Abstract
The type of free fatty acids (FFAs), saturated or unsaturated, is critical in the development of insulin resistance (IR), since the degree of saturation correlates with IR. We compared the effects of the saturated FFA palmitate, the unsaturated FFA oleate, and a mixture of each on the production of mitochondrial reactive oxygen species (mtROS), mitochondrial DNA (mtDNA) damage, mitochondrial function, apoptosis, and insulin-signaling pathway in skeletal muscle cells. Only palmitate caused a significant increase of mtROS production, which correlated with concomitant mtDNA damage, mitochondrial dysfunction, induction of JNK, apoptosis, and inhibition of insulin signaling. Blocking de novo synthesis of ceramide abolished the effects of palmitate on mtROS production, viability, and insulin signaling. Oleate alone did not cause mtROS generation and mtDNA damage, and its addition to palmitate prevented palmitate-induced mtDNA damage, increased total ATP levels and cell viability, and prevented palmitate-induced apoptosis and inhibition of insulin-stimulated Akt (Ser473) phosphorylation. The peroxisome proliferator activator receptor-γ coactivator 1α (PGC-1α) protein level and promoter activity were decreased at concentrations of palmitate ≥0.5 mM, whereas addition of oleate increased both PGC-1α level and promoter activity. Expression of the mitochondrial transcription factor (TFAM) was significantly diminished after palmitate but not oleate treatment. Addition of the ROS scavenger, N-acetylcystein (NAC), to palmitate restored both the expression and promoter activity of PGC-1α as well as TFAM expression. We propose that 1) mtROS generation is the initial event in the induction of mitochondrial dysfunction and consequent apoptosis and the inhibition of insulin signaling and that 2) oleate ameliorates palmitate-induced mitochondrial dysfunction and thus may contribute to the prevention of palmitate-induced IR.
Keywords: insulin resistance, free fatty acids, mitochondrial dysfunction, reactive oxygen species, mitochondrial biogenesis
accumulation of muscular free fatty acids (FFAs) has been implicated in the insulin resistance (IR) seen in type 2 diabetes (4). Additionally, mitochondrial dysfunction and increased mitochondrial oxidative stress has been proposed to play an important role in insulin resistance (IR) and type 2 diabetes (7, 15, 16, 19, 26, 29, 31, 44–46). Previously, it has been shown that saturated FFAs cause IR (22, 51), whereas unsaturated FFAs improve insulin sensitivity (45, 42) in diabetic patients. Also, studies in vitro have demonstrated that the saturated FFA palmitate induced IR in skeletal muscle cells (6, 13, 20, 38, 43, 49), whereas the unsaturated FFA oleate improved insulin sensitivity (9, 13, 17, 43). A recent study showed that palmitate-induced IR and apoptosis were positively related, whereas oleate did not induce apoptosis and improved insulin sensitivity in L6 skeletal muscle cells (49). However, the mechanisms by which these two common dietary FFAs cause different effects on IR and apoptosis have not been fully elucidated. Potential candidates mediating the effects of the saturated FFA palmitate on IR include 1) an increase in production of ceramide (6, 38) and accumulation of diacylglycerol, leading to activation of PKCθ (23), 2) mitochondrial dysfunction (20, 39) and increased oxidative stress (21, 28, 39), 3) activation of proinflammatory NF-κB and mitogen-activated kinases (8, 53), and 4) decreased peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) activation (12, 53) and reduced mitochondrial gene expression and cellular oxygen consumption rates (12). Compelling evidence in the literature has suggested that all of the above-mentioned factors linking palmitate-induced IR are somehow connected. Indeed, a very recent study has shown that mitochondrial superoxide production is a common feature of many different models of IR, including saturated fat-induced IR in skeletal muscle cells (21). Previously, we showed that the saturated FFA palmitate increased the production of superoxide and nitric oxide (NO), induced mitochondrial DNA (mtDNA) damage, and triggered mitochondrial dysfunction, which caused apoptosis in L6 skeletal muscle cells (39). Our current study was designed to address specifically the effects of saturated and unsaturated FFAs on mitochondrial reactive oxygen species (mtROS) production, mtDNA damage, and mitochondrial function and their relationship to the induction of apoptosis and IR in skeletal muscle cells. We found that the different effects of these FFAs on IR correlated with their ability to induce mitochondrial oxidative stress, mtDNA damage, and consequent mitochondrial dysfunction in skeletal muscle cells. Also, our results demonstrated that de novo synthesis of ceramide is involved in the palmitate-induced mtROS generation, mitochondrial dysfunction, and insulin signaling. Moreover, we discovered that palmitate-induced oxidative stress contributed to the loss in the insulin-stimulated phosphorylation of Akt and to downregulation of PGC-1α and mitochondrial transcription factor (TFAM), which regulate mitochondrial biogenesis. On the basis of these data and considering the correlation between mtROS production, mitochondria dysfunction, apoptosis, and IR, we conclude that palmitate-induced oxidative stress is an initial event contributing to IR in skeletal muscle by triggering mitochondrial dysfunction, which leads to apoptosis.
MATERIALS AND METHODS
Reagents and plasmids.
Dulbecco's modified Eagle's medium (DMEM) was from Invitrogen (Carlsbad, CA), Fetal bovine serum (FBS) was from Hyclone (Logan, UT). Palmitate, 2-bromopalmitate, oleate, fumonisin B1, C2-ceramide, C2-dihydroceramide, SP-600125, insulin (from bovine pancreas), penicillin-streptomycin, and N-acetylcystein (NAC) were from Sigma (St. Louis, MO). The plasmid containing the 5′-flanking sequence of the mouse PGC-1α gene (2-kilobase promoter, −2,533 to +78 relative to transcriptional start site) and luciferase gene was purchased from Addgene (plasmid no. 8887; www.addgene.com). This PGC-1α promoter construct has been used extensively by other investigators (1, 12). The pEGFP-N1 vector (catalog no. 6085-1, GenBank accession no. U55762) was from Clontech Laboratories (Palo Alto, CA).
Cell culture and treatment.
Rat L6 skeletal muscle cells were obtained from ATCC (Manassas, VA). Cells were grown in DMEM supplemented with 10% FBS and 50 μg/ml penicillin-streptomycin in 5% CO2 at 37°C. For these studies, L6 myoblasts were plated in culture dishes or 24-well plates and used at the myotube stage of differentiation, as described previously (39). Stock concentrations of fumonisin B1, C2-ceramide, C2-dihydroceramide, and SP-600125 were dissolved in dimethylsulfoxide (DMSO). Control cultures not treated with fumonisin B1, C2-ceramide, C2-dihydroceramide, and SP-600125 received the same concentration of DMSO as in the compound-treated cultures. A stock concentration of palmitate and oleate and a mixture (oleate-palmitate, 2:1 molar ratio, termed o/p mixture in all later experiments) was dissolved in 50% ethanol; 2-bromopalmitate was dissolved in absolute ethanol. All FFAs were used for treatment of L6 myotubes after conjugation with the appropriate concentration of FFA-free BSA (0.5% BSA for 0.1 mM, 1% BSA for 0.25 mM, and 2% BSA for 0.5 mM of FFA). This gave a final molar ratio of FFA/BSA of ∼2:1, which is close to the ratio observed in human serum (47). For consistency with our previous study (39), cultures with 0.75 and 1 mM FFA were incubated in the medium containing 2% BSA. Control cells were treated with drug diluent only (corresponding concentrations of BSA and ethanol in the DMEM medium). Cultures containing either fumonisin B1 (50 μM) or NAC (5 mM) were pretreated for 30 min prior to palmitate addition. In the Akt (Ser473) phosphorylation experiments, L6 myotubes were incubated with different concentrations of FFAs for 16 h and then serum starved for 2 h and incubated with 100 nM of insulin for 15 min.
Measurement of nitric oxide production.
L6 myotubes were exposed to varying concentrations of oleate or the o/p mixture for 6 h in serum-free culture medium. Control cells received drug diluent only. Nitric oxide (NO) production was measured as described previously (39). Briefly, after treatment, aliquots of medium were collected, and nitrite production was evaluated in duplicate using the Griess reaction (0.5% sulfanylamide and 0.05% naphthalene diamine dihydrochloride in 2.5% orthophosphoric acid). Nitrite values were determined using varying concentrations of sodium nitrite as a standard.
Mitochondrial ROS production.
L6 myotubes were exposed to palmitate, oleate, or the o/p mixture for 6 and 24 h and then analyzed for mtROS production. MitoSOX Red, a mitochondrial superoxide indicator for live-cell imaging (Invitrogen, Carlsbad, CA), was employed to analyze mitochondrial superoxide generation within L6 myotube mitochondria. This agent is live-cell permeant and is rapidly and selectively targeted to the mitochondria. Once in the mitochondria, MitoSOX Red reagent is readily oxidized by superoxide but not by other ROS or reactive nitrogen species. Cells were incubated with 5 μM MitoSOX Red during the final 1 h of treatment, as described previously (21). After incubation with MitoSOX Red, cells were washed twice with phosphate-buffered saline and plates read in microplate fluorescence reader (Fluoroscan Ascent; Labsystem, Franklin, MA) at 525 (excitation) and 620 nm (emission). The increase in ROS production was calculated as the percentage increased compared with control.
Assay for mtDNA damage.
L6 myotubes were exposed to palmitate, oleate, or the o/p mixture, as described above. DNA isolation and quantitative Southern blots were performed as described previously (39, 40). The resultant band images were scanned and analyzed using Molecular Analyst (Bio-Rad, Hercules, CA) and Fujifilm Image Gauge Version 2.2 (FujiFilm USA, Stamford, CT) software. Break frequency was determined using the Poisson expression (s = −lnPo, where s is the number of breaks per fragment and Po is the fraction of fragments free of breaks) (39, 40).
Measurement of ATP levels.
Cells were grown in 24-well culture plates and treated with FFAs for the indicated times. To determine the total cellular ATP level, an ATP bioluminescence assay kit (Roche, Mannheim, Germany) was used as described (39, 40). This kit employs a well-established technique that uses the ATP dependency of the light-emitting luciferase catalyzed oxidation of luciferin for the measurement of extremely low concentrations of ATP (11). The emitted light is linearly related to the ATP concentration and was measured using a luminometer. Values are displayed as a percentage of untreated control.
Cell viability.
The CellTiter 96 assay (Promega), a colorimetric method for determining the number of viable cells by assessing mitochondrial function, was performed at the indicated time after exposure to different concentrations of palmitate, oleate, and the o/p mixture, as described previously (10). The reagent contains a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), inner salt]?> and an electron-coupling reagent (phenazine methosulfate). MTS is bioreduced by the dehydrogenase enzymes found in metabolically active cells into a formazan product soluble in tissue culture medium. The quantity of formazan product measured by the amount of 490 nm absorbance is directly proportional to the number of living cells in culture. The reagent is added to culture wells, and the cells are incubated for 2 h. Optical density was read at 490 nm in a microplate reader. Data are displayed as a percentage of untreated controls.
Protein isolation and Western blot analysis.
Proteins extracts for total cellular fractions were isolated in cell lysis buffer (Cell Signaling Technology, Beverly, MA) supplemented with 0.1 mg of PMSF and a 1/100 dilution of protease and phosphatase inhibitor cocktails (Sigma). The cells were scraped to dislodge them from the bottom of the dishes and then passed four times through a 27-gauge needle attached to the 1cc-gauge syringe (Becton Dickinson, Franklin Lakes, NJ). Samples were centrifuged for 10 min at 14,000 g, and the supernatants were used for Western blots. Protein concentrations were determined using the Bio-Rad protein dye microassay. SDS-polyacrylamide gel electrophoresis and transfer of separated proteins to PVDF membranes were performed as described previously (39). Blocking and antibody immunoblotting were performed in 5% nonfat dry milk and Tris-buffered saline with 0.1% Tween-20 (TBS-T). TBS-T and TBS were used for washing. The antibodies used were actin (Sigma), caspase-3, phospho-Akt (Ser473), total Akt, phospho-SAP/JNK (T183/Y185), total SAP/JNK (Cell Signaling Technology), PGC-1α, and TFAM (Santa Cruz Biotechnology, Santa Cruz, CA). Complexes formed were detected with horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG antibodies (Promega, Madison, WI) using chemiluminescent reagents (SuperSignal; Pierce, Rockford, IL). Where indicated, the resultant band images were scanned and analyzed using Fujifilm Image Gauge Version 2.2 software.
Transfection and reporter analysis.
L6 myotubes were cotransfected with a mixture of PGC-1α promoter and pEGFP-N1 plasmids using Fugene HD Transfection Reagent (Roche, Mannheim, Germany). Twenty four hours after transfection, cells were treated with different concentrations of FFAs for an additional 24 h. Forty eight hours after transfection, plates were read in a microplate fluorescence reader (Fluoroscan Ascent; Labsystem, Franklin, MA) at 487 nm (excitation) and 525 nm (emission). After reading, cells were lysed and luciferase activity was determined using a luciferase activity kit (Promega). Luciferase activity was normalized for green fluorescent protein fluorescence intensity. Data are indicated as a percentage of untreated controls.
Statistical analysis.
Data are expressed as means ± SE. Differences between two groups were assessed using unpaired, two-tailed Student's t-test. Data involving more than two groups were performed using one-way ANOVA (GraphPad Prism) followed by Bonferroni analysis where appropriate. Statistical significance was determined at the 0.05 level.
RESULTS
Only palmitate induced mtROS production in L6 myotubes.
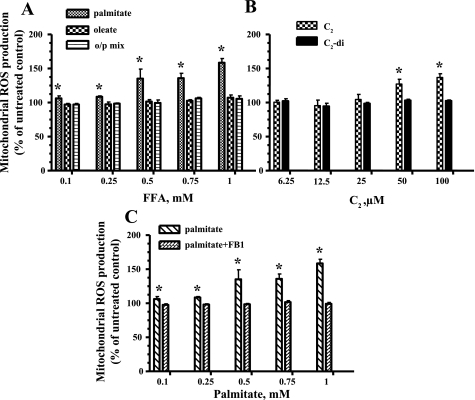
Myotubes were exposed to different concentrations of the saturated FFA palmitate (16:0) and the unsaturated FFA oleate (18:1) or the o/p mixture for 6 and 24 h. Only palmitate significantly increased mtROS in L6 myotubes after 24 h of incubation (Fig. 1A). We were not able to detect significant mtROS generation after 6-h incubation with the FFAs (data not shown). To determine the involvement of ceramide in the generation of mtROS, we exposed L6 myotubes to C2-ceramide, a cell-permeable analog of ceramide or metabolically inactive control (C2-dihydroceramide). As shown in Fig. 1B, C2-ceramide mimicked the effects of palmitate on the generation of mtROS. Addition of fumonisin B1, a selective inhibitor of de novo synthesis of ceramide, abrogated the effects of palmitate on mtROS production (Fig. 1C). These data confirmed that palmitate induced mtROS production through de novo synthesis of ceramide.
Fig. 1.
Effects of free fatty acids (FFAs) and ceramide on mitochondrial reactive oxygen species (mtROS) production in L6 myotubes. After 24 h of treatment, cells were analyzed in a fluorescent plate reader, and the increase in ROS production was calculated as a %increase compared with control. Mitochondrial superoxide production in L6 myotubes treated with the indicated concentrations of the different FFAs (A), the indicated concentrations of C2-ceramide (C2) or metabolically inactive C2-dihydroceramide (C2-di) (B), or the indicated concentrations of palmitate in the presence of 50 μM fumonisin B1 (FB1; C). The mean results ± SE are shown (n ≥ 3). *P < 0.05. o/p mix, Oleate-palmitate mixture.
Oleate prevented palmitate-induced mtDNA damage in L6 myotubes.
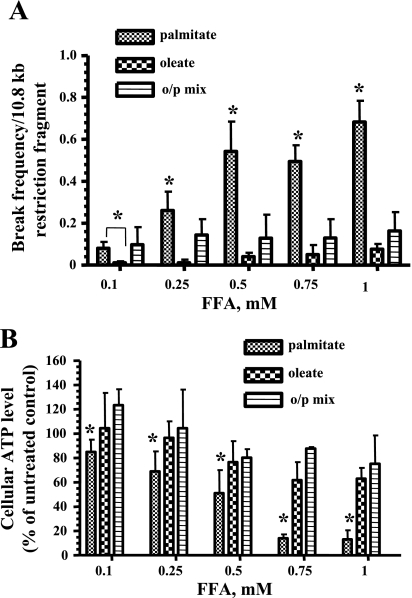
L6 myotubes were exposed to different concentrations of palmitate, oleate, or the o/p mixture (0.1–1 mM) for 6 h, as described in materials and methods. Quantitative alkaline Southern blot analysis was performed using a mtDNA specific probe. As shown in Fig. 2A, only palmitate caused significant mtDNA damage, even at low concentrations, whereas oleate alone did not induce significant damage, and the addition of oleate to palmitate prevented the palmitate-induced mtDNA damage (Fig. 2A).
Fig. 2.
Oleate protects L6 myotubes against palmitate-induced mitochondrial DNA (mtDNA) damage and decline in ATP level. A: break frequency per 10.8 kb fragment of mtDNA after 6 h of treatment with the indicated concentrations of palmitate, oleate, or the o/p mixture. Break frequency was determined as described in materials and methods. Intensity of the band was determined by densitometry. B: L6 myotubes were treated with the indicated concentration of palmitate, oleate, or o/p mix for 24 h, and ATP production was measured. The mean results ± SE are shown (n ≥ 3). *P < 0.05.
Addition of oleate attenuated the palmitate-induced decrease in ATP levels and mitochondrial viability in L6 myotubes.
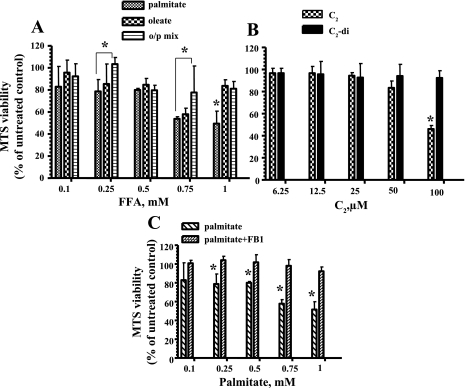
To determine whether the protection from palmitate-induced mtDNA damage by oleate correlates with increased ATP levels and enchanced viability, we next analyzed total ATP levels and mitochondrial viability after treatment with the different FFAs. Total cellular ATP levels were measured 24 h after exposure to different concentrations of palmitate, oleate, or the o/p mixture (0.1–1 mM). Only palmitate was found to reduce ATP levels in a dose-dependent fashion (Fig. 2B). Mitochondrial viability was evaluated in L6 myotube cultures treated with palmitate, oleate, or the o/p mixture for 24 h. As shown in Fig. 3A, only palmitate was significantly toxic to L6 myotubes. Also, we found that C2-ceramide decreased mitochondrial viability in L6 myotubes (Fig. 3B). Addition of fumonisin B1 to palmitate protected L6 myotubes from toxic effect of palmitate (Fig. 3C), confirming that de novo synthesis of ceramide contributed to the palmitate-induced decreased of mitochondrial viability in L6 myotubes.
Fig. 3.
Cell viability in L6 myotubes after treatment with FFAs and ceramide. A: only palmitate significantly decreased viability in L6 myotubes. B: C2-ceramide but not C2-di diminished mitochondrial viability in L6 myotubes. C: FB1 blocked de novo synthesis of ceramide and enhanced viability in L6 myotubes. The mean results ± SE are shown (n ≥ 3). *P < 0.05.
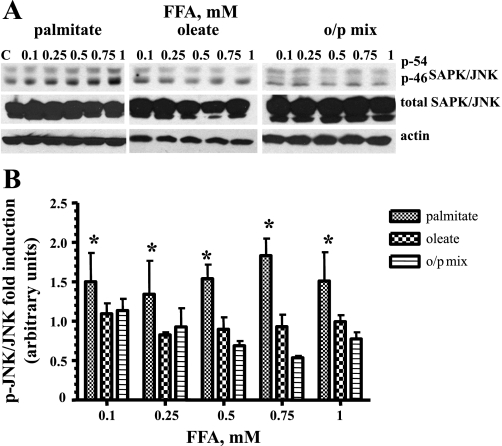
Only palmitate induced activation of the stress-associated protein kinase JNK in L6 myotubes.
Because the activation of stress-activated protein kinase JNK has been implicated in both the modulation of apoptosis (48) and IR (32, 44), we compared the phosporylation/activation level of JNK after incubation with palmitate, oleate, and the o/p mixture. As shown in Fig. 4, only palmitate significantly induced phosphorylation of JNK kinase. Furthermore, addition of oleate suppressed palmitate-induced JNK phosphorylation (Fig. 4), providing direct evidence that addition of oleate diminishes palmitate-induced oxidative stress in L6 myotubes.
Fig. 4.
Effects of palmitate, oleate, and the o/p mixture on JNK activation in skeletal muscle cells. L6 myotubes were exposed to the indicated concentrations of palmitate, oleate, or o/p mix for 24 h. Total cell lysates were isolated and analyzed by Western blot with the indicated antibodies. A: representative blots are shown. B: the values from densitometry from at least 3 (p-JNK) independent experiments were normalized to the level of total JNK and expressed as fold of difference compared with the corresponding untreated controls ± SE (n ≥ 3). *P < 0.001.
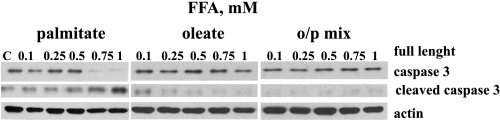
Only palmitate induced caspase-3 cleavage in L6 myotubes.
Previously, we have shown that palmitate induced apoptosis in L6 myotubes (39). We next sought to determine whether oleate prevented palmitate-mediated apoptosis using Western blot analysis of caspase-3 activation. Myotubes were exposed to different concentrations of palmitate, oleate, or the o/p mixture (0.1–1 mM) for 24 h. Following 24 h of palmitate treatment, activated caspase-3 was found in L6 myotubes (Fig. 5), whereas the same concentrations of oleate or the o/p mixture did not cause cleavage of this caspase.
Fig. 5.
Oleate prevents palmitate-induced apoptosis in skeletal muscle cells. L6 myotubes were treated with the indicated concentrations of palmitate, oleate, or o/p mix for 24 h. Caspase-3 antibodies were used to recognize the full-length (35 kDa) and cleaved (17 kDa) fragments of caspase-3. Representative blots from 3 independent experiments are shown. Equal loading was confirmed using anti-actin antibody.
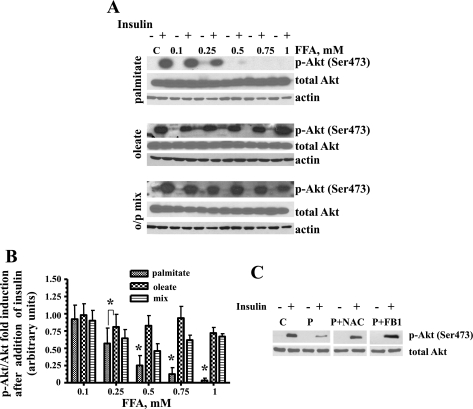
Effects of palmitate, oleate, and the o/p mixture on insulin-induced Akt (Ser473) phosphorylation in L6 myotubes.
We compared phosphorylated Akt levels in L6 myotubes treated with the different FFAs. L6 myotubes were treated with palmitate, oleate, or the o/p mixture for 16 h and then serum starved for 2 h and incubated in the presence or absence of 100 nM insulin for 15 min. Representative blots for Akt (Ser473) and total Akt protein expression levels are shown in Fig. 6A. Only palmitate significantly and dose-dependently reduced Akt (Ser473) phosphorylation levels in L6 myotubes (Fig. 6, A and B). To determine whether ROS generation is involved in palmitate-induced decrease of Akt (Ser473) phosphorylation, the effect of an antioxidant, NAC (a precursor compound for glutathione formation), was used. As shown in Fig. 6C, NAC attenuated palmitate-induced inhibition of Akt (Ser473) phosphorylation. To evaluate whether de novo synthesis of ceramide was also involved in the decrease of Akt (Ser473) phosphorylation, we utilized fumonisin B1. The results shown in Fig. 6C indicate that fumonisin B1 abolished palmitate-induced reduction in Akt (Ser473) phosphorylation.
Fig. 6.
Oleate, N-acetylcysteine (NAC), and FB1 ameliorated palmitate-mediated inhibition of insulin-induced Akt (Ser473) phosphorylation in skeletal muscle cells. A and B: L6 myotubes were exposed to the indicated concentrations of palmitate, oleate, or o/p mix for 16 h and then serum starved for 2 h and incubated in the presence or absence of 100 nM insulin for 15 min. Total cell lysates were isolated and analyzed by Western blot analysis with the indicated antibodies. A: representative blots from at least 3 independent experiments are shown. B: the values from densitometry from at least 3 (p-Akt) independent experiments were normalized to the level of total Akt and expressed as fold of difference after addition of insulin compared with the corresponding untreated controls ± SE (n ≥ 3). *P < 0.001. C: L6 myotubes were incubated with 1 mM palmitate (P) alone or with 1 mM palmitate in the presence of either 5 mM NAC or 50 μM FB1. Western blot analysis was performed using p-Akt and total Akt antibodies. C, control.
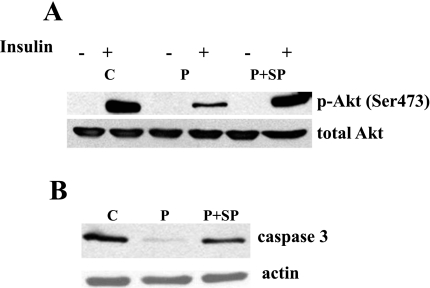
Palmitate-induced activation of JNK contributes to both palmitate-induced inhibition of insulin signaling and palmitate-induced apoptosis.
To determine whether palmitate-induced JNK activation (Fig. 5) is involved in insulin signaling and apoptosis, we used a potent and selective inhibitor of JNK, SP-600125 (3). First, L6 myotubes were treated with 1 mM palmitate and different concentrations (10 and 25 μM) of SP-600125 for 24 h. We found that 25 μM of SP-600125 reversed the palmitate-induced phosporylation of JNK (data not shown). Next, we determined that coincubation of L6 myotubes with 25 μM of SP-600125 improved insulin-stimulated Akt (Ser473) phosphorylation (Fig. 7A) and prevented caspase-3 activation (Fig. 7B) in L6 myotubes exposed to 1 mM palmitate.
Fig. 7.
Effect of a JNK inhibitor on palmitate-induced inhibition of insulin-stimulated Akt (Ser473) phosphorylation and apoptosis in L6 myotubes. To evaluate insulin signaling, L6 myotubes were incubated with 1 mM palmitate in the presence or absence of 25 μM JNK inhibitor SP-600125 (SP) for 16 h prior to serum starvation and stimulation with insulin. To assess apoptosis, L6 myotubes were incubated with 1 mM palmitate with or without 25 μM JNK inhibitor SP for 24 h. Representative blots are shown. Western blots analyses were performed using p-Akt and total Akt antibodies (A) and caspase-3 and actin antibodies (B).
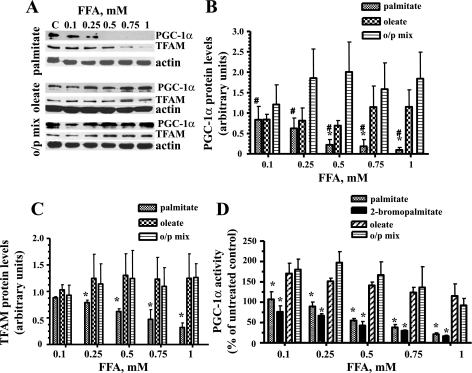
Palmitate decreased the expression of both TFAM and PGC-1α transcription factors and downregulated promoter activity of PGC-1α in L6 myotubes.
To evaluate the effect of different FFAs on the expression of PGC-1α and TFAM, L6 myotubes were treated with palmitate, oleate, or the o/p mixture (0.1–1 mM) for 24 h. Palmitate decreased both the PGC-1α and TFAM total protein levels in L6 myotubes (Fig. 8, A–C). In contrast, oleate increased the concentrations of both of these proteins. Interestingly, treatment of skeletal muscle cells with the o/p mixture increased PGC-1α protein levels more profoundly than treatment with oleate alone (Fig. 8B). Since PGC-1α operates in an autoregulatory loop (18), we next evaluated the promoter activity of PGC-1α after treatment with the different FFAs. We performed transient transfection of L6 myotubes with a mixture of PGC-1α promoter and pEGFP-N1 plasmids. Cells were treated with different concentrations of palmitate, oleate, or the o/p mixture, and PGC-1α promoter activity was determined as described above. Higher concentrations of palmitate (0.5–1 mM) significantly decreased PGC-1α promoter activity, whereas treatment with oleate and the o/p mixture increased its activity (Fig. 8D). Palmitoylation could be one possible mechanism mediating the effect of palmitate on the reduction of PGC-1α promoter activity. To test whether protein palmitoylation was implicated in the palmitate-induced inhibition of the promoter activity of PGC-1α, we used 2-bromopalmitate, an inhibitor of FFA β-oxidation in mitochondria (5), which also blocks palmitate incorporation into proteins (52). 2-Bromopalmitate reproduced the inhibitory effect of palmitate on PGC-1α promoter activity (Fig. 8D) and decreased the PGC-1α protein level at concentrations ≥0.5 mM (data not shown). Thus, palmitoylation of proteins was not involved in the decline of the of PGC-1α promoter activity after palmitate treatment.
Fig. 8.
Effects of palmitate, oleate, or o/p mix on the expression of mitochondrial transcription factor (TFAM) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and on the promoter activity of PGC-1α in skeletal muscle cells. L6 myotubes were exposed to the indicated concentrations of palmitate, oleate, or o/p mix for 24 h. A: total cell lysates were isolated and analyzed by Western blot with the indicated antibodies. Representative blots are shown. B: the values from densitometry from at least 3 independent Western blot experiments for PGC-1α were normalized to the level of actin and presented as fold of difference compared with the corresponding untreated controls ± SE (n ≥ 3). #P < 0.01 vs. o/p mix; *P < 0.05 vs. oleate. C: densitometry results for TFAM protein normalized for actin level and presented as fold of difference compared with the corresponding untreated controls ± SE (n ≥ 3); *P < 0.05 vs. palmitate. D: L6 myotubes were transiently transfected with a mixture of PGC-1α promoter and pEGFP-N1 plasmids. Cells were treated with the indicated concentrations of palmitate, 2-bromopalmitate, oleate, or o/p mix, and the PGC-1α promoter activity was determined as described in materials and methods. Luciferase expression was normalized to green fluorescent protein (GFP) fluorescence intensity. Data are indicated as a percentage of the untreated controls. Means ± SE are shown (n ≥ 3). *P < 0.01 vs. both oleate and o/p mix.
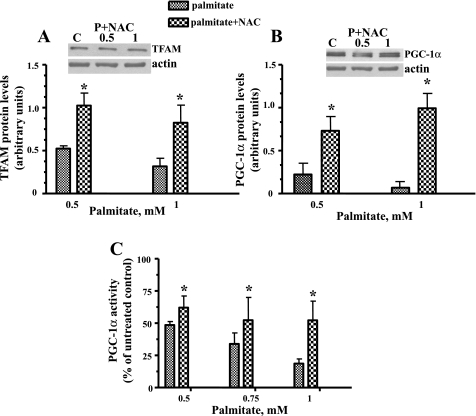
An ROS scavenger blocked the palmitate-induced decline in both TFAM and PGC-1α protein levels and increased palmitate-induced inhibition of PGC-1α promoter activity.
To determine whether ROS generation is involved in the palmitate-induced decrease in both TFAM and PGC-1α protein levels, the effect of an antioxidant, NAC (a precursor compound for glutathione formation), on the expression of these proteins was evaluated. Western blotting revealed that, when compared with untreated L6 myotubes, expression of both TFAM and PGC-1α was significantly increased in NAC-preincubated cells (Fig. 9, A and B). Also, NAC partially restored the palmitate-induced decline in PGC-1α promoter activity (Fig. 9C), suggesting that oxidative stress is involved in the downregulation of both PGC-1α promoter activity and protein production.
Fig. 9.
ROS scavenger reversed palmitate-induced downregulation of both TFAM and PGC-1α protein level and PGC-1α promoter activity. L6 myotubes were pretreated in the presence or absence of 5 mM NAC for 30 min and then exposed to the indicated concentrations of palmitate. NAC significantly increased the palmitate-induced decline in TFAM (A) and PGC-1α expression (B). C: L6 myotubes were transiently transfected with a mixture of PGC-1α promoter and pEGFP-N1 plasmids. Cells were preincubated in the presence or absence of 5 mM NAC for 30 min and then exposed to the indicated concentrations of palmitate, and PGC-1α promoter activity was determined. Luciferase expression was normalized to GFP fluorescence intensity. Data are indicated as a percentage of untreated controls. Means ± SE are shown (n ≥ 3). *P < 0.01 vs. palmitate.
DISCUSSION
The consumption of a Western diet that is high in saturated fats significantly worsens IR (30, 36), whereas diets rich in mono- and polyunsaturated FFAs have a less pronounced effect or even improve insulin sensitivity (35, 41). Therefore, supplementing diets with unsaturated fat may have a favorable effect on the prevention of IR and development of type 2 diabetes. Although several different mechanisms for the beneficial effect of oleate on insulin signaling have been proposed (9, 37, 43), the exact mechanisms remain to be elucidated. The present study was designed to further clarify the molecular basis for the different effects of saturated and unsaturated FFAs on the development of IR in skeletal muscle cells. We have shown previously that palmitate induced oxidative stress, mitochondrial dysfunction, and apoptosis in L6 myotubes (39). We wanted to extend that study to explore the unsaturated FFA oleate, which has been shown to improve insulin sensitivity in L6 skeletal muscle cells (13, 17). Although we have used the saturated FFA palmitate previously (39), we chose to use it in this study as an example of a common saturated FFA to compare its effect to that of oleate, the most common unsaturated FFA. In addition to using palmitate and oleate alone, we have used a mixture of both FFAs, since palmitate in vivo is always present in a mixture with unsaturated FFAs, mostly oleate. It is worth noting that it is difficult to say what the ratio of oleate to palmitate in the plasma is, since this ratio can change substantially with diet and disease. A number of novel results were obtained. First, we identified that the unsaturated FFA oleate, as opposed to the saturated FFA palmitate, did not induce 1) NO and mtROS production and 2) mtDNA damage and mitochondrial dysfunction in rat L6 skeletal muscle cells. Moreover, addition of oleate prevented palmitate-induced apoptosis and the inhibition of insulin-stimulated Akt (Ser473) phosphorylation. Second, we found that de novo synthesis of ceramide was involved in the effects of palmitate on mtROS production, viability, and insulin signaling. Third, we found that palmitate-induced activation of JNK contributed to both palmitate-induced inhibition of insulin signaling and palmitate-induced apoptosis. Additionally, we showed that palmitate decreased and oleate increased the expression of two major mitochondrial transcription factors, PGC-1α and TFAM, that regulate mitochondrial biogenesis. Also, palmitate radically decreased and oleate increased the promoter activity of PGC-1α. Moreover, we identified that palmitate-induced downregulation of those transcription factors, as well as the promoter activity of PGC-1α, is mediated by oxidative stress, since the ROS scavenger NAC significantly restored expression of both TFAM and PGC-1α and the promoter activity of PGC-1α.
Previously, we and others have shown that palmitate induced the generation of ROS in skeletal muscle cells (21, 28, 39). In addition, it has been shown that ROS production occurred through activation of the NAPDH oxidase system (28) and also by the alteration of mitochondrial electron transport chains (21, 28). In this study, we found that oleate, as opposed to palmitate, did not stimulate production of NO (data not shown) and did not induce mtROS or mtDNA damage in L6 myotubes. Moreover, we found that palmitate induced mtROS generation through the de novo synthesis of ceramide. In addition, we demonstrated that ceramide treatment led to the increase of mtROS generation. Previously, it has been reported that ceramide accumulated with palmitate treatment and caused apoptosis in L6 myotubes (49). Also, ceramide has been shown to inhibit insulin-induced Akt phosphorylation in L6 myotubes (24). Ceramide has been directly connected with mitochondrial oxidative stress; ceramide triggers mitochondrial oxidative stress, and oxidative stress in turn promotes further ceramide synthesis (2). Our results showed mtROS generation after palmitate but not oleate treatment, which is in agreement with this hypothesis and thus supports the findings of the previous study (37, 43), which demonstrated that oleate completely prevented palmitate-induced IR in skeletal muscle cells by blocking palmitate-induced ceramide synthesis (37, 43). The increase in mtROS generation in our study was similar to the results obtained by another group (21), which used similar concentrations of palmitate for the treatment of L6 myotubes.
Despite the fact that we found significant NO production and mtDNA damage after 6 h of incubation with palmitate, we were only able to detect an increase in mtROS after 24-h incubation with palmitate. We believe it is likely that palmitate-induced NO production, which occurred at an earlier point after palmitate treatment, could be a major cause for the palmitate-induced mtDNA damage, which in turn could further heighten production of mitochondrial-derived ROS, which were detected at the later time point. Another possible explanation for this could be the greater sensitivity of the methods used for evaluating mtDNA damage and NO production. In our previous study, we found that palmitate induced significant mtDNA damage that correlated with a decline in ATP levels, loss of viability, and increased apoptosis in L6 myotubes (39). In the current study, we also found that palmitate, as opposed to oleate, caused mtDNA damage and mitochondrial dysfunction at even lower doses. At the same time, addition of oleate to palmitate led to increased mtDNA integrity, improved ATP levels, and increased mitochondrial function. Also, addition of oleate to palmitate prevented palmitate-induced apoptosis and inhibition of insulin-stimulated Akt (Ser473) phosphorylation. Furthermore, we showed that ROS scavenger NAC ameliorated palmitate-induced reduction of Akt (Ser473) phosphorylation, suggesting that oxidative stress is involved in the palmitate-induced inhibition of Akt phosphorylation in L6 myotubes. Given that palmitate induced ROS generation in L6 myotubes (21, 28, 39), and because JNK kinase is activated in response to a variety of stress inducers, including ROS (for review, see Ref. 14), we evaluated a link between palmitate treatment and JNK activation. We found that palmitate increased the phosphorylation/activation status of JNK in L6 myotubes. Oleate alone did not activate JNK, and addition of oleate decreased palmitate-induced activation of JNK kinase. Activation of JNK has been implicated in the modulation of apoptosis (48) and IR (44). Also, a recent study showed that palmitate-induced, mitochondria-derived ROS may be major contributors to JNK activation and IR in hepatocytes (32). Similarly to this study (32), we also found that palmitate-induced JNK activation is an important contributor to palmitate-induced apoptosis and IR resistance in L6 skeletal muscle cells.
One of the potential mechanisms for the protective effect of oleate against palmitate-induced mitochondrial dysfunction and inhibition of insulin signaling may involve upregulation of the mitochondrial biogenesis factors PGC-1α and TFAM. Previously, Crunkhorn et al. (12) reported that palmitate decreased PGC-1α mRNA levels, whereas addition of oleate reversed palmitate-induced reduction in PGC-1α mRNA levels in mouse C2C12 myotubes. Our results showed that the decline in the PGC-1α protein in L6 myotubes after palmitate treatment (0.75 mM; Fig. 6B) was about twofold greater than in the study by Coll et al. (8), which was done using C2C12 myotubes treated with the same concentration of palmitate. Also, we found that palmitate, but not oleate, downregulated the expression of the mitochondrial transcription factor TFAM. Moreover, addition of a ROS scavenger to palmitate restored both expression and promoter activity of PGC-1α as well as TFAM expression, confirming that palmitate-induced oxidative stress contributed to the downregulation of these transcription factors. It has been found that altered expression of TFAM and PGC-1α modulated mitochondrial biogenesis (33), and overexpression of PGC-1α protected cells from mitochondrial dysfunction (27) and oxidative stress (50). Also, it has been shown that TFAM plays a crucial role in maintaining mtDNA as a main component of the nucleoid (25). Hence, we suggest that ROS-induced downregulation of TFAM and PGC-1α expression may contribute further to palmitate-induced mitochondrial dysfunction and oxidative stress by decreasing mitochondrial biogenesis and thus establishing a vicious cycle of events in which palmitate-induced oxidative stress causes mitochondrial dysfunction, which causes a concomitant increase in ROS production. Increased ROS production leads to a decline in both TFAM and PGC-1α and an even more profound mitochondrial dysfunction. We postulate that oleate rescued palmitate-induced mitochondrial dysfunction, initiation of apoptosis, and inhibition of insulin signaling by activation of PGC-1α and TFAM expression, which caused subsequent activation of mitochondrial biogenesis and an increase in mitochondrial function. Additional investigation will be required to clarify this point and the mechanisms involved. Our results suggesting the possibility that oleate induces mitochondrial biogenesis are supported by recent observations that oleate-increased mitochondrial β-oxidation is one of the mechanisms contributing to the prevention of palmitate-induced IR in skeletal muscle cells (9).
Taken altogether, our findings, which established a link between palmitate, but not oleate, induced mtROS production, consequent mitochondrial dysfunction, and IR, have further extended those of previous studies, which explored the protective role of oleate against palmitate-induced IR (9, 17, 37, 43). On the basis of these studies (9, 17, 37, 43), and considering very recent work that showed that mitochondrial superoxide production is a common feature of many different models of IR, including saturated fat-induced IR in skeletal muscle cells (21), we propose that palmitate-induced mtROS production is an upstream mechanism in the induction of mitochondrial dysfunction and consequent apoptosis and the inhibition of insulin signaling in skeletal muscle cells. Oleate supplementation has the ability to protect mitochondria and its genome from palmitate-induced oxidative stress and, therefore, may enhance mitochondrial function, protect against apoptosis, and increase insulin sensitivity. In this regard, the present study enhances the understanding of the mechanisms involved in IR. Identification of the pathways that regulate mitochondrial function and mtROS production can provide new potential therapeutic strategies for prevention of IR and other factors contributing to the development of type 2 diabetes.
GRANTS
This research was supported by National Institutes of Health Grants DK-073808 (to L. Rachek) and ES-03456 (to G. Wilson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to Viktoriya Solodushko for providing technical expertise on Western blot analysis of phosphoproteins and to Dr. Valentina Grishko for helpful suggestions and discussion.
REFERENCES
- 1.Amat R, Planavila A, Chen SL, Iglesias R, Giralt M, Villarroya F. SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD. J Biol Chem 284: 21872–21880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med 31: 717–728, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681–13686, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, Coleman E, Smith C. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest 88: 960–966, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chase JF, Tubbs PK. Specific inhibition of mitochondrial fatty acid oxidation by 2-bromopalmitate and its coenzyme A and carnitine esters. Biochem J 129: 55–65, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys 419: 101–109, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chow L, From A, Seaquist E. Skeletal muscle insulin resistance: the interplay of local lipid excess and mitochondrial dysfunction. Metabolism 59: 70–85, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 283: 11107–11116, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Coll T, Jové M, Rodríguez-Calvo R, Eyre E, Palomer X, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Palmitate-mediated downregulation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha in skeletal muscle cells involves MEK1/2 and nuclear factor-kappaB activation. Diabetes 55: 2779–2787, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun 3: 207–212, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods 160: 81–88, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Crunkhorn S, Dearie F, Mantzoros C, Gami H, daSilva WS, Espinoza D, Faucette R, Barry K, Bianco AC, Patti ME. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem 282: 15439–15450, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J 399: 473–481, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23: 599–622, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Fleischman A, Kron M, Systrom DM, Hrovat M, Grinspoon SK. Mitochondrial function and insulin resistance in overweight and normal-weight children. J Clin Endocrinol Metab 94: 4923–4930, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fridlyand LE, Philipson LH. Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obes Metab 8: 136–145, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gao D, Griffiths HR, Bailey CJ. Oleate protects against palmitate-induced insulin resistance in L6 myotubes. Br J Nutr 102: 1557–1563, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci USA 100: 7111–7116, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernández-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, Finucane F, Liesa M, Chiellini C, Naon D, Zorzano A, Nolan JJ. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1{alpha}/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care 33: 645–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirabara SM, Curi R, Maechler P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J Cell Physiol 222: 187–194, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA 106: 17787–17792, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunnicutt JW, Hardy RW, Williford J, McDonald JM. Saturated fatty acid-induced insulin resistance in rat adipocytes. Diabetes 43: 540–545, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Itani SI, Zhou Q, Pories WJ, MacDonald KG, Dohm GL. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes 49: 1353–1358, 2000 [DOI] [PubMed] [Google Scholar]
- 24.JeBailey L, Wanono O, Niu W, Roessler J, Rudich A, Klip A. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes 56: 394–403, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion 7: 39–44, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280: 33588–33598, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lambertucci RH, Hirabara SM, SilveiraLdos R, Levada-Pires AC, Curi R, Pithon-Curi TC. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J Cell Physiol 216: 796–804, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Maron DJ, Fair JM, Haskell WL. Saturated fat intake and insulin resistance in men with coronary artery disease. The Stanford Coronary Risk Intervention Project Investigators and Staff. Circulation 84: 2020–2027, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, vandeWeijer T, Sels JP, Schrauwen P, Hesselink MK. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 59: 572–579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto K, Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem 284: 14809–14818, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314–317, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Pagel-Langenickel I, Bao J, Pang L, Sack MN. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev 31: 25–51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parillo M, Rivellese AA, Ciardullo AV, Capaldo B, Giacco A, Genovese S, Riccardi G. A high-monounsaturated-fat/low-carbohydrate diet improves peripheral insulin sensitivity in non-insulin-dependent diabetic patients. Metabolism 41: 1373–1378, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Parker DR, Weiss ST, Troisi R, Cassano PA, Vokonas PS, Landsberg L. Relationship of dietary saturated fatty acids and body habitus to serum insulin concentrations: the Normative Aging Study. Am J Clin Nutr 58: 129–136, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Pickersgill L, Litherland GJ, Greenberg AS, Walker M, Yeaman SJ. Key role for ceramides in mediating insulin resistance in human muscle cells. J Biol Chem 282: 12583–12589, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J 382: 619–629, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rachek LI, Musiyenko SI, LeDoux SP, Wilson GL. Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in l6 rat skeletal muscle cells. Endocrinology 148: 293–299, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Rachek LI, Thornley NP, Grishko VI, LeDoux SP, Wilson GL. Protection of INS-1 cells from free fatty acid-induced apoptosis by targeting hOGG1 to mitochondria. Diabetes 55: 1022–1028, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr 23: 447–456, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Ryan M, McInerney D, Owens D, Collins P, Johnson A, Tomkin GH. Diabetes and the Mediterranean diet: a beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM 93: 85–91, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Sabin MA, Stewart CE, Crowne EC, Turner SJ, Hunt LP, Welsh GI, Grohmann MJ, Holly JM, Shield JP. Fatty acid-induced defects in insulin signalling, in myotubes derived from children, are related to ceramide production from palmitate rather than the accumulation of intramyocellular lipid. J Cell Physiol 211: 244–252, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 53: 1412–1417, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Schrauwen P, Schrauwen-Hinderling V, Hoeks J, Hesselink MK. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta 1801: 266–271, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Spector AA. Fatty acid binding to plasma albumin. J Lipid Res 16: 165–179, 1975 [PubMed] [Google Scholar]
- 48.Tan J, Kuang W, Jin Z, Jin F, Xu L, Yu Q, Kong L, Zeng G, Yuan X, Duan Y. Inhibition of NFkappaB by activated c-Jun NH2 terminal kinase 1 acts as a switch for C2C12 cell death under excessive stretch. Apoptosis 14: 764–770, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Turpin SM, Lancaster GI, Darby I, Febbraio MA, Watt MJ. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab 291: E1341–E1350, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res 66: 562–573, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Vessby B, Unsitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nalsen C, Berglund L, Louheranta A, Rasmussen BM, Calvert GD, Maffetone A, Pedersen E, Gustafsson IB, Storlien LH. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia 44: 312–319, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem 275: 261–270, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Weigert C, Brodbeck K, Staiger H, Kausch C, Machicao F, Haring HU, Schleicher ED. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J Biol Chem 279: 23942–23952, 2004 [DOI] [PubMed] [Google Scholar]