keratinocyte growth factor (KGF) has myriad effects on alveolar epithelial cells with the potential to prevent lung injury and improve repair including: mitogenic activity, stimulating cell migration, promoting surfactant production, and improving lung fluid clearance (9, 24). In fact, KGF pretreatment has proven to have a protective effect in animal models of pulmonary fibrosis and acute lung injury. Thus, there has been considerable interest in pursuing KGF as a therapeutic approach to treating human lung disease.
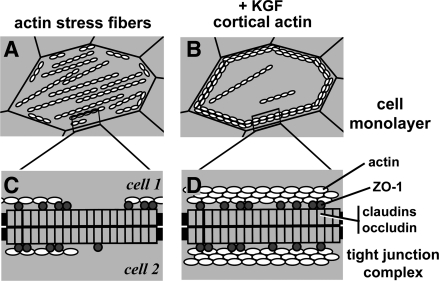
Recently, LaFemina et al. (17) demonstrated that KGF enhanced the barrier function of primary rat alveolar epithelial cells in culture. This effect was primarily due to cytoskeletal reorganization induced by KGF. Cells cultured in the absence of KGF had actin organized into stress fibers traversing through the cytoplasm; however, filamentous actin in cells treated with KGF was primarily peri-junctional (Fig. 1). This has a two-pronged effect on alveolar epithelial barriers, since the disassembly of stress fibers helps stabilize intercellular cell contacts by reducing cell contractile forces, and peri-junctional bundles of actin directly fortify intercellular junctions (15).
Fig. 1.
Cytoskeletal rearrangement induced by keratinocyte growth factor (KGF) treatment of alveolar epithelial cells. A and B: diagram indicating the orientation of actin filaments in cells cultured in control medium forming stress fibers (A) or in the presence of KGF, which are predominantly peri-junctional, cortical actin (B). C and D: attachment of transmembrane tight junction proteins (claudins, occludin) to the actin cytoskeleton via the scaffold protein zonula occludens-1 (ZO-1). Increased peri-junctional bundles of actin fibers provide more ZO-1 attachment sites, which stabilize tight junction strands, thus promoting their barrier function. Not depicted in this diagram are other classes of scaffold and transmembrane proteins, which also crosslink tight junctions to the cytoskeleton.
Several classes of intercellular junctions interact with actin, including tight junctions, which directly regulate diffusion across paracellular barriers between cells (3). Peripheral scaffold proteins, primarily zonula occludens (ZO)-1 and ZO-2, act as intermediaries to crosslink actin with transmembrane tight junction proteins, including claudins and occludin (Fig. 1). There are nearly two dozen mammalian claudins; the subset of claudins expressed by a given cell dictates the extent and charge selectivity of tight junction permeability (4). However, claudins do not provide a functional paracellular barrier unless they are assembled into tight junctions tethered to the actin cytoskeleton (22, 27).
Alveolar epithelial cells have a particularly complex pattern of claudin expression (6, 16) that is regulated in response to sepsis, inflammation, and acute lung injury (7, 11, 26). Given this, LaFemina et al. (17) performed a careful analysis of claudin expression using highly purified type I and type II alveolar epithelial cells isolated directly from rat lung. At the level of mRNA, the three major claudins expressed by both type I and type II cells were claudin-3, claudin-4, and claudin-18. However, at least seven different claudins were detectable by immunoblot. The pattern of claudin expression for alveolar epithelial cells derived from cultured type II cells was comparable to bona fide type I cells, which further confirms the utility of this approach to study most aspects of alveolar epithelial barrier function.
Importantly, the direct analysis of bona fide type I and type II cells enabled confirmation of previous reports that claudin-3 is highly enriched in type II cells compared with type I cells (16, 23). In fact, by immunoblot, type II cells contained 17 times more claudin-3 protein than type I cells (17). The functional significance of enhanced claudin-3 expression by type II cells remains obscure at present. Considering that in the normal alveolus most tight junctions formed by type II cells will also involve type I cells, one intriguing possibility is that the presence of claudin-3 in heterotypic junctions might confer unique permeability characteristics for type II-type I cell junctions. Future work is needed to determine whether this is the case.
LaFemina et al. (17) found that KGF significantly improved the barrier both to rapid ion diffusion [measured as increased transepithelial resistance (TER)] and to the paracellular diffusion pathway used by small molecules. Since TER is largely controlled by the claudin composition of tight junction strands, it was surprising that the increase in TER induced by KGF-treated alveolar epithelial cells was not associated with any changes in claudin expression. The ability of KGF to enhance alveolar epithelial cell TER was also unexpected given previous studies demonstrating that KGF did not prevent a decrease in airway epithelial TER induced by hydrogen peroxide (5). However, KGF does enable airway epithelial cells treated with hydrogen peroxide to retain the barrier to paracellular albumin diffusion, by stabilizing the actin cytoskeleton (5, 25), comparable to the effect on alveolar epithelial cells (17).
One possible explanation for the effect of KGF on alveolar epithelial TER is that the increase in cortical actin induced by KGF may induce changes to the assembly of tight junctions with the potential to alter claudin function. However, these changes must be subtle, since they were undetectable by total cell immunoblot analysis of tight junction protein expression or at the level of immunofluorescence microscopy.
Moreover, actin remodeling and cell contractility through the Rho kinase and myosin light chain kinase (MLCK) pathways have an adverse effect on tight junctions (15). In fact, KGF improves airway barrier function through inhibition of Rho kinase activity (10). Whether KGF improves alveolar epithelial barrier function by inhibiting Rho kinase remains to be determined. Recent studies where cultured human alveolar epithelial cells were challenged with a mixture of proinflammatory hormones (cytomix) to disrupt tight junctions suggest that MLCK may play a more prominent role than Rho kinase in modulating alveolar barrier function (11). In that study, cytomix caused a significant, specific increase in claudin-18 internalization by alveolar epithelial cells, which was antagonized by MLCK inhibitors, but not Rho kinase inhibitors.
KGF generally promotes maintenance of the type II cell phenotype (1, 14, 19, 20), so it was unexpected that KGF did not induce cultured alveolar epithelial cells to have a pattern of claudin expression more in line with type II cells; the comparable level of claudin-3 expression in either the presence or absence of KGF was particularly striking (17). However, since KGF preserved the expression of a type II cell marker (RT270), and prevented expression of a type I cell marker (RT140), claudin expression is clearly dissociable from other aspects of alveolar epithelial phenotype. This also underscores the concept that type I and type II cells are likely to represent extreme endpoints of a spectrum of alveolar epithelial phenotypes (12, 13) and that the effect of KGF on alveolar epithelial cell phenotype will be influenced by cell microenvironment (e.g., extent and type of injury) or as mimicked by cell culture conditions.
Studies using mesenchymal stem cells (MSCs) have provided another clue for the potential of KGF to prevent acute lung injury. Using an isolated, perfused human lung model challenged with endotoxin, MSCs administered intratracheally 1 h after endotoxin prevented disruption of the pulmonary endothelial barrier and promoted ENaC-mediated alveolar fluid clearance (18). Conditioned medium from MSCs had a similar effect. However, MSCs treated with siRNA to interfere with KGF production blunted this effect, consistent with a protective effect of KGF. The results of LaFemina et al. (17) suggest that KGF may also promote alveolar barrier function; whether this occurs in the intact, stressed lung remains to be determined. It will also be important to define the therapeutic window of opportunity for KGF to be effective, since clinical efficacy ideally requires patients to be treated hours to days after the initial onset of acute lung injury.
MSCs produce several other cytokines in addition to KGF (8, 21). In fact, a recent study identified a key role for angiopoietin-1 secreted by MSCs in protection of alveolar epithelial barrier function from the effects of proinflammatory hormones by stabilizing claudin-18 incorporation into tight junctions (11). This raises the possibility that KGF may require other cofactors for optimal activity. If this is the case, one can envision combination therapy using tailored hormone mixtures. However, the potential for MSCs to home to sites of active injury may provide a more effective approach to targeted, localized paracrine tissue repair (8, 21). Moreover, strategies based on engineering MSCs to have increased KGF secretion represent another alternative to systemic hormone treatment. Aguilar et al. (2) recently showed that MSCs transfected with a tetracycline-inducible KGF construct partially protected mice from bleomycin-induced pulmonary fibrosis; transfected hematopoietic stem cells had significantly higher protective potential. Given recent progress using natural MSCs, it is tempting to speculate that MSCs engineered for inducible KGF expression might prove useful in treatment of acute lung injury.
GRANTS
This work was supported by Emory Alcohol and Lung Biology Center (NIH Grants P50-AA-013757 and HL-083120).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Abraham V, Chou ML, DeBolt KM, Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 276: L825–L834, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Aguilar S, Scotton CJ, McNulty K, Nye E, Stamp G, Laurent G, Bonnet D, Janes SM. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS One 4: e8013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol 295: F867–F876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boardman KC, Aryal AM, Miller WM, Waters CM. Actin re-distribution in response to hydrogen peroxide in airway epithelial cells. J Cell Physiol 199: 57–66, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Chen SP, Zhou B, Willis BC, Sandoval AJ, Liebler JM, Kim KJ, Ann DK, Crandall ED, Borok Z. Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J Appl Physiol 98: 322–328, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Cohen TS, Gray Lawrence G, Margulies SS. Cultured alveolar epithelial cells from septic rats mimic in vivo septic lung. PLoS One 5: e11322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cribbs SK, Matthay MA, Martin GS. Stem cells in sepsis and acute lung injury. Crit Care Med. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298: L715–L731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai LP, Sinclair SE, Chapman KE, Hassid A, Waters CM. High tidal volume mechanical ventilation with hyperoxia alters alveolar type II cell adhesion. Am J Physiol Lung Cell Mol Physiol 293: L769–L778, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem 285: 26211–26222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 288: L179–L189, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez RF, Allen L, Dobbs LG. Rat alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol 297: L1045–L1055, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isakson BE, Lubman RL, Seedorf GJ, Boitano S. Modulation of pulmonary alveolar type II cell phenotype and communication by extracellular matrix and KGF. Am J Physiol Cell Physiol 281: C1291–C1299, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol 177: 512–524, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koval M, Ward C, Findley MK, Roser-Page S, Helms MN, Roman J. Extracellular matrix influences alveolar epithelial claudin expression and barrier function. Am J Respir Cell Mol Biol 42: 172–180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaFemina MJ, Rokkam D, Chandrasena A, Pan J, Bajaj A, Johnson M, Frank JA. Keratinocyte growth factor enhances barrier function without altering claudin expression in primary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol (September 10, 2010). doi:10.1152/ajplung.00233.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 106: 16357–16362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason RJ, Lewis MC, Edeen KE, McCormick-Shannon K, Nielsen LD, Shannon JM. Maintenance of surfactant protein A and D secretion by rat alveolar type II cells in vitro. Am J Physiol Lung Cell Mol Physiol 282: L249–L258, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Qiao R, Yan W, Clavijo C, Mehrian-Shai R, Zhong Q, Kim KJ, Ann D, Crandall ED, Borok Z. Effects of KGF on alveolar epithelial cell transdifferentiation are mediated by JNK signaling. Am J Respir Cell Mol Biol 38: 239–246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sueblinvong V, Weiss DJ. Cell therapy approaches for lung diseases: current status. Curr Opin Pharmacol 9: 268–273, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell 20: 3930–3940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol 29: 62–70, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 282: L924–L940, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Waters CM, Savla U, Panos RJ. KGF prevents hydrogen peroxide-induced increases in airway epithelial cell permeability. Am J Physiol Lung Cell Mol Physiol 272: L681–L689, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin 4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol 297: L219–L227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci USA 107: 8237–8241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]