGGGTGGGACG-3′; O. aries TIMP2, forward 5′-CCCGGACGAGTGCCTCTGGA-3′ and reverse 5′-CGCAGGAGCCGTCGCTTCTC-3′; and O. aries TIMP3, forward 5′-CCTCTCCCAGCGCAAGGGGT-3′ and reverse 5′-GCCACCCTTCTGCCGGATGC-3′. Each reaction yielded amplicons between 80 and 200 bp. PCR conditions were as follows: 20 s at 95°C, 20 s at 56°C, and 20 s at 72°C for 35 cycles. After amplification, a melting curve was used to confirm product purity, and the PCR products were electrophoresed to confirm the targeted sizes. Results are expressed relative to tubulin.
Electrophoretic mobility shift assay.
Nuclear proteins were prepared using a Nuclear Extract Kit (Active Motif, Carlsbad, CA) following the manufacturer's instruction. The following oligonucleotide sequences were used as probes in gel-shift assays: Smad-binding element (SBE) forward, 5′-GGAGTATGTCTAGACTGACAATGTAC-3′ and reverse, 5′-GTACATTGTCAGTCTAGACATACTCC-3′ (39). Briefly, biotin-labeled oligonucleotides were heat-treated at 90–95°C for 3 min, followed by rapid cooling on ice just before incubation with proteins. Solutions were subjected to electrophoresis in native polyacrylamide gel and DNA electroblotted at 380 mA for 60 min to a positively charged nylon membrane (BiodyneB Membrane) in a MiniProtean II electroblotter (Bio-Rad) using 0.5× TBE buffer (450 mM Tris, 450 mM boric acid, and 10 mM EDTA, pH 8.3). The DNA was cross-linked to the membrane by 312 nm ultraviolet radiation for 10 min; the membrane was washed and then incubated in chemiluminenscent solution, and bands were visualized by exposure to X-ray film (14).
Statistical analyses.
Each animal was an experimental unit. Data were analyzed as a complete randomized design using GLM of SAS. Differences in mean values were compared by Tukey's multiple-comparison test, and means ± SE were reported. Statistical significance was considered as P < 0.05.
RESULTS
TGF-β signaling pathway.
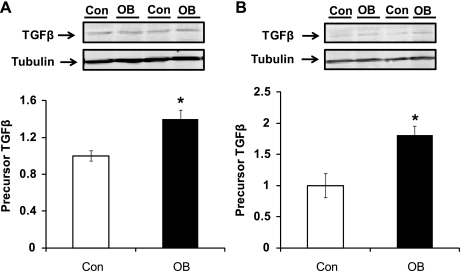
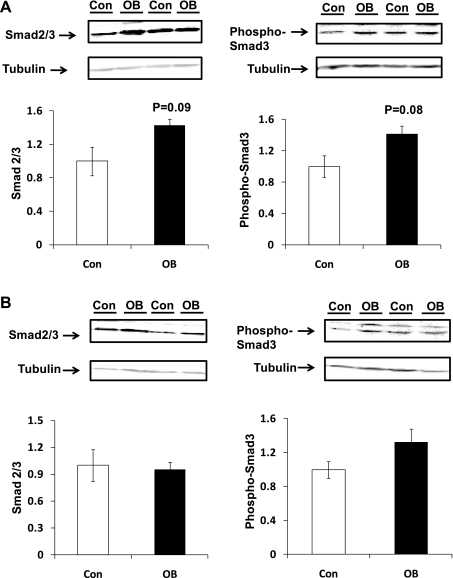
TGF-β protein concentration was 39.9 ± 9.9% higher (P < 0.05) at 75 days gestation (dG) in OB fetal myocardium than in Con myocardium and 80.5 ± 15.5% (P < 0.05) higher at 135 dG (Fig. 1). Smad2/3 are the key downstream mediators of the TGF-β signaling pathway. At 75 dG, Smad2/3 and phosphorylated Smad3 showed a trend toward increase (P = 0.08 and P = 0.09, respectively) in myocardium of fetuses of MO ewes compared with Con myocardium. At 135 dG, no difference in Smad2/3 or in their phosphorylation was observed between Con and OB groups (Fig. 2).
Fig. 1.
Transforming growth factor-β (TGFβ) content in fetal ventricular myocardium of sheep fed a control (Con, open bars) and obesogenic (OB, filled bars) diet. A: 75 days gestation (dG); B: 135 dG. *P < 0.05; mean ± SE; n = 5 sheep.
Fig. 2.
Smad signaling in fetal ventricular myocardium of Con (open bars) and OB (filled bars) sheep. A: Smad2/3 and phospho-Smad2/3 in fetal myocardium tended to increase in OB compared with Con fetal myocardium at 75 dG; B: no difference of Smad2/3 and phospho-Smad2/3 was detected in fetal myocardium between OB and Con fetal myocardium at 135 dG. Data are means ± SE; n = 5.
p38 phosphorylation.
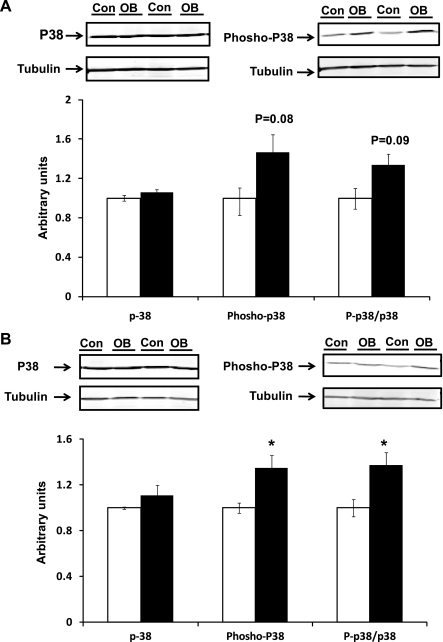
p38 phosphorylates Smad3 at Ser203 and Ser207 residues, which dramatically enhances its transactivation potential (17). No difference in total p38 protein expression was observed between OB and Con fetal groups at either 75 and 135 dG (Fig. 3). However, phosphorylation of p38 tended to be higher in OB samples (P = 0.08) at 75 dG. At 135 dG, phosphorylation of p38 was 34.7 ± 11.2% higher (P < 0.05) in the 135 dG OB group. The ratio of phospho-p38 to p38 in 135 dG samples was also higher (37.6 ± 10.8%, P < 0.05) in OB compared with Con fetal myocardium (Fig. 3).
Fig. 3.
p38 and its phosphorylation in fetal myocardium of Con (open bars) and OB (filled bars) sheep. A: an increase in p38 phosphorylation was observed in OB compared with Con fetal myocardium at 75 dG; B: an increase in p38 phosphorylation was observed in OB compared with Con fetal myocardium at 135 dG. *P < 0.05; mean ± SE; n = 5.
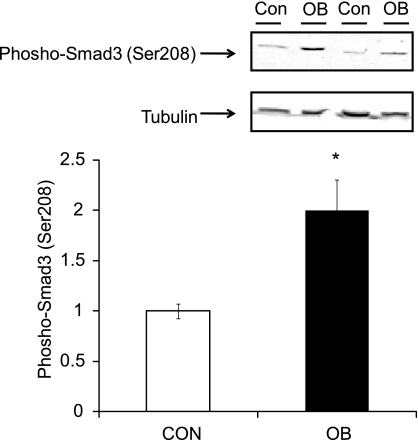
To confirm the effect of p38 in Smad signaling, phosphorylation of Smad3 at Ser208 was measured (Fig. 4). An increase (99.6 ± 30.8%, P < 0.05) in phospho-Smad3 (Ser208) was observed in OB compared with Con fetal myocardium at 135 dG.
Fig. 4.
Phospho-Smad3 (Ser208) in fetal ventricular myocardium of Con (open bar) and OB (filled bar) sheep at 135 dG. An increase in phospho-Smad3 (Ser208) was observed in OB compared with Con fetal myocardium. *P < 0.05; mean ± SE; n = 5.
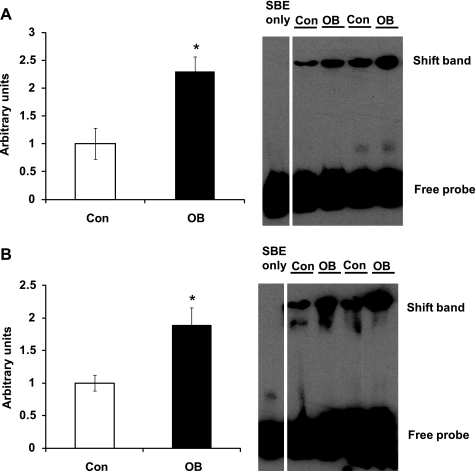
Smads binding to SBE in OB fetal myocardium.
The TGF-β and p38 signaling phosphorylate Smad2/3, which enhances their binding to SBE of targeted genes. To analyze whether there was a difference in protein interaction with the SBE, we extracted nucleoproteins and conducted electrophoretic mobility shift assays using a DNA fragment corresponding to the SBE. Samples from 75 dG OB fetal myocardium showed 130.2 ± 26.0% (P < 0.05) more DNA shift than that from Con samples (Fig. 5). Consistently, the 135 dG OB samples had 88.3 ± 27.8% (P < 0.05) more DNA shift than Con fetal myocardium samples (Fig. 5).
Fig. 5.
Electrophoretic mobility shift assay (EMSA) of nucleoproteins extracted from fetal myocardium of Con (open bars) and OB (filled bars) sheep. A: EMSA for Smad-binding element (SBE) showing an increased shift of DNA in OB compared with Con fetal myocardium at 75 dG; B: EMSA for SBE showing an increased shift of DNA in OB compared with Con fetal myocardium at 135 dG. *P < 0.05; mean ± SE; n = 5.
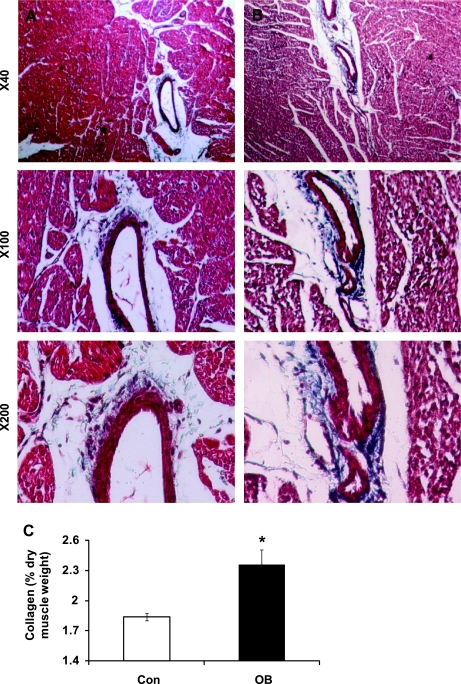
Collagen concentration in OB and Con fetal ventricular muscle.
The upregulated TGF-β and p38 signaling induces fibrosis as evidenced by accumulation of collagen. To test whether there is a difference in collagen accumulation, we analyzed the collagen content in 135 dG fetal myocardium. Our data from the Masson trichrome staining revealed overt myocardial fibrosis in OB fetal myocardium compared with the Con group (Fig. 6, A and B).
Fig. 6.
Collagen content in fetal myocardium of Con (open bars) and OB (filled bars) sheep. A: representative images of fetal myocardium from Con sheep at different magnifications; B: representative images of fetal myocardium from OB sheep at different magnifications; C: collagen content calculated based on the concentration of hydroxyproline showing an increase in collagen concentration in the myocardium of 135 dG fetuses of MO ewes. *P < 0.05; mean ± SE; n = 5.
The amino acid hydroxyproline is present only in significant amounts in collagen. Using hydroxyproline content of myocardium as an indicator, collagen concentration was greater in the OB (P < 0.05) than the Con fetal myocardium (2.42 ± 0.16 vs. 1.87 ± 0.04%) (Fig. 6C).
The ratio of collagen types III/I is often considered as an important marker for myocardial stiffness and is altered in some models of heart disease. However, the ratio in collagen III/I mRNA expression did not differ between the two groups examined here (1.26 ± 0.19 for Con vs. 1.32 ± 0.10 for OB).
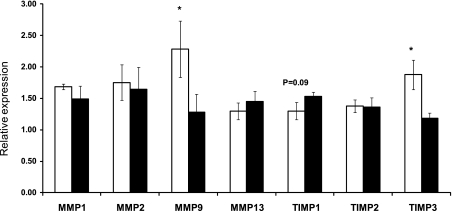
Relative mRNA expression of MMPs and TIMPs in day 75 fetal myocardium samples.
MMPs and TIMPs are important regulators in collagen metabolism. MMP9 (2.28 ± 0.45 vs. 1.28 ± 0.28, P < 0.05) and TIMP3 (1.88 ± 0.23 vs. 1.18 ± 0.08, P < 0.05) were lower in OB sheep compared with Con sheep. TIMP1 in the OB group tended to increase compared with Con (P = 0.09) (Fig. 7). No difference in mRNA expression of MMP1, MMP2, MMP13, and TIMP2 was noted between OB and Con groups.
Fig. 7.
Relative matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) expression in fetal myocardium of Con (open bars) and OB (filled bars) sheep at 75 dG. MMP9 and TIMP3 were higher in the Con group compared with OB sheep. TIMP1 in the OB group tended to increase compared with Con. *P < 0.05; mean ± SE; n = 5.
DISCUSSION
Sheep and humans process many similarities in fetal development; therefore, the sheep is frequently employed for studying normal and abnormal pregnancy (1, 2, 6, 12, 23, 31). Humans and sheep differ in many aspects from common laboratory rodents in pregnancy and fetal development. Rodents are polytocous and generate a larger biomass of conception products relative to maternal weight compared with pregnant women, an important issue in nutritional studies. In our previous studies in sheep pregnancy, we observed that MO induced inflammation in fetal skeletal muscle as demonstrated by the upregulation of nuclear factor κ light-chain enhancer of activated B cells and Jun NH2-terminal kinase (JNK) pathways (32, 38), changes that were accompanied by enhanced fibrosis (14). Inflammation was detected in the fetal myocardium of the same cohort of sheep as shown by the enhanced phosphorylation of JNK, which correlated with impaired contractile function of fetal myocardia in response to high workload stress (35). These data prompted us to conduct the current study to assess fibrosis in fetal myocardium and associated changes in TGF-β and p38 signaling.
TGF-β-mediated myocardial fibrosis plays a pivotal role in the impaired cardiac contractile especially restrictive cardiomyopathy during diastole, probably as a result of increased collagen concentration, which gives rise to myocardial stiffening and loss of myocardial compliance (16). In certain cardiomyopathies, inflammation induces the expression of TGF-β, which then leads to connective tissue expansion during myocardial regeneration (5, 30). We analyzed TGF-β and the phosphorylation of key downstream signaling mediators in fetal myocardium at both mid- and late gestation. Although there was no difference in Smad2/3 content, the phosphorylation of Smad3 at Ser423/425 was higher in OB compared with Con fetal myocardium.
p38 is a kinase essential for Smad-dependent gene transcription (8). p38 phosphorylates Smad3 at Ser203 and Ser207 residues, which are required for the full transactivation potential of Smad3 (17). Inhibition of p38 abolishes the TGF-β response (21). In this study, enhanced p38 phosphorylation was detected in OB compared with Con fetal myocardium, and the phosphorylation of Smad3 at Ser203, a site phosphorylated by p38, was also increased. The enhancement of both Smad and p38 phosphorylation would be expected to enhance the transcription activity of promoters containing SBE. We used an electrophoretic mobility shift assay to analyze the binding of Smads to the conserved SBE (GTCTAGAC) (39). As expected, an enhanced interaction of Smads with SBE DNA fragment was detected in OB compared with Con fetal myocardium. Correspondingly, a higher accumulation of collagen was observed in OB fetal myocardium. To our knowledge, this is the first report demonstrating enhanced accumulation of collagen in fetal myocardium induced by MO via a mechanism of TGF-β/p38-mediated gene expression.
MMPs are crucial for cardiac remodeling (33). The activity of MMPs is inhibited by the TIMPs (22). The balance between MMP and TIMP is essential for numerous physiological processes (24). In the current study, the overall difference in the mRNA expression of MMPs and TIMPs was minor between Con and OB groups, with a decrease in both MMP9 and TIMP3 mRNA expression in OB compared with the Con group. The rationale for such decrease is unclear at this time. Nonetheless, the lack of difference in MMPs and TIMPs indicates that the enhanced fibrosis in OB fetal myocardium is mainly the result of the increased collagen synthesis instead of collagen degradation and remodeling, which are closely associated with fibrosis in adult myocardium (33). Therefore, the increase in collagen accumulation in fetal myocardium appears to be distinctly different from cardiac remodeling in adult myocardium.
Because of their different mechanical properties, the myocardial collagen III/I ratio affects myocardial stiffness (19), which is associated with pathological, hypertension-induced cardiac hypertrophy in rats (4). In this study, myocardial collagen III/I ratio in fetal myocardium was not altered because of MO, which again indicates that fetal fibrosis as a result of MO might be different from cardiac fibrosis and remodeling occurring in the aged heart.
Cardiac fibrosis is associated with many cardiomyopathies, including conditions induced by obesity, diabetes, and aging (28, 36). In particular, myocardial extracellular matrix remodeling is considered an important factor in the development of ventricular hypertrophy and is essential for the adaptive and maladaptive changes associated with the various myopathic anomalies (13). Our observations indicate that MO induces changes in myocardium at an early developmental stage, which typically is not observed until later in life. Such changes would be expected to impair cardiac function of offspring. An earlier study from our group using the same cohort of sheep revealed impairment of cardiac function in OB fetal heart at late gestation (35). Nonetheless, the mechanism of action contributing to impaired cardiac contractility in the offspring was essentially unknown at the time. Given that MO is known to increase cardiovascular diseases in offspring, our observation that MO induces collagen accumulation in fetal myocardium should shed some light on the missing link between MO and compromised cardiac function in offspring as measured by the Langendorff preparation (35).
In conclusion, our findings demonstrate that MO activates TGF-β and p38, which enhances Smad-mediated signaling and collagen accumulation in fetal myocardium. Such a change would tend to impair the contractile function of offspring hearts and predisposes them to cardiac dysfunction.
GRANTS
This work was supported by National Institutes of Health Grants P20-RR-016474 and 1R03-HD-057506.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Anthony RV, Scheaffer AN, Wright CD, Regnault TR. Ruminant models of prenatal growth restriction. Reprod Suppl 61: 183–194, 2003 [PubMed] [Google Scholar]
- 2.Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, Broughton Pipkin F, Stephenson T, Symonds ME. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology 144: 3575–3585, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 22: 1257–1266, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Burgess ML, Buggy J, Price RL, Abel FL, Terracio L, Samarel AM, Borg TK. Exercise- and hypertension-induced collagen changes are related to left ventricular function in rat hearts. Am J Physiol Heart Circ Physiol 270: H151–H159, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology 65: 826–834, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Das UG, Schroeder RE, Hay WW, Jr, Devaskar SU. Time-dependent and tissue-specific effects of circulating glucose on fetal ovine glucose transporters. Am J Physiol Regul Integr Comp Physiol 276: R809–R817, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Decologne N, Kolb M, Margetts PJ, Menetrier F, Artur Y, Garrido C, Gauldie J, Camus P, Bonniaud P. TGF-beta1 induces progressive pleural scarring and subpleural fibrosis. J Immunol 179: 6043–6051, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Dziembowska M, Danilkiewicz M, Wesolowska A, Zupanska A, Chouaib S, Kaminska B. Cross-talk between Smad and p38 MAPK signalling in transforming growth factor beta signal transduction in human glioblastoma cells. Biochem Biophys Res Commun 354: 1101–1106, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, Heath JK. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J 6: 1899–1904, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa F, Matsuzaki K, Mori S, Tahashi Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki Y, Nishizawa M, Fujisawa J, Inoue K. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology 38: 879–889, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Gosselin LE, Williams JE, Deering M, Brazeau D, Koury S, Martinez DA. Localization and early time course of TGF-beta 1 mRNA expression in dystrophic muscle. Muscle Nerve 30: 645–653, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Hay WW, Jr, Sparks JW, Wilkening RB, Battaglia FC, Meschia G. Fetal glucose uptake and utilization as functions of maternal glucose concentration. Am J Physiol Endocrinol Metab 246: E237–E242, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Hayden MR, Chowdhury N, Govindarajan G, Karuparthi PR, Habibi J, Sowers JR. Myocardial myocyte remodeling and fibrosis in the cardiometabolic syndrome. J Cardiometab Syndr 1: 326–333, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Yan X, Zhu MJ, McCormick RJ, Ford SP, Nathanielsz PW, Du M. Enhanced transforming growth factor-β signaling and fibrogenesis in ovine fetal skeletal muscle of obese dams at late gestation. Am J Physiol Endocrinol Metab 298: E1254–E1260, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, Ji L, Iwamoto Y, Li E, Schneider M, Russell KS, Fu XY. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA 100: 12929–12934, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kai H, Kuwahara F, Tokuda K, Imaizumi T. Diastolic dysfunction in hypertensive hearts: roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens Res 28: 483–490, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J Biol Chem 280: 1024–1036, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kaya Z, Goser S, Buss SJ, Leuschner F, Ottl R, Li J, Volkers M, Zittrich S, Pfitzer G, Rose NR, Katus HA. Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis. Circulation 118: 2063–2072, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapiere CM, Nusgens B, Pierard GE. Interaction between collagen type I and type III in conditioning bundles organization. Connect Tissue Res 5: 21–29, 1977 [DOI] [PubMed] [Google Scholar]
- 20.Li J, Campanale NV, Liang RJ, Deane JA, Bertram JF, Ricardo SD. Inhibition of p38 mitogen-activated protein kinase and transforming growth factor-beta1/Smad signaling pathways modulates the development of fibrosis in adriamycin-induced nephropathy. Am J Pathol 169: 1527–1540, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer-Ter-Vehn T, Gebhardt S, Sebald W, Buttmann M, Grehn F, Schlunck G, Knaus P. p38 inhibitors prevent TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci 47: 1500–1509, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Morgan J, Rouche A, Bausero P, Houssaini A, Gross J, Fiszman MY, Alameddine HS. MMP-9 overexpression improves myogenic cell migration and engraftment. In: Muscle Nerve (2010/08/25 ed.), 2010 [DOI] [PubMed] [Google Scholar]
- 23.Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-gamma, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology 148: 878–885, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Murphy G, Docherty AJ. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol 7: 120–125, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Nathanielsz PW. Animal models that elucidate basic principles of the developmental origins of adult diseases. Ilar J 47: 73–82, 2006 [DOI] [PubMed] [Google Scholar]
- 26.NRC National Research Council. Nutrient Requirements of Sheep. Washington, DC: National Academy Press, 1985 [Google Scholar]
- 27.Ponten A, Folestad EB, Pietras K, Eriksson U. Platelet-derived growth factor D induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice. Circ Res 97: 1036–1045, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Pugh KG, Wei JY. Clinical implications of physiological changes in the aging heart. Drugs Aging 18: 263–276, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Reusens B, Ozanne SE, Remacle C. Fetal determinants of type 2 diabetes. Curr Drug Targets 8: 935–941, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Salvadori C, Peters IR, Day MJ, Engvall E, Shelton GD. Muscle regeneration, inflammation, and connective tissue expansion in canine inflammatory myopathy. Muscle Nerve 31: 192–198, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Sebert SP, Hyatt MA, Chan LL, Patel N, Bell RC, Keisler D, Stephenson T, Budge H, Symonds ME, Gardner DS. Maternal nutrient restriction between early and midgestation and its impact upon appetite regulation after juvenile obesity. Endocrinology 150: 634–641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong JF, Yan X, Zhu MJ, Ford SP, Nathanielsz PW, Du M. Maternal obesity downregulates myogenesis and beta-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab 296: E917–E924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: “Embracing the MMP-independent-side of the family.” J Mol Cell Cardiol 48: 445–453, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med 21: 149–157, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Ma H, Tong C, Zhang H, Lawlis GB, Li Y, Zang M, Ren J, Nijland MJ, Ford SP, Nathanielsz PW, Li J. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J 24: 2066–2076, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Song Y, Wang Q, Kralik PM, Epstein PN. Causes and characteristics of diabetic cardiomyopathy. Rev Diabet Stud 3: 108–117, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys 93: 440–447, 1961 [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Tong JF, Zhu MJ, Ford SP, Nathanielsz PW, Du M. Upregulation of TLR4/NF-κB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology 151: 380–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell 1: 611–617, 1998 [DOI] [PubMed] [Google Scholar]