Abstract
The objective of this study was to assess the response of a large animal model to high dietary fat and fructose (HFFD). Three different metabolic assessments were performed during 13 wk of feeding an HFFD (n = 10) or chow control (CTR, n = 4) diet: oral glucose tolerance tests (OGTTs; baseline, 4 and 8 wk), hyperinsulinemic-euglycemic clamps (HIEGs; baseline and 10 wk) and hyperinsulinemic-hyperglycemic clamps (HIHGs, 13 wk). The ΔAUC for glucose during the OGTTs more than doubled after 4 and 8 wk of HFFD feeding, and the average glucose infusion rate required to maintain euglycemia during the HIEG clamps decreased by ≈30% after 10 wk of HFFD feeding. These changes did not occur in the CTR group. The HIHG clamps included experimental periods 1 (P1, 0–90 min) and 2 (P2, 90–180 min). During P1, somatostatin, basal intraportal glucagon, 4 × basal intraportal insulin, and peripheral glucose (to double the hepatic glucose load) were infused; during P2, glucose was also infused intraportally (4.0 mg·kg−1·min−1). Net hepatic glucose uptake during P1 and P2 was −0.4 ± 0.1 [output] and 0.2 ± 0.8 mg·kg−1·min−1 in the HFFD group, respectively, and 1.8 ± 0.8 and 3.5 ± 1.0 mg·kg−1·min−1 in the CTR group, respectively (P < 0.05 vs. HFFD during P1 and P2). Glycogen synthesis through the direct pathway was 0.5 ± 0.2 and 1.5 ± 0.4 mg·kg−1·min−1 in the HFFD and CTR groups, respectively (P < 0.05 vs. HFFD). In conclusion, chronic consumption of an HFFD diminished the sensitivity of the liver to hormonal and glycemic cues and resulted in a marked impairment in NHGU and glycogen synthesis.
Keywords: impaired glucose tolerance, glycogen synthesis, hyperinsulinemic euglycemic clamp, hyperinsulinemic hyperglycemic clamp, portal signal
chronic consumption of a western diet, characterized by foods rich in sugar and abundant in total and saturated fat, has been suggested to play a role in the development of type 2 diabetes (9, 37, 38). Numerous studies have delineated the effects of dietary fat on whole body insulin sensitivity. For example, 3 days of high-fat feeding (59% of kcal from fat) was sufficient to produce hepatic insulin resistance in rats, as evidenced by a diminished ability of hyperinsulinemia to suppress hepatic glucose production (HGP) in the absence of an alteration in peripheral (skeletal muscle and white adipose tissue) insulin sensitivity (20, 22, 31). These findings were supported in a canine model, in which hepatic insulin resistance was also found to be the primary metabolic consequence associated with 12 wk of moderate-fat (44% of kcal from fat) feeding (18). However, other studies have demonstrated that peripheral insulin resistance precedes liver resistance in response to high dietary fat consumption. Rocchini et al. (30) observed a significant reduction in insulin-mediated whole body glucose uptake after 1 wk of high-fat feeding in dogs (regular diet supplemented with 0.9 kg/day cooked beef fat), whereas the ability of insulin to suppress HGP was retained for the duration of the study (6 wk). Similarly, Kim et al. (17) reported a significant decrease in insulin-stimulated whole body glucose uptake, with only a tendency toward reduced suppression of HGP by insulin in dogs fed a high-fat diet (54% kcal as fat) for 6 wk. Thus, the temporal development of hepatic and/or peripheral insulin resistance in the context of high fat feeding remains unclear.
Foods rich in fructose are also a key component of a Western diet. Given that the contribution of fructose to dietary carbohydrate intake has increased significantly in the U.S. and has paralleled the rapid rise in obesity and diabetes (24, 39), several studies have characterized the effect of high dietary fructose on whole body insulin sensitivity. Pagliassotti et al. (28, 36) demonstrated in the rat that the quantity of dietary sucrose (a disaccharide comprising glucose and fructose in a 1:1 ratio) influenced the time course of the development of insulin resistance in a tissue-specific manner, in that consumption of a diet high in sucrose (68% of kcal as sucrose) resulted in hepatic and peripheral insulin resistance within 8 wk, whereas consumption of a diet lower in sucrose (18% of kcal as sucrose) required at least 16 wk to elicit hepatic insulin resistance, with no change in peripheral insulin sensitivity. Follow-up studies demonstrated that fructose was the primary mediator of sucrose-induced impairments in insulin action and glucose intolerance in vivo (28, 36).
A finding shared among the high-fat or high-fructose feeding studies is that the liver is particularly vulnerable to the effects of dietary nutrients in excess. The nutritional induction of hepatic insulin resistance has a large impact on whole body glucose metabolism, given that liver acts as a dynamic regulator of glucose homeostasis in both the fed and fasted states. Thus, the glucose intolerance characteristic of individuals with diabetes is partly attributable to a defect in the ability of the liver to switch from glucose production to glucose storage following a meal (3, 13, 21).
While the effects of high dietary fat or fructose on insulin's ability to suppress HGP have been extensively studied, their effects on hepatic glucose uptake and disposition have not been delineated. In addition, previous studies have generally utilized supraphysiological quantities of fructose (e.g., ≈60% of kcal as fructose) to investigate its physiological and cellular effects (5, 12, 19). As a result, the combined effects of dietary fat and fructose, in quantities that mimic a Western diet, on the temporal development of glucose intolerance and hepatic and/or peripheral insulin resistance are not known. Likewise, it is not known whether high-fat/high-fructose (HFFD) feeding coupled with an experimental reduction in β-cell mass (partial pancreatectomy) augments glucose intolerance to a larger extent than diet alone. The objective of the present study was to investigate how the combination of high dietary fat and fructose influences 1) the temporal development of insulin resistance and impaired glucose tolerance in the presence or absence of a compromised endocrine pancreas, and 2) the ability of the liver to take up glucose under conditions that mimic the postprandial state (hyperinsulinemia, hyperglycemia, and the portal glucose feeding signal).
RESEARCH DESIGN AND METHODS
Animals, Surgical Procedures, and Experimental Timeline
The protocol was approved by the Vanderbilt University Animal Care and Use Committee, and all facilities met the standards published by the American Association for the Accreditation of Laboratory Animal Care. Fourteen adult male dogs (25.7 ± 0.9 kg at baseline) consumed a standard meat (Kal Kan, Vernon, CA) and laboratory chow diet (CTR; PMI Nutrition canine diet 5006, St. Louis, MO) with total metabolizable energy of 1,900 kcal/day [31% protein, 26% fat, and 43% carbohydrate (virtually all in the form of digestible starch)] for 2 wk to ensure weight stabilization and acclimation. Following this period, oral glucose tolerance tests (OGTTs) and hyperinsulinemic euglycemic (HIEG) clamps were performed on each dog. These studies were used for a baseline (BL) metabolic assessment of glucose tolerance and insulin sensitivity. The following week, each dog underwent a laparotomy in which a sham operation (Sh; n = 8) or a partial pancreatectomy (Px, ≈65% removal; n = 6) was performed. Partial pancreatectomies were carried out with the intent to develop a large animal model of type 2 diabetes (when coupled with an HFFD) that would mimic the natural progression and metabolic characteristics of the human disease. Briefly, the pancreaticoduodenal artery and vein were isolated along with isolation of the right lobe of the pancreas from its mesenteric connections. The right lobe was ligated and transected at the union of the right lobe caudal extremity and the distal duodenum while preserving the venous and arterial vasculature of the duodenum. The pancreaticoduodenal artery and vein were ligated and transected at the distal end of the lobe, and the right pancreatic lobe was removed. Next, the pancreaticosplenic arteries and veins were identified and isolated. The left lobe was ligated and transected at the union with the pylorus in the apical portion of the pancreas. The mesenteric and omental connections and the arterial and venous vasculature supplying the left lobe were ligated and transected, and the left pancreatic lobe was removed. Approximately 35% of the pancreas remained in place with intact exocrine and biliary tree function.
Postoperatively, each dog was randomly assigned to either the control (CTR: n = 4) or HFFD group (HFFD-Sh: n = 4; HFFD-Px: n = 6) in which 22% of the energy was derived from protein, 52% from fat and 26% from carbohydrate, the majority of which (17% of the total energy in the diet) was derived from fructose (PMI Nutrition TestDiet, St. Louis, MO). During the first wk of feeding, dogs assigned to the HFFD diet ate ≈ 3,700 kcal/day; however, caloric consumption decreased by ≈15% after 1 wk on the diet, and by wk 10, caloric consumption had stabilized at ≈ 1,700 kcal/day.
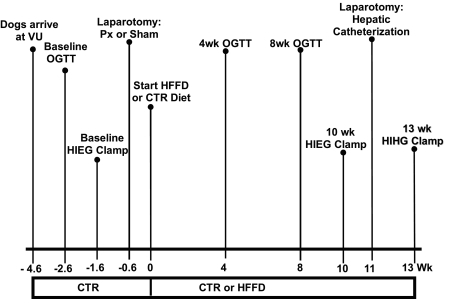
OGTTs were repeated after 4 and 8 wk, and another hyperinsulinemic euglycemic (HIEG) clamp was performed after 10 wk of CTR or HFFD feeding. At week 11, each dog underwent a laparotomy to implant sampling catheters into the femoral artery, the hepatic portal vein, and a hepatic vein and to place infusion catheters into a splenic and jejunal vein (1). At the same time, ultrasonic flow probes (Transonic Systems, Ithaca, NY) were placed around the hepatic artery and the portal vein, as described previously (1). At week 13, a hyperinsulinemic hyperglycemic clamp (HIHG; see below) was performed on each dog, and net hepatic glucose balance was measured. All dogs were healthy, as indicated by 1) leukocyte count <18,000/mm3, 2) hematocrit >35%; and 3) good appetite and normal stools. The experimental timeline is shown in Fig. 1.
Fig. 1.
Experimental timeline. Numbers below horizontal line indicate week in which an experiment or surgery was conducted relative to initiation of experimental diets (CTR or HFFD). CTR, standard meat and laboratory chow diet; HFFD, high-fat/high-fructose diet; VU, Vanderbilt University; OGTT, oral glucose tolerance test; HIEG, hyperinsulinemic euglycemic clamp; Px, partial pancreatectomy; HIHG, hyperinsulinemic hyperglycemic clamp.
Experimental Design
OGTTs.
The OGTTs were conducted in 24-h-fasted dogs that had been fed one can of meat immediately prior to fasting. Following a 10-min basal control period, Polycose (0.9 g/kg body wt; Polycose, Abbott Nutrition, Columbus, OH) was administered orally, and plasma glucose, insulin, C-peptide, and glucagon concentrations were monitored over the following 180 min.
HIEGs.
The HIEGs were conducted in 18-h-fasted dogs that had been fed one can of meat immediately prior to fasting. The HIEGs consisted of a 90-min equilibration period (−120 to −30 min), a 30-min basal control period (−30 to 0 min), and a 120-min experimental period (0 to 120 min). At time 0, a constant infusion of somatostatin (0.8 μg·kg−1·min−1; Bachem, Torrance, CA) was started in a peripheral vein to suppress endogenous insulin and glucagon secretion. Insulin (2.0 mU·kg−1·min−1; Lilly, Indianapolis, IN) and glucagon (0.7 ng·kg−1·min−1; Novo Nordisk, Princeton, NJ) were then replaced via infusion into a peripheral vein with a goal of increasing arterial insulin 10-fold while clamping glucagon at a basal value. In addition, at time 0, a variable intravenous infusion of 50% dextrose was started in a leg vein to maintain euglycemia (≈100 mg/dl) throughout the study.
HIHGs.
The HIHGs were conducted in 18-h-fasted dogs that had been fed one can of meat immediately prior to fasting. The HIHGs consisted of a 100-min equilibration period (−120 to −20 min), a 20-min basal control period (−20 to 0 min), and a 180-min experimental period divided into 2 subperiods (P1, 0–90 min; P2, 90–180 min). At −120 min, a priming dose of [3-3H]glucose (38 μCi) was given, followed by a constant infusion of [3-3H]glucose (0.38 μCi/min). At time 0, a constant infusion of somatostatin (0.8 μg·kg−1·min−1, Bachem) was started in a peripheral vein, and insulin and glucagon were then replaced intraportally at 4 × basal (1.2 mU·kg−1·min−1, Lilly) and basal (0.55 ng·kg−1·min−1, Novo Nordisk) rates, respectively. At time 0, a variable infusion of D50 was started in a leg vein to double the hepatic glucose load by clamping the plasma glucose level at ≈220 mg/dl. During the second experimental subperiod (90–180 min), 20% dextrose was infused in the portal vein at a constant rate (4.0 mg·kg−1·min−1) to activate the portal glucose signal, and the peripheral glucose infusion rate (GIR) was adjusted as necessary to clamp the hepatic glucose load (HGL) to that in P1. At the end of the study, the animal was anesthetized with pentobarbital sodium and a laparotomy was performed. The hormone and glucose infusions were continued while liver sections were freeze-clamped in situ and stored at −70°C for terminal glycogen analysis. Two dogs in the HFFD-Px group had to be dropped from the cohort, one because of catheter failure and one because of an infusion error.
Metabolite and Glycogen Analyses
Hematocrit levels, glucose, glucagon, insulin, C-peptide and NEFA levels in plasma, and lactate, alanine, and glycerol concentrations in blood were determined using standard procedures as previously described (2, 10, 27). Plasma triglyceride (TG) levels were measured using a serum TG determination kit (Sigma-Aldrich, St. Louis, MO). Hepatic glycogen levels were determined in a subset of dogs (HFFD, n = 5; CTR, n = 4) using the amyloglucosidase method described by Keppler and Decker (16). The remaining HFFD-fed dogs (n = 3) were utilized for other purposes following the HIHG experiments, which precluded the acquisition of liver tissue.
Calculations
The trapezoidal rule was used for determination of area under the curve (AUC), which was then adjusted for the baseline AUC in each group (ΔAUC). Net hepatic substrate balances (NHB) were calculated with the A-V difference method using the formula NHB = Loadout − Loadin, where Loadout = [H]·HF and Loadin = [A]·AF + [P]·PF. [A], [P], and [H] represent substrate concentrations in femoral artery, portal vein, and hepatic vein blood or plasma, respectively, and AF, PF, and HF represent blood or plasma flow (as measured using ultrasonic flow probes) through the hepatic artery, the portal vein, and the hepatic vein, respectively. With this calculation, positive values reflect net hepatic production and negative values represent net hepatic uptake. To avoid any potential errors arising from incomplete mixing of the intraportally infused glucose in the portal vein blood, net hepatic glucose balance (NHGB) and hepatic glucose load (HGL) were also calculated by an indirect (I) method (2, 32). Thus, the load of glucose entering the liver was calculated as
where GA is the arterial plasma glucose concentration, GIRPO is the portal glucose infusion rate, and GUG is the uptake of glucose by the gastrointestinal tract, calculated as previously described (25). The load of a glucose exiting the liver was calculated as
where GH represents the hepatic vein plasma glucose concentration. Indirect NHGB was thus calculated as
NHGB and HGL calculated using the indirect method did not differ significantly from those obtained using the direct calculation, but only the data derived from the indirect method are reported in this paper. Net hepatic fractional substrate extraction (NHFX), hepatic sinusoidal hormone concentrations, nonhepatic glucose uptake (non-HGU), net hepatic carbon retention, and direct glycogen synthesis were calculated as described previously (2, 32). The rates of glucose appearance (Ra) and disappearance (Rd) were calculated using Steele's steady-state equation (34). Tracer was infused in all four HIHG clamps in the CTR group, and in five of the eight in the HFFD group (3 of the dogs were utilized for other purposes following the HIHG studies, so [3-3H]glucose was not infused); however, we experienced a technical problem with the tracer infusion pump in one of the HIHG clamps. As a result, we were left with four dogs in the HFFD group. Only values obtained during the steady-state period of the HIHG clamp were used for the determination of glucose turnover (e.g., 180–210 min of P1, 270–300 min of P2). Endogenous glucose production (endo Ra) was calculated as Ra − (peripheral GIR + [portal GIR × 1 − net hepatic fractional glucose extraction]) (25). Hepatic glucose uptake was estimated (est HGU) by subtracting NHGU from endo Ra.
Statistical Analyses
Data are presented as means ± SE. For OGTTs, statistical comparisons within CTR, HFFD-Sh, or HFFD-Px groups were carried out using one-way ANOVA (SigmaStat, San Jose, CA). For HIEGs and HIHGs, statistical comparisons between groups and over time were carried out using two-way repeated-measures ANOVA (group × time). The Student-Newman-Keuls multiple comparisons test was used post hoc when significant (P < 0.05) F ratios were obtained. To test for differences in fasting plasma factors and body weight before and after HFFD or CTR feeding, paired t-tests were performed.
RESULTS
OGTTs
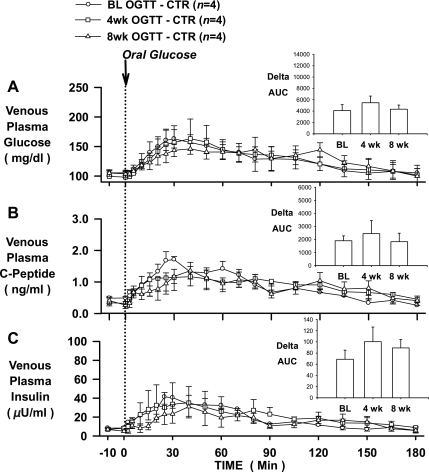
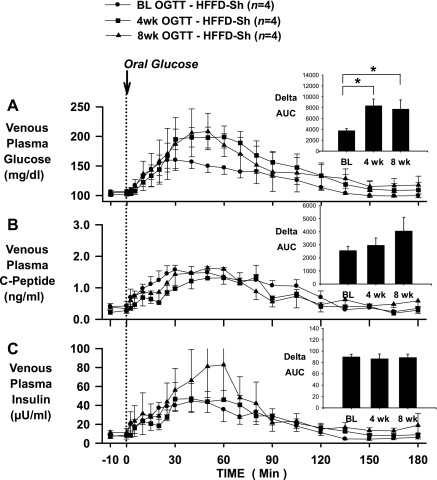
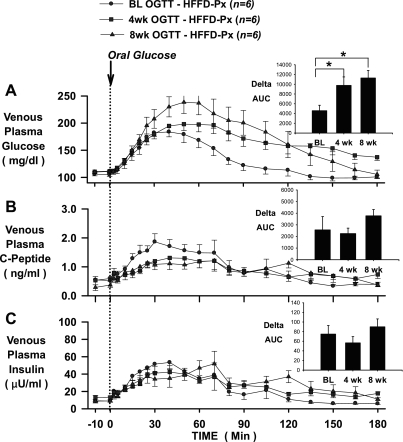
In the CTR group, glucose tolerance (assessed by the ΔAUC for glucose over 180 min), insulin secretion (assessed by the ΔAUC for C-peptide over 180 min), and plasma insulin levels (assessed by the ΔAUC for insulin over 180 min) seen in response to an oral glucose challenge were not different at baseline or after 4 or 8 wk of feeding (Fig. 2), demonstrating the reproducibility of normal glucose tolerance in dogs maintained on a standard meat and chow diet. On the other hand, the glycemic response to an oral glucose challenge was significantly increased after HFFD feeding, as indicated by 123 and 113% increases in the ΔAUC for glucose in the HFFD-Sh and HFFD-Px groups, respectively, after 4 wk of feeding, and 106 and 147% increases in the HFFD-Sh and HFFD-Px groups, respectively, after 8 wk of feeding (Figs. 3A and 4A). The deterioration of glucose tolerance in the HFFD groups was attributable in part to a β-cell defect, given that a compensatory increase in insulin secretion, as indicated by C-peptide levels, failed to occur whether or not the pancreas was compromised (Figs. 3B and 4B). As a result, the ΔAUC for insulin at 4 and 8 wk was not significantly different from that observed in the baseline OGTT (Figs. 3C and 4C) despite increased plasma glucose. In addition, although fasting plasma glucagon concentrations were similar between diet groups at BL, 4 and 8 wk (Table 1), the magnitude of the decrease in plasma glucagon during the 8-wk OGTT was significantly less in both HFFD groups compared with CTR (pg/ml; HFFD-Px: 7 ± 3 and HFFD-Sh: 13 ± 5 vs. CTR: 29 ± 8, P < 0.05), suggestive of impaired α-cell function after HFFD feeding.
Fig. 2.
OGTTs conducted in 24-h-fasted dogs at baseline (BL; circles), and after 4 (squares) and 8 (triangles) wk of feeding a CTR diet (n = 4). Polycose was administered orally (0.9 g/kg), and plasma glucose (A), C-peptide (B), and insulin (C) concentrations were measured over 180 min. Insets: AUCs over 180 min for glucose (A), C-peptide (B), and insulin (C). Data are means ± SE.
Fig. 3.
OGTTs conducted in 24-h-fasted dogs at baseline (BL; circles), and after 4 (squares) and 8 (triangles) wk of feeding a HFFD to sham-operated dogs (HFFD-Sh; n = 4). Polycose was administered orally (0.9 g/kg), and plasma glucose (A), C-peptide (B), and insulin (C) concentrations were measured over 180 min. Insets: AUCs over 180 min for glucose (A), C-peptide (B), and insulin (C). Data are means ± SE. *P < 0.05 vs. baseline ΔAUC.
Fig. 4.
OGTTs conducted in 24-h-fasted dogs at baseline (BL; circles), and after 4 (squares) and 8 (triangles) wk of feeding a HFFD to partially pancreatectomized dogs (HFFD-Px; n = 6). Polycose was administered orally (0.9 g/kg), and plasma glucose (A), C-peptide (B), and insulin (C) concentrations were measured over 180 min. Insets: AUCs over 180 min for glucose (A), C-peptide (B), and insulin (C). Data are means ± SE. *P < 0.05 vs. baseline ΔAUC.
Table 1.
Venous plasma glucagon concentrations during OGTTs
| Experimental Period, min |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Basal Period, −10 to 0 min | 30 | 60 | 90 | 120 | 150 | 180 |
| Plasma Glucagon, pg/ml | |||||||
| CTR | |||||||
| BL | 38 ± 11 | 34 ± 9 | 31 ± 7 | 29 ± 8 | 29 ± 7 | 35 ± 6 | 31 ± 8 |
| 4 wk | 40 ± 4 | 37 ± 3 | 39 ± 3 | 33 ± 5 | 30 ± 4 | 32 ± 4 | 35 ± 3 |
| 8 wk | 65 ± 7 | 58 ± 8 | 55 ± 14 | 52 ± 8 | 57 ± 9 | 46 ± 2 | 50 ± 4 |
| HFFD-Sh | |||||||
| BL | 46 ± 11 | 54 ± 12 | 43 ± 7 | 45 ± 12 | 44 ± 14 | 47 ± 11 | 43 ± 10 |
| 4 wk | 42 ± 9 | 41 ± 11 | 43 ± 9 | 40 ± 11 | 42 ± 11 | 47 ± 11 | 42 ± 10 |
| 8 wk | 46 ± 5 | 38 ± 4 | 47 ± 14 | 44 ± 12 | 47 ± 12 | 41 ± 12 | 42 ± 12 |
| HFFD-Px | |||||||
| BL | 42 ± 9 | 42 ± 10 | 44 ± 13 | 41 ± 9 | 37 ± 9 | 41 ± 9 | 39 ± 7 |
| 4 wk | 47 ± 9 | 38 ± 10 | 44 ± 13 | 43 ± 8 | 38 ± 8 | 39 ± 8 | 40 ± 7 |
| 8 wk | 43 ± 10 | 45 ± 10 | 46 ± 9 | 48 ± 11 | 48 ± 9 | 46 ± 8 | 48 ± 11 |
Values are means ± SE; control (CTR), n = 4; high-fat/high-fructose-fed, sham operated (HFFD-Sh), n = 4; HFFD, partially pancreatecomized (HFFD-Px), n = 6. BL, baseline. Dogs were 24-h fasted prior to study.
HIEG Clamps
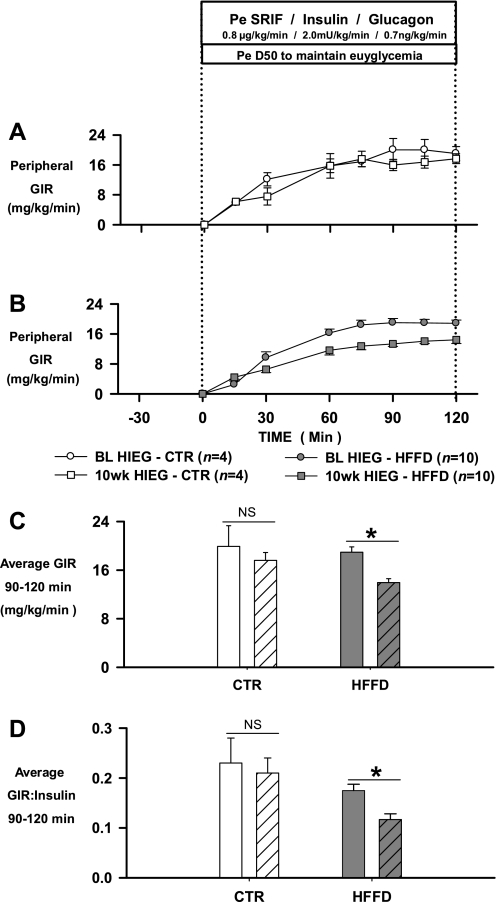
HIEG clamp studies were performed at BL and 10 wk. Plasma glucagon concentrations were clamped at basal levels, while plasma insulin concentrations were elevated 10-fold from basal (Table 2). Plasma C-peptide levels fell to zero in each group secondary to somatostatin infusion. In the CTR group, the average (90–120 min) GIR required to maintain euglycemia was 19.9 ± 3.4 and 17.6 ± 1.3 mg·kg−1·min−1 during the BL- and 10-wk-HIEGs, respectively (Fig. 5, A and C). On the other hand, the average (90–120 min) GIR (mg·kg−1·min−1) required to maintain euglycemia in the HFFD group was decreased by 26% (BL, 18.9 ± 0.9; 10 wk, 13.9 ± 0.7, P < 0.05 vs. BL) after 10 wk of HFFD feeding (Fig. 5, B and C). Because the reduction in GIR after HFFD feeding was similar between the HFFD-Sh and HFFD-Px groups, the data from both groups were combined in Fig. 5 (see Fig. legend). Despite use of the same insulin infusion rate (2.0 mU·kg−1·min−1), the steady-state plasma insulin concentration was higher in the HFFD group at week 10 compared with BL (BL, 112 ± 16; 10 wk, 128 ± 10 μU/ml; Table 2). As a result, a 33% decrease in the GIR to insulin ratio was evident in the HFFD group after 10 wk of feeding (Fig. 5D). As expected, the elevation in insulin elicited a rapid reduction in plasma NEFA levels, while plasma TGs tended to drift down throughout the experiment in each group at BL and 10 wk (Table 2). There were no differences in plasma lipid levels between the CTR and HFFD groups at any time point.
Table 2.
Venous plasma glucose, insulin, glucagon, free fatty acid, and triglyceride concentrations during hyperinsulemic euglycemic clamps
| Experimental Period, min | |||||
|---|---|---|---|---|---|
| Group | Basal Period, −30 to 0 min | 30 | 60 | 90 | 120 |
| Plasma glucose, mg/dl | |||||
| CTR | |||||
| BL | 102 ± 3 | 102 ± 1 | 101 ± 6 | 105 ± 7 | 104 ± 2 |
| 10 wk | 104 ± 4 | 93 ± 8 | 90 ± 5 | 113 ± 5 | 99 ± 3 |
| HFFD | |||||
| BL | 108 ± 2 | 99 ± 4 | 97 ± 2 | 106 ± 3 | 104 ± 3 |
| 10 wk | 110 ± 2 | 104 ± 4 | 103 ± 2 | 101 ± 2 | 105 ± 3 |
| Plasma insulin, μU/ml | |||||
| CTR | |||||
| BL | 8 ± 1 | 76 ± 5† | 82 ± 8† | 84 ± 7† | 85 ± 6† |
| 10 wk | 7 ± 1 | 87 ± 13† | 83 ± 6† | 85 ± 6† | 83 ± 8† |
| HFFD | |||||
| BL | 10 ± 1 | 104 ± 7† | 110 ± 7† | 113 ± 7† | 114 ± 5† |
| 10 wk | 11 ± 1 | 122 ± 9† | 125 ± 10† | 128 ± 10† | 126 ± 11† |
| Plasma glucagon, pg/ml | |||||
| CTR | |||||
| BL | 34 ± 5 | 42 ± 8 | 41 ± 3 | 42 ± 3 | 40 ± 3 |
| 10 wk | 47 ± 11 | 44 ± 6 | 45 ± 5 | 44 ± 3 | 48 ± 9 |
| HFFD | |||||
| BL | 41 ± 5 | 49 ± 5 | 48 ± 6 | 50 ± 6 | 48 ± 6 |
| 10 wk | 43 ± 5 | 52 ± 4 | 49 ± 5 | 48 ± 6 | 46 ± 6 |
| Plasma free fatty acids, μmol/l | |||||
| CTR | |||||
| BL | 821 ± 49 | 166 ± 30† | 56 ± 7† | 48 ± 10† | 39 ± 13† |
| 10 wk | 775 ± 113 | 127 ± 26† | 97 ± 21† | 71 ± 21† | 62 ± 19† |
| HFFD | |||||
| BL | 758 ± 97 | 168 ± 52† | 105 ± 13† | 71 ± 11† | 54 ± 9† |
| 10 wk | 751 ± 72 | 146 ± 21† | 113 ± 27† | 80 ± 10† | 61 ± 8† |
| Plasma triglycerides, μmol/l | |||||
| CTR | |||||
| BL | 1195 ± 81 | 1,202 ± 107 | 1,044 ± 104 | 937 ± 106 | 841 ± 96† |
| 10 wk | 1244 ± 239 | 1,304 ± 188 | 1,122 ± 142 | 979 ± 146 | 958 ± 182 |
| HFFD | |||||
| BL | 870 ± 100 | 858 ± 105 | 779 ± 117 | 669 ± 75† | 625 ± 58† |
| 10 wk | 1021 ± 77 | 979 ± 89 | 812 ± 75† | 736 ± 58† | 700 ± 72† |
Values are means ± SE; CTR, n = 4; HFFD, n = 10. Dogs were 18-h fasted prior to study.
P < 0.05 vs. basal period.
Fig. 5.
Mean glucose infusion rates (GIR; A and B) during HIEG clamps conducted in 18-h-fasted dogs at baseline (BL; circles) and after 10 wk (squares) of feeding a CTR diet (n = 4; A) or a HFFD to Sh or Px (n = 10; B) dogs. Average GIR (C) and GIR-to-insulin ratios (D) during 90–120 min of HIEGs conducted at BL (filled bars) and after 10 wk (patterned bars) of feeding a CTR or HFFD diet. Data from HFFD-Sh and HFFD-Px groups were combined in B–D because the reduction in GIR (in mg·kg−1·min−1) after 10 wk of HFFD feeding was similar between groups (HFFD-Sh, BL: 18.5 ± 1.7, 10 wk: 13.9 ± 0.7; HFFD-Px, BL: 19.2 ± 1.3, 10 wk: 14.1 ± 1.1). Data are means ± SE. *P < 0.05 vs. baseline.
HIHG Clamps
Hormone concentrations.
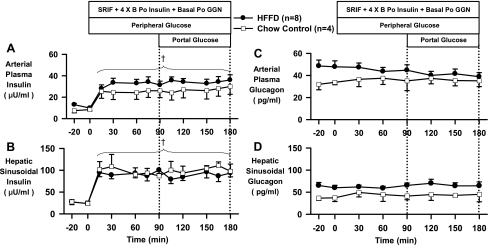
After 13 wk of HFFD feeding, the fasting plasma arterial insulin (μU/ml: CTR, 8 ± 2; HFFD, 11 ± 1, P = 0.06) and glucagon (pg/ml: CTR, 33 ± 4; HFFD, 48 ± 5, P = 0.06) concentrations tended to be elevated relative to those in the CTR group (Fig. 6 and Table 3). During the HIHG clamp, glucagon was maintained at a basal level while insulin was increased three- to fourfold (Fig. 6).
Fig. 6.
Arterial plasma insulin (A) and glucagon (C), and hepatic sinusoidal insulin (B) and glucagon (D) during basal (−20 to 0 min) and experimental periods (0 to 180 min) of HIHG clamps conducted in 18-h-fasted dogs after 13 wk of feeding CTR (n = 4; □) or HFFD (n = 8; ●) diet. Data from HFFD-Sh and HFFD-Px groups were combined in this figure because there were no differences between groups for these clamped parameters. Data are means ± SE. †P < 0.05 vs. basal period (HFFD and CTR groups); *P < 0.05 vs. CTR group.
Table 3.
Body weight, fasting plasma glucose, and plasma insulin
| Week of CTR or HFFD Feeding |
||||
|---|---|---|---|---|
| Group | BL | 4 wk | 8 wk | 13 wk |
| Body weight, kg | ||||
| CTR | 25 ± 2 | 24 ± 1 | 25 ± 2 | 26 ± 1 |
| HFFD | 26 ± 1 | 28 ± 1 | 29 ± 1 | 29 ± 1 |
| Plasma glucose, mg/dl | ||||
| CTR | 106 ± 5 | 99 ± 9 | 105 ± 6 | 108 ± 1 |
| HFFD | 106 ± 2 | 107 ± 3 | 108 ± 2 | 106 ± 1 |
| Plasma insulin, μU/ml | ||||
| CTR | 8 ± 2 | 8 ± 1 | 6 ± 1 | 8 ± 2 |
| HFFD | 8 ± 1 | 11 ± 1* | 10 ± 1 | 11 ± 1* |
Values are means ± SE; CTR, n = 4; HFFD, n = 10. Data from the HFFD-Sh and HFFD-Px groups were combined because there was no difference between groups for each parameter. Dogs were 24-h-fasted prior to plasma collection.
P < 0.05 vs. BL via paired t-test.
Plasma glucose concentrations and hepatic glucose load.
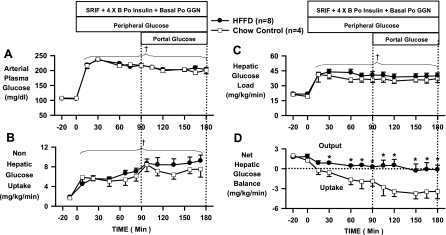
During the control period, arterial plasma glucose concentrations were 108 ± 1 and 106 ± 1 mg/dl in the CTR and HFFD groups, respectively (Fig. 7A and Table 3). During P1, arterial plasma glucose was increased to 218 ± 3 mg/dl in both groups to double the hepatic glucose load. The arterial plasma glucose concentrations were reduced slightly in both groups during P2 (mg/dl: CTR, 205 ± 4; HFFD, 199 ± 6) to maintain a doubling of the hepatic glucose load in the presence of portal glucose infusion. As a result, the hepatic glucose loads (mg·kg−1·min−1: CTR, 36 ± 3; HFFD, 40 ± 3) were similar throughout the experiment in both groups (Fig. 7C).
Fig. 7.
Arterial blood glucose (A), nonhepatic glucose uptake (Non-HGU; B), hepatic glucose load (C), and net hepatic glucose balance (NHGB; D) during basal (−20 to 0 min) and experimental periods (0 to 180 min) of HIHG clamps conducted in 18-h-fasted dogs after 13 wk of feeding a CTR (n = 4; □) or HFFD (n = 8; ●) diet. Negative values for NHGB indicate net hepatic uptake; positive values indicate net hepatic production. Data from HFFD-Sh and HFFD-Px groups were combined for HIHG analyses because there was no difference in NHGB (mg·kg−1·min−1) between groups [average during last 30 min of 2 subperiods (P1, 0–90 min; P2, 90–180 min); HFFD-Sh: −0.1 ± 0.5; HFFD-Px: 0.3 ± 0.8]. Data are means ± SE. †P < 0.05 vs. basal period (HFFD and CTR groups); *P < 0.05 vs. CTR group.
NHGB.
Net hepatic glucose output (NHGO) in the control period (mg·kg−1·min−1: CTR, 1.6 ± 0.2; HFFD, 1.8 ± 0.3) was unaffected by diet (Fig. 7D). Since NHGB after 13 wk of HFFD feeding was similar in the HFFD-Px and HFFD-Sh groups (see Fig. 7 legend), the data from both groups were combined in Figs. 6 and 7. When challenged with hyperinsulinemia and hyperglycemia (P1), the CTR group switched from net glucose output to net glucose uptake, reaching an average NHGU rate of −1.8 ± 0.8 mg·kg−1·min−1 (last 30 min of P1; Fig. 7D). The livers of HFFD group, on the other hand, displayed an inability to consume glucose in the presence of hyperinsulinemia and hyperglycemia, as indicated by an average NHGO rate of 0.4 ± 0.1 mg·kg−1·min−1 (last 30 min of P1, P < 0.05 vs. CTR; Fig. 7D). When the portal glucose signal was activated by infusing glucose into the portal vein (P2), there was a doubling of NHGU in the CTR group (−3.5 ± 1.0 mg·kg−1·min−1 during the last 30 min of P2; Fig. 7D). In contrast, portal glucose delivery in the HFFD group was unable to cause significant NHGU (−0.2 ± 0.8 mg·kg−1·min−1 during the last 30 min of P2, P < 0.05 vs. CTR; Fig. 7D).
Glucose Turnover, GIR, and Non-HGU
In the CTR group during hyperinsulinemia and hyperglycemia (P1), endo Ra significantly decreased (mg·kg−1·min−1: Basal, 2.2 ± 0.2; P1, 0.6 ± 0.5, P < 0.05 vs. basal), and the estimated rate of hepatic glucose uptake (est HGU) increased (mg·kg−1·min−1: Basal, 0.6 ± 0.3; P1, 2.5 ± 1.2, NS) relative to the basal period (Table 4). In the presence of the portal glucose signal (P2), there was no further suppression of endo Ra (mg·kg−1·min−1: P2, 0.8 ± 0.6), but the rate of est HGU significantly increased (mg·kg−1·min−1: P2, 4.3 ± 1.3, P < 0.05 vs. basal). However, in the HFFD group, a significant decline in endo Ra was not observed until P2 (mg·kg−1·min−1: Basal, 2.4 ± 0.2; P1, 1.7 ± 0.2; P2, 0.4 ± 0.2, P < 0.05 vs. Basal). Despite the presence of the portal glucose signal, est HGU did not increase significantly (mg·kg−1·min−1: Basal, 0.8 ± 0.3; P1, 1.4 ± 0.3; P2, 1.3 ± 0.7, NS; Table 4).
Table 4.
Tracer-determined rate of endogenous glucose appearance (Endo Ra), net hepatic glucose balance (NHGB), estimated hepatic glucose uptake (Est HGU = Endo Ra–NHGB), and rate of glucose disappearance (Glucose Rd) during hyperinsulinemic hyperglycemic clamps in a subset of dogs
| Experimental Period | |||
|---|---|---|---|
| Group | Basal Period | Period 1 | Period 2 |
| Endo Ra | |||
| CTR | 2.2 ± 0.2 | 0.6 ± 0.5† | 0.8 ± 0.6† |
| HFFD | 2.4 ± 0.2 | 1.7 ± 0.2 | 0.4 ± 0.2† |
| NHGB | |||
| CTR | 1.6 ± 0.2 | −1.9 ± 0.8† | −3.5 ± 0.9† |
| HFFD | 1.6 ± 0.1 | 0.3 ± 0.1†* | −0.9 ± 0.9† |
| Est HGU | |||
| CTR | 0.6 ± 0.3 | 2.5 ± 1.2 | 4.3 ± 1.3† |
| HFFD | 0.8 ± 0.3 | 1.4 ± 0.3 | 1.3 ± 0.7 |
| Glucose Rd | |||
| CTR | 2.2 ± 0.2 | 7.3 ± 0.8† | 10.0 ± 1.5† |
| HFFD | 2.4 ± 0.2 | 7.0 ± 0.9† | 8.7 ± 1.3† |
Values are means ± SE in mg·kg–1·min–1; CTR, n = 4; HFFD, n = 4. Negative values for balance data indicate net hepatic uptake. Dogs were 18-h fasted prior to study.
P < 0.05 vs. CTR;
P < 0.05 vs. basal period.
The GIR and the rate of non-HGU increased over time in both groups in response to hyperinsulinemia, hyperglycemia, and portal glucose delivery (Table 5 and Fig. 7B). Given that NHGU was reduced by ≈3.0 mg·kg−1·min−1 in the HFFD group compared with CTR, one might have expected a comparable decrease (≈27%) in GIR in the HFFD group if peripheral insulin sensitivity was unchanged. However, the decrease in GIR was somewhat less than that (1.9 mg·kg−1·min−1, or 17% decrease), indicating that a small increase occurred in the average non-HGU rate (mg·kg−1·min−1: CTR, 6.6 ± 1.1; HFFD, 7.9 ± 0.8). Furthermore, glucose Rd did not differ significantly among the CTR and HFFD groups during either experimental period (mg·kg−1·min−1: CTR P1, 7.3 ± 0.8; P2, 10 ± 1.5; HFFD P1, 7.0 ± 0.9; P2, 8.7 ± 1.3; Table 4). In fact, the difference in Rd between groups in P2 (1.3 mg·kg−1·min−1) was similar to the difference in GIR in P2 (1.9 mg·kg−1·min−1). These data thus clearly indicate that the defect in glucose uptake occurred in the liver and not peripheral tissues.
Table 5.
Total hepatic blood flow, total glucose infusion rate, arterial blood lactate and glycerol concentrations, arterial plasma NEFA concentration, net hepatic lactate, glycerol, and NEFA balance, and net hepatic carbon retention during hyperinsulinemic hyperglycemic clamps
| Experimental Period |
|||
|---|---|---|---|
| Group | Basal Period | Period 1 | Period 2 |
| Total hepatic blood flow, ml·kg–1·min–1 | |||
| CTR | 23 ± 3 | 21 ± 3 | 22 ± 3 |
| HFFD | 27 ± 2 | 25 ± 2 | 26 ± 1 |
| Total glucose infusion rate, mg·kg–1·min–1 | |||
| CTR | 0.0 ± 0.0 | 7.7 ± 0.9† | 11.0 ± 1.2† |
| HFFD | 0.0 ± 0.0 | 6.0 ± 0.6† | 9.1 ± 1.0† |
| Arterial blood lactate, μmol/l | |||
| CTR | 295 ± 10 | 703 ± 113† | 829 ± 58† |
| HFFD | 296 ± 17 | 469 ± 35†* | 731 ± 72† |
| Net hepatic lactate balance, μmol·kg–1·min–1 | |||
| CTR | −6.4 ± 0.9 | 6.3 ± 2.5† | 3.6 ± 2.1† |
| HFFD | −6.9 ± 0.8 | −3.6 ± 1.1†* | −4.1 ± 0.8†* |
| Arterial blood glycerol, μmol/l | |||
| CTR | 81 ± 17 | 33 ± 5† | 34 ± 7† |
| HFFD | 96 ± 7 | 42 ± 7† | 44 ± 9† |
| Net hepatic glycerol balance, μmol·kg–1·min–1 | |||
| CTR | −1.6 ± 0.5 | −0.6 ± 0.1 | −0.7 ± 0.1 |
| HFFD | −2.3 ± 0.3 | −0.8 ± 0.1 | −1.0 ± 0.2 |
| Arterial plasma NEFA, μmol/l | |||
| CTR | 828 ± 117 | 122 ± 22† | 62 ± 10† |
| HFFD | 813 ± 81 | 127 ± 34† | 101 ± 38† |
| Net hepatic NEFA balance, μmol·kg–1·min–1 | |||
| CTR | −2.4 ± 0.9 | −0.4 ± 0.1† | −0.1 ± 0.1† |
| HFFD | −3.0 ± 0.4 | −0.2 ± 0.1† | −0.2 ± 0.1† |
| Net hepatic carbon retention, mg glucose equivalents·kg–1·min–1 | |||
| CTR | −1.0 ± 0.1 | 0.7 ± 0.7† | 2.6 ± 0.9† |
| HFFD | −1.2 ± 0.2 | −0.1 ± 0.1† | 0.5 ± 0.8† |
Values are means ± SE; CTR, n = 4; HFFD, n = 8. Negative values for balance data indicate net hepatic uptake; negative values for carbon retention indicate net hepatic glycogen breakdown. Dogs were 18-h fasted prior to study.
P < 0.05 vs. CTR;
P < 0.05 vs. basal period.
Lactate, Net Hepatic Carbon Retention and Glycogen Metabolism
During the control period, arterial blood lactate concentrations and net hepatic lactate uptake were similar in both groups (Table 5). However, in response to hyperinsulinemia, hyperglycemia, and portal glucose delivery, there was a significant increase in the arterial blood lactate concentrations in the CTR group that resulted from a switch in net hepatic lactate balance from uptake to output (Table 5). In contrast, a switch from net hepatic lactate uptake to output did not occur at any time in the HFFD group (Table 5). Likewise, net hepatic carbon retention (mg glucose equivalents·kg−1·min−1), an index of glycogen accretion, was lower in the HFFD group than in the CTR group during P1 [CTR, 1.2 ± 0.7; HFFD, −0.1 ± 0.1 (glycogen breakdown)]. In response to portal glucose delivery, there was an increase in net hepatic carbon retention (mg glucose equivalents·kg−1·min−1) in the CTR group (1.9 ± 0.3) coincident with a doubling in NHGU; however, this was not evident in the HFFD group (0.6 ± 0.8), consistent with an inability of the portal signal to activate NHGU (Table 5). Although the terminal hepatic glycogen content was not significantly lower in the HFFD group (mg glycogen/g liver: CTR, 46 ± 2; HFFD, 38 ± 5), glycogen synthesis via the direct pathway was (mg·kg−1·min−1: CTR, 1.5 ± 0.4; HFFD, 0.5 ± 0.2, P = 0.03), consistent with a decrease in NHGU and net hepatic carbon retention in the HFFD group.
Fat Metabolism
Arterial plasma NEFA and glycerol concentrations were similar between the HFFD and CTR groups during the control period (Table 5). In response to the elevation in arterial insulin (P1 and P2), an equivalent decrease in arterial plasma NEFA and blood glycerol concentrations was observed in the two groups. The net hepatic uptake rates of NEFA and glycerol were reduced in parallel to the changes in the levels of NEFA and glycerol in the blood (Table 5).
DISCUSSION
The purpose of this study was to investigate, in a large animal model, how consumption of an HFFD, coupled with a compromised pancreatic mass, influenced the temporal development of impaired glucose tolerance, whole body insulin resistance, and the ability of the liver to take up and store glucose in the presence of conditions that mimic the postprandial state. Utilization of the canine model enabled the longitudinal assessment of perturbations in glucose metabolism and insulin sensitivity at the whole body and organ level, including the measurement of NHGU. Herein, we report the novel finding that 13 wk of HFFD feeding rendered the liver incapable of NHGU despite the presence of hyperinsulinemia, hyperglycemia, and the portal glucose feeding signal.
HFFD and the Liver
The CTR group displayed normal glucose tolerance, as well as a rapid induction of NHGU in the presence of hyperinsulinemia and hyperglycemia, a response that was augmented even further in the presence of the portal glucose signal. Coincident with stimulation of NHGU, a significant increase in net hepatic carbon retention and direct glycogen synthesis was observed. On the other hand, consumption of an HFFD resulted in impaired glucose tolerance in a relatively short period of time (4 wk), as indicated by more than a doubling in the ΔAUC for glucose in response to an oral glucose challenge in both the HFFD-Sh and HFFD-Px groups. Given the vital role of the liver in the disposition of an oral glucose load (26), these data raise the possibility that a reduction in hepatic insulin sensitivity and/or hepatic glucose effectiveness (GE) contributed to the attenuation in glucose tolerance after 4 wk of HFFD feeding. This is supported by our data from the HIHG clamps, which demonstrated that 13 wk of HFFD feeding rendered the liver incapable of switching from net glucose output to net glucose uptake in response to a combined increase in glucose and insulin. Glucose tracer kinetics indicated that this was attributable to a defect in both the suppression of glucose production and in the augmentation of hepatic glucose uptake. Consequently, net hepatic carbon retention and glycogen synthesis through the direct pathway were markedly diminished relative to the rates evident in the CTR group. Altogether, these data demonstrate that HFFD feeding elicits a robust metabolic phenotype characterized by a diminished ability of hyperinsulinemia and hyperglycemia to suppress hepatic glucose production, as well as an inability of the liver to consume glucose and synthesize glycogen under conditions that mimic the postprandial state.
HFFD and Nonhepatic Tissues (Adipose and Skeletal Muscle)
It was evident from the OGTTs that some degree of whole body insulin resistance or reduced GE contributed to the attenuation in glucose tolerance, given that greater hyperglycemia existed in the HFFD group compared with the CTR group even though their insulin levels were virtually equivalent. Thus, HIEG experiments were conducted to assess changes in whole body insulin sensitivity more precisely. Consistent with IGT, whole body insulin resistance was evident in the HFFD group, as indicated by a significant decrease (≈5.0 mg·kg−1·min−1) in the GIR required to maintain euglycemia after 10 wk of feeding. Since tracers were not infused during the HIEG clamps, it is not known whether the reduction in GIR was due to an impairment in the ability of insulin to suppress HGP, stimulate glucose uptake in the liver and/or peripheral tissues (skeletal muscle and adipose tissue), or some combination of the two. The possibility exists that the reduction in GIR may have been accounted for solely by a decrease in hepatic, not peripheral, insulin sensitivity. For example, the GIR decreased by ≈5.0 mg·kg−1·min−1 in the HFFD group, and by ≈2.0 mg·kg−1·min−1 in the CTR group after 10 wk of feeding. The decrease in GIR in the CTR group was not statistically significant and did not impact glucose tolerance, whole body insulin sensitivity, or NHGU. If the decrease in GIR in the HFFD group were adjusted for the decrease observed in the CTR group, the signal size of the reduction in whole body glucose utilization in the HFFD group would be ≈3.0 mg·kg−1·min−1. Given that HGP under basal conditions is ≈2.5 mg·kg−1·min−1, and that the liver can take up a small amount of glucose even under euglycemic conditions (≈1.0 mg·kg−1·min−1), we think it is likely that the reduction in GIR seen after 10 wk of HFFD feeding was explained primarily by a defect at the liver.
Consistent with this notion, as stated earlier, the ability of hyperinsulinemia and hyperglycemia to suppress endo Ra and stimulate HGU was significantly impaired after 13 wk of HFFD feeding. On the other hand, the ability of insulin to stimulate skeletal muscle glucose uptake was normal if not improved, given that non-HGU was modestly elevated (20%) in the HFFD group compared with CTR during the HIHG clamps. The increase in non-HGU was likely related to the fact that the arterial insulin concentration was slightly higher in the HFFD group than in the CTR group during insulin infusion. Indeed, when non-HGU was expressed relative to the arterial insulin concentration, the ratios were similar in the HFFD and CTR groups (HFFD, 0.25 ± 0.03; CTR, 0.28 ± 0.08). Furthermore, although NHGU during P2 was decreased by 3.3 mg·kg−1·min−1 in the HFFD group compared with the CTR group, the reductions in whole body glucose Rd (1.3 mg·kg−1·min−1) and GIR (1.9 mg·kg−1·min−1) were nearly equivalent in magnitude and less than the change in NHGU, indicating that the ability of insulin and/or glucose to stimulate non-HGU was enhanced despite a marked impairment in hepatic glucose uptake.
Previous studies have suggested that diet-induced impairments in insulin action during euglycemia can be compensated for by enhanced GE during hyperglycemia. For example, Commerford et al. (6) demonstrated in rodents that consumption of a high-fat or high-sucrose diet resulted in a decrease in insulin's ability to suppress HGP and stimulate whole body glucose uptake during an HIEG clamp, but during an HIHG clamp, these parameters were restored to the rates observed in chow-fed controls. This was also demonstrated to be the case in insulin-resistant, normoglycemic relatives of type 2 diabetic patients, in which glucose disposal was increased during a hyperglycemic pancreatic clamp due in part to enhanced GE in the skeletal muscle (11). Thus, it is possible that increased non-HGU in the HFFD group during the HIHG clamps was reflective of enhanced GE in the skeletal muscle in the presence of hyperglycemia.
With regard to adipose tissue, the ability of insulin to suppress lipolysis after 13 wk of HFFD feeding was also similar between the HFFD and CTR groups, as demonstrated by rapid and comparable reductions in arterial NEFA and glycerol concentrations in the presence of a fourfold rise in plasma insulin. As a result, net hepatic NEFA uptake was similar between groups and did not provide an explanation for the deficit in NHGU in the HFFD group.
HFFD and the Endocrine Pancreas
Consumption of an HFFD resulted in significantly augmented glycemia in response to an oral glucose challenge in both the HFFD-Sh and HFFD-Px groups after 4 and 8 wk of feeding. Based on the ΔAUCs for C-peptide and insulin, it appeared as if the impairment in glucose tolerance was due in part to a β-cell defect, given that insulin secretion was not enhanced relative to baseline studies despite a greater than twofold increase in glucose. To lend support to the observation that HFFD feeding impaired glucose tolerance due in part to a β-cell defect, a meta-analysis of all OGTTs performed over the last 3 yr in our laboratory was conducted. Consistent with the findings of the current paper, HFFD feeding increased the ΔAUC for glucose in response to an oral glucose challenge by 121% in sham-pancreatectomized (n = 5; P < 0.05 vs. baseline OGTT) and 130% in pancreatectomized (n = 10; P < 0.05 vs. baseline OGTT) dogs. On the other hand, the rise in insulin secretion (as indicated by the ΔAUC for C-peptide following HFFD feeding vs. that observed during the baseline OGTT) was virtually unchanged in the either group (7% decrease in the sham group; 5% increase in pancreatectomized group). Although a decrease in β-cell mass and/or function could explain insufficient glucose-stimulated insulin secretion (GSIS) in response to an oral glucose challenge, the β-cell responses were similar in both HFFD groups regardless of partial pancreatic resection, suggesting that a defect in β-cell function, not mass, was associated with the lack of hyperinsulinemic compensation. In fact, it appears as though the surgical reduction (≈65%) in pancreatic mass was without significant effect, which is consistent with earlier data. For example, Frey et al. (8) suggested that the development of diabetes following a partial pancreatectomy was directly related to the extent of pancreatic resection. In patients with otherwise normal pancreatic function, up to 80% of the pancreatic parenchyma could be removed without a change in metabolic status. On the other hand, near-total (80–95%) or total pancreatectomy resulted in 100% of the patients developing diabetes postoperatively (8, 33). Studies conducted in rodents demonstrated that removal of 85–95% of the pancreas was required before hyperglycemia ensued, and even then, there was a heterogeneous hyperglycemic response that correlated with the extent of pancreatic resection (4, 15). Our hope was that the dietary insult might trigger a further reduction in β-cell mass in HFFD-fed dogs, leading to the development of a diabetic phenotype, but that did not occur.
Recently, Ionut et al. (14) characterized the development of IGT in a canine model of high-fat diet-induced obesity before and after streptozotocin (STZ)-induced β-cell destruction. In the absence of STZ, glucose tolerance was retained during high-fat feeding due to a compensatory increase in insulin secretion, whereas high-fat feeding coupled with an intermediate dose of STZ (18.5 mg/kg) resulted in impaired glucose tolerance due to a 77–93% reduction in β-cell function secondary to β-cell destruction (14). In the present study, however, insulin secretion following an oral glucose challenge was impaired in both the HFFD-Sh and -Px groups, resulting in augmented glycemia. There are some key differences between the studies conducted by Ionut et al. (14) and those presented in this paper. First, we utilized a surgical (partial pancreatectomy) rather than chemical (STZ) approach to create a pure model of compromised pancreatic mass so that any toxic, off-target effects of STZ could be avoided (e.g., generation of highly reactive ions and DNA strand break within the β-cells) (29, 40). In addition, after the initial hypercaloric phase, dogs in the present study consumed ≈1,600–2,100 kcal/day, whereas caloric consumption by dogs in the study of Ionut et al. (14) exceeded 5,000 kcal/day. Last, we utilized a high-fat and high-fructose feeding paradigm to emulate consumption of a Western diet. High-fructose feeding has been associated with a reduction in β-cell mass and an increase in the percentage of apoptotic cells, whereas high-fat feeding has been associated with a reduction in β-cell glucose oxidation and insufficient GSIS (23, 35). It is possible that an interaction with the fructose component of the diet modified the impact of high dietary fat on whole body insulin sensitivity and β-cell function in the current study. Just as STZ precipitated the development of impaired glucose tolerance secondary to β-cell destruction (14), so might an increase in dietary fructose impair β-cell function and glucose tolerance in the context of high-fat feeding.
In addition, the composition of dietary fat has been shown to directly influence β-cell function in that unsaturated fat impairs, whereas saturated fat enhances, GSIS (7). Although the composition of dietary fat was not described in the paper by Ionut et al. (14), it is possible that their diet contained a higher concentration of saturated fat and/or a lower concentration of unsaturated fat than that used in the current study (22% saturated/28% unsaturated as %total energy), which might have altered β-cell responses in the presence of a glucose challenge (7). Altogether, the phenotypic differences among our models are likely related to differences in dietary constituents, caloric consumption, and the manner in which β-cell dysfunction was induced.
In conclusion, a defect in the ability of the liver to transition from glucose production to glucose uptake has been implicated in the development of IGT and hyperglycemia with type 2 diabetes (3, 13, 21). Likewise, a marked reduction in splanchnic glucose uptake and hepatic glycogen synthesis following a meal has been observed in individuals with either type 1 or type 2 diabetes (3, 13, 21). In the present study, we utilized a large-animal model to demonstrate that chronic consumption of an HFFD diminishes the sensitivity of the liver to hormonal (insulin) and glycemic (hyperglycemia and the portal glucose signal) cues and results in a marked impairment in NHGU and glycogen synthesis. Thus, consumption of dietary fat and fructose in excess might play a role in the etiology of IGT, insulin resistance, and diabetes through their hepatospecific effects. Future studies will need to be conducted to identify the hepatocellular signaling defects that link high dietary fat and fructose to impaired NHGU in vivo.
GRANTS
This research was supported in part by a National Institutes of Health Grant RO1-DK-18243 and the Diabetes Research and Training Center Grant DK-020593.
DISCLOSURES
No conflicts of interest relevant to this article were reported by the authors.
ACKNOWLDGMENTS
We greatly appreciate the technical assistance and support of Margaret Lautz, Jon Hastings, Patsy Raymer, and the Diabetes Research and Training Center Hormone Assay and Analytical Services Core. Part of this work was presented at the 69th Annual Meeting of the American Diabetes Association, New Orleans, June, 2009. Dr. Cherrington is the Jacquelyn A. Turner and Dr. Dorothy J. Turner Chair in Diabetes Research.
REFERENCES
- 1.Adkins-Marshall BA, Myers SR, Hendrick GK, Williams PE, Triebwasser K, Floyd B, Cherrington AD. Interaction between insulin and glucose-delivery route in regulation of net hepatic glucose uptake in conscious dogs. Diabetes 39: 87–95, 1990 [DOI] [PubMed] [Google Scholar]
- 2.An Z, DiCostanzo CA, Moore MC, Edgerton DS, Dardevet DP, Neal DW, Cherrington AD. Effects of the nitric oxide donor SIN-1 on net hepatic glucose uptake in the conscious dog. Am J Physiol Endocrinol Metab 294: E300–E306, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, Jensen MD, Schwenk WF, Rizza RA. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes 49: 272–283, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest 71: 1544–1553, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catena C, Giacchetti G, Novello M, Colussi G, Cavarape A, Sechi LA. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am J Hypertens 16: 973–978, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Commerford SR, Bizeau ME, McRae H, Jampolis A, Thresher JS, Pagliassotti MJ. Hyperglycemia compensates for diet-induced insulin resistance in liver and skeletal muscle of rats. Am J Physiol Regul Integr Comp Physiol 281: R1380–R1389, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Dobbins RL, Szczepaniak LS, Myhill J, Tamura Y, Uchino H, Giacca A, McGarry JD. The composition of dietary fat directly influences glucose-stimulated insulin secretion in rats. Diabetes 51: 1825–1833, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Frey CF, Child CG, Fry W. Pancreatectomy for chronic pancreatitis. Ann Surg 184: 403–413, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med 164: 2235–2240, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Galassetti P, Chu CA, Neal DW, Reed GW, Wasserman DH, Cherrington AD. A negative arterial-portal venous glucose gradient increases net hepatic glucose uptake in euglycemic dogs. Am J Physiol Endocrinol Metab 277: E126–E134, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Henriksen JE, Levin K, Thye-Ronn P, Alford F, Hother-Nielsen O, Holst JJ, Beck-Nielsen H. Glucose-mediated glucose disposal in insulin-resistant normoglycemic relatives of type 2 diabetic patients. Diabetes 49: 1209–1218, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension 10: 512–516, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Hwang JH, Perseghin G, Rothman DL, Cline GW, Magnusson I, Petersen KF, Shulman GI. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J Clin Invest 95: 783–787, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ionut V, Liu H, Mooradian V, Castro AV, Kabir M, Stefanovski D, Zheng D, Kirkman EL, Bergman RN. Novel canine models of obese pre-diabetes and of mild type 2 diabetes. Am J Physiol Endocrinol Metab 298: E38–E48, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem 274: 14112–14121, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Keppler D, Decker K. Glycogen: determination with amyloglycosidase. In: Methods of Enzymatic Analysis, edited by Bergmeyer HU. New York: Verlag Chemie Weinheim, Academic, 1974, p. 1127–1131 [Google Scholar]
- 17.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab 292: E1590–E1598, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kim SP, Ellmerer M, VanCitters GW, Bergman RN. Primacy of hepatic insulin resistance in the development of the metabolic syndrome induced by an isocaloric moderate-fat diet in the dog. Diabetes 52: 2453–2460, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Koo HY, Miyashita M, Cho BH, Nakamura MT. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochem Biophys Res Commun 390: 285–289, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 40: 1397–1403, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, DallaMan C, Cobelli C, Cline GW, Shulman GI, Waldhausl W, Roden M. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 53: 3048–3056, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Lanthier N, Molendi-Coste O, Horsmans Y, van Rooijen N, Cani PD, Leclercq IA. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol 298: G107–G116, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Maiztegui B, Borelli MI, Raschia MA, Del Zotto H, Gagliardino JJ. Islet adaptive changes to fructose-induced insulin resistance: beta-cell mass, glucokinase, glucose metabolism, and insulin secretion. J Endocrinol 200: 139–149, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 139: 1228S–1235S, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Myers SR, McGuinness OP, Neal DW, Cherrington AD. Intraportal glucose delivery alters the relationship between net hepatic glucose uptake and the insulin concentration. J Clin Invest 87: 930–939, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagliassotti MJ, Cherrington AD. Regulation of net hepatic glucose uptake in vivo. Annu Rev Physiol 54: 847–860, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Pagliassotti MJ, Holste LC, Moore MC, Neal DW, Cherrington AD. Comparison of the time courses of insulin and the portal signal on hepatic glucose and glycogen metabolism in the conscious dog. J Clin Invest 97: 81–91, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagliassotti MJ, Prach PA. Quantity of sucrose alters the tissue pattern and time course of insulin resistance in young rats. Am J Physiol Regul Integr Comp Physiol 269: R641–R646, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Rerup CC. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev 22: 485–518, 1970 [PubMed] [Google Scholar]
- 30.Rocchini AP, Marker P, Cervenka T. Time course of insulin resistance associated with feeding dogs a high-fat diet. Am J Physiol Endocrinol Metab 272: E147–E154, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279: 32345–32353, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Satake S, Moore MC, Igawa K, Converse M, Farmer B, Neal DW, Cherrington AD. Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes 51: 1663–1671, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Slezak LA, Andersen DK. Pancreatic resection: effects on glucose metabolism. World J Surg 25: 452–460, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 187: 15–24, 1956 [DOI] [PubMed] [Google Scholar]
- 35.Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest 117: 246–257, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thresher JS, Podolin DA, Wei Y, Mazzeo RS, Pagliassotti MJ. Comparison of the effects of sucrose and fructose on insulin action and glucose tolerance. Am J Physiol Regul Integr Comp Physiol 279: R1334–R1340, 2000 [DOI] [PubMed] [Google Scholar]
- 37.van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med 136: 201–209, 2002 [DOI] [PubMed] [Google Scholar]
- 38.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 25: 417–424, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med 10: 160, 2008 [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson GL, Patton NJ, McCord JM, Mullins DW, Mossman BT. Mechanisms of streptozotocin- and alloxan-induced damage in rat B cells. Diabetologia 27: 587–591, 1984 [DOI] [PubMed] [Google Scholar]