Abstract
Glucose-stimulated insulin secretion (GSIS) by β-cells requires the generation of ATP from oxidation of pyruvate as well as generation of coupling factors involving three different pyruvate cycling shuttles. The roles of several key enzymes involved in pyruvate cycling in β-cells have been documented using isolated islets and β-cell clonal lines. To investigate the role of the pyruvate dehydrogenase (PDH) complex (PDC) in GSIS, a murine model of β-cell-specific PDH deficiency (β-PDHKO) was created. Pancreatic insulin content was decreased in 1-day-old β-PDHKO male pups and adult male mice. The plasma insulin levels were decreased and blood glucose levels increased in β-PDHKO male mice from neonatal life onward. GSIS was reduced in isolated islets from β-PDHKO male mice with about 50% reduction in PDC activity. Impairment in a glucose tolerance test and in vivo insulin secretion during hyperglycemic clamp was evident in β-PDHKO adults. No change in the number or size of islets was found in pancreata from 4-wk-old β-PDHKO male mice. However, an increase in the mean size of individual β-cells in islets of these mice was observed. These findings show a key role of PDC in GSIS by pyruvate oxidation. This β-PDHKO mouse model represents the first mouse model in which a mitochondrial oxidative enzyme deletion by gene knockout has been employed to demonstrate an altered GSIS by β-cells.
Keywords: pyruvate cycling, β-cell hypertrophy
insulin secretion by pancreatic islet β-cells is influenced by a variety of effectors, with glucose being the primary and most important stimulus. Insulin secretion requires metabolism of glucose in the β-cell with an associated increase in total intracellular ATP and an increase in the cytosolic ATP/ADP ratio (7, 18, 36). Increases in the cytosolic ATP concentration result in the sequential closure of KATP channels, depolarization of the plasma membrane, opening of voltage-sensitive L-type Ca2+ channels, Ca2+ influx, and insulin secretion (15, 34). The uptake of glucose and its phosphorylation to glucose 6-phosphate are catalyzed by high Km glucose transporter 2 and the rate-limiting high Km glucokinase, respectively. These “glucosensors” ensure an increase in glucose phosphorylation, since blood glucose concentrations rise after a carbohydrate-rich meal. Pyruvate derived from glucose is channeled predominantly into its mitochondrial metabolism because of low levels of lactate dehydrogenase activity and of the monocarboxylate transporter-1 for lactate in the plasma membrane of islets (23, 24, 26, 34). Another link between the cytosolic and mitochondrial metabolism of glucose is the transfer of NADH via the glycerol 3-phosphate shuttle and the malate-aspartate shuttle for ATP production (8, 22).
Evidence shows that metabolites derived from mitochondrial metabolism of pyruvate generated primarily from glucose and to a limited extent from other nutrients (such as amino acids) play a critical role in metabolism-secretion coupling of insulin secretion by β-cells (24, 26). Pyruvate is converted to acetyl-CoA and oxaloacetate by the action of the pyruvate dehydrogenase complex (PDC) and pyruvate carboxylase (PC), respectively. Approximately 50% of glucose-derived pyruvate is converted to acetyl-CoA by PDC, and the remainder of the pyruvate flux is mediated by PC for anaplerosis to generate the tricarboxylic acid (TCA) cycle intermediates for “pyruvate cycling” in β-cells (24, 26). Hence, PDC plays a decisive role in the generation of ATP and several coupling factors generated in the mitochondria (e.g., GTP, glutamate, etc.) and in the cytosol (e.g., malonyl-CoA, NADPH, etc.) using the mitochondria-derived compounds for enhancing insulin secretion by β-cells. The roles of PC (12, 42) as well as several key enzymes such as malic enzyme (10, 29), ATP-citrate lyase (10), acetyl-CoA carboxylase (33), and cytosolic-NADP-isocitrate dehydrogenase (31) in glucose-stimulated insulin secretion (GSIS) have been investigated extensively using isolated islets and cultured β-cell clonal lines. However, several of these observations have been contradicted for pyruvate carboxylase (14), cytosolic malic enzyme (1, 32), and ATP-citrate lyase (25). It should be emphasized that these investigations were performed using clonal β-cells or cultured islets treated by transient siRNA transfection or stable shRNA plasmids. Surprisingly, there are no investigations on PC or other key enzymes involved in pyruvate cycling in β-cells in vivo using gene knockout technology. Although ∼50% of the pyruvate derived from glucose is decarboxylated to acetyl-CoA by the PDC reaction, the role of PDC in GSIS has not been investigated extensively.
Mammalian PDC is composed of multiple copies of three catalytic components, one binding protein, and dedicated kinases and phosphatases (28). PDC activity is intricately regulated by phosphorylation (inactivation)/dephosphorylation (activation) of pyruvate dehydrogenase (PDH) (α2β2 heterotetramer) component by dedicated families of PDH kinases (PDK1-4) and PDH phosphatases (PDP1–2) (11, 28, 39). In mammals, PDHα is encoded by two genes, an X-linked allele (Pdha1 in mouse) that is expressed in somatic cells and an autosomal, intronless paralogue (Pdha2 in mouse) that is expressed only in postmeiotic spermatogenic cells (2, 6).
Using several experimental approaches, such as expression of PDC regulatory enzymes (PDK and PDP) (27), the use of dichloroacetate as PDK inhibitor (27), reduction in PDC activity using a PDH-specific siRNA approach (42), and measurements of pyruvate-carbon flux via PDC using isotopomer analysis (3, 20), it has been reported that PDC played no role or only a minor regulatory role in GSIS by cultured clonal β-cells and isolated islets. These analyses relied upon either indirect measurements or in vitro activity measurements. The findings of these studies remain controversial. No studies are reported using stably modified cell preparations or in vivo alterations in PDC activity. The approach employed in one study (27) was overexpression of a specific PDH kinase or phosphatase to regulate phosphorylation or dephosphorylation status of PDC and not to suppress or delete expression of one of the genes involved in the catalytic components of PDC. We have developed a murine model that carries a mutation in the X-linked Pdha1 gene encoding the subunit of the PDH component of PDC (16). Availability of a murine model with targeted Pdha1 mutation has allowed us to investigate the consequences of an isolated β-cell-specific PDC deficiency on insulin secretion in male mice. The findings presented here show that chronic PDC deficiency in β-cells impairs GSIS both in vivo and in vitro.
MATERIALS AND METHODS
Generation of pancreatic β-cell-specific PDH-deficient male mice.
All animal experiments were conducted in accordance with the Guide for the Use and Care of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo. A strategy using the Cre-loxP system was employed to introduce a silent mutation in the Pdha1 gene (2 loxP sites introduced into intronic sequences flanking exon 8) that could then be induced in vivo to produce a null mutation (16). To generate a β-cell-specific deletion of the Pdha1 gene, females (genotype Pdha1flox8/Pdha1flox8) were bred with transgenic male mice harboring a Cre recombinase transgene driven by the rat insulin gene II promoter (The Jackson Laboratory, Bar Harbor, ME). Previous studies have shown that Cre recombinase is expressed strongly in pancreatic β-cells and weakly in certain brain regions (certain neurons of the hypothalamus) of these transgenic Cre mice, and its expression was readily detected in these cells at 11.5 days postcoitus (5, 9).
The progeny were tested for the presence or absence of the Cre transgene by polymerase chain reaction (PCR) analysis using tail DNA samples on postnatal day 15, except for 1-day-old neonates (16, 37). Genotyping of the progeny identified the presence of the Cre transgene in both sexes. To generate control male mice (β-PDHCT), females (genotype: Pdha1flox8/Pdha1flox8) were bred with wild-type males (genotype: Pdha1wt/Y). Newborns were reared by their dams and weaned on postnatal day 21 onto a rodent laboratory chow (%calorie distribution: 70 carbohydrate, 10.9 fat, and 19.1 protein) and water ad libitum and remained on this regimen until they were euthanized. The tissue-specific genotype was determined by PCR analysis (37) using tissue DNA samples. Genomic DNA was isolated with Omni Prep kit (Bio-WORLD, Dublin, OH) from isolated islets, liver, and skeletal muscle. The presence of Pdha1 alleles (Pdha1wt, Pdha1flox8, and Pdha1Δex8) and Cre transgene was determined by PCR analysis (37) using the specific sets of primers (Pdha1 sense primer 5′-AGCAGCCAGCA CGGACTACT-3′, antisense primer 5′-GCAGCCAAACAGATTACACC-3′, and Cre primers sense 5′-GCATTTCTGGGGATTGCTTA-3′ and antisense 5′-CCCGGCAA AACAGGTAGTTA-3′), as described previously (37).
Insulin measurements.
Trunk blood was collected and centrifuged and the serum stored at −20°C until being assayed for insulin using a commercially available radioimmunoassay kit (Millipore, Billerica, MA). To measure pancreatic insulin content, weighed pieces of pancreata were homogenized by sonication in acid-ethanol solution (38) and centrifuged, and the supernatants stored at −20°C until they were assayed for insulin. For 1-day-old mice, the whole pancreas from each male mouse (sex based on genotype) was treated as indicated above, and the insulin content was expressed as the quantity of insulin per pancreas.
Isolation of islets and GSIS studies.
Islets were isolated by collagenase digestion (41). Briefly, the carefully trimmed pancreas was minced and digested with collagenase (Crescent Chemical, Islandia, NY), and the islets were picked manually under a stereomicroscope. Five islets per tube were preincubated for 30 min in 250 μl of Krebs-Ringer bicarbonate (KRB) buffer containing 16 mM HEPES, 5.5 mM glucose, and 0.01% bovine serum albumin, pH 7.4, at 37°C under an atmosphere of 95% O2-5% CO2 in a shaking water bath. The islets were resuspended in fresh KRB buffer containing 5.5 mM glucose with or without added 11.2 mM glucose (16.7 mM final), 10 mM α-ketoisocaproate, or 25 mM KCl, and an aliquot was withdrawn at 60 min for determination of secreted insulin (38).
PDC activity determination.
Isolated islets were homogenized in 50 mM MOPS buffer, pH 7.4, 80 mM KCl, 2 mM MgCl2, 0.5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, and 0.1 μg/ml leupeptin, freeze-thawed in liquid nitrogen three times, and centrifuged at 600 g for 10 min. To measure total PDC activity, the supernatant was dephosphorylated (to completely activate PDC) with purified recombinant rat PDH phosphatase 1 (37). Total PDC activity was determined by production of 14CO2 from [1-14C]pyruvic acid (GE Healthcare, Piscataway, NJ) (19).
β-Cell morphometric analysis.
Morphometric analysis of pancreata from 33- and 108-day-old β-PDHCT and β-cell-specific PDH-deficient (β-PDHKO) male mice was performed using a Carl Zeiss transmitted light microscope at a magnification of ×250 or ×400. Pancreata were fixed in 10% formalin and embedded in paraffin using a standardized protocol, and sections of 5 μm were cut and mounted on Superfrost-plus slides (Fisher Scientific, Toronto, ON, Canada). Immunohistochemistry was performed to localize insulin using a modified avidin-biotin peroxidase method, as described previously (40). Tissue sections were counterstained with Carrazi's hematoxylin and mounted under glass coverslips with Eukit (Ruth Wagener Enterprises, Newmarket, ON, Canada). Automatic image analysis of the pancreatic sections for calculation of tissue areas was performed with Northern Eclipse, version 6.0, morphometric analysis software (Empix Imaging, Mississauga, ON, Canada). The number and average area of small (<5,000 μm2), medium, and large (>10,000 μm2) islets was calculated for each group from five sections per pancreas taken at 50–60 section intervals to represent the head region of the pancreas. The minimum size for an islet was taken to be a cluster of three or more insulin-positive cells. The mean area of individual β-cells was also measured from insulin-immunoreactive cells. Approximately 100 cells were selected from at least five islets within each pancreas.
Glucose tolerance test.
Intraperitoneal glucose tolerance test was performed on nonanesthetized, overnight-fasted mice receiving a glucose dose of 1.5 mg/g body wt (37). Tail blood glucose levels were measured using a glucometer (Ascencia Elite; Bayer, Mishiwaka, IN) before (0 min) and 15, 30, 45, 60, 90, and 120 min after an intraperitoneal injection of glucose.
In vivo insulin secretion using hyperglycemic clamp.
These experiments were conducted following the standardized procedures established at the Yale Mouse Metabolic Phenotyping Center and approved by the Institutional Animal Care and Use Committee of Yale University (43). The procedure involved chronic cannulation of the right jugular vein to establish a chronic catheter ∼7 days earlier for intravenous infusion of glucose during the clamp. On the day of the clamp experiment, an overnight-fasted mouse was placed in a restrainer for the experiment to be conducted in an awake and minimally stressed state. The tail was tethered using tape for 2 h prior to the start of experiment for acclimatization. The blood samples were obtained from the tail vessels requiring a small tail cut. To assess in vivo insulin secretion, a 90-min hyperglycemic clamp was conducted with a primed and variable infusion of 20% glucose to raise and maintain plasma glucose concentrations at ∼25 mM. Blood samples (20 μl) were collected at 5- to 10-min intervals during the acute phase and a 15-min interval during the late phase for the measurement of plasma glucose and insulin concentrations. Plasma glucose concentration was measured immediately using a Beckman Glucose Analyzer II (Beckman, Fullerton, CA), and plasma insulin levels were assessed by radioimmunoassay using a kit from Linco Research (St. Charles, MO). The area under the curve of plasma insulin profiles was assessed to determine glucose-induced insulin secretion in vivo (i.e., pancreatic β-cell function). Acute and late insulin responses were defined as area under curve of 0- to 30- and 30- to 90-min insulin profiles, respectively.
Statistics.
The results are expressed as means ± SE of the number of independent observations as indicated. The significance of the difference between the two groups was determined by Student's t-test, and a P value of <0.05 was considered significant.
RESULTS
Generation of β-PDHKO male mice.
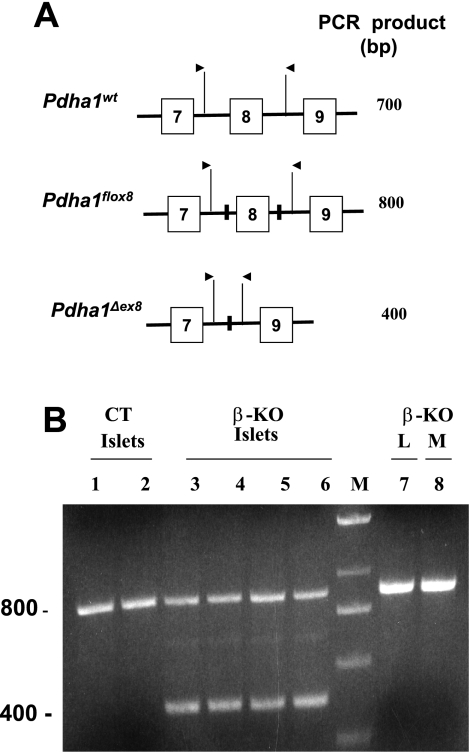
β-Cell-specific PDC deficiency in mice was induced by breeding homozygous female mice (genotype: Pdha1flox8/Pdha1flox8) carrying the targetable mutation with transgenic homozygous male mice harboring a Cre recombinase transgene driven by the rat insulin gene II promoter (genotype: Pdha1wt/Y; Crebetacell). No embryonic lethality was observed for male and female progeny of this mating because the average litter size was comparable with the litter size of mating of floxed females (genotype: Pdha1flox8/Pdha1flox8) with wild-type males (genotype: Pdha1wt/Y). PCR analysis of DNA prepared from islets isolated from β-PDHKO male mice (age 12 wk) showed the presence of the deleted Pdha1 gene (Pdha1Δex8, 400 bp) and the Pdha1flox8 (800-bp fragment) (Fig. 1, A and B, lanes 3–6) and the Cre transgene (168-bp fragment; results not shown) in the β-PDHKO islets. In addition to β-cells, islets also contained α-, γ-, and δ-cells that did not express the Cre gene in β-PDHKO mice. However, the functional Pdha1flox8 gene was present in the latter cell types, resulting in the detection of the 800-bp fragment in DNA isolated from islets from β-PDHKO male mice (Fig. 1B, lanes 3–6). On the other hand, only the Pdha1flox8 (800-bp fragment) was observed in islets from control (β-PDHCT) male mice (Fig. 1B, lanes 1 and 2). DNA prepared from liver and skeletal muscle samples from β-PDHKO male mice showed only the presence of Pdha1flox8 allele (800 bp), indicating the absence of Cre expression in these tissues (Fig. 1B, lanes 7 and 8).
Fig. 1.
A schematic representation of 3 partial Pdha1 alleles (A) and PCR analysis (B) of tissue DNA preparations of 4-mo-old β-PDHCT control (CT) and β-cell-specific PDH-deficient (β-KO) male progeny. In A, the presence of the inserted loxP sequence is indicated by a solid vertical bar in introns. The locations and direction of sense and antisense primers are depicted by arrows. The expected sizes of PCR products are also listed. The results in B show the presence of Pdha1flox8 allele (800 bp) and Pdha1Δex8 allele (400 bp). As expected, islets isolated from 2 CT mice (lanes 1 and 2) had only the flox allele, whereas islets isolated from 4 different β-KO mice (lanes 3–6) showed the presence of both the Pdha1flox8 and Pdha1Δex8 alleles, indicating deletion in β-cells. Liver and skeletal muscle taken from a β-KO mouse (reported in lane 3) had only the flox allele (lanes 7 and 8). M denotes a DNA ladder marker lane.
To decipher whether a deficiency of PDC activity in β-cells affected body weight gains in β-PDHKO male mice, body weights of mice in the postweaning period were determined. Although the body weights of β-PDHKO male mice were significantly lower on postnatal day 30 compared with age-matched control β-PDHCT male mice (15.0 ± 0.7 vs. 18.7 ± 0.7 g, respectively), this difference in body weight was not detected between the two groups by age 4 mo [β-PDHCT: 30.7 ± 1.3 g (n = 8); β-PDHKO: 30.7 ± 1.1 g (n = 13)].
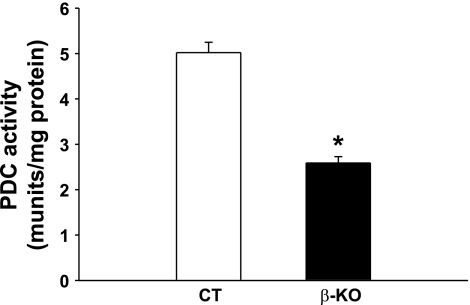
To demonstrate that the targeted deletion in the Pdha1 gene in islets of β-PDHKO male mice resulted in a corresponding reduction in its enzyme activity, PDC activity was determined in islets isolated from 3-mo-old male mice. Total PDC activity was markedly decreased (∼50%) in islets from β-PDHKO male mice compared with the age-matched β-PDHCT male mice (Fig. 2). One of the reasons for remaining residual PDC activity in islets of β-PDHKO male mice is the activity derived from other cell types constituting an islet. Such a possibility is indicated by the presence of the 800-bp fragment (Fig. 1B) corresponding to the wild-type Pdha1 gene allele in islets of the β-PDHKO male mice. It is also plausible that Pdha1 gene deletion did not occur in all of the β-cells.
Fig. 2.
“Total” pyruvate dehydrogenase complex (PDC) activity in islets from β-KO and CT mice at 4 mo of age. Results are means ± SE (n = 4). *P < 0.05.
Development of hyperglycemia in β-PDHKO male mice.
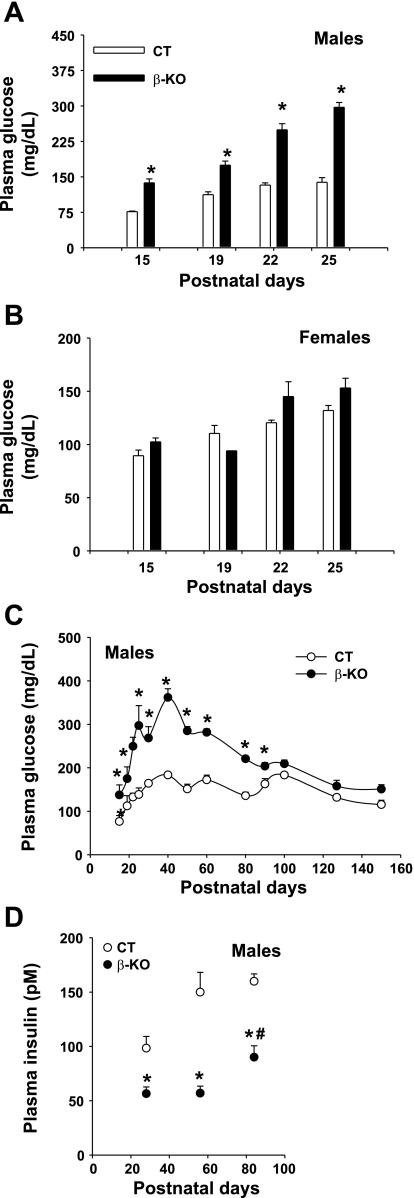
To determine the effect of PDC deficiency in β-cells on plasma glucose levels in the β-PDHKO mice during postnatal life, tail blood glucose levels were determined in randomly fed mice at various ages. During the suckling period (from postnatal days 15–25), blood glucose levels were increased significantly in β-PDHKO male mice (∼1.5- to 2-fold) compared with the levels in age-matched β-PDHCT male mice (Fig. 3A). As expected based on random inactivation of one of the two X alleles in heterozygous progeny females (maternally derived Pdha1flox8 allele and paternally derived Pdha1wt allele), there were no significant differences in blood glucose levels in age-matched β-PDHKO female mice compared with corresponding wild-type female mice during the suckling period (Fig. 3B). Due to heterozygous genotype, β-PDHKO female mice were not investigated further in this study. In the postweaning period up to postnatal day 90, blood glucose levels were significantly higher in β-PDHKO male mice compared with age-matched β-PDHCT male mice; however, this significant difference in blood glucose levels was not maintained in older β-PDHKO male mice (Fig. 3C).
Fig. 3.
A and B: tail blood glucose levels in randomly fed male and female mice, respectively, during the first 25 postnatal days. C: tail blood glucose levels in randomly fed male mice from postnatal day 15 to 150. D: plasma insulin levels in 28-, 56-, and 84 day-old β-KO and CT male mice. Open bars and ○, CT mice; filled bars and ●, β-KO mice. Results are means ± SE (n = 6–9 for blood glucose and 5–6 for plasma insulin levels). *P < 0.05 for comparison between age-matched CT and β-KO mice; #P < 0.05 for comparison of plasma insulin levels between β-KO mice at age 56 and 84 postnatal days.
Altered pancreatic and plasma insulin levels in β-PDHKO male mice.
To determine whether a deficiency of PDC activity in pancreatic islets in β-PDHKO male mice affected levels of insulin in these mice, plasma insulin levels in β-PDHKO male mice and age-matched β-PDHCT males were measured in the postweaning period. Plasma insulin levels were reduced significantly (∼45%) in β-PDHKO male mice on postnatal days 28, 56, and 84 (Fig. 3D). Interestingly, there was a significant increase in plasma insulin levels of 84-day-old β-PDHKO mice compared with that of 56-day-old β-PDHKO mice (Fig. 3D). This may explain in part the observed near-normalization of blood glucose levels in these mice after 90 days of age (Fig. 3C).
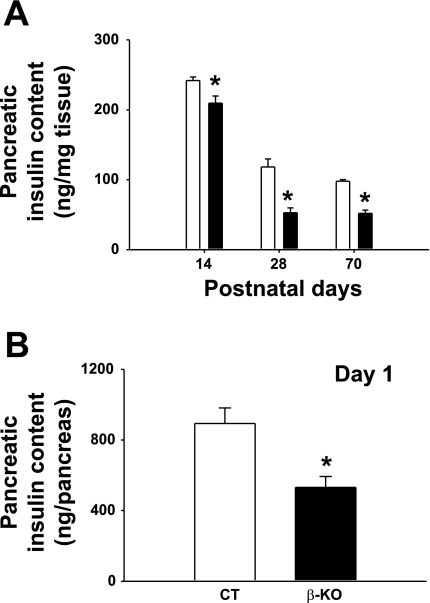
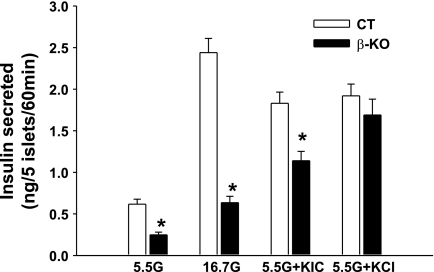
Pancreatic insulin content was also markedly reduced in β-PDHKO male mice on the postnatal days 14, 28, and 70 (Fig. 4A). Of particular interest is the observation that even on postnatal day 1 (within 24 h after birth) pancreatic insulin content in newborn male pups was significantly reduced (Fig. 4B), and this defect persisted up to postnatal age 70 days (the last time point investigated). The observation that the insulin secretion response of islets from 70-day-old β-PDHKO adult male mice to a glucose stimulus (at both 5.5 and 16.7 mM) was markedly reduced at 60 min indicated that β-cell-specific PDH deletion impacted the functional capacity of islets from the β-PDHKO male mice (Fig. 5). The insulin secretion response by islets from β-PDHKO male mice to 25 mM KCl was not significantly different from that of β-PDHCT, indicating a pre-KATP channel defect despite decreased insulin content (Fig. 5). The insulin secretion by islets from β-PDHKO mice to 10 mM α-ketoisocaproate (KIC) plus 5.5 mM glucose was lower compared with that by islets from β-PDHCT mice. Although the catabolism of KIC in the mitochondria generates acetyl-CoA, the lack of acetyl-CoA production from glucose-derived pyruvate by islets from β-PDHKO mice might account for a lesser than normal response (Fig. 5).
Fig. 4.
Effects of pyruvate dehydrogenase (PDH) deletion on pancreatic insulin content in 14-, 28-, and 70-day-old (A) and 1-day-old (B) CT and β-KO male mice. Results are means ± SE [n as indicated: 1 (n = 10), 14 (n = 10), 28 (n = 5), and 70 days (n = 4)]. *P < 0.05.
Fig. 5.
Effects of PDH deletion on insulin secretion. Glucose-stimulated insulin secretion in the presence of 5.5 or 16.7 mM glucose (G), 5.5 mM G + 25 mM KCl, and 5.5 mM G + 10 mM α-ketoisocaproate for 60 min by islets isolated from pancreata of 10-wk-old CT and β-KO male mice. Results are means ± SE (n = 8–10). *P < 0.05.
Islet morphometric analysis.
When islet morphometric analysis was performed, no change was seen in the number of different sizes of islets [average islet area (μm2): 1,604 ± 135 and 1,533 ± 224 for small-, 6,838 ± 268 and 6,966 ± 485 for medium-, and 24,867 ± 4,897 and 31,313 ± 5,352 for large-sized islets for β-PDHCT and β-PDHKO male mice, respectively] in β-PDHKO mice compared with controls at postnatal age 33 days and also at 108 days (results not shown). However, there was a significant increase in the mean size of individual β-cells in islets from 33-day-old β-PDHKO compared with β-PDHCT male mice (average β-cell size: 191 ± 8.4 μm2 for β-PDHCT and 228 ± 10.8 μm2 for β-PDHKO, P < 0.05). In contrast, no difference was seen in the mean size of individual β-cells between β-PDHKO (223 ± 4 μm2) and β-PDHCT (223 ± 17 μm2) animals at 108 days, suggesting a possibility of glycemic stress effect on β-cell hypertrophy.
In vivo studies.
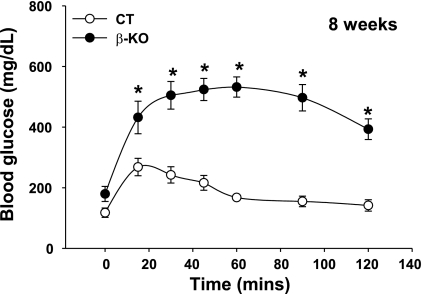
Glucose tolerance test was performed on β-PDHCT (control) and β-PDHKO male mice at age 8 wk by intraperitoneal glucose injection (1.5 g/kg body wt) after an overnight fast. The fasting blood glucose levels (time 0) between the two groups were not significantly different (Fig. 6). Compared with the response of blood glucose levels of β-PDHCT male mice over a 120-min period, the response of 8-wk-old β-PDHKO male mice was abnormal with significant levels of hyperglycemia over the same period (Fig. 6).
Fig. 6.
Intraperitoneal glucose tolerance test in 8-wk-old β-KO and CT male mice. The results are means ± SE (n = 4 or 5). *P < 0.05.
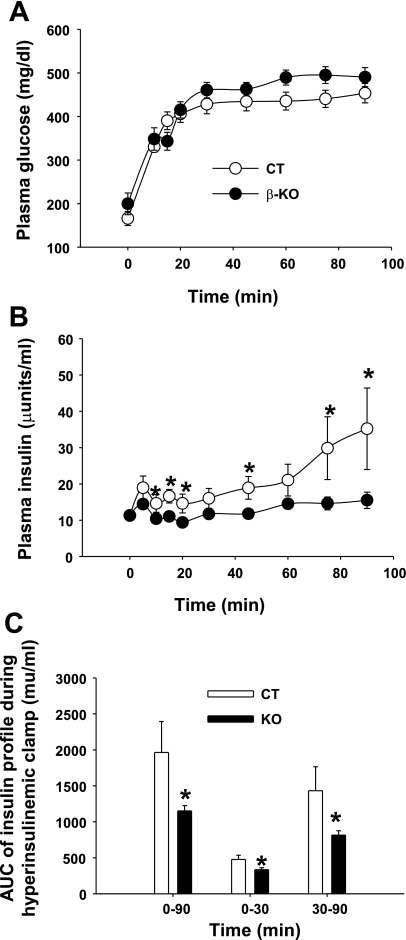
To confirm that the insulin secretion function was impaired in vivo, hyperglycemic clamp studies were conducted in β-PDHKO and β-PDHCT male mice at 4 mo of age after an overnight fast (Fig. 7). The blood glucose was rapidly increased and maintained at ∼25 mM throughout the clamp (Fig. 7A). Analysis of plasma insulin levels at multiple time points during the clamp period clearly indicated significant impairment of the insulin secretion response in vivo of β-PDHKO mice manifested by a significant decrease in the area under the curve for insulin secretion during both acute (0–30 min) and late phases (30–120 min) compared with β-PDHCT mice (Fig. 7, B and C).
Fig. 7.
Hyperglycemic clamp analysis in β-KO and CT mice at the age of 16 wk. A: A 90-min clamp was conducted with a primed and variable infusion of glucose to raise and maintain plasma glucose concentrations at ∼25 mM in β-KO (n = 13) and CT (n = 8) mice. B: plasma insulin was measured at the indicated time points. C: values indicate the area under the curve (AUC) for insulin secretion throughout the experiment (0–90 min) or during acute (0–30 min) and late (30–90 min) phases of secretion. *P < 0.05, using unpaired 2-tailed t-test in B.
DISCUSSION
The generation of ATP from acetyl-CoA oxidation due to the metabolism of pyruvate in the mitochondria of β-cells together with the secondary signals generated from the TCA cycle intermediates plays a critical role in the coupling of glucose metabolism with insulin secretion (15, 24, 26). Pyruvate is nearly equally metabolized by PDC and PC in the mitochondria, and pyruvate cycling in β-cells has been shown to involve three different cycles (namely the pyruvate-malate, pyruvate-citrate, and pyruvate-isocitrate cycles). The roles of PC and other key enzymes involved in these cycles have been investigated extensively for GSIS using isolated islets and cultured clonal β-cell lines (10, 12, 14, 17, 29, 31–33, 42). The results presented here show in vivo and in vitro evidence for a key role played by PDC in insulin secretion in β-cells because of its central role in the production of cellular ATP and the participation in the operation of the three pyruvate cycling pathways. Furthermore, our β-PDHKO mouse model represents the first mouse model in which a mitochondrial oxidative enzyme involved in glucose oxidation has been deleted to investigate insulin secretion by β-cells.
GSIS by β-cells requires glucose metabolism to increase intracellular ATP content and also generation of coupling factors (such as malonyl-CoA, NADPH, GTP, α-ketoglutarate, etc.) (15, 24, 26). The pathways involved in meeting these requirements in β-cells obligate that a sizeable amount of pyruvate formed from glucose metabolism via glycolysis is further metabolized via PDC in the mitochondria. The importance of this requirement for insulin secretion is clearly evident from the results presented in this study. A deletion of the Pdha1 gene in β-cells resulted only in a reduction in PDH (and hence, PDC) activity in islets from β-PDHKO male mice, with a significant impairment in GSIS in both in vitro and in vivo conditions. It is possible that some acetyl-CoA can be generated from endogenous substrates such as fatty acids and some amino acids. However, these endogenous substrates were not sufficient to compensate for the loss of acetyl-CoA generated by PDC in vivo due to its deficiency. The oxidation of acetyl-CoA derived from pyruvate in the TCA cycle coupled with the respiration/oxidative phosphorylation processes results in the generation of ATP. This process is likely to be reduced in β-cells from β-PDHKO male mice. Additionally, the formation of the TCA cycle intermediates in the mitochondria (anaplerosis) and their metabolism in the cytosol via three different cycles (cataplerosis) result in the formation of several coupling factors required for insulin secretion. Interestingly, these processes depend, in part, on the initial action of PDC in the mitochondria.
Pancreatic β-cells possess a high level of PC (35). A large body of evidence indicates that PC plays an important role for the synthesis of the TCA cycle intermediates, which are involved in the transport of the reducing equivalents and acetyl groups out of mitochondria to generate NADPH and acetyl-CoA via cataplerosis in the cytosol (15, 24, 26). The pyruvate-malate cycle involving PC in the mitochondria and malic enzyme in the cytosol regenerates pyruvate, producing NADPH in the cytosol. This cycle does not require the participation of acetyl-CoA carbons directly in this pathway. However, acetyl-CoA acts as an allosteric activator of PC and hence, regulates the carbon flux via PC.
The formation of citrate, isocitrate, and α-ketoglutarate in the mitochondria requires both acetyl-CoA and oxaloacetate from pyruvate via the actions of PDC and PC, respectively. The transport of citrate from the mitochondria to the cytosol and its metabolism in the cytosol via the pyruvate-citrate shuttle generate both NADPH and acetyl-CoA, which is converted to malonyl-CoA, as metabolic coupling factors. Cytosolic malonyl-CoA acting as a coupling factor inhibits carnitine palmitoyl-CoA transferase I, resulting in an increase in cytosolic long-chain acyl-CoA (30). A glucose-induced increase in cytosolic malonyl-CoA concentration requires first the action of PDC to generate acetyl-CoA from pyruvate in the mitochondria and subsequently its transport as citrate to regenerate acetyl-CoA for the synthesis of malonyl-CoA in the cytosol. Thus, the role of PDC is central for the formation of acetyl-CoA from glucose-derived pyruvate in the mitochondria.
The pyruvate-isocitrate cycle can generate NADPH in the cytosol via NADP-isocitrate dehydrogenase. For this shuttle to operate, the formation of acetyl-CoA from pyruvate by PDC is critical for the formation of citrate and then isocitrate. It is interesting to note that since the cytosolic malic enzyme is lacking in mouse islets (21), pyruvate cycling via the pyruvate-isocitrate cycle may play an even greater role for NADPH generation in mouse islets.
In β-PDHKO male mice, there was no effect on the distribution of the number of different sizes of islets, but rather, there was an increase in β-cell size in islets on postnatal day 33 but not on postnatal day 108. We speculate that increased β-cell size may be a compensatory response to chronic hyperglycemia observed in younger β-PDHKO mice (Fig. 3C). It is likely that the oxidation in β-cells of β-PDHKO male mice of fatty acids derived from milk fat as well as the increased availability of ketone bodies (4, 13) can provide energy for the ontogeny of islets during the suckling period but will not support preproinsulin gene expression and insulin secretion by β-cells.
In summary, our study is the first to employ a gene knockout approach to PDC, the key enzyme involved in regulating pyruvate oxidation and TCA cycle flux for GSIS in β-cells. Acetyl-CoA derived from pyruvate oxidation via PDC is important in the functioning of the three pyruvate cycling pathways for the production of the coupling factors involved in insulin secretion by β-cells. Studies so far reported for GSIS involving key enzymes for pyruvate cycling are performed using transiently or stably transfected clonal β-cells or cultured islets only, and the findings often contradict the putative roles of several key enzymes in GSIS. Limitations of these approaches have recently been pointed out by Brown et al. (1). The results presented here from both in vitro and in vivo studies demonstrate the central role played by PDC in GSIS by β-cells.
GRANTS
This study was supported in part by US Public Health Service Grant DK-20478 and University at Buffalo Biochemistry Department funds.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to Dr. S. G. Laychock of the Department of Pharmacology and Toxicology, University at Buffalo, for critical reading of the manuscript.
REFERENCES
- 1.Brown LJ, Longacre MJ, Hasan NM, Kendrick MA, Stoker SW, MacDonald MJ. Chronic reduction of the cytosolic or mitochondrial NAD(P)-malic enzyme does not affect insulin secretion in a rat insulinoma cell line. J Biol Chem 284: 35359–35367, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown RM, Head RA, Boubriak II, Leonard JV, Thomas NH, Brown GK. Mutations in the gene for the E1beta subunit: a novel cause of pyruvate dehydrogenase deficiency. Hum Genet 115: 123–127, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Cline GW, Lepine RL, Papas KK, Kibbey RG, Shulman GI. 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem 279: 44370–44375, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Cremer JE, Heath DF. The estimation of rates of utilization of glucose and ketone bodies in the brain of the suckling rat using compartmental analysis of isotopic data. Biochem J 142: 527–544, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, Oz OK, Pourbahrami T, Lu CY, Richardson JA, Karsenty G, Li C. Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol 24: 258–269, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahl HH, Brown RM, Hutchison WM, Maragos C, Brown GK. A testis-specific form of the human pyruvate dehydrogenase E1 alpha subunit is coded for by an intronless gene on chromosome 4. Genomics 8: 225–232, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Detimary P, Gilon P, Henquin JC. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem J 333: 269–274, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, Yamauchi N, Kubota N, Murayama S, Aizawa T, Akanuma Y, Aizawa S, Kasai H, Yazaki Y, Kadowaki T. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 283: 981–985, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Gannon M, Shiota C, Postic C, Wright CV, Magnuson M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 26: 139–142, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Guay C, Madiraju SR, Aumais A, Joly E, Prentki M. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem 282: 35657–35665, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Harris RA, Popov KM, Zhao Y, Kedishvili NY, Shimomura Y, Crabb DW. A new family of protein kinases—the mitochondrial protein kinases. Adv Enzyme Regul 35: 147–162, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Hasan NM, Longacre MJ, Stoker SW, Boonsaen T, Jitrapakdee S, Kendrick MA, Wallace JC, MacDonald MJ. Impaired anaplerosis and insulin secretion in insulinoma cells caused by small interfering RNA-mediated suppression of pyruvate carboxylase. J Biol Chem 283: 28048–28059, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins RA, Williamson DH, Krebs HA. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J 122: 13–18, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen MV, Joseph JW, Ilkayeva O, Burgess S, Lu D, Ronnebaum SM, Odegaard M, Becker TC, Sherry AD, Newgard CB. Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J Biol Chem 281: 22342–22351, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 295: E1287–E1297, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MT, Mahmood S, Hyatt SL, Yang HS, Soloway PD, Hanson RW, Patel MS. Inactivation of the murine pyruvate dehydrogenase (Pdha1) gene and its effect on early embryonic development. Mol Genet Metab 74: 293–302, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Joseph JW, Odegaard ML, Ronnebaum SM, Burgess SC, Muehlbauer J, Sherry AD, Newgard CB. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J Biol Chem 282: 31592–31600, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kennedy HJ, Pouli AE, Ainscow EK, Jouaville LS, Rizzuto R, Rutter GA. Glucose generates sub-plasma membrane ATP microdomains in single islet beta-cells. Potential role for strategically located mitochondria. J Biol Chem 274: 13281–13291, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Kerr DS, Ho L, Berlin CM, Lanoue KF, Towfighi J, Hoppel CL, Lusk MM, Gondek CM, Patel MS. Systemic deficiency of the first component of the pyruvate dehydrogenase complex. Pediatr Res 22: 312–318, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, Sherry AD. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci USA 99: 2708–2713, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonald MJ. Differences between mouse and rat pancreatic islets: succinate responsiveness, malic enzyme, and anaplerosis. Am J Physiol Endocrinol Metab 283: E302–E310, 2002 [DOI] [PubMed] [Google Scholar]
- 22.MacDonald MJ. Evidence for the malate aspartate shuttle in pancreatic islets. Arch Biochem Biophys 213: 643–649, 1982 [DOI] [PubMed] [Google Scholar]
- 23.MacDonald MJ. High content of mitochondrial glycerol-3-phosphate dehydrogenase in pancreatic islets and its inhibition by diazoxide. J Biol Chem 256: 8287–8290, 1981 [PubMed] [Google Scholar]
- 24.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab 288: E1–E15, 2005 [DOI] [PubMed] [Google Scholar]
- 25.MacDonald MJ, Smith AD, 3rd, Hasan NM, Sabat G, Fahien LA. Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. J Biol Chem 282: 30596–30606, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9: 193–205, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Nicholls LI, Ainscow EK, Rutter GA. Glucose-stimulated insulin secretion does not require activation of pyruvate dehydrogenase: impact of adenovirus-mediated overexpression of PDH kinase and PDH phosphate phosphatase in pancreatic islets. Biochem Biophys Res Commun 291: 1081–1088, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Patel MS, Korotchkina LG. The biochemistry of the pyruvate dehydrogenase complex. Biochem Mol Biol Edu 31: 5–15, 2003 [Google Scholar]
- 29.Pongratz RL, Kibbey RG, Shulman GI, Cline GW. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J Biol Chem 282: 200–207, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 267: 5802–5810, 1992 [PubMed] [Google Scholar]
- 31.Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, Becker TC, Sherry AD, Newgard CB, Jensen MV. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem 281: 30593–30602, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ronnebaum SM, Jensen MV, Hohmeier HE, Burgess SC, Zhou YP, Qian S, MacNeil D, Howard A, Thornberry N, Ilkayeva O, Lu D, Sherry AD, Newgard CB. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J Biol Chem 283: 28909–28917, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronnebaum SM, Joseph JW, Ilkayeva O, Burgess SC, Lu D, Becker TC, Sherry AD, Newgard CB. Chronic suppression of acetyl-CoA carboxylase 1 in beta-cells impairs insulin secretion via inhibition of glucose rather than lipid metabolism. J Biol Chem 283: 14248–14256, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutter GA. Visualising insulin secretion. The Minkowski Lecture 2004. Diabetologia 47: 1861–1872, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem 272: 18572–18579, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Schuit FC, Huypens P, Heimberg H, Pipeleers DG. Glucose sensing in pancreatic beta-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes 50: 1–11, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Sidhu S, Gangasani A, Korotchkina LG, Suzuki G, Fallavollita JA, Canty JM, Jr, Patel MS. Tissue-specific pyruvate dehydrogenase complex deficiency causes cardiac hypertrophy and sudden death of weaned male mice. Am J Physiol Heart Circ Physiol 295: H946–H952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinivasan M, Aalinkeel R, Song F, Mitrani P, Pandya JD, Strutt B, Hill DJ, Patel MS. Maternal hyperinsulinemia predisposes rat fetuses for hyperinsulinemia, and adult-onset obesity and maternal mild food restriction reverses this phenotype. Am J Physiol Endocrinol Metab 290: E129–E134, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Sugden MC, Holness MJ. Therapeutic potential of the mammalian pyruvate dehydrogenase kinases in the prevention of hyperglycaemia. Curr Drug Targets Immune Endocr Metabol Disord 2: 151–165, 2002 [PubMed] [Google Scholar]
- 40.Thyssen S, Arany E, Hill DJ. Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology 147: 2346–2356, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Xia M, Laychock SG. Insulin secretion, myo-inositol transport, and Na(+)-K(+)-ATPase in glucose-desensitized rat islets. Diabetes 42: 1392–1400, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Han J, Long YS, Epstein PN, Liu YQ. The role of pyruvate carboxylase in insulin secretion and proliferation in rat pancreatic beta cells. Diabetologia 51: 2022–2030, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105: 745–755, 2001 [DOI] [PubMed] [Google Scholar]