Abstract
Insulin resistance is highly prevalent in Asian Indians and contributes to worldwide public health problems, including diabetes and related disorders. Surrogate measurements of insulin sensitivity/resistance are used frequently to study Asian Indians, but these are not formally validated in this population. In this study, we compared the ability of simple surrogate indices to accurately predict insulin sensitivity as determined by the reference glucose clamp method. In this cross-sectional study of Asian-Indian men (n = 70), we used a calibration model to assess the ability of simple surrogate indices for insulin sensitivity [quantitative insulin sensitivity check index (QUICKI), homeostasis model assessment (HOMA2-IR), fasting insulin-to-glucose ratio (FIGR), and fasting insulin (FI)] to predict an insulin sensitivity index derived from the reference glucose clamp method (SIClamp). Predictive accuracy was assessed by both root mean squared error (RMSE) of prediction as well as leave-one-out cross-validation-type RMSE of prediction (CVPE). QUICKI, FIGR, and FI, but not HOMA2-IR, had modest linear correlations with SIClamp (QUICKI: r = 0.36; FIGR: r = −0.36; FI: r = −0.27; P < 0.05). No significant differences were noted among CVPE or RMSE from any of the surrogate indices when compared with QUICKI. Surrogate measurements of insulin sensitivity/resistance such as QUICKI, FIGR, and FI are easily obtainable in large clinical studies, but these may only be useful as secondary outcome measurements in assessing insulin sensitivity/resistance in clinical studies of Asian Indians.
Keywords: quantitative insulin-sensitivity check index
insulin resistance is typically defined as decreased sensitivity or responsiveness to metabolic actions of insulin such as insulin-mediated glucose disposal (17). Insulin resistance plays a major pathophysiological role in type 2 diabetes and is associated with obesity, hypertension, dyslipidemia, coronary heart disease, and the metabolic syndrome (18). Asian Indians are highly susceptible to diabetes, accelerated atherosclerosis, and ischemic heart disease (9, 28). Globally, India has the largest number of individuals with diabetes. Moreover, Asian Indians have the highest prevalence of diabetes (in percentage terms) among Asian Americans in the United States (20, 28). Not surprisingly, insulin resistance is widespread among both native and migrant Asian Indians (23, 24). Consequently, accurately quantifying insulin sensitivity/resistance is important in this vulnerable population. Among the many available methods for measuring insulin sensitivity in humans, the hyperinsulinemic euglycemic “glucose clamp” is widely accepted as the reference method because it directly measures whole body glucose disposal at a given level of insulinemia under steady-state conditions (17). However, the glucose clamp is labor intensive, technically demanding, and time consuming, making it impractical for use in large epidemiological and clinical studies. As a consequence, a number of simple surrogate indices of insulin sensitivity/resistance derived from fasting blood insulin and glucose concentrations [e.g., quantitative insulin sensitivity check index (QUICKI), homeostasis model assessment (HOMA), 1/fasting insulin] or oral glucose tolerance tests have been developed (17). These surrogate indices are extensively employed in large clinical studies.
Some surrogate measurements of insulin sensitivity have been thoroughly validated against the reference standard glucose clamp method (17). However, ethnicity and BMI may affect validity of fasting-based surrogate indices of insulin sensitivity (2, 13). Surrogate estimates of insulin sensitivity, including QUICKI and HOMA-insulin resistance (HOMA-IR), are often used to assess insulin resistance in Asian-Indian and South Asian populations (7, 14, 16). However, these surrogates have not undergone formal validation analyses specifically in Asian Indian populations. Therefore, in the present study, we examined linear correlations between several simple surrogate indices [(QUICKI), HOMA2-IR, fasting insulin-to-glucose ratio (FIGR), and fasting insulin (FI)] and an insulin sensitivity index derived from the reference glucose clamp method (SIClamp) in a cohort of Asian-Indian men with a wide range of insulin sensitivity/resistance. Importantly, we also performed calibration model analysis to robustly evaluate the absolute accuracy of these surrogates to predict results from the reference glucose clamp.
EXPERIMENTAL PROCEDURES
Study participants.
In this cross-sectional study, we used data from 70 Asian-Indian men ranging in age from 18 to 73 yr who underwent hyperinsulinemic euglycemic glucose clamp procedures at one of two institutions: Mayo Clinic College of Medicine (Rochester, MN) or St. John's Medical College and Research Institute (Bangalore, India). Data from some of these subjects have been reported previously (19, 26). All clinical studies were approved by the Institutional Review Boards of the above-mentioned institutions. All procedures followed were in accordance with each institution's guidelines, and all participants gave written, informed consent prior to their participation. In the present study, for these Asian Indians, nonobese subjects were defined as having a BMI <23 kg/m2, whereas subjects with BMI ≥23 were considered obese (6, 29). Subjects were considered to be diabetic or have impaired fasting glucose if they met the American Diabetes Association criteria for type 2 diabetes (3). Nondiabetic participants were in good health, had no first-degree relatives with type 2 diabetes, and were not taking any medications. Diabetic subjects who were being treated with medications were studied after oral hypoglycemic medications were discontinued (3 wk for thiazolidinediones and 5 days for sulfonylurea and metformin). Participants with serum creatinine concentrations >1.5 mg/dl, on medications that may impact on energy metabolism, with liver function abnormalities, or with active coronary artery disease were excluded from our analyses.
Hyperinsulinemic euglycemic glucose clamp.
Insulin sensitivity was evaluated by glucose clamp, as described previously (19, 26). Briefly, participants were admitted to the Research Center on the evening before the study. After an overnight fast of ≥10 h, clamp studies were performed beginning at ∼7 AM. After a 10-min priming dose, insulin (Humulin; Eli Lilly) was infused at a constant rate (ranging from 40 to 60 mU·m2·min−1 at the 2 institutions). Plasma glucose concentrations from arterialized blood samples were measured at the bedside every 5–10 min with a glucose analyzer. An intravenous infusion of dextrose was adjusted to maintain the plasma glucose concentration between 85 and 90 mg/dl. Blood samples were also collected every 20–30 min for measuring plasma insulin concentrations. We defined the steady-state period of the clamp as an ≥40-min period (1–2 h after the beginning of the insulin infusion) where the coefficient of variation for plasma glucose, plasma insulin, and glucose infusion rate was <5%. Mean parameter values during the steady-state period were used to calculate SIClamp [defined as M/ΔI corrected for body weight, where M is the steady-state glucose infusion rate (mg/min) and ΔI is the difference between basal and steady-state plasma insulin concentrations (μU/ml)].
Fasting-based surrogate indices.
QUICKI was calculated as defined previously from fasting glucose and insulin values (12). QUICKI = 1/[log(I0) + log(G0)], where I0 is fasting insulin (μU/ml) and G0 is fasting glucose (mg/dl). Because QUICKI is the reciprocal of the log-transformed product of fasting glucose and insulin, it is a dimensionless index without units. The authors who originally developed the HOMA model recommended that HOMA2, the correctly solved computer model, be used when HOMA is compared with other models (27). HOMA2-IR was calculated by using the updated computer model for HOMA-2 indices (27). FIGR was calculated as the ratio of insulin expressed in microunits per milliliter to glucose expressed as milligrams per deciliter.
Laboratory assays.
Routine assays for serum lipids, plasma glucose, and plasma insulin were performed in the respective clinical centers. Fasting plasma insulin concentrations were measured using an electrochemiluminescent method (Roche Diagnostics, Mannheim, Germany) (26) or two-site immunoenzymatic assay performed on the Access automated immunoassay system (Beckman, Chaska, MN) (19), as described previously. The intra- and interassay coefficients of variation were <5 and <10%, respectively, for either assay using trilevel lyophilized serum controls (Bio-Rad Laboratories, Irvine, CA). The average relative percent difference between the two methods was 5.5%.
Calibration model analysis of surrogate indices.
We chose a standard calibration model to evaluate the predictive accuracy of various simple surrogate indices of insulin sensitivity. Calibration model is particularly appropriate when an expensive, inconvenient, or laborious but accurate measurement method such as the glucose clamp is compared with an inexpensive and quick but indirect method such as the surrogate index of insulin sensitivity. In a typical regression model, y = f (x; θ) + ε, x is the input variable (e.g., SIClamp), y is the response variable (e.g., QUICKI), θ is an unknown parameter, and ε is the random error. Using an estimated model, y = f (x; θ̂), to predict QUICKI (new y*) for a given value of SIClamp (x*) is regression. Conversely, calibration is when the surrogate method is regressed on the accurate measurement method and new x* (SIClamp) is predicted for a given y* (surrogate index). Thus, calibration is inverse regression. If the value of the accurate measurement method (x) is prespecified as part of an experimental design, this is called classical or controlled calibration. If both x and y are random, the process is called random calibration. Since QUICKI (or other surrogates) and SIClamp are measured with error from a patient population, random calibration is the more appropriate method to use. Calibration model analyses were performed as described previously (5). Here we fitted a calibration model xi = α + βyi + εi, where xi is SIClamp, yi is the surrogate index, and εi is the random error for the ith subject. It was assumed that the random error had Gaussian distribution with mean of 0 and a constant variance. Although SIClamp was measured with error, it was assumed for our model that the measurement error of SIClamp (determined from a robust, direct, and data-intensive protocol) was very small relative to measurement error of simple surrogates determined from single fasting measurements (e.g., QUICKI) (15). Therefore, to simplify the analysis, we neglected the measurement error for SIClamp in our calibration model.
For each surrogate index, two types of predicted residuals were considered. The first type of residual is the difference between measured SIClamp (xi for the ith subject) and fitted SIClamp, x̂i = α + β̂yi. That is, the residual, ei = xi − x̂i, is derived from the calibration model with all subjects included in the estimation of model parameters α and β. The second type of residual considered is a cross-validation type predicted residual e(i) = xi − x̂(i), where xi is still the measured SIClamp but x̂(i) is the predicted SIClamp from the calibration model that excludes the ith subject. The subscript (i) means “with the ith subject deleted.” From these two types of residuals, criterion functions were used to evaluate prediction accuracy: square root of the mean squared error of prediction and leave-one-out cross-validation-type root mean squared error of prediction . Smaller values of RMSE and CVPE indicate better predictive power. CVPE is more robust than RMSE because CVPE uses an estimate that excludes the ith subject when predicting results for the ith subject. This is relevant to clinical situation in which data for each new patient is based on a model obtained from previous patients.
Statistical analyses.
Pearson correlation coefficients (r) and respective P values were calculated to assess the statistical significance of the model using a linear least-squares fit method to obtain the linear regression. To compare predictive accuracy of QUICKI and other surrogates in terms of CVPE and RMSE, we performed hypothesis testing with the one-sided alternative hypothesis that QUICKI had a smaller RMSE or CVPE than another surrogate using a bootstrap percentile method, with 60,000 replications performed for each comparison. The bootstrap method is appropriate because the RMSEs (or CVPEs) corresponding to QUICKI and other surrogates were derived from the same group of subjects and thus correlated. The P values calculated from comparison of RMSE and CVPE were for pairwise comparisons. For example, QUICKI and HOMA were compared with respect to CVPE, a bootstrap percentile method with 60,000 replications was used to get a sample of 60,000 differences in CVPE [CVPE (HOMA) − CVPE (QUICKI)], and then a P value for one-sided superiority testing was estimated as the proportion of the bootstrap replications less than zero. One-sided hypothesis testing was used because multiple previous studies in humans have demonstrated the superiority of QUICKI as a surrogate index of insulin sensitivity from a variety of perspectives supporting an a priori expectation. P < 0.05 was considered to indicate statistical significance. The R statistical platform was used for statistical analyses and implementation of the random calibration model (http://www.r-project.org).
RESULTS
Baseline clinical characteristics of our study subjects are shown in Table 1. Data from a total of 70 men (aged 18–73 yr) who underwent glucose clamp studies were included in this analysis. BMI may affect the validity of fasting-based surrogate indices of insulin sensitivity (13). Therefore, men with a wide range of BMI (16–31) were included in this study. The majority of the men in this study were nonobese with BMI <23, with obese individuals (BMI >23) comprising 27% (n = 18) of the study population. SIClamp varied from 1.6 to 17 [10−2 mg·kg−1·min−1 (μU/ml)] in our overall cohort that included some men with type 2 diabetes (n = 9) or impaired fasting glucose (n = 8). There was a significant but modest correlation between BMI and SIClamp; r = −0.30, P < 0.05.
Table 1.
Clinical characteristics of Asian-Indian men in this study
| Clinical Parameters | Value |
|---|---|
| Age, yr | 30 ± 14 |
| BMI, kg/m2 | 21 ± 4 |
| Systolic blood pressure, mmHg | 117 ± 12 |
| Diastolic blood pressure, mmHg | 75 ± 9 |
| Fasting plasma glucose, mg/dl | 103 ± 34 |
| Fasting plasma insulin, μU/ml | 10.0 ± 6.4 |
| QUICKI | 0.347 ± 0.043 |
| HOMA2-IR | 1.36 ± 0.97 |
| Insulin/glucose, (μU/ml)·mg−1·dl−1 | 0.09 ± 0.05 |
| Clamp plasma insulin, μU/ml | 83 ± 25 |
| Glucose infusion rate, mg·kg−1·min−1 | 4.13 ± 2.29 |
| SIClamp, 10−2 mg·kg−1·min−1·(μU/ml)−1 | 6.1 ± 3.2 |
| Total cholesterol, mg/dl | 128 ± 33 |
| LDL, mg/dl | 60 ± 13 |
| HDL, mg/dl | 35 ± 7 |
| Triglycerides, mg/dl | 101 ± 41 |
Data are means ± SD; n = 70. QUICKI, quantitative insulin sensitivity check index; HOMA2-IR, homeostasis model assessment 2 of insulin resistance; SIClamp, reference glucose clamp method.
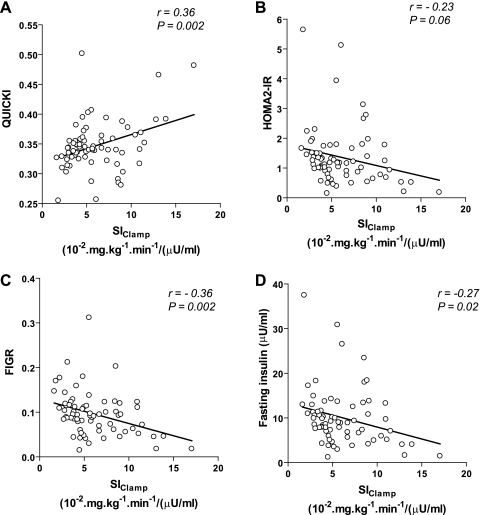
When we compared relationships between surrogate indices and SIClamp, simple linear regression analysis showed modest but significant correlations between SIClamp and QUICKI, FIGR, or FI (r = −0.27 to −0.36) but not with HOMA2-IR (Fig. 1). QUICKI and FIGR tended to have slightly stronger correlations with SIClamp than either FI or HOMA2-IR. However, log transformation of HOMA2-IR improves the strength of correlation with SIClamp from 0.23 to 0.36. Validation studies of HOMA and clinical studies that use HOMA-IR to assess insulin resistance routinely do not log-transform HOMA-IR or HOMA2-IR. Thus, we used untransformed HOMA2-IR for evaluating absolute accuracy.
Fig. 1.
Linear correlations between surrogate indices of insulin sensitivity/resistance and reference glucose clamp method (SIClamp). The solid line represents the linear least-squares fit between each calculated surrogate index of insulin sensitivity/resistance and measured SIClamp. Correlation coefficients (r) and corresponding P values are shown in each part. A: results derived from quantitative insulin sensitivity check index (QUICKI). B: results derived from homeostasis model assessment 2 of insulin resistance (HOMA2-IR). C: results derived from fasting insulin-to-glucose ratio (FIGR). D: results derived from fasting insulin concentrations.
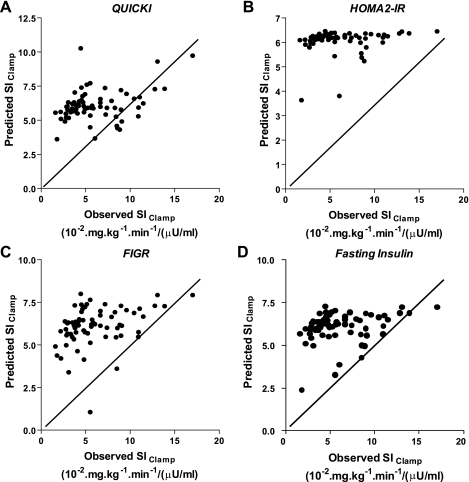
We next evaluated the absolute accuracy of various surrogate indices using calibration model analysis. We regressed experimentally determined SIClamp on each surrogate index and fitted these data to a calibration model, as described in experimental procedures. We determined model parameters α and β for each surrogate index for the entire cohort. We then used the fitted calibration model (using leave-one-out cross-validation analysis) to generate plots for each surrogate index, comparing predicted SIClamp values (generated from each surrogate index) with actual values for SIClamp derived from glucose clamp studies for each subject in our entire cohort (Fig. 2). If a surrogate index perfectly predicted SIClamp, results for each individual would fall on a straight line with a slope of 1 and a y-intercept of 0. By inspection, HOMA2-IR had very poor predictive accuracy, with a wide range of actual values for SIClamp corresponding to very similar predicted values of SIClamp generated from calibration analysis of HOMA2-IR data. Of note, none of the predicted values from HOMA2-IR coincided with the ideal line of perfect predictive accuracy (Fig. 2B). By contrast, QUICKI, FIGR, and FI all appeared to have comparable (albeit modest) predictive accuracy for SIClamp. To quantitatively assess predictive accuracy for each surrogate index, residuals (measured SIClamp − predicted SIClamp) generated from random calibration analysis for each surrogate were used to calculate CVPE and RMSE (Table 2). No significant differences were noted among CVPE or RMSE when these parameters were compared between QUICKI and the other surrogate indices.
Fig. 2.
Comparison between measured SIClamp and predicted SIClamp from various surrogate indices of insulin sensitivity/resistance. Predicted SIClamp shown for each surrogate index was calculated using the leave-one-out cross-validation analysis of the calibration model as described in experimental procedures. The solid line indicates ideal predictive accuracy. A: results derived from QUICKI. B: results derived from HOMA2-IR. C: results derived from FIGR. D: results derived from fasting insulin concentrations.
Table 2.
CVPE and RMSE estimates of error calculated from calibration analysis of surrogate indices of insulin sensitivity/resistance
| CVPE | P Value | RMSE | P Value | |
|---|---|---|---|---|
| QUICKI | 3.16 | 3.02 | ||
| HOMA2-IR | 3.18 | 0.61 | 3.14 | 0.85 |
| Fasting insulin | 3.16 | 0.58 | 3.11 | 0.78 |
| FIGR | 3.07 | 0.48 | 3.02 | 0.47 |
RMSE, root mean squared error; CVPE, cross-validation-type RMSE of prediction; FIGR, fasting insulin-to-glucose ratio. CVPE and RMSE were calculated from calibration analysis of various surrogate indices of insulin sensitivity as described in experimental procedures. P values correspond to comparisons between QUICKI and alternative surrogate indices.
DISCUSSION
Asian Indians, even relatively lean individuals, have a high prevalence of insulin resistance, and they are at high risk for developing type 2 diabetes and atherogenic cardiovascular disease (9, 28). Hence, developing simple, reliable, and accurate methods for quantifying insulin sensitivity is of great interest in this particular population so that preventive measures can be implemented in these people who constitute ∼18% of the world's population. Surrogate indices of insulin sensitivity/resistance based on fasting concentrations of plasma glucose and insulin are widely used in large epidemiological studies, prospective clinical trials, and clinical research studies with a variety of patient populations and ethnic groups (17). These surrogate indices (some more than others) generally provide a reliable, reproducible, and accurate index of insulin sensitivity with positive predictive power for the development of diabetes (5, 10, 12, 17, 27). Not surprisingly, these surrogate indices are often used to assess insulin sensitivity/resistance in Asian Indians. However, to date, formal extensive and robust direct validation studies of these indices in this or any other Asian population are lacking. Only a few studies have examined linear correlations between surrogate indices of insulin sensitivity and the reference glucose clamp method in Asians (11, 30). Correlation coefficients per se are not informative regarding the absolute accuracy of a method and may be misleading with respect to positive predictive power. Therefore, evaluation of predictive accuracy is a necessary component of thorough validation. In the present study, for the first time, we evaluated the predictive absolute accuracy of various surrogate indices of insulin sensitivity/resistance in Asian-Indian men, using calibration model analysis and glucose clamp estimates as the reference standard for comparison.
The subjects included in this study had a wide range of BMI and insulin sensitivity/resistance. This is especially important because Asian-Indian men tend to be insulin resistant at BMIs that are considered normal for Northern Europeans (i.e., 25), and young, lean Asian-Indian men are substantially more insulin resistant compared with lean men from other ethnic groups (22, 23). Strikingly, in Asian-American adults with BMI between 18.5 and 22.9 kg/m2, Asian Indians have the highest ethnic-specific diabetes prevalence rate (∼7%) (20). This is noteworthy given that a majority of adult Asian Indians (∼55%) have a BMI <23 kg/m2 (6). The approximate average body fat for a BMI of 23 is 26% in middle-aged Asian Indian men (4).
BMI influences the relationship between surrogate indices and direct measurements of insulin sensitivity (13). Therefore, one of the strengths of the present validation study is that our cohort has a wide range of insulin sensitivity and BMI. Nevertheless, the linear correlations between surrogate indices (QUICKI, FIGR, and FI) and the reference standard, SIClamp, were modest (r = −0.27 to −0.36), with HOMA2-IR performing especially poorly. In a number of other published studies, correlations between clamp estimates and surrogates, including QUICKI and HOMA-IR, are substantially better than we observed in the present study (r = ∼0.60–0.90) (5, 11, 12, 17, 25, 27, 30). Similarly, in other studies, QUICKI and log (HOMA) are excellent at predicting insulin sensitivity (SIClamp) (5, 12).
The discrepancies between our correlation results and those of others (including some of our own previous studies) may be due to a variety of factors. Subgroup analyses in some studies demonstrate that correlations between clamp or SSPG (another direct measurement of insulin sensitivity) and surrogate indices of insulin sensitivity/resistance are lower (r = ∼0.30–0.45) in individuals with a low BMI (≤25 kg/m2) (11, 13, 15, 25). Moreover, in subjects with low BMI, a modification of QUICKI that includes incorporation of free fatty acid (FFA) levels into the index improves correlations between clamp and QUICKI in nonobese individuals (21). Unfortunately, FFA levels were not measured in this cohort to test this hypothesis. One potential explanation for these finding is that individuals with low BMI have high insulin sensitivity with extremely low fasting insulin levels that may be at the limit of detection of current insulin assays. Thus, there is more error when measurement of low insulin levels is attempted. Another possibility is that in lean subjects with low fasting insulin levels there is more heterogeneity among the multiple contributors to insulin sensitivity. Although the majority of men in the present study, as in the general Asian-Indian population, were nonobese with a BMI <23, these subjects were insulin resistant with elevated mean fasting insulin levels well within the sensitivity of our insulin assay. Therefore, it seems unlikely that the differences in correlation magnitudes between our present study and previous studies are due to low fasting insulin levels associated with low BMI. On the other hand, perhaps a low BMI per se results in lower correlations between clamp and surrogate measurements for reasons that are not well understood.
The clamps used in our cohort had insulin infusion rates ranging from 40 to 60 mU·m2·min−1. In our Asian-Indian cohort, which tended to be insulin resistant even at a BMI <23, it is unlikely that an insulin infusion rate of 40–60 mU·m2·min−1 was completely suppressing hepatic glucose production. This would introduce error into estimates of SIClamp and tend to worsen correlations with surrogate indices. Thus, future validation studies should consider using a higher dose of insulin (e.g., 120 mU·m2·min−1) to suppress endogenous glucose production and insulin secretion. Of interest, none of the surrogate measurements distinguishes between hepatic and peripheral insulin sensitivity. Ideally, our analysis should be done on clamps that use a single fixed insulin infusion rate so that values for SIClamp are strictly comparable. However, the dose of insulin infusion used in this study was specific to the two institutions (19, 26). We examined whether study site influenced the relationships between surrogate indices and SIClamp. There were no significant differences in the strength of the correlation coefficients for linear relationships between surrogate indices and SIClamp from the two study sites (Fisher's z-transformation, P > 0.61). In addition, there was no significant study site-by-SIClamp interaction for any of the surrogate insulin sensitivity indices (P > 0.30). Moreover, we have observed previously that correlations between QUICKI and clamp-derived glucose disposal rates did not improve with higher doses of insulin infusion in lean individuals (15). These results together suggest that differences in SIClamp from studies that used insulin infusion rates between 40 and 60 mU·m2·min−1 are unlikely to have introduced enough error to fully account for the differences between correlation results in the present study and previous studies.
Another reason for discrepancies between our correlation results and those of other studies may have to do with the fact that surrogate indices based on fasting glucose and insulin concentrations primarily reflect hepatic insulin sensitivity/resistance, whereas SIClamp is a direct measurement primarily of muscle insulin sensitivity (17). Asian-Indian men are genetically predisposed to develop hepatic steatosis and hepatic insulin resistance at a lower BMI than other ethnic groups (22). Typically, hepatic and skeletal muscle insulin sensitivity/resistance track together. However, in some circumstances, hepatic and muscle insulin sensitivity may be discordant (1). This has clinical relevance because hepatic and muscle insulin resistance play different pathophysiological roles in the development of impaired glucose tolerance and diabetes (8). Thus, hepatic insulin resistance in nondiabetic Asian-Indian men that is discordant with skeletal muscle insulin sensitivity may be a potential explanation for weaker correlations between surrogate measurements of insulin sensitivity/resistance. This hypothesis deserves further investigation in future studies that include measurement of hepatic glucose production using the isotope dilution technique during fasting and hyperinsulinemic conditions (during euglycemic hyperinsulinemic clamp) to calculate indices of hepatic insulin resistance.
Using calibration model analysis, fasting-based surrogate indices were reasonable at predicting SIClamp. There were no significant differences in CVPE and RMSE (the 2 criterion functions measuring predictive accuracy) among any of the surrogates tested.
A limitation of the present study is its moderate sample size. However, performing larger numbers of glucose clamps (e.g., >100) is extremely difficult logistically. Indeed, the present study includes a larger cohort of Asian-Indian men who have undergone glucose clamp studies than any other previously published study that we are aware of. Since our study included only men, our results may not be applicable to women. Currently, there is a lack of standardized insulin assays. Data for this study were obtained from two institutions that used different insulin assays. Consequently, the use of different insulin assays may introduce error to affect the relationships between surrogate indices and SIClamp. However, a measurement of precision error, the average relative percent difference between the two methods, was very low at 5.5%. Furthermore, the Roche Diagnostics assay results were equivalent to the Beckman immunoassay results by a paired t-test (P = 0.30). These results suggest that the different insulin assays used in this study are concordant and unlikely to have affected the results of this study. Nevertheless, in future validation studies it is preferable to measure insulin concentrations using a single assay method in the same laboratory.
In summary, in Asian-Indian men we demonstrated that QUICKI, FIGR, and FI, but not HOMA2-IR, modestly correlate with insulin sensitivity determined by SIClamp. Among the surrogate indices examined, we observed comparable limited ability to accurately predict insulin sensitivity as determined by glucose clamp. We conclude that QUICKI, FIGR, and FI may be useful secondary outcome measurements in assessing insulin sensitivity/resistance in clinical studies of Asian Indians in certain contexts. Although obtaining surrogate indices of insulin sensitivity/resistance is convenient and feasible, design and interpretation of clinical studies in Asian Indians must carefully consider the limited ability of simple surrogate indices to robustly predict insulin resistance in this specific population.
GRANTS
This work was supported, in part, by the Intramural Research Program of the National Center for Complementary and Alternative Medicine, Bethesda, MD; the Ajinomoto Amino Acid Research Program (to A. V. Kurpad); Public Service Grants T32-DK-07352-28 and KL2-RR-084151 (to B. A. Irving); and Public Service Grants UL1-RR-24150 (Mayo Clinic Center for Clinical and Translational Research) and R01-DK-41973 and Centers for Disease Control and Prevention Grant 10 awarded through the American Association of Physicians of Indian Origin (to K. S. Nair).
DISCLOSURES
There are no potential conflicts of interest or disclosures relevant to this article.
REFERENCES
- 1.Abdul-Ghani MA, Matsuda M, DeFronzo RA. Strong association between insulin resistance in liver and skeletal muscle in non-diabetic subjects. Diabet Med 25: 1289–1294, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez JA, Bush NC, Hunter GR, Brock DW, Gower BA. Ethnicity and weight status affect the accuracy of proxy indices of insulin sensitivity. Obesity (Silver Spring) 16: 2739–2744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 32, Suppl 1: S62–S67, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat DS, Yajnik CS, Sayyad MG, Raut KN, Lubree HG, Rege SS, Chougule SD, Shetty PS, Yudkin JS, Kurpad AV. Body fat measurement in Indian men: comparison of three methods based on a two-compartment model. Int J Obes (Lond) 29: 842–848, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes 54: 1914–1925, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Deepa M, Farooq S, Deepa R, Manjula D, Mohan V. Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47). Eur J Clin Nutr 63: 259–267, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Deepa R, Velmurugan K, Arvind K, Sivaram P, Sientay C, Uday S, Mohan V. Serum levels of interleukin 6, C-reactive protein, vascular cell adhesion molecule 1, and monocyte chemotactic protein 1 in relation to insulin resistance and glucose intolerance—the Chennai Urban Rural Epidemiology Study (CURES). Metabolism 55: 1232–1238, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Faerch K, Borch-Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia 52: 1714–1723, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Ghaffar A, Reddy KS, Singhi M. Burden of non-communicable diseases in South Asia. BMJ 328: 807–810, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanley AJ, Williams K, Gonzalez C, D'Agostino RB, Jr, Wagenknecht LE, Stern MP, Haffner SM, San Antonio Heart Study; Mexico City Diabetes Study; Insulin Resistance Atherosclerosis Study Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes 52: 463–469, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Kang ES, Yun YS, Park SW, Kim HJ, Ahn CW, Song YD, Cha BS, Lim SK, Kim KR, Lee HC. Limitation of the validity of the homeostasis model assessment as an index of insulin resistance in Korea. Metabolism 54: 206–211, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85: 2402–2410, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Abbasi F, Reaven GM. Impact of degree of obesity on surrogate estimates of insulin resistance. Diabetes Care 27: 1998–2002, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Lear SA, Kohli S, Bondy GP, Tchernof A, Sniderman AD. Ethnic variation in fat and lean body mass and the association with insulin resistance. J Clin Endocrinol Metab 94: 4696–4702, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Mather KJ, Hunt AE, Steinberg HO, Paradisi G, Hook G, Katz A, Quon MJ, Baron AD. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab 86: 5457–5464, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Mente A, Razak F, Blankenberg S, Vuksan V, Davis AD, Miller R, Teo K, Gerstein H, Sharma AM, Yusuf S, Anand SS, Study of the Health Assessment And Risk Evaluation; Study of the Health Assessment And Risk Evaluation in Aboriginal Peoples Investigators Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care 33: 1629–1634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294: E15–E26, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 57: 1166–1175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oza-Frank R, Ali MK, Vaccarino V, Narayan KM. Asian Americans: diabetes prevalence across U.S. and World Health Organization weight classifications. Diabetes Care 32: 1644–1646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perseghin G, Caumo A, Caloni M, Testolin G, Luzi L. Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in nonobese individuals. J Clin Endocrinol Metab 86: 4776–4781, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, Cobelli C, Shulman GI. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci USA 103: 18273–18277, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab 86: 5366–5371, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet 375: 408–418, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Ruige JB, Mertens IL, Bartholomeeusen E, Dirinck E, Ferrannini E, Van Gaal LF. Fasting-based estimates of insulin sensitivity in overweight and obesity: a critical appraisal. Obesity (Silver Spring) 14: 1250–1256, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Unni US, Ramakrishnan G, Raj T, Kishore RP, Thomas T, Vaz M, Kurpad AV. Muscle mass and functional correlates of insulin sensitivity in lean young Indian men. Eur J Clin Nutr 63: 1206–1212, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053, 2004 [DOI] [PubMed] [Google Scholar]
- 29.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Komatsu M, Tahara H, Shoji T, Okuno Y, Nishizawa Y. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care 26: 2426–2432, 2003 [DOI] [PubMed] [Google Scholar]